1. Introduction
Today, three-dimensional (3D) printing technologies hold great promise in preparing customized (“tailor-made”) medicines and personalized drug delivery systems (DDS) to improve the patient experience and clinical outcomes [
1,
2,
3]. It is well known that conventional manufacturing and a “one-size-fits-all” concept are not suitable for all patient groups and for all medical conditions or diseases [
4,
5]. Pharmaceutical 3D-printing could enable the production of small batches of products on demand, which could improve the efficiency and flexibility of hospitals and pharmacies [
6]. Therefore, pharmaceutical 3D-printing technologies are expected to make a revolutionary contribution to the new approach to personalized medicine. In personalized drug therapy and treatment, each patient is given a medicine that meets their needs in a suitable dosage, composition, and dosage form (
Figure 1).
Oral pharmaceutical solid dosage forms (i.e. tablets, capsules, and granules) are widely used due to their simplicity and low invasiveness in both human and veterinary drug treatments. Traditionally, oral pharmaceutical dosage forms are manufactured batchwise on an industrial scale using various well-known technologies and a relatively large number of excipients. The manufacturing process of solid dosage forms, such as tablets, can be very complex, involving series of sequential steps (granulating, drying, mixing, tableting, coating), while 3D-printing enables different steps to be merged for certain active pharmaceutical ingredient (API) and printing ink combinations [
7]. Within the last 10-15 years, a number of promising 3D-printing technologies have been introduced for pharmaceutical applications. A revolution in and the widespread use of 3D-printing technology are expected [
8].
The automated semi-solid extrusion (SSE) material deposition method is a new, modified form of 3D-printing technology for pharmaceutical applications. Automated SSE material deposition, like traditional 3D-printing methods, enables significant flexibility in designing and preparing oral solid dosage forms, making it a suitable technology for preparing personalized dosage forms for pharmaceutical applications [
9]. Automated SSE material deposition is the method of choice for pharmaceutical polymer-based printing and dose dispensing due to its simplicity, precision, efficiency, low operating temperature, and suitability for preparing even high drug-loaded preparations [
10,
11]. The advantages of automated SSE material deposition include accessibility in the preparation of customized medications and standardized workflows [
11]. Recently, automated SSE material deposition was successfully used in the preparation of customized immediate-release clopidogrel tablets intended for pediatric applications [
12]. The technology also provides an opportunity to design and generate oral chewable dosage forms. Chewable tablets are administered without water, thus increasing patient compliance. Moreover, chewable tablets are not constrained by size, as they are designed to be chewed before they are swallowed [
13].
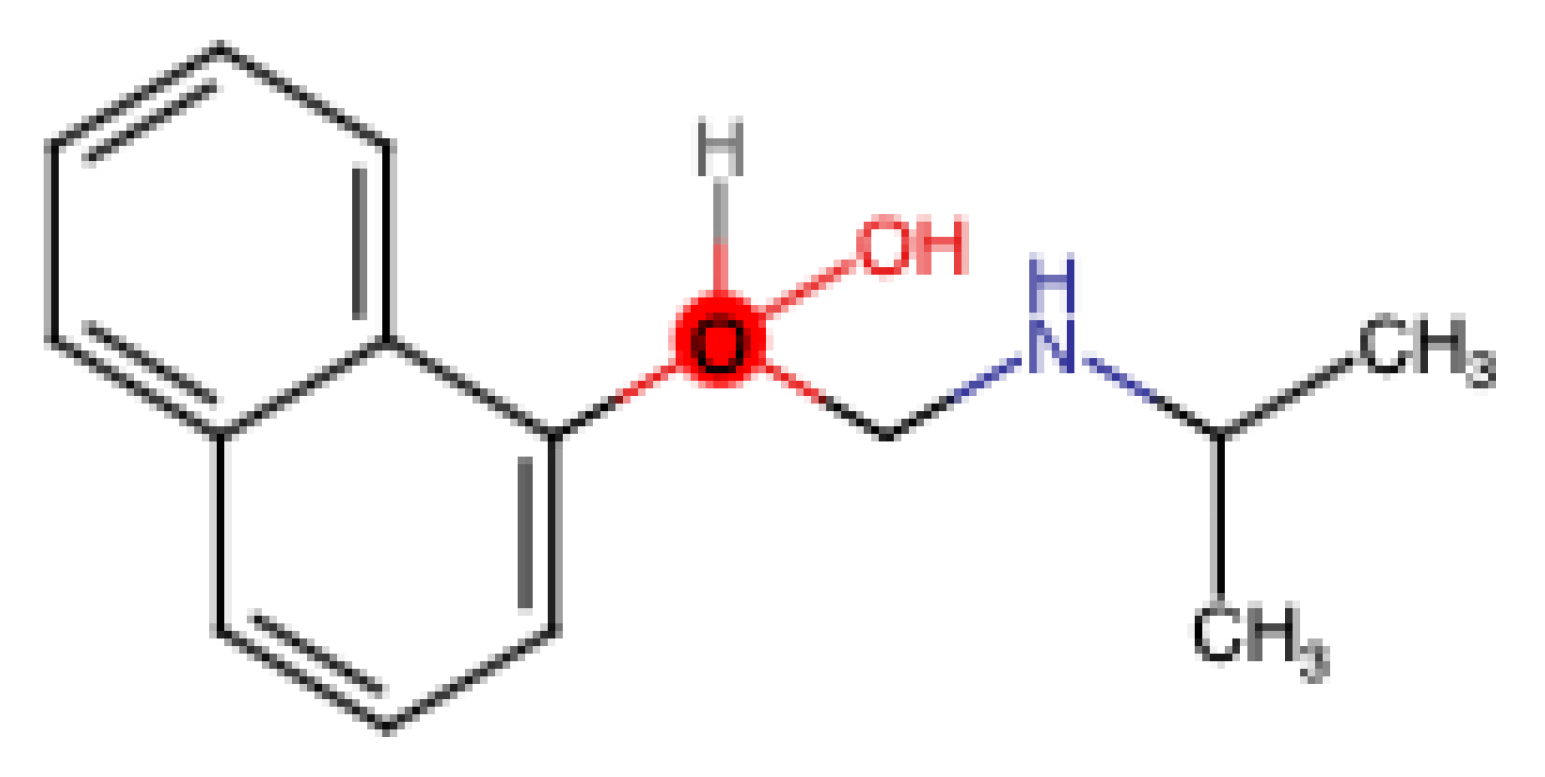
Propranolol (
Figure 2) is a non-selective beta blocker used in the treatment of common diseases such as high blood pressure and heart arrhythmia, and less often for the treatment of performance anxiety and essential tremors. Since children are not just“small adults”, personalized drug therapy and treatment are needed with pediatric patients. For example, the initial daily dose of propranolol for infants depends on the treatment, ranging from 1 mg/kg to 1.5 mg/kg [
14]. Therefore, individualized dosing of propranolol for special patient groups is of the utmost importance. The pediatric need for immediate-release low-dose propranolol formulations makes this API an ideal candidate for use as an example to be formulated in orodispersible DDSs [
15].
To date, only a few research works have been published on the pharmaceutical 3D-printing of propranolol drug preparations. Zhu and colleagues [
16] developed 3D-printed gummy chewable propranolol tablets for pediatric administration using a printing ink containing gelatin and carrageenan in extrusion-based printing. The binary semi-solid printing mixtures of gelatin and carrageenan were found to improve the thermal stability, printability, administration, and drug release properties of the printed gummy tablets. Jovanovic et al. [
17] used SSE 3D-printing to prepare gelatin-based mucoadhesive buccal films loaded with propranolol HCl for pediatric and geriatric administration. The inclusion of synthetic polymers such as povidone and polyvinyl alcohol (PVA) in the gelatin-based printing ink enhanced the printing performance, mechanical and mucoadhesive properties, and prolonged drug release behavior of the buccal films. Such 3D-printed oral DDS developed for propranolol hydrochloride successfully bypassed the first-pass metabolism and provided the prolonged effects of API with reduced dose variability. More recently, Alqahtani et al. [
18] introduced a gastric floating tablet with prolonged gastric floating time and a sustained drug release profile printed using a 3D-printing technique based on fused deposition modelling. In summary, the abovementioned 3D-printed drug preparations (chewable tablets, semi-solid buccal films, and a gastro-retentive floating system) enabled drug release to be controlled and therapeutic effects to be extended, thus improving oral bioavailability and patient compliance. Such personalized drug carriers offer effective solutions to pharmaceutical challenges such as the poor bioavailability of API and inadequate patient adherence, and could therefore significantly extend possibilities for individualized treatments.
The aim of this study was to develop automated SSE material deposition technology for preparing novel customized chewable gel tablets intended for pediatric applications, and to investigate the printing performance and final properties of such tablets. The chewable gel tablets loaded with propranolol hydrochloride (-HCl) at three different content levels (3.0 mg, 4.0 mg, and 5.0 mg) were generated using a commercial Cura-Blend™ polymeric printing gel. The effects of API on the viscosity of the printing gel and the printing performance of the gel tablets were investigated. The physical appearance, dimensions, mass and mass variation, solid-state properties, mechanical properties, and in-vitro drug release of the gel tablets were studied.
2. Materials and Methods
2.1. Materials
CuraBlend™ (CurifyLabs Oy, Finland) was used as a polymeric printing gel within the automated SSE material deposition method. CuraBlendTM consists of gelatin, cocoa butter, and purified water, as well as small amounts of additives supporting the SSE 3D-printing. The pre-mixture of CuraBlendTM was prepared in CurifyLabs Oy (batch B10623, prepared on May 2, 2023), and it was used within one week for SSE 3D-printing.
Propranolol HCl (Thermo Scientific, Japan) powder (purity 99%) was used as the API in the chewable gel tablets. Hydrochloric acid (≥ 37%, Sigma-Aldrich, Germany) and purified water (Millipore Milli-Ro 12 Plus, Merck KGaA, Germany) were used for preparing the dissolution medium. All reagents used in the HPLC analysis were of European Pharmacopoeia (Ph.Eur.) quality and ordered from Sigma-Aldrich (Germany).
2.2. Methods
2.2.1. Viscosity Measurements of Semi-Solid Printing Mixtures
The viscosity of the semi-solid mixtures (printing gels) was studied at 45 ± 5 °C with a Brookfield Ametek DVNext viscosimeter (Brookfield Engineering, USA). The measurements were performed in triplicate with the reference CuraBlend™ semi-solid printing mixture (without API) and CuraBlend™ semi-solid printing mixture loaded with propranolol HCL (1% w/w). The measurements were performed using a constant rotation speed of 0.5 rpm, a shear rate of 1.000, and torque of 18-18.9%.
2.2.2. Automated Semi-Solid Extrusion (SSE) Material Deposition
The automated MiniLab SSE material deposition system (Natural Machines, Spain) was used for preparing the chewable gel tablets. Several printing cycles were performed. The automated SSE material deposition process took place at 42 °C and at ambient relative humidity (RH 32%).
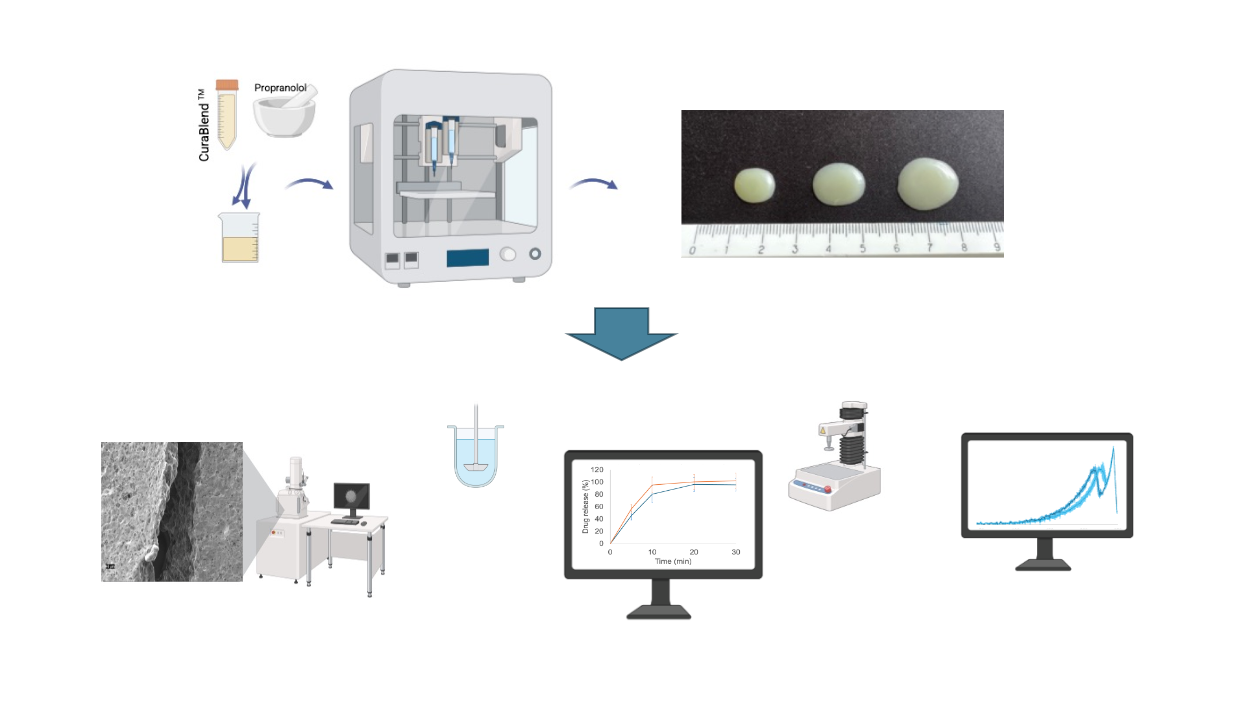
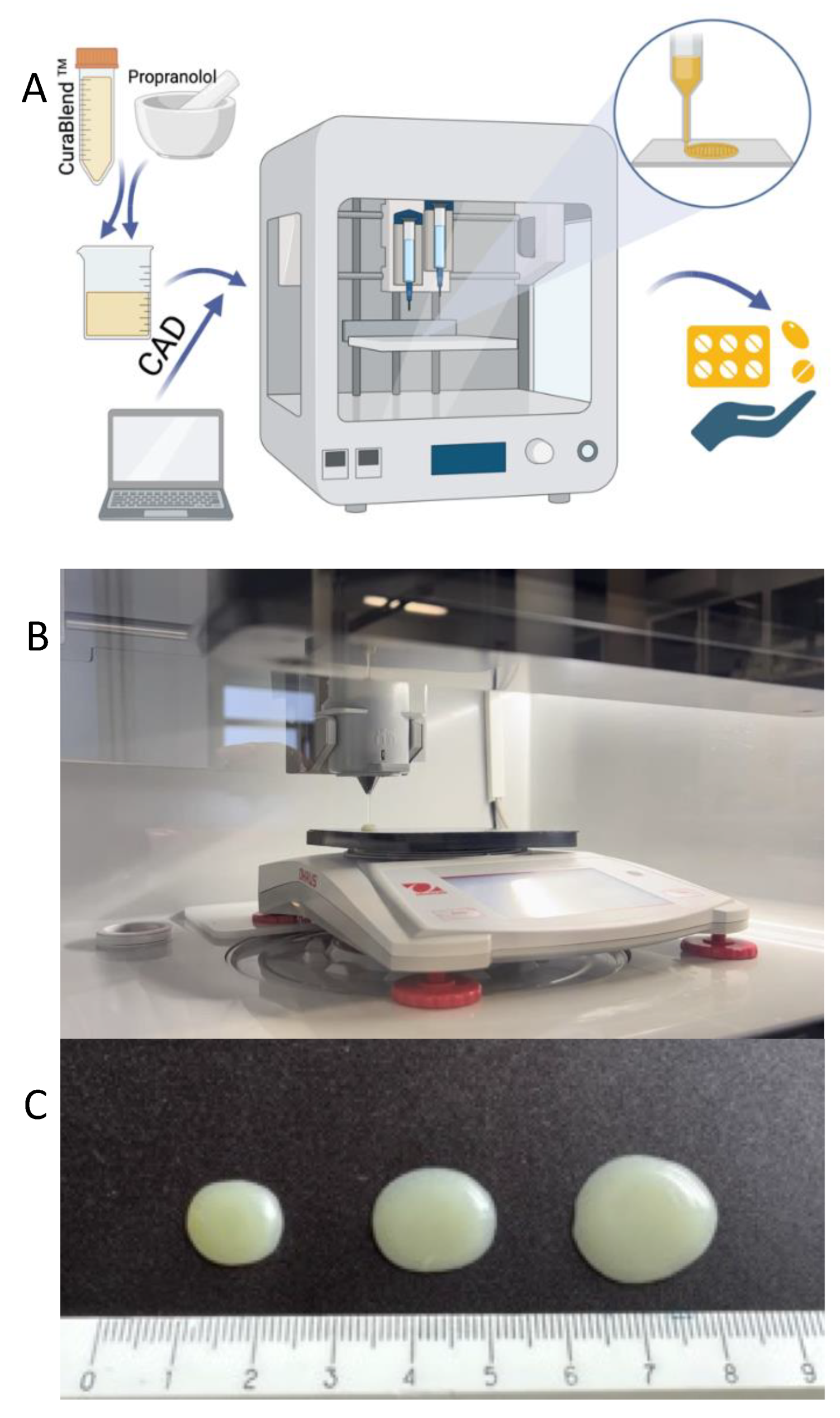
A schematic illustration of the automated SSE material deposition process is given in
Figure 3A. For preparing the reference gel tablets (without API), the printing mixture was first heated to a temperature of 42-45 °C in a water bath. When the mixture reached
the predetermined temperature and a semi-solid state with a uniform flow, it was transferred to the printing syringe. The syringe was closed and placed in the preheated syringe
slot of the MiniLab 3D SSE material deposition system. The syringe was kept in the system for 15 minutes to ensure the uniform temperature of the printing mixture throughout the
syringe. The number of gel tablets and the amount of API (determined based on the weight of the tablets) were entered as printing parameters into the printer management program (Printer Web App, CurifyLabs Oy, Finland). At the beginning of every SSE material deposition process, checks were made to ensure that the printing mixture exited the syringe freely and that there was no blockage or other obstruction at the opening of the syringe.
Figure 3B shows the automated SSE material deposition system in operation, while
Figure 3C shows the representative gel tablets loaded with 1% (w/w) propranolol HCl.
For preparing the API-loaded gel tablets, the printing mixture was heated to a temperature of 42-45 °C in a water bath. The required amount of propranolol HCl was weighed, taking into account that the target concentration of API in the gel tablets is 1% (w/w), and the content of API was adjusted based on the tablet weight. When the semi-solid printing mixture reached the predetermined temperature (42-45 °C), the required amount of API was weighed and added to the mixture, and finally mixed until the mixture was homogeneous. The mixture was then transferred to a printing syringe, and the process continued as described previously with the reference gel tablets.
A total of eight to nine reference gel tablets (without an API load) were printed for the control preparations of the contents of each gel tablet (3.0 mg, 4.0 mg, and 5.0 mg), with the target weight of these reference gel tablets being 300.0 mg, 400.0 mg, and 500.0 mg, respectively. A total of 75 gel tablets with API content of 3.0 mg (n = 25), 4.0 mg (n = 25) or 5.0 mg (n = 25) were prepared, and the target weight of these drug-loaded gel tablets was 300.0 mg, 400.0 mg, and 500.0 mg, respectively.
Figure 3C shows the representative gel tablets for each gel tablet size. For a short-term stability study, we printed an additional twelve (12) gel tablets with an API load of 3.0 mg and 5.0 mg.
After printing, the gel tablets were placed in a refrigerator (+2-8 °C) to solidify for 30 minutes. The physical appearance, mass and mass variation, and dimensions (length, weight, and height) of the gel tablets were studied after the solidification period. After the measurements, the gel tablets were carefully packed in a blister pack and stored in the refrigerator (+2-8 °C) for future studies.
2.2.3. Physical Appearance, Mass, and Dimensions of Gel Tablets
The physical appearance (i.e. layout, shape, and potential printing defects) of the gel tablets was visually inspected. The average weight and weight variation of the gel tablets were determined using an analytical balance Denver Instrument APX-200 (Cole-Parmer Instrument Company LLC, USA), and the dimensions (length, width, height) were measured with a digital micrometer Ironside 150 mm (Ironside International, France). Measuring the height of the gel tablets was somewhat challenging, since the freshly printed gel tablets were soft and delicate. Therefore, the measurement of height needed to be conducted with care, i.e. without accidentally compressing the surface of the gel tablet.
2.2.4. Scanning Electron Microscopy
The surface structure and morphology of the gel tablets were investigated using a high-resolution scanning electron microscope (SEM; Zeiss EVO® 15 MA, Germany). The samples were mounted on aluminum stubs with a conductive carbon film, and were magnetron-sputter coated with a 3-nm platinum layer in an argon atmosphere before microscopy.
2.2.5. Fourier Transform-Infrared Spectroscopy
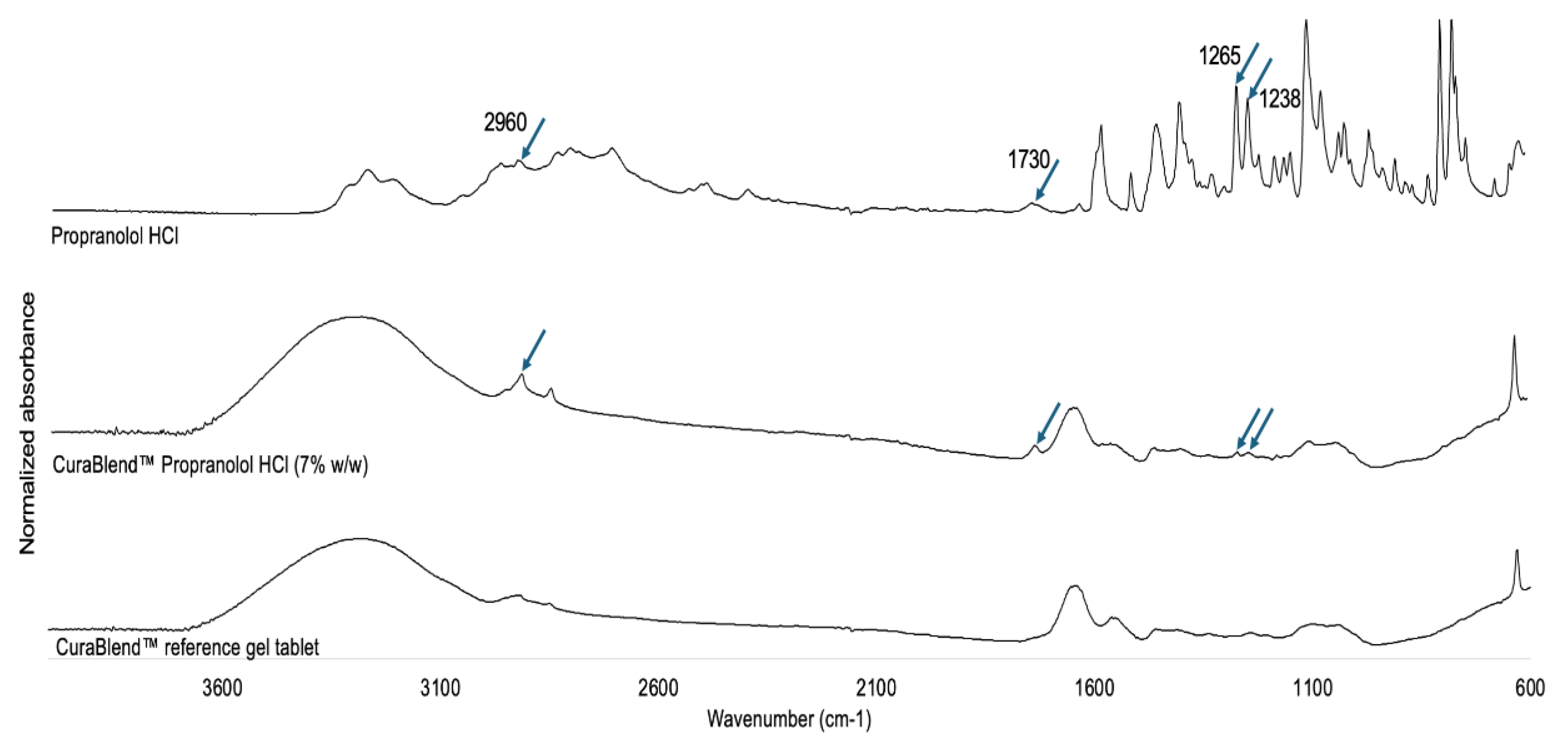
The physical solid-state properties of the gel tablets were investigated with Fourier transform-infrared (FTIR) spectroscopy using a Shimadzu IRPrestige-21 spectrometer (Shimadzu Europa GmbH, Germany) with Specac Golden Gate ATR (Specac Ltd, UK). The FTIR spectra of pure propranolol HCl powder, the CuraBlend™ printing mixture, and the CuraBlend™ printing mixture loaded with API (7% w/w) were used as the references for the FTIR spectra of the printed gel tablets. The CuraBlend™ loaded with 7% API (instead of a 1% API load) was selected for FTIR spectroscopy studies because of the sensitivity of the method. The samples were scanned over a range of 4000–600 cm−1 at a resolution of 4 cm−1 for 20 scans.
2.2.6. X-Ray Powder Diffraction
The X-ray powder diffraction (XRPD) patterns of the raw materials and 3D-printed gel tablets were obtained using an X-ray diffractometer (D8 Advance, Bruker AXS GmbH, Germany). The XRPD experiments were carried out in symmetrical reflection mode (Bragg-Brentano geometry) with CuKα radiation (1.54 Å). The angular range was from 5° 2-theta to 35° 2-theta with steps of 0.2° 2-theta. The scattered intensities were measured with a LynxEye 1-dimensional detector with 165 channels. The operating voltage and current were 40 kV and 40 mA, respectively.
2.2.7. Mechanical Tests
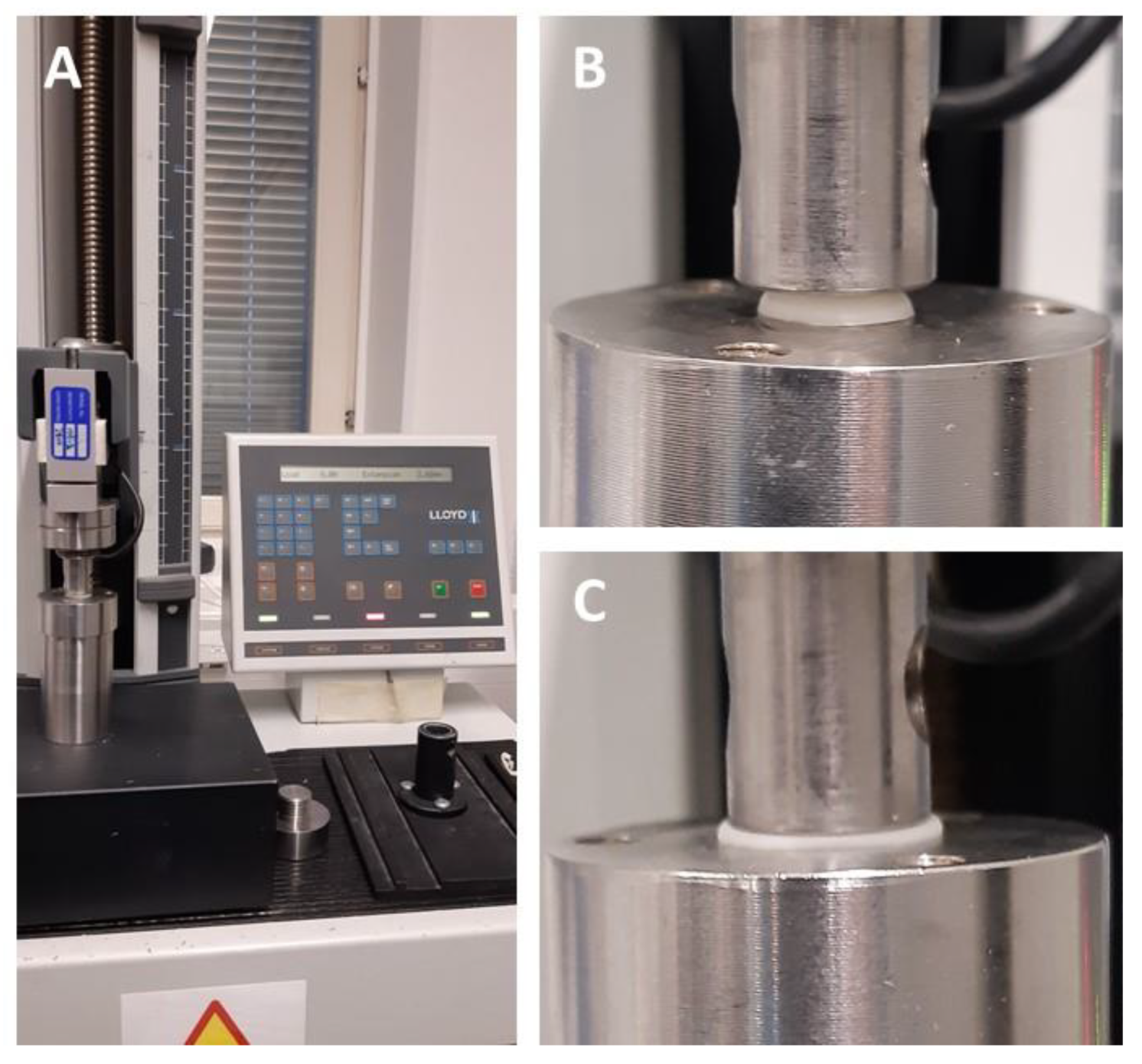
The mechanical properties of the CuraBlend™ gel tablets were studied with a Lloyd LRX material testing device (Lloyd LRX, Lloyd Ltd., UK) equipped with Nexygen software for measuring and data analysis (
Figure 4A).
Figure 4B and 4C are representative photographs of the starting point and end-phase of the mechanical test performed for the CuraBlend™ gel tablets, respectively. The constant speed of the crosshead in the tests was 1.000 mm/min. The gauge length used in the measurements was 5.00 mm, and the area was 3.00 × 4.00 mm. The test was automatically terminated at a load of 100 N. The number of parallel gel tablets used for the measurements was six (6). All mechanical tests were conducted at ambient room temperature and in conditions of relative humidity (22 ± 2 °C).
2.2.8. Dissolution Test In Vitro
The
in-vitro dissolution of the gel tablets was investigated using a Sotax AT 7 Smart dissolution test apparatus (Sotax AG, Switzerland) equipped with an Ismatec IPC 8 ISM 931 peristaltic pump (Cole-Parmer Instrument Company LLC, USA) and a Specord 200 Plus spectrophotometer (Analytik Jena GmbH, Germany). A comparison of the dissolution test method described in the United States Pharmacopoiea (USP 35) for Propranolol Hydrochloride Tablets and our modified in-house dissolution test method is shown in
Table 1. The dissolution medium was 900 ml of 0.063 M hydrochloric acid, HCl (36.7 ± 0.2 °C), and the rotating rate of both paddles and baskets was 100 rpm. A wavelength of 298 nm was used in the UV-Vis detector. The parallel number of samples used in each dissolution test was 4-6. In the dissolution tests of the gel tablets, samples were taken at regular intervals (5 min, 10 min, 20 min, 30 min). In the additional dissolution test of the gel tablets with a drug load of 5.0 mg, the samples were taken at 1 min, 2 min, 4 min, 8 min, 16 min and 32 min analogously to that described previously. For sampling, 1.0 ml of solution was pipetted into Eppendorf tubes, and the tubes were subsequently centrifuged at 15000 g for 5 minutes. Then, 0.8 ml of the solution was pipetted into glass vials, and the vials were placed in a refrigerator (+2-8 °C).
Figure 5 is a schematic illustration of the dissolution test protocol used in this study. Since there is no official or specific dissolution testing protocol for 3D-printed or automated SSE material deposited gel tablets, we used both the paddle and basket methods in our in-house dissolution test (modified from the USP 35 dissolution test method).
2.2.9. High-Performance Liquid Chromatography
The drug content and in-vitro drug release of the gel tablets were determined by means of high-performance liquid chromatography (HPLC). The HPLC system consisted of a Shimadzu HPLC Prominence modular system (Shimadzu Europa GmbH, Germany), which was composed of two LC-20AD high-pressure pumps, a Nexera X2 SIL-30AC autosampler, a SPD-M20A detector, and a CTO-20AC column oven. A SphereClone (TM) 250 x 4.6 mm column (Phenomenex, USA) was used.
A modified Ph. Eur. HPLC method was used for the quantitative analysis of propranolol HCl (Ph.Eur. 11.2, 2023). The stationary phase sorbent was octadecylsilyl silica gel. The mobile phase eluent consisted of tetrabutylammonium dihydrogen phosphate R, sodium lauryl sulfate R, sulfuric acid, water for chromatography, and acetonitrile. The pH of the eluent was adjusted to 3.3 with dilute sodium hydroxide solution R. The flow rate was 1.0 ml/min (which was modified, since according to Ph. Eur. the flow rate should be 1.8 ml/min). The amount of injected sample was 20 µl and the analysis was performed at a temperature of 30 °C. An analytical wavelength of 292 nm was used on the spectrophotometric detector.
2.2.10. Statistical analysis
Microsoft Excel 2021 Pro was used for data processing, statistical calculations, graphs, and figures. The chemical structure of propranolol (
Figure 2) was created with Chemaxon Marvin. BioRender.com was used for generating
Figure 1 and
Figure 3A. CurifyLabs Minilabs control software was used to control the printer, and a WinTDS control program was used for the dissolution test.
3. Results
3.1. Viscosity of Semi-Solid Printing Mixtures
The viscosity of the semi-solid printing mixture loaded with propranolol HCl (1% w/w) was 2623 ± 860 cP (n = 3) at 42 °C, while the viscosity of the reference printing mixture (without API) was 3485 ± 199 cP (n = 3). The inclusion of API (1% w/w) in the printing mixture decreased viscosity and increased fluidity, thus enhancing the spreading of the mixture on the printed (material deposition) bed. Due to the higher fluidity, the printing mixture even tended to come out of the printing syringe, which resulted in changes in the mass and dimensions of the gel tablets. We found that if the printing mixture was kept in the water bath during preheating for too long a period, or if a slightly higher temperature was used, the viscosity (and/or density) of the printing mixture changed. This in turn changed the mass of the mixture, which was expected to flow out during automated SSE material deposition.
3.2. Physical Appearance of 3D-Printed Gel Tablets
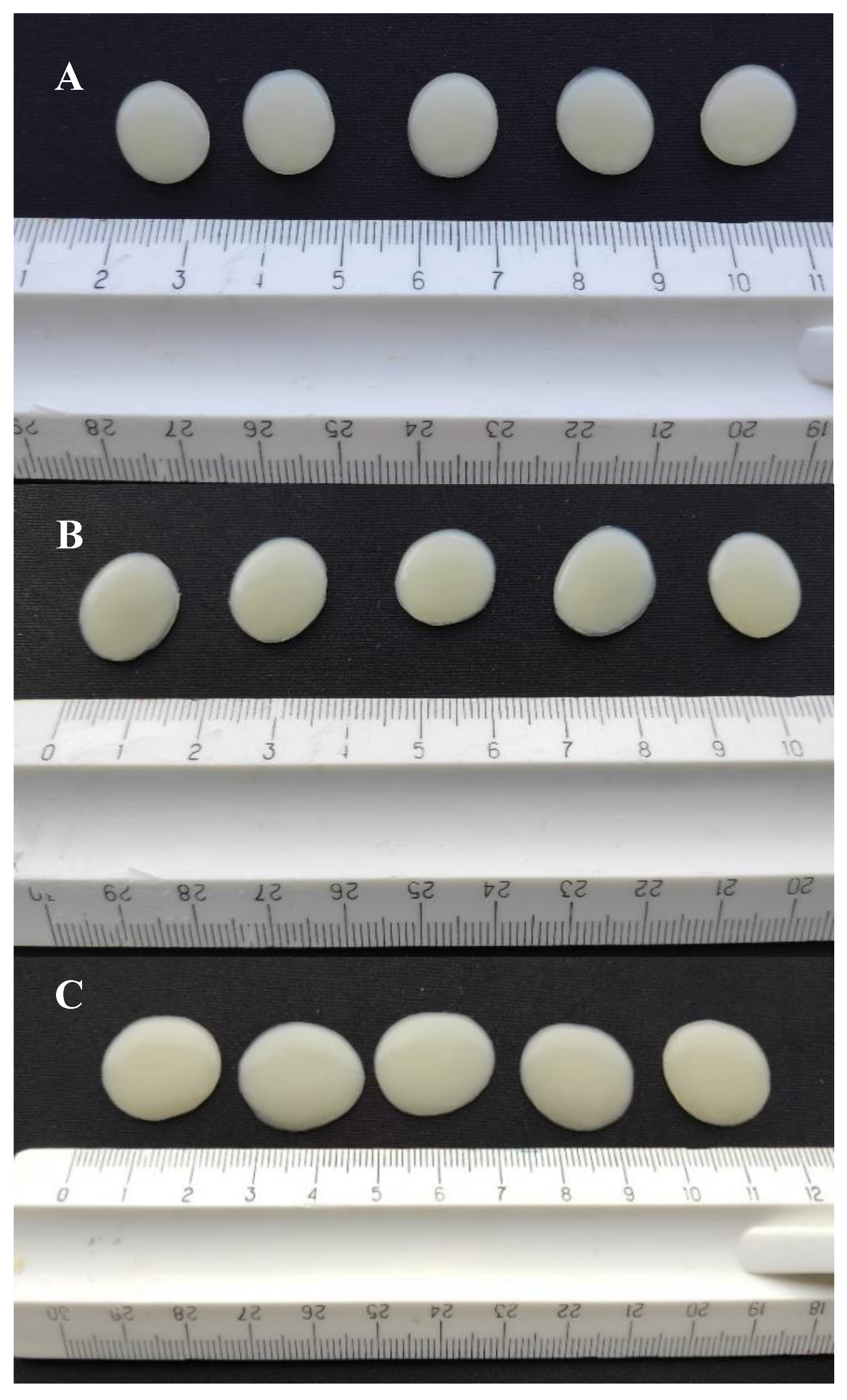
The gel tablets prepared using the automated SSE material deposition method were mostly round or slightly oval in shape (
Figure 6). The surface of the tablets was soft, elastic, and smooth. The printing mixture was evenly distributed, being slightly thinner at the edges and thicker in the middle. As expected, the gel tablets loaded with 3.0 mg of API (with a target tablet weight of 300.0 mg) presented the smallest diameter (size), while the gel tablets loaded with 5.0 mg of API (500.0 mg) presented the largest diameter and size (
Figure 6 and
Table 2).
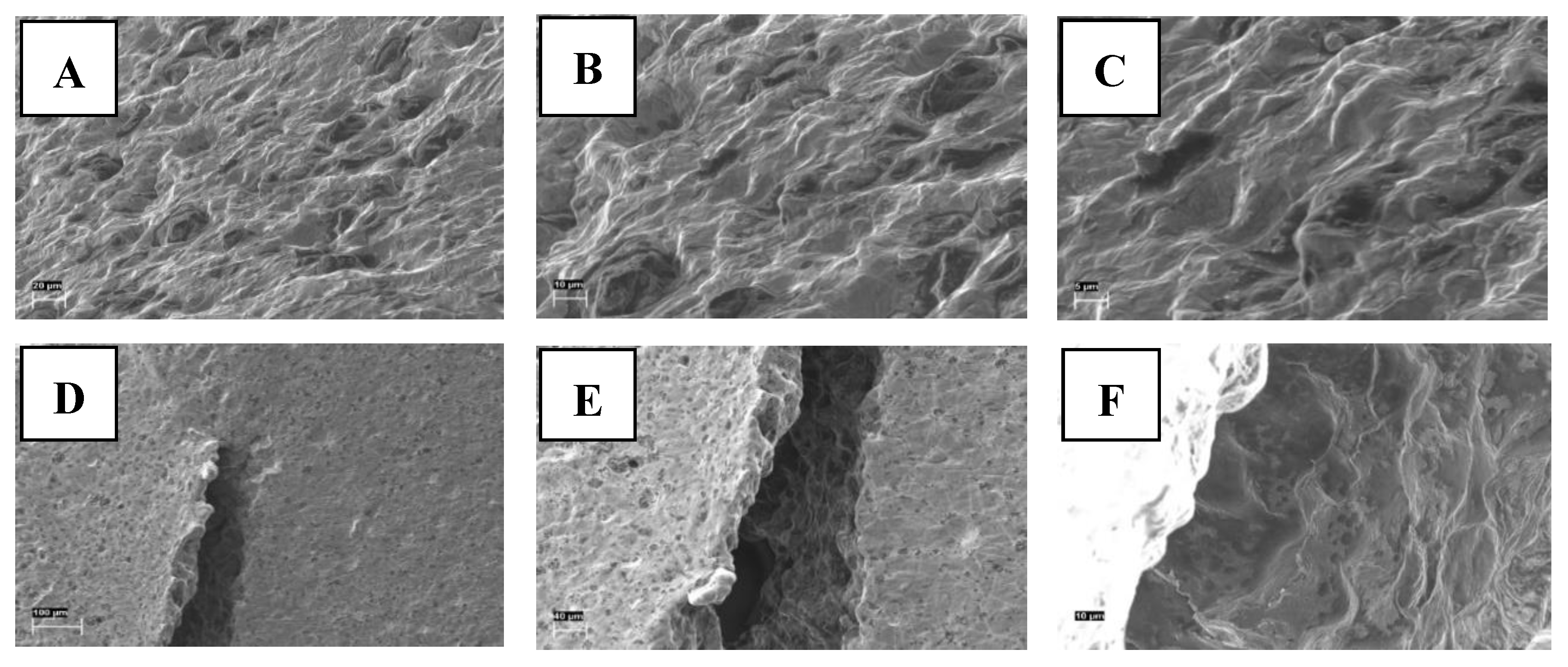
The SEM images taken from the cross-section and upper surface of the gel tablets loaded with 1% (w/w) API are shown in
Figure 7. The tablets presented a porous internal structure as seen in the SEM images on the cross-section of the API-loaded gel tablet (
Figure 7A-C). The surface of the gel tablet was relatively smooth, with tiny pores on it. However, we observed small cracks with a diameter of 100-200 μm on the surface of the tablets as surface defects (
Figure 7D-F), thus suggesting the presence of internal stress and strain in these tablets.
Figure 7E also reveals that the crack wall on the surface (i.e. the internal structure of the gel tablets) has a porous structure.
3.3. Weight and Weight Variation of Gel Tablets
The MiniLab control program of our automated SSE material deposition system was programmed to generate the gel tablets with an exact specified mass. The program was set for printing the gel tablets with a 1% (w/w) API content and for printing the desired number of the tablets. We used three different contents of API (3.0 mg, 4.0 mg, and 5.0 mg) corresponding to the (gel tablet) target masses of 300.0 mg, 400.0 mg, and 500.0 mg, respectively. The amount of mixture coming out from the printing syringe was regulated by pressure applied to the piston (non-changeable by printer user) and the range of movement of the piston, which was programmed based on the volume and expected viscosity of the mixture to be printed out.
The weights of the gel tablets (n = 25) were recorded on an analytical balance 30 minutes after printing. This was followed by measuring the diameter and height of the tablets (
Table 2). The weight and diameter of the gel tablets prepared from the API-loaded (1% w/w) CuraBlend™ semi-solid mixtures were larger than those of the reference gel tablets printed from the CuraBlend™ mixture without API (propranolol HCl). The gel tablets were oval to round in shape, and the diameter of the tablets loaded with 3.0 mg, 4.0 mg or 5.0 mg of API was 0.5 cm, 1.5 cm, and 2.0 cm, respectively (
Figure 6 and
Table 2). Interestingly, the gel tablets loaded with API exhibited a significantly larger diameter and smaller height compared to the reference gel tablets without API (
Table 2). This is obviously due to the lower viscosity of the API-loaded printing mixtures (as described in sub-chapter 3.1), thus enhancing the spreading of the mixture on the printed bed.
The gel tablets were not blistered to MediCup immediately after printing. Since the humidity in the laboratory was relatively low, it is evident that the gel tablets may have dried (i.e. lost their moisture) and solidified after printing, causing their weight to decrease. In this study, we did not investigate the effects of storage in blister packaging on the weight of the tablets.
3.4. Physicochemical Changes in the API Loaded in the Gel Tablets
Since automated extrusion-based material deposition is a novel method for additive manufacturing, we were also interested in revealing potential process-induced transformations (PITs) in the formulations of propranolol hydrochloride used in this technology.
Figure 8 shows the FTIR spectra for propranolol hydrochloride in powder form, the gel tablet loaded with 7% (w/w) API, and the reference gel tablet without API. As seen in
Figure 8, the FTIR spectra of the reference gel tablet and the gel tablet loaded with 7% (w/w) API are quite similar. However, the FTIR spectrum of the gel tablet loaded with API (propranolol HCl) has four distinguishable peaks that coincide with the corresponding spectrum of propranolol hydrochloride powder at 2960 cm
-1, 1730 cm
-1, 1265 cm
-1, and 1238 cm
-1 which are not present in the FTIR spectrum of the reference gel tablet. The characteristic peaks within 2960-2715 cm
-1 were caused by a secondary amine group, while the individual peak at approximately 1250 cm
-1 was caused by aryl alkyl ether [
19]. The results suggest the absence of PIT related to the use of propranolol HCl in this gel tablet formulation. It is evident that the low intensity of the two characteristic peaks for API (in the FTIR spectrum of the gel tablet) was due to the low concentration of API in these formulations intended for pediatric drug therapy.
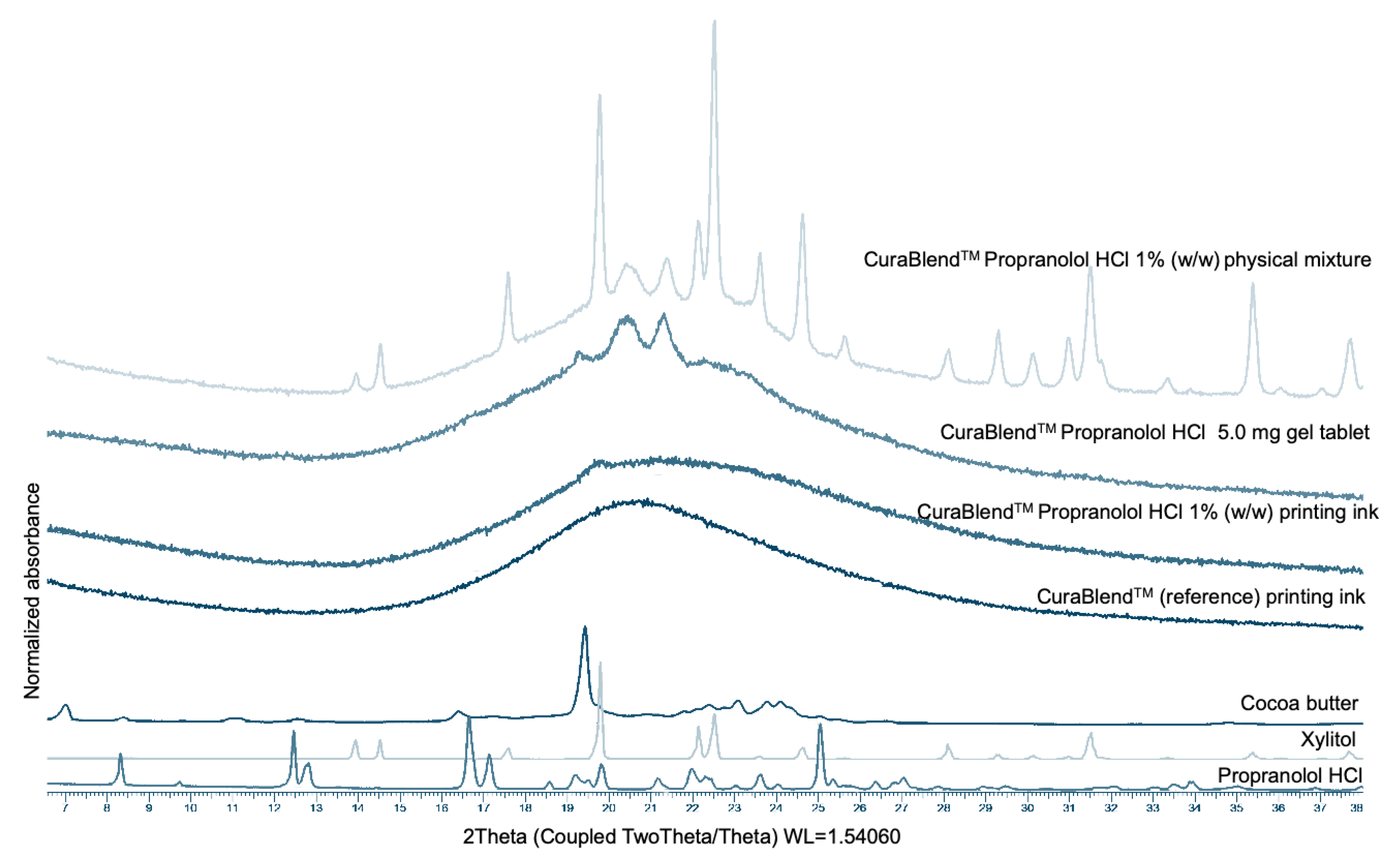
Figure 9 presents the XRPD patterns of the pure materials (propranolol HCl and key excipients), the CuraBlend™ printing ink (with and without API), the physical mixture of CuraBlend™ and propranolol HCl (1% w/w), and the printed propranolol (5.0 mg) gel tablets. The spectrum of propranolol HCl shows numerous sharp peaks due to its crystalline state, as has been seen in previous studies [
20]. The XRPD pattern for the physical mixture of CuraBlend™ and propranolol HCl 1% (m/m) showed the peaks characteristic of xylitol and cocoa butter, which are the key excipients in CuraBlend™ printing ink. The characteristic peaks for propranolol HCl, however, were not detectable in this diffractogram, obviously due to the low concentration of API. The XRPD pattern of the CuraBlend™ reference printing ink (without API) and CuraBlend™-API 1% (m/m) printing ink displayed an “amorphous halo” characteristic of amorphous materials like gelatin (which is the main component of CuraBlend™). The printed propranolol 5.0 mg gel tablets presented mainly an “amorphous halo” with some coincidental peaks with the XRPD pattern of the physical mixture of CuraBlend™ and propranolol HCl 1% (m/m) (
Figure 9).
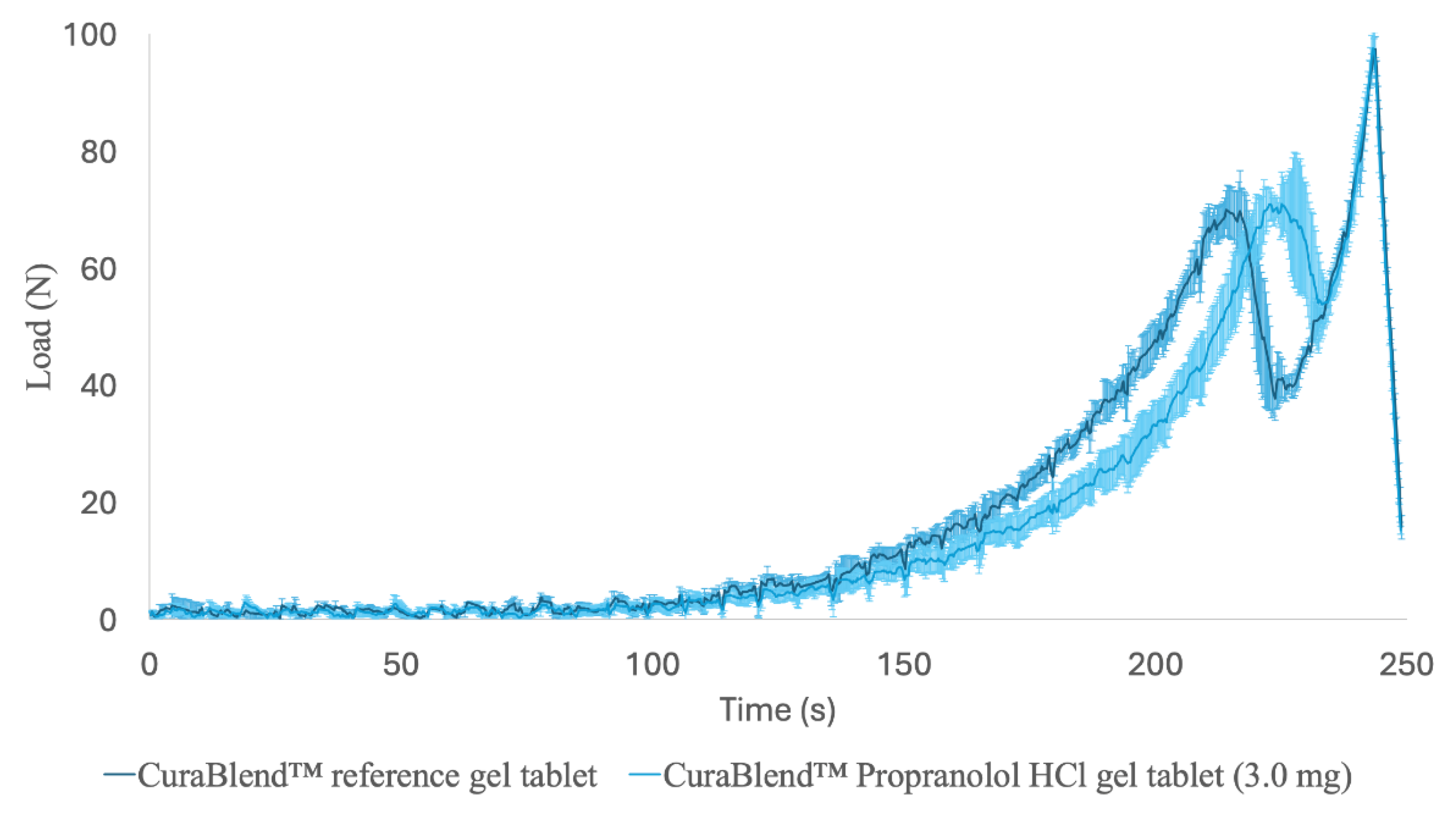
3.5. Mechanical Properties
The printed gel tablets were elastic, soft, and jelly-like chewable drug preparations. The characteristic compression force-time profiles for the CuraBlend™ reference gel tablets (without API) and the propranalol HCl-loaded (3.0 mg) CuraBlend™ gel tablets are shown in
Figure 10. These curves show the resistance of the gel tablet under a constantly increasing load (N) as a function of time (min). The maximum load needed for the final deformation (“breaking down”) of the CuraBlend™ reference gel tablets was very uniform, ranging from 65 N to 75 N. The maximum load needed for the final deformation of the propranolol HCl-loaded (3.0 mg) CuraBlend™ gel tablets was even more uniform and slightly higher, ranging from 73 N to 80 N. The time for the maximum load at break (Tmax) was different between the reference and API-loaded gel tablets (3.5 min
vs 3.7 min).
3.6. Dissolution In Vitro
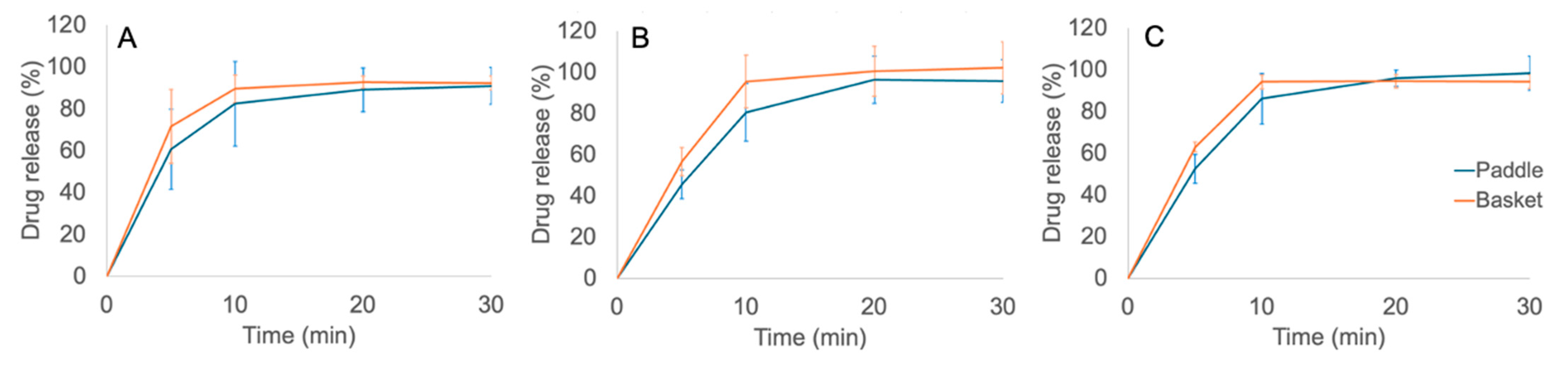
As seen in
Figure 11, the drug release of the printed gel tablets was completed within 10-15 minutes, thus confirming the immediate-release dissolution of these drug preparations. According to the United States Pharmacopoeia (USP 35), no less than 75% of the API load should be released within 30 minutes at a rotation speed of 100 rpm, in order to comply with the in-vitro dissolution requirements of oral immediate-release tablets. As seen in
Figure 11, the drug release was 100% within 30 minutes with these printed gel tablets. Therefore, the dissolution of the gel tablets prepared using the automated SSE material deposition method complied with the corresponding requirements of USP 35. As seen in
Figure 11, we did not find any significant differences in the dissolution profiles of the printed gel tablets between the basket and paddle methods. The
in-vitro dissolution of API (propranolol HCl) was slightly faster when the tablets were tested using the basket method compared to the paddle method. This is obviously due to the slight differences in the sampling point, mixing pattern and capacity between basket and paddle dissolution methods. In the basket method, the sample is taken closer to the printed gel tablet in the dissolution vessel than it is in the paddle method. Moreover, it is evident that rotating baskets do not stir the dissolution medium as intensively as rotating paddles. Consequently, the concentration of API is initially higher in the closed vicinity of the baskets.
4. Conclusions
Automated extrusion-based material deposition is a feasible method for preparing customized chewable gel tablets intended for pediatric drug therapy. The method enables elastic and small-sized gel tablets to be prepared based on a CuraBlendTM polymeric mixture and loaded with 1% (w/w) propranolol HCl. The viscosity of the semi-solid CuraBlendTM polymeric mixture is dependent on the concentration of propranolol HCl, which in turn affects the printing performance of the gel tablets. The inclusion of propranolol HCl in CuraBlendTM decreases the viscosity of the mixture, but this does not significantly impair the printing performance of the semisolid mixture. The small weight variation among the printed gel tablets (less than 5%) suggests the uniform and stable performance of the formulation using the automated extrusion-based material deposition method. The inclusion of API in the CuraBlendTM polymeric ink mixture slightly enhances the mechanical properties of the printed gel tablets. The dissolution test in vitro verifies that the printed gel tablets are immediate-release drug preparations. More research is needed to identify the key material and process parameters using the automated extrusion-based material deposition method and to reveal the effects of these parameters on the performance and stability of gel tablets.
Author Contributions
Conceptualization, K.R., U.P., I.L., and J.H.; methodology, A.M., S.A., I.L., H.R., and J.A.; validation, A.M., S.A., H.R., and J.A.; investigation, K.R., A.M., U.P., H.R., and J.A.; resources, J.H., L.P., and N.S.T.; writing—original draft preparation, K.R., J.H., and U.P.; writing—review and editing, J.H., U.P., I.L., L.P., S.A., and N.S.T.; visualization, K.R. and U.P.; supervision, U.P., I.L., and J.H.; project administration, J.H. and N.S.T.; funding acquisition, J.H. and N.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded as part of the CurifyLabs research project (a project with the University of Tartu, VMVFA22189).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
MSc (Pharm) Karin Lombiots is kindly acknowledged for her skillful laboratory work.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered potential competing interests. This study was conducted within the framework of a joint research project between the University of Tartu (Institute of Pharmacy) and CurifyLabs Oy, Finland. The project was funded by CurifyLabs Oy, Finland. The authors (S.A. and N.S.T.) with affiliations to CurifyLabs Oy receive their salaries from the company, and they were involved in the design of the study, in the collection, analysis, and interpretation of data, and in the writing of the manuscript. S.A. reports a relationship with CurifyLabs Oy which includes employment. N.S.T. reports a relationship with CurifyLabs Oy which includes board membership, employment, and equity or stocks. All of the other authors declare that they have no known competing financial interests or personal relationships which may have appeared to influence the work reported in this paper.
References
- Seoane-Viaño, I.; Trenfield, S. J.; Basit, A. W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Advanced Drug Delivery Reviews 2021, 174, 553-575. [CrossRef]
- Vaz, V. M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M. J.; Sánchez-Pintos, P.; Giraldez-Montero, J. M.; Januskaite, P.; Duran-Piñeiro, G.; Bóveda, M. D.; Alvarez-Lorenzo, C.; Basit, A. W.; Goyanes, A.; Couce, M. L. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. International Journal of Pharmaceutics 2024, 657, 124140. [CrossRef]
- Ilyes, K. The future of pharmaceutics: 3D-printing?. Romanian Journal of Pharmaceutical Practice 2020, 13, 138-192. [CrossRef]
- Jain, K.; Shukla, R.; Yadav, A.; Ujjwal, R. R.; Singh Flora, S. J. 3D Printing in Development of Nanomedicines. Nanomaterials 2021, 11, 420. [CrossRef]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [CrossRef]
- Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. [CrossRef]
- Mathiyalagan, R.; Sjöholm, E.; Manandhar, S.; Lakio, S.; Rosenholm, J. M.; Kaasalainen, M.; Wang X.; Sandler, N. Personalizing oral delivery of nanoformed piroxicam by semi-solid extrusion 3D printing. European Journal of Pharmaceutical Sciences 2023, 188, 106497. [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Rajan Prakash, D.; Lindfors, L.; Wang, Q.; Wang, X.; Ojala, S.; Sandler, N. 3D-Printed Veterinary Dosage Forms—A Comparative Study of Three Semi-Solid Extrusion 3D Printers. Pharmaceutics 2020, 12, 1239. [CrossRef]
- Sandler Topelius, N.; Shokraneh, F.; Bahman, M.; Lahtinen, J.; Hassinen, N.; Airaksinen, S.; Verma, S.; Hrizanovska, L.; Lass, J.; Paaver, U.; et al. Automated Non-Sterile Pharmacy Compounding: A Multi-Site Study in European Hospital and Community Pharmacies with Pediatric Immediate Release Propranolol Hydrochloride Tablets. Pharmaceutics 2024, 16, 678. [CrossRef]
- Shokraneh, F.; Filppula, A. M.; Tornio, A.; Aruväli, J.; Paaver, U.; Sandler Topelius, N. Automated extrusion-based dispensing: Personalized dosing and quality control of clopidogrel tablets for pediatric care. European Journal of Pharmaceutical Sciences 2025, 204, 106967. [CrossRef]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [CrossRef]
- Prasad, A.; Kumar Sinha, A.; Kumar, B.; Prasad, A.; Kumari, M. Individualized dosing of oral propranolol for treatment of infantile hemangioma: a prospective study. Pan African Medical Journal 2019, 32, 155.
- Vakili, H.; Nyman, J. O.; Genina, N.; Preis M.; Sandler, N. Application of a colorimetric technique in quality control for printed pediatric orodispersible drug delivery systems containing propranolol hydrochloride. International Journal of Pharmaceutics 2016, 511, 606-618. [CrossRef]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: an Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [CrossRef]
- Jovanović, M.; Petrović, M.; Cvijić, S.; Tomić, N.; Stojanović, D.; Ibrić, S.; Uskoković, P. 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics 2021, 13, 2143. [CrossRef]
- Alqahtani, A.A.; Mohammed, A.A.; Fatima, F.; Ahmed, M.M. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets. Polymers 2023, 15, 3554. [CrossRef]
- Suksaeree, J.; Saingam, W.; Monton, C.; Pichayakorn, W. FT-IR Study Between Propranolol Hydrochloride and Pharmaceutically Acceptable Excipients in Physical Mixture. Bulletin of Health Science and Technology 2015, 13, 17-24.
- Cirri, M.; Mura, P.; Benedetti, S.; Buratti, S. Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics 2023, 15, 2217. [CrossRef]
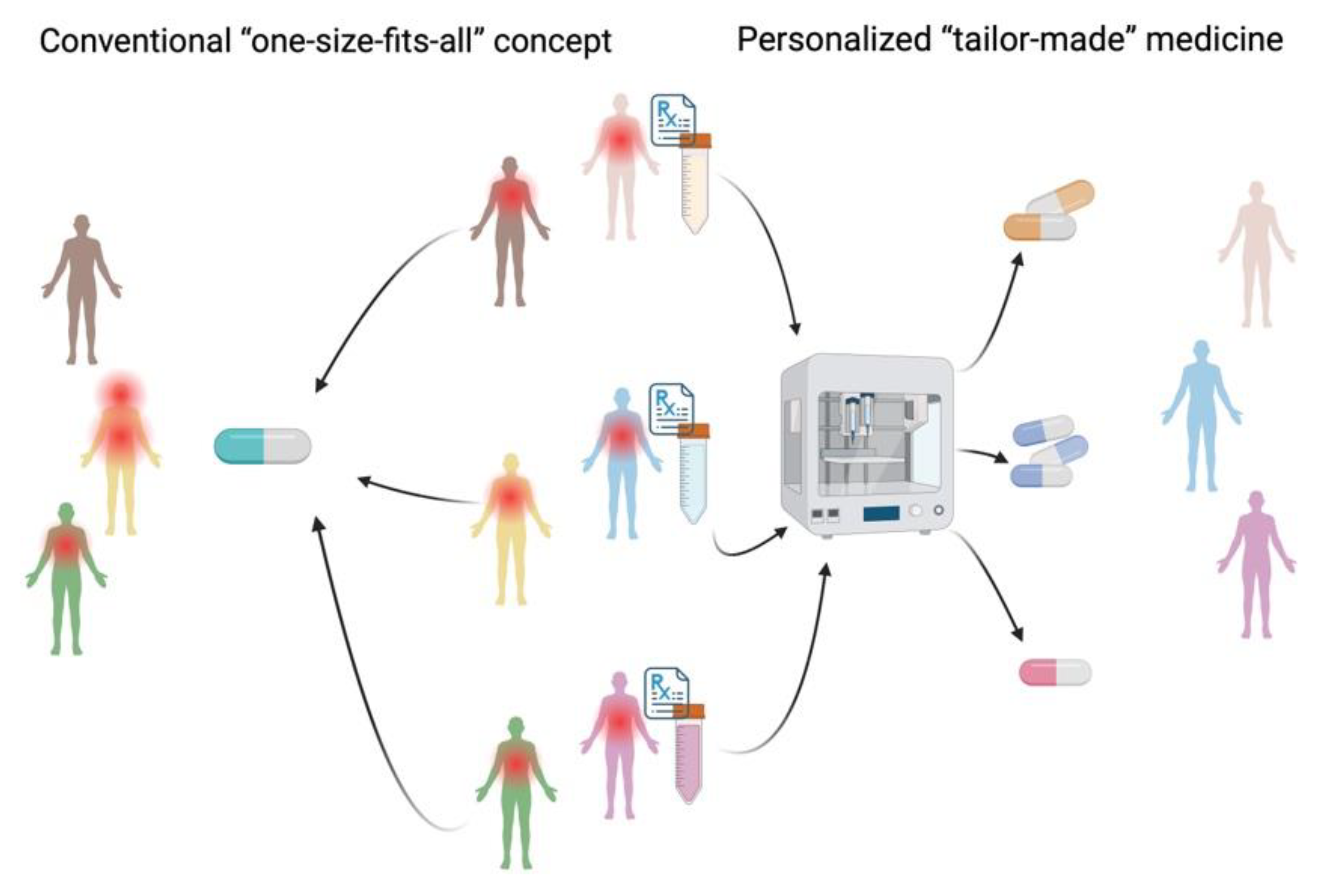
Figure 1.
Comparison of a conventional “one-size-fits-all” concept and personalized “tailor-made” concept using pharmaceutical 3D-printing. Created with BioRender.com.
Figure 1.
Comparison of a conventional “one-size-fits-all” concept and personalized “tailor-made” concept using pharmaceutical 3D-printing. Created with BioRender.com.
Figure 2.
Chemical structure of propranolol.
Figure 2.
Chemical structure of propranolol.
Figure 3.
(A) Schematic illustration of the automated semi-solid extrusion (SSE) material deposition process. Key: CAD = computer-aided design; (B) Photograph of the automated SSE material deposition system and pre-filled syringe based on the predetermined amount or weight of material; (C) A representative photograph of the gel tablets prepared using the automated SSE material deposition method. The gel tablets are loaded with 1% (w/w) propranolol HCl. From left to right, the target weight of the gel tablets is 300.0 mg, 400.0 mg, and 500.0 mg.
Figure 3.
(A) Schematic illustration of the automated semi-solid extrusion (SSE) material deposition process. Key: CAD = computer-aided design; (B) Photograph of the automated SSE material deposition system and pre-filled syringe based on the predetermined amount or weight of material; (C) A representative photograph of the gel tablets prepared using the automated SSE material deposition method. The gel tablets are loaded with 1% (w/w) propranolol HCl. From left to right, the target weight of the gel tablets is 300.0 mg, 400.0 mg, and 500.0 mg.
Figure 4.
(A) A Lloyd LRX material tester equipped with Nexygen software for force-time measurement and data analysis. The representative photographs in (B) and (C) depict the start and end-phase, respectively, of the mechanical test of CuraBlend™ gel tablets (n = 6) in a Lloyd LRX tester.
Figure 4.
(A) A Lloyd LRX material tester equipped with Nexygen software for force-time measurement and data analysis. The representative photographs in (B) and (C) depict the start and end-phase, respectively, of the mechanical test of CuraBlend™ gel tablets (n = 6) in a Lloyd LRX tester.
Figure 5.
Schematic illustration of the dissolution test protocol of the gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method. .
Figure 5.
Schematic illustration of the dissolution test protocol of the gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method. .
Figure 6.
The gel tablets (n = 5) prepared using the automated semi-solid extrusion (SSE) material deposition method. Key: (A) Reference gel tablets (without any API); (B) Gel tablets with 3.0 mg of propranolol HCl; (C) Gel tablets with 5.0 mg of propranolol HCl. .
Figure 6.
The gel tablets (n = 5) prepared using the automated semi-solid extrusion (SSE) material deposition method. Key: (A) Reference gel tablets (without any API); (B) Gel tablets with 3.0 mg of propranolol HCl; (C) Gel tablets with 5.0 mg of propranolol HCl. .
Figure 7.
Scanning electron microscopy (SEM) images of the cross-section (A-C) and surface (with a potential crack or defect) (D-F) of the gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method, and loaded with 1% (w/w) propranolol HCl. Key: Magnification scale bar 20 µm, 10 µm, 5 µm (A-C), and 100 µm, 40 µm, 10 µm (D-F), respectively.
Figure 7.
Scanning electron microscopy (SEM) images of the cross-section (A-C) and surface (with a potential crack or defect) (D-F) of the gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method, and loaded with 1% (w/w) propranolol HCl. Key: Magnification scale bar 20 µm, 10 µm, 5 µm (A-C), and 100 µm, 40 µm, 10 µm (D-F), respectively.
Figure 8.
Fourier transform-infrared (FTIR) spectra for (A) propranolol HCl in powder form, for (B) the gel tablet generated using the automated semi-solid extrusion (SSE) material deposition method and loaded with 7% (w/w) propranolol HCl, and for (C) the reference gel tablet without active substance (propranolol HCl).
Figure 8.
Fourier transform-infrared (FTIR) spectra for (A) propranolol HCl in powder form, for (B) the gel tablet generated using the automated semi-solid extrusion (SSE) material deposition method and loaded with 7% (w/w) propranolol HCl, and for (C) the reference gel tablet without active substance (propranolol HCl).
Figure 9.
X-ray powder diffraction (XRPD) patterns for the pure materials (propranolol HCl and key excipients), the CuraBlend™ printing ink (with and without API), the physical mixture of CuraBlend™ and propranolol HCl (1% m/m), and the printed propranolol HCl (5.0 mg) gel tablets.
Figure 9.
X-ray powder diffraction (XRPD) patterns for the pure materials (propranolol HCl and key excipients), the CuraBlend™ printing ink (with and without API), the physical mixture of CuraBlend™ and propranolol HCl (1% m/m), and the printed propranolol HCl (5.0 mg) gel tablets.
Figure 10.
The characteristic force-time profiles in a uniaxial tablet compression test for (A) the printed CuraBlend™ reference gel tablets (without API) (n = 6) and (B) the printed CuraBlend™-propranolol HCl (3.0 mg) gel tablets (n = 6). The mechanical strength values were measured with a Lloyd LRX material tester using a constant speed (1.000 mm/min) for the crosshead.
Figure 10.
The characteristic force-time profiles in a uniaxial tablet compression test for (A) the printed CuraBlend™ reference gel tablets (without API) (n = 6) and (B) the printed CuraBlend™-propranolol HCl (3.0 mg) gel tablets (n = 6). The mechanical strength values were measured with a Lloyd LRX material tester using a constant speed (1.000 mm/min) for the crosshead.
Figure 11.
In-vitro drug release curves for the printed CuraBlend™-propranolol HCl gel tablets prepared using the automated extrusion-based material deposition method (n = 6). The printed gel tablets contained (A) 3.0 mg, (B) 4.0 mg, and (C) 5.0 mg of propranolol HCl. The dissolution tests were performed using both the basket (orange curve) and paddle (blue curve) methods. .
Figure 11.
In-vitro drug release curves for the printed CuraBlend™-propranolol HCl gel tablets prepared using the automated extrusion-based material deposition method (n = 6). The printed gel tablets contained (A) 3.0 mg, (B) 4.0 mg, and (C) 5.0 mg of propranolol HCl. The dissolution tests were performed using both the basket (orange curve) and paddle (blue curve) methods. .
Table 1.
Comparison of the dissolution test method described in the United States Pharmacopoiea (USP 35) for Propranolol Hydrochloride Tablets and the modified in-house dissolution test method used for these gel tablets.
Table 1.
Comparison of the dissolution test method described in the United States Pharmacopoiea (USP 35) for Propranolol Hydrochloride Tablets and the modified in-house dissolution test method used for these gel tablets.
| Parameter |
USP 35 |
Modified (in-house) |
| Apparatus |
Basket |
Paddle and basket |
| Rotation speed |
100 rpm |
100 rpm |
| Dissolution medium |
0.063 M HCl, 900 ml |
0.063M HCl, 900 ml |
| Temperature |
36.7 ± 0.2 °C |
36.7 ± 0.2 °C |
| Time |
60 min |
45 min |
| Analytical wavelength |
298 nm |
298 nm |
Table 2.
Average weight, diameter, and height of gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method and loaded with propranolol HCl at a content of 3.0 mg, 4.0 mg, and 5.0 mg (n = 25).
Table 2.
Average weight, diameter, and height of gel tablets prepared using the automated semi-solid extrusion (SSE) material deposition method and loaded with propranolol HCl at a content of 3.0 mg, 4.0 mg, and 5.0 mg (n = 25).
| Response |
Gel tablet prepared using the automated SSE material deposition method |
| |
Tablet strength 3.0 mg (mean ± SD) |
Tablet strength 4.0 mg (mean ± SD) |
Tablet strength 5.0 mg (mean ± SD) |
| Ref. gel tablet (without API) |
CuraBlend™ gel tablet loaded with API (1%) |
Ref. |
CuraBlend™
gel tablet loaded with API (1%) |
Ref. |
CuraBlend™
gel tablet loaded with API (1%) |
| Weight (mg) |
280 ± 8.0 |
282 ± 8.3 |
375 ±10.6 |
378 ± 7.6 |
468 ±13.1 |
481 ± 20.8 |
| Diameter (mm) |
12.7 ± 0.2 |
14.8 ± 0.7 |
14.7 ± 0.3 |
17.1 ± 0.8 |
16.5 ± 0.3 |
20.2 ± 1.0 |
| Height (mm) |
2.7 ± 0.1 |
2.1 ± 0.2 |
2.7 ± 0.1 |
2.1 ± 0.1 |
2.7 ± 0.1 |
2.0 ± 0.1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).