Submitted:
02 April 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
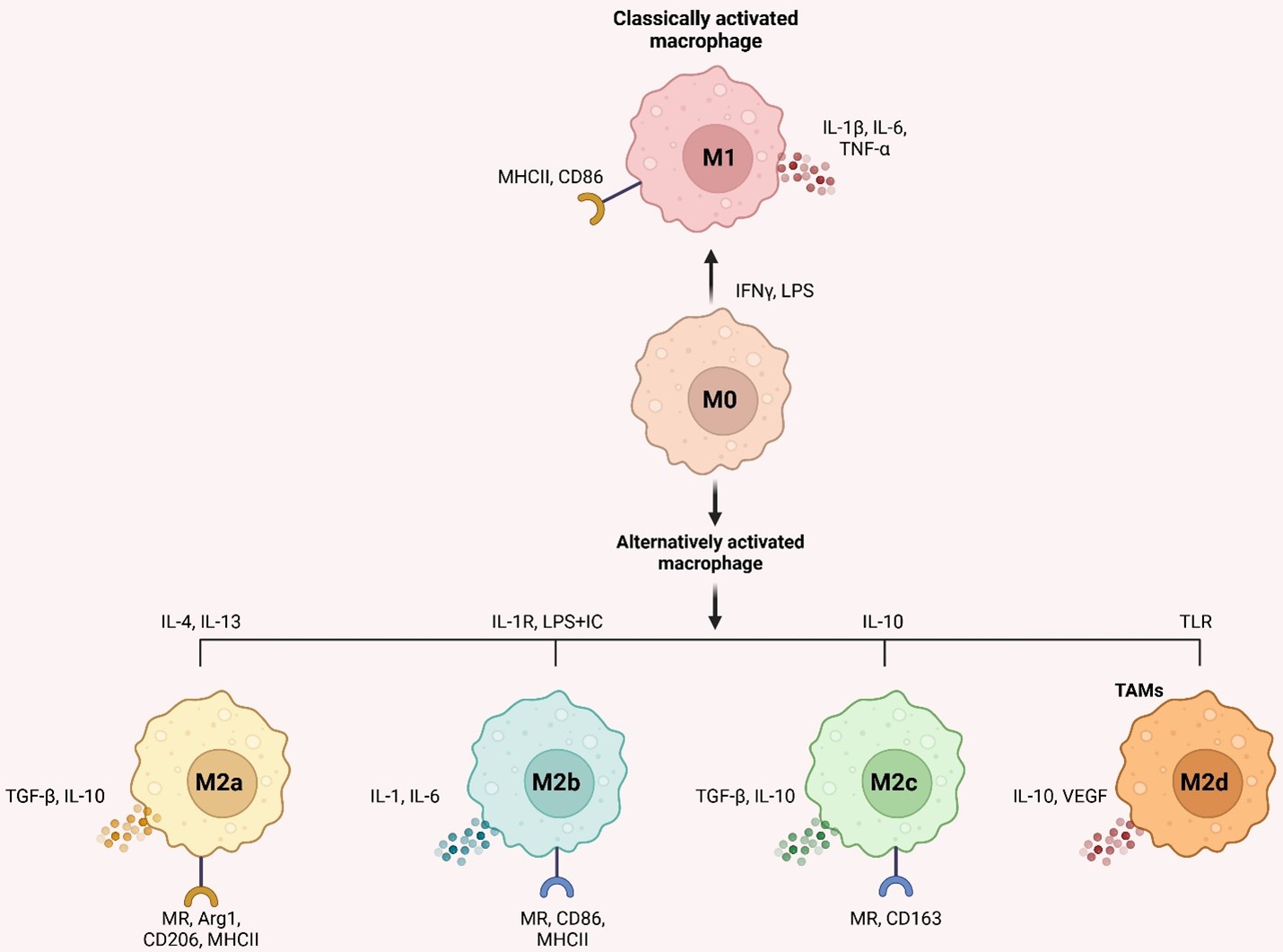
2. Macrophage Origin and Polarization
Macrophage Polarization and Differentiation in TME
3. Macrophages Inside the TME Accelerate Tumor Development
3.1. Implications of M1 Type TAM on Regression of Tumors
3.2. M2 Type TAM's Role in Driving Tumor Growth
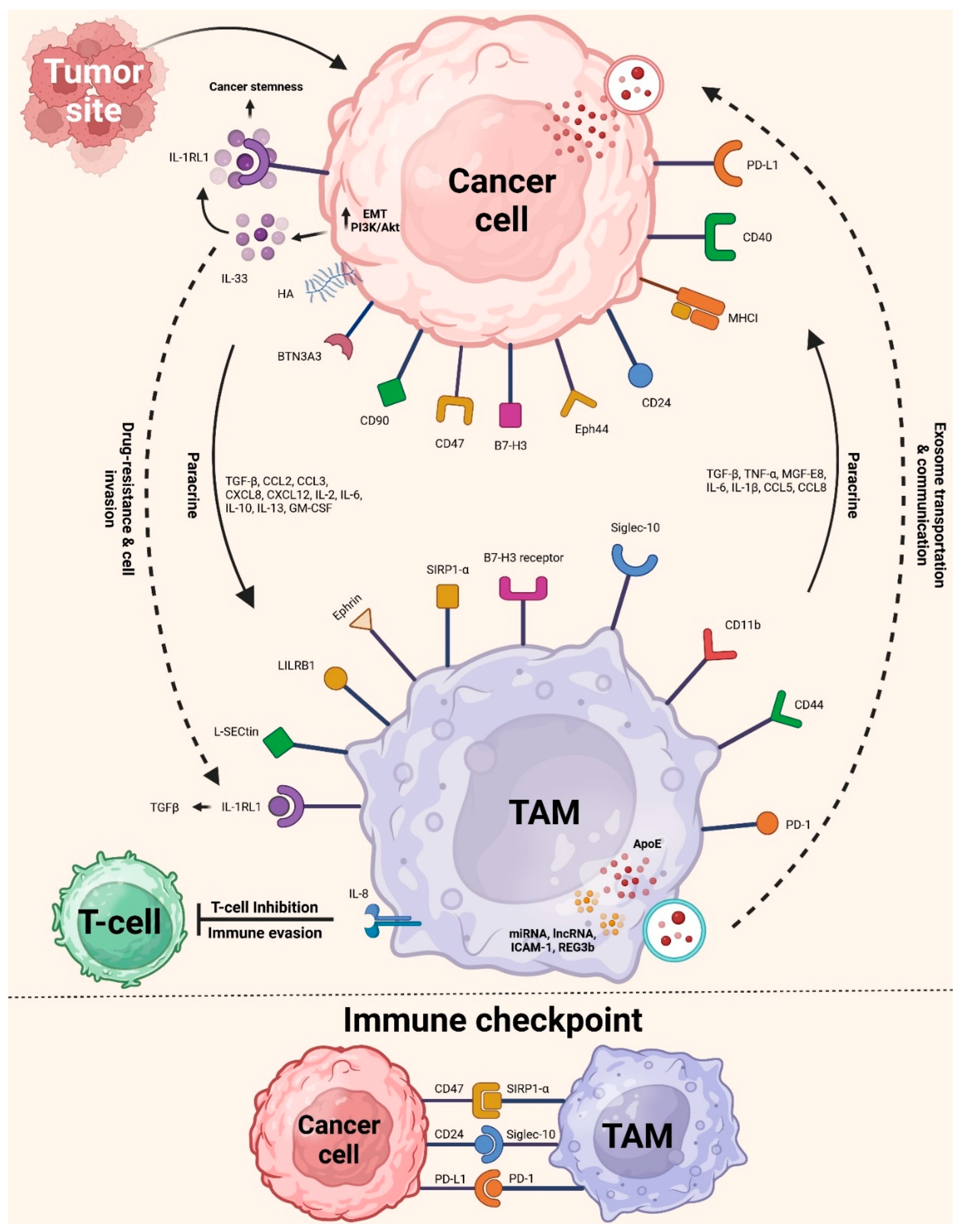
4. Crosstalk Between TAMs and Tumor Cells
5. Macrophage in Immunoregulation
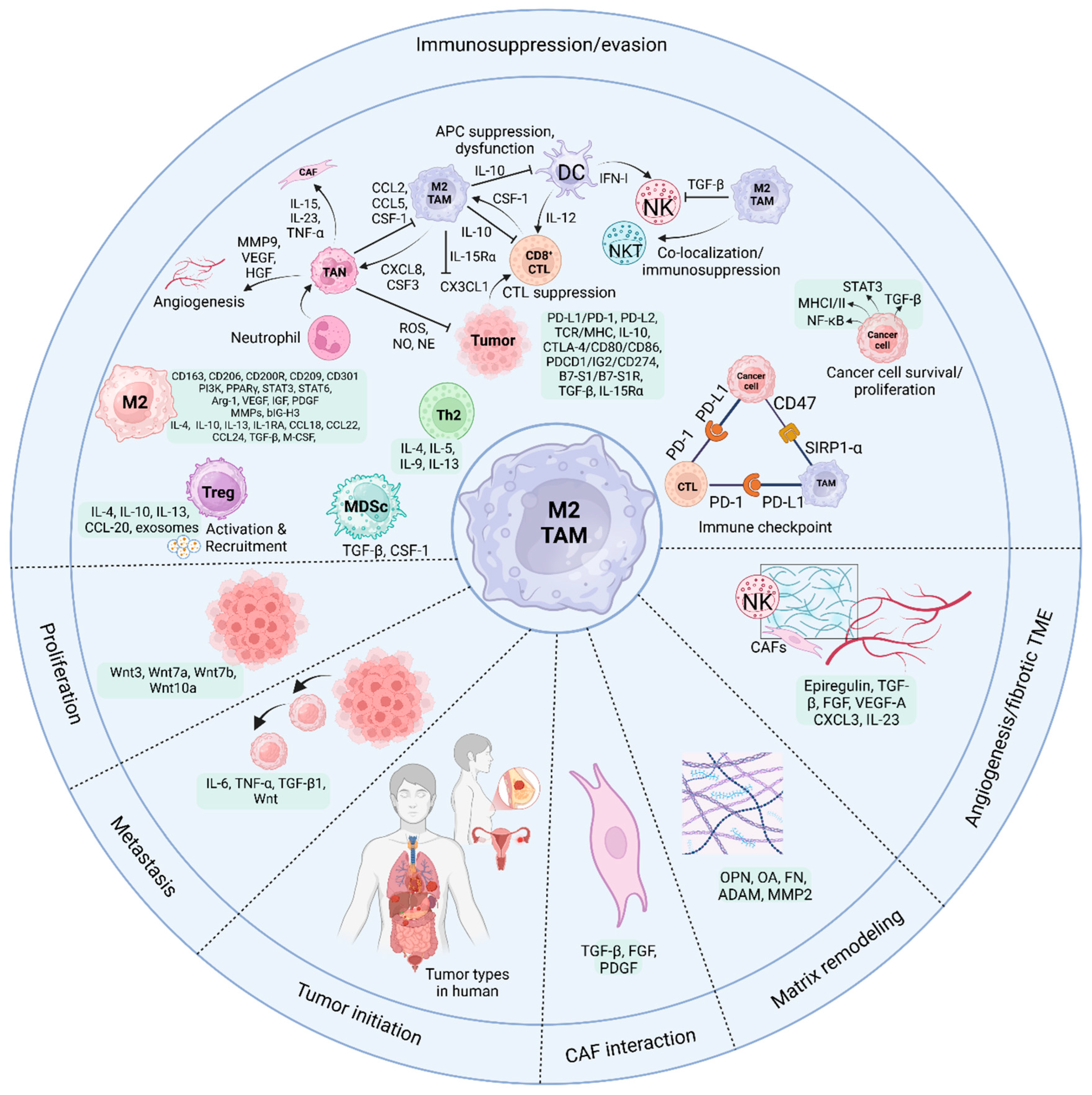
5.1. TAMs and CD8+ CTLs
5.2. TAMs and NK Cells
5.3. TAMs and NKT Cells
5.4. TAMs and CD4 T Cells
5.5. TAMs and DCs
5.6. TAMs and Neutrophils
5.7. TAMs and MDSCs
5.8. TAMs and γδ T Cells
6. Immunotherapy Employing Macrophages and Anti-PD-1/PD-L1
6.1. Effects of TAMs on PD-1/PD-L1 Expression
6.2. TAMs and Resistance to Anti-PD-1
6.3. Macrophage Immune Responses to Anti-PD-1/PD-L1 Therapy
7. Targeting TAM Immunotherapy
7.1. Reduced TAM Levels
7.2. TAM Reprogramming
7.3. Therapy Using Macrophages
7.4. Integrating Anti-PD-1 Treatment with Macrophage Targeting in Cancer
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L., et al., Cancer statistics, 2025. CA Cancer J Clin. 2025, 75, 10–45.
- Siegel, R.L., et al., Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48.
- Shi, D.; Gao, L.; Wan, X.-C.; Tian, T.; Hu, J.; Zhang, Q.-L.; Su, Y.-F.; Zeng, Y.-P.; Hu, Z.-J.; Yu, B.-H.; et al. Clinicopathologic features and abnormal signaling pathways in plasmablastic lymphoma: a multicenter study in China. BMC Med. 2022, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Liu, J.; Fan, Q.; et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy. Cell Death Discov. 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Guan, F., et al., Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct Target Ther. 2025, 10, 93.
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 1–32. [Google Scholar] [CrossRef]
- Feng, X., et al., IFN-τ Maintains Immune Tolerance by Promoting M2 Macrophage Polarization via Modulation of Bta-miR-30b-5p in Early Uterine Pregnancy in Dairy Cows. Cells 2025, 14.
- He, Y.; Hong, Q.; Chen, S.; Zhou, J.; Qiu, S. Reprogramming tumor-associated macrophages in gastric cancer: a pathway to enhanced immunotherapy. Front. Immunol. 2025, 16, 1558091. [Google Scholar] [CrossRef]
- Wu, Y.; Park, J.; Xu, E.; Kim, D.; Lee, J.; Oh, Y.-K. MicroRNA-induced reprogramming of tumor-associated macrophages for modulation of tumor immune microenvironment. J. Control. Release 2025, 381, 113593. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. New Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England). 2021, 398, 27–40.
- Li, J.-W.; Deng, C.; Zhou, X.-Y.; Deng, R. The biology and treatment of Epstein-Barr virus-positive diffuse large B cell lymphoma, NOS. Heliyon 2023, 10, e23921. [Google Scholar] [CrossRef]
- Goc, J.; Lv, M.; Bessman, N.J.; Flamar, A.-L.; Sahota, S.; Suzuki, H.; Teng, F.; Putzel, G.G.; Eberl, G.; Withers, D.R.; et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 2021, 184, 5015–5030.e16. [Google Scholar] [CrossRef]
- Scholler, N.; Perbost, R.; Locke, F.L.; Jain, M.D.; Turcan, S.; Danan, C.; Chang, E.C.; Neelapu, S.S.; Miklos, D.B.; Jacobson, C.A.; et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat. Med. 2022, 28, 1872–1882. [Google Scholar] [CrossRef]
- Hirz, T.; Mei, S.; Sarkar, H.; Kfoury, Y.; Wu, S.; Verhoeven, B.M.; Subtelny, A.O.; Zlatev, D.V.; Wszolek, M.W.; Salari, K.; et al. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat. Commun. 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef]
- Chan, J.M.; Quintanal-Villalonga, Á.; Gao, V.R.; Xie, Y.; Allaj, V.; Chaudhary, O.; Masilionis, I.; Egger, J.; Chow, A.; Walle, T.; et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell 2021, 39, 1479–1496.e18. [Google Scholar] [CrossRef]
- Park, M.; Kim, Y.S.; Song, H. Macrophages: a double-edged sword in female reproduction and disorders. Exp. Mol. Med. 2025, 57, 285–297. [Google Scholar] [CrossRef]
- Kloosterman, D.J.; Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 2023, 186, 1627–1651. [Google Scholar] [CrossRef]
- Lösslein, A.K.; Henneke, P. Macrophage Differentiation and Metabolic Adaptation in Mycobacterial Infections. Annu. Rev. Immunol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Verona, F.; Di Bella, S.; Schirano, R.; Manfredi, C.; Angeloro, F.; Bozzari, G.; Todaro, M.; Giannini, G.; Stassi, G.; Veschi, V. Cancer stem cells and tumor-associated macrophages as mates in tumor progression: mechanisms of crosstalk and advanced bioinformatic tools to dissect their phenotypes and interaction. Front. Immunol. 2025, 16, 1529847. [Google Scholar] [CrossRef] [PubMed]
- López-Collazo, E.; Hurtado-Navarro, L.; L, E. Cell fusion as a driver of metastasis: re-evaluating an old hypothesis in the age of cancer heterogeneity. Front. Immunol. 2025, 16, 1524781. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yi, Y.; Han, C.; Shi, B. NF-κB signaling pathway in tumor microenvironment. Front. Immunol. 2024, 15, 1476030. [Google Scholar] [CrossRef]
- Daniel, B., et al., The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res 2018, 46, 4425–4439. [CrossRef]
- Liu, H.; Amakye, W.K.; Ren, J. Codonopsis pilosula polysaccharide in synergy with dacarbazine inhibits mouse melanoma by repolarizing M2-like tumor-associated macrophages into M1-like tumor-associated macrophages. Biomed. Pharmacother. 2021, 142, 112016. [Google Scholar] [CrossRef]
- Vergadi, E., et al., Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol 2017, 198, 1006–1014. [CrossRef]
- Zhao, Y.; Yu, Z.; Ma, R.; Zhang, Y.; Zhao, L.; Yan, Y.; Lv, X.; Zhang, L.; Su, P.; Bi, J.; et al. lncRNA-Xist/miR-101-3p/KLF6/C/EBPα axis promotes TAM polarization to regulate cancer cell proliferation and migration. Mol. Ther. - Nucleic Acids 2021, 23, 536–551. [Google Scholar] [CrossRef]
- Singer, M.; Zhang, Z.; Dayyani, F.; Zhang, Z.; Yaghmai, V.; Choi, A.; Valerin, J.; Imagawa, D.; Abi-Jaoudeh, N. Modulation of Tumor-Associated Macrophages to Overcome Immune Suppression in the Hepatocellular Carcinoma Microenvironment. Cancers 2024, 17, 66. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Snijckers, R.P.M.; Foks, A.C. Adaptive immunity and atherosclerosis: aging at its crossroads. Front. Immunol. 2024, 15, 1350471. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, C.; Li, B. Effects of macrophages in OSCC progression. Front. Immunol. 2025, 15, 1517886. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Davidson, D.; Li, R.; Zhong, M.-C.; Qian, J.; Chen, J.; Veillette, A. Inflammatory macrophages exploit unconventional pro-phagocytic integrins for phagocytosis and anti-tumor immunity. Cell Rep. 2021, 37, 110111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, Q.; Han, M.; Ye, F.; Wang, M.; Qiu, Y.; Wang, J.; Gao, M.; Hou, F.; Wang, H. FBXO38 regulates macrophage polarization to control the development of cancer and colitis. Cell. Mol. Immunol. 2023, 20, 1367–1378. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, Z.; Guo, X.; Ma, R.; Wang, X.; Zhou, P.; Li, C.; Tang, Z.; Zhao, R.; Gao, P. CAP2 promotes gastric cancer metastasis by mediating the interaction between tumor cells and tumor-associated macrophages. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 1–35. [Google Scholar] [CrossRef]
- Park, M.D. , et al. , TREM2 macrophages drive NK cell paucity and dysfunction in lung cancer. Nat Immunol 2023, 24, 792–801. [Google Scholar]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Dalla, E.; Leader, A.M.; LeBerichel, J.; Nikolic, J.; Morales, B.M.; Brown, M.; Chang, C.; Troncoso, L.; Chen, S.T.; et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 2021, 595, 578–584. [Google Scholar] [CrossRef]
- Leader, A.M.; Grout, J.A.; Maier, B.B.; Nabet, B.Y.; Park, M.D.; Tabachnikova, A.; Chang, C.; Walker, L.; Lansky, A.; Le Berichel, J.; et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 2021, 39, 1594–1609.e12. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Gao, J., et al., PANoptosis: bridging apoptosis, pyroptosis, and necroptosis in cancer progression and treatment. Cancer Gene Ther 2024, 31, 970–983. [CrossRef]
- Toledo, B.; Chen, L.Z.; Paniagua-Sancho, M.; Marchal, J.A.; Perán, M.; Giovannetti, E. Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy. J. Hematol. Oncol. 2024, 17, 1–34. [Google Scholar] [CrossRef]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-associated macrophages: an effective player of the tumor microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef]
- Tomassetti, C.; Insinga, G.; Gimigliano, F.; Morrione, A.; Giordano, A.; Giurisato, E. Insights into CSF-1R Expression in the Tumor Microenvironment. Biomedicines 2024, 12, 2381. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, C.; Zeng, C.; Sun, C.; Xia, L.; Wang, G.; Chen, X.; Zhang, B.; Liu, J.; Ding, Z.-Y. Cancer stem cells and niches: challenges in immunotherapy resistance. Mol. Cancer 2025, 24, 1–22. [Google Scholar] [CrossRef]
- Yao, J.; Ji, L.; Wang, G.; Ding, J. Effect of neutrophils on tumor immunity and immunotherapy resistance with underlying mechanisms. Cancer Commun. 2024, 45, 15–42. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, J.; Ruan, S.; Zhang, B.; Zhu, H.; Que, Y.; Ying, S.; Li, X.; Hu, Y.; Song, Z. Overcoming immunotherapy resistance in gastric cancer: insights into mechanisms and emerging strategies. Cell Death Dis. 2025, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dong, S.; Huang, R.; Chen, X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers 2023, 15, 726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, L.; Wang, Y.; Zhang, J.; Wu, Q.; Zhao, P.; Shi, Y.; Wang, P.; Fang, L. Deciphering the role of CD47 in cancer immunotherapy. J. Adv. Res. 2023, 63, 129–158. [Google Scholar] [CrossRef]
- Brady, R.V.; Thamm, D.H. Tumor-associated macrophages: Prognostic and therapeutic targets for cancer in humans and dogs. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; George, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018, 19, 76–84. [Google Scholar] [CrossRef]
- Xu, H., et al., Immunoglobulin-like transcript 5 polarizes M2-like tumor-associated macrophages for immunosuppression in non-small cell lung cancer. Int J Cancer, 2025.
- Zhang, Y.; Wang, B.; Chen, J.; Li, T. Role of exosomal miRNAs and macrophage polarization in gastric cancer: A novel therapeutic strategy. Eur. J. Pharmacol. 2025, 990, 177268. [Google Scholar] [CrossRef]
- Väyrynen, J.P., et al., The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol Res 2021, 9, 8–19. [CrossRef]
- Robinson, S.D.; Filippopoulou, C.; Besta, S.; Samuels, M.; Betrán, A.L.; Abu Ajamieh, M.; Vella, V.; Jones, W.; Giamas, G. Spatial biology – unravelling complexity within the glioblastoma microenvironment. Trends Mol. Med. 2025. [Google Scholar] [CrossRef]
- Gangadaran, P.; Onkar, A.; Rajendran, R.L.; Goenka, A.; Oh, J.M.; Khan, F.; Nagarajan, A.K.; Muthu, S.; Krishnan, A.; Hong, C.M.; et al. Noninvasive in vivo imaging of macrophages: understanding tumor microenvironments and delivery of therapeutics. Biomark. Res. 2025, 13, 1–25. [Google Scholar] [CrossRef]
- Cai, D.X.; Tian, F.; Zhang, D.K.; Tu, J.F.; Wang, Y.H. CRABP2 (Cellular Retinoic Acid Binding Protein 2D): A novel biomarker for the diagnosis and prognosis involved in immune infiltration of lung adenocarcinoma. J. Cancer 2025, 16, 1631–1646. [Google Scholar] [CrossRef]
- Qi, Y.-Q.; Xiong, F.; Chen, Y.-J. The correlation between tumor-associated macrophages and the prognosis of east Asian hepatocellular carcinoma patients: A systematic review and meta-analysis. Pathol. - Res. Pr. 2023, 252, 154919. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Vuletić, A.; Martinović, K.M. Divide and Conquer—Targeted Therapy for Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 1396. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Baghel, K.S.; Tewari, B.N.; Shrivastava, R.; Malik, S.A.; Lone, M.U.-D.; Jain, N.K.; Tripathi, C.; Kanchan, R.K.; Dixit, S.; Singh, K.; et al. Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1β dependent upregulation ofMYO3Agene in breast cancer cells. OncoImmunology 2016, 5, e1196299–e1196299. [Google Scholar] [CrossRef]
- Jahandideh, A.; Yarizadeh, M.; Masjedi, M.N.-K.; Fatehnejad, M.; Jahandideh, R.; Soheili, R.; Eslami, Y.; Zokaei, M.; Ahmadvand, A.; Ghalamkarpour, N.; et al. Macrophage’s role in solid tumors: two edges of a sword. Cancer Cell Int. 2023, 23, 1–25. [Google Scholar] [CrossRef]
- Mei, J.; Xiao, Z.; Guo, C.; Pu, Q.; Ma, L.; Liu, C.; Lin, F.; Liao, H.; You, Z.; Liu, L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 2016, 7, 34217–34228. [Google Scholar] [CrossRef]
- Jin, R.; Neufeld, L.; McGaha, T.L. Linking macrophage metabolism to function in the tumor microenvironment. Nat. Cancer 2025, 6, 239–252. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Y.; Pu, K.; Tang, W. Nanomaterial-enabled metabolic reprogramming strategies for boosting antitumor immunity. Chem. Soc. Rev. 2024, 54, 653–714. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Bao, C.; Ma, Q.; Ying, X.; Wang, F.; Hou, Y.; Wang, D.; Zhu, L.; Huang, J.; He, C. Histone lactylation in macrophage biology and disease: from plasticity regulation to therapeutic implications. EBioMedicine 2024, 111, 105502. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, Y.; Gong, Q.; Luo, K. Metformin-based nanomedicines for reprogramming tumor immune microenvironment. Theranostics 2025, 15, 993–1016. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zheng, L.; Qi, C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol. Cancer 2025, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, B.; Bello, A.B.; Moon, J.J.; Arai, Y.; Lee, S.-H. Regenerative Functions of Regulatory T Cells and Current Strategies Utilizing Mesenchymal Stem Cells in Immunomodulatory Tissue Regeneration. Tissue Eng. Regen. Med. 2025, 22, 167–180. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S.; Kim, W.; Lee, E.H.; Kim, S.; Kim, T.; Shin, E.-A.; Pyo, K.-H.; Lee, H.; Jin, S.H.; et al. Isoxazole-based molecules restore NK cell immune surveillance in hepatocarcinogenesis by targeting TM4SF5 and SLAMF7 linkage. Signal Transduct. Target. Ther. 2025, 10, 1–18. [Google Scholar] [CrossRef]
- Fanijavadi, S.; Thomassen, M.; Jensen, L.H. Targeting Triple NK Cell Suppression Mechanisms: A Comprehensive Review of Biomarkers in Pancreatic Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 515. [Google Scholar] [CrossRef]
- Imani, S.; Kaboli, P.J.; Babaeizad, A.; Maghsoudloo, M. Neoantigen mRNA vaccines and A 2 A receptor antagonism: A strategy to enhance T cell immunity. Hum. Vaccines Immunother. 2025, 21, 2458936. [Google Scholar] [CrossRef]
- Griffiths, J.I.; Cosgrove, P.A.; Medina, E.F.; Nath, A.; Chen, J.; Adler, F.R.; Chang, J.T.; Khan, Q.J.; Bild, A.H. Cellular interactions within the immune microenvironment underpins resistance to cell cycle inhibition in breast cancers. Nat. Commun. 2025, 16, 2132. [Google Scholar] [CrossRef]
- Li, Z.; Duan, D.; Li, L.; Peng, D.; Ming, Y.; Ni, R.; Liu, Y. Tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for hepatocellular carcinoma: recent research progress. Front. Pharmacol. 2024, 15, 1382256. [Google Scholar] [CrossRef]
- Toghraie, F.S.; Bayat, M.; Hosseini, M.S.; Ramezani, A. Tumor-infiltrating myeloid cells; mechanisms, functional significance, and targeting in cancer therapy. Cell. Oncol. 2025, 1–32. [Google Scholar] [CrossRef]
- Hensler, M.; Kasikova, L.; Fiser, K.; Rakova, J.; Skapa, P.; Laco, J.; Lanickova, T.; Pecen, L.; Truxova, I.; Vosahlikova, S.; et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J. Immunother. Cancer 2020, 8, e000979. [Google Scholar] [CrossRef]
- Trebska-McGowan, K.; Chaib, M.; Alvarez, M.A.; Kansal, R.; Pingili, A.K.; Shibata, D.; Makowski, L.; Glazer, E.S. TGF-β Alters the Proportion of Infiltrating Immune Cells in a Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2022, 26, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, S.; Xia, L.; Sun, Z.; Chan, K.M.; Bernards, R.; Qin, W.; Chen, J.; Xia, Q.; Jin, H. Hepatocellular carcinoma: signaling pathways and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 1–43. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.-B.; Sun, R.; Wang, N.; Weng, X.-Q.; Xu, T.-Y.; Fu, D.; Feng, Y.; Xu, P.-P.; Cheng, S.; et al. Dual targeting PD-L1 and 4-1BB to overcome dendritic cell-mediated lenalidomide resistance in follicular lymphoma. Signal Transduct. Target. Ther. 2025, 10, 1–13. [Google Scholar] [CrossRef]
- Xiong, Z.; Huang, Y.; Cao, S.; Huang, X.; Zhang, H. A new strategy for the treatment of advanced ovarian cancer: utilizing nanotechnology to regulate the tumor microenvironment. Front. Immunol. 2025, 16, 1542326. [Google Scholar] [CrossRef]
- Ricci, J.-E. Tumor-induced metabolic immunosuppression: Mechanisms and therapeutic targets. Cell Rep. 2025, 44, 115206. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Liu, J.; Guo, L.; Guo, Q.; Liu, W. Bone marrow immune cells and drug resistance in acute myeloid leukemia. Exp. Biol. Med. 2025, 250, 10235. [Google Scholar] [CrossRef]
- E Menjivar, R.; Nwosu, Z.C.; Du, W.; Donahue, K.L.; Hong, H.S.; Espinoza, C.; Brown, K.; Velez-Delgado, A.; Yan, W.; Lima, F.; et al. Arginase 1 is a key driver of immune suppression in pancreatic cancer. eLife 2023, 12. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 1–33. [Google Scholar] [CrossRef]
- Chi, H.; Pepper, M.; Thomas, P.G. Principles and therapeutic applications of adaptive immunity. Cell 2024, 187, 2052–2078. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, H.; Zhao, P. Compliant immune response of silk-based biomaterials broadens application in wound treatment. Front. Pharmacol. 2025, 16, 1548837. [Google Scholar] [CrossRef] [PubMed]
- Kersten, K.; Hu, K.H.; Combes, A.J.; Samad, B.; Harwin, T.; Ray, A.; Rao, A.A.; Cai, E.; Marchuk, K.; Artichoker, J.; et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 2022, 40, 624–638.e9. [Google Scholar] [CrossRef] [PubMed]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. 2018, 115, E4041–E4050. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 1–19. [Google Scholar] [CrossRef]
- Soriano-Cruz, M.; Vázquez-González, W.G.; Molina-Vargas, P.; Faustino-Trejo, A.; Chávez-Rueda, A.K.; Legorreta-Haquet, M.V.; Aguilar-Ruíz, S.R.; Chávez-Sánchez, L. Exosomes as Regulators of Macrophages in Cardiovascular Diseases. Biomedicines 2024, 12, 2683. [Google Scholar] [CrossRef]
- Shen, L.; Li, Y.; Zhao, H. Fibroblast growth factor signaling in macrophage polarization: impact on health and diseases. Front. Immunol. 2024, 15, 1390453. [Google Scholar] [CrossRef]
- Ho, P.-C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.-C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.-K.; Berestjuk, I.; Hoeve, J.T.; et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat. Cancer 2024, 5, 1045–1062. [Google Scholar] [CrossRef]
- Sato, T.; Sugiyama, D.; Koseki, J.; Kojima, Y.; Hattori, S.; Sone, K.; Nishinakamura, H.; Ishikawa, T.; Ishikawa, Y.; Kato, T.; et al. Sustained inhibition of CSF1R signaling augments antitumor immunity through inhibiting tumor-associated macrophages. J. Clin. Investig. 2025, 10. [Google Scholar] [CrossRef]
- Luan, X.; Lei, T.; Fang, J.; Liu, X.; Fu, H.; Li, Y.; Chu, W.; Jiang, P.; Tong, C.; Qi, H.; et al. Blockade of C5a receptor unleashes tumor-associated macrophage antitumor response and enhances CXCL9-dependent CD8+ T cell activity. Mol. Ther. 2023, 32, 469–489. [Google Scholar] [CrossRef]
- Xie, M., et al., FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol 2023, 79, 109–125. [CrossRef] [PubMed]
- Bader, J.E.; Wolf, M.M.; Lupica-Tondo, G.L.; Madden, M.Z.; Reinfeld, B.I.; Arner, E.N.; Hathaway, E.S.; Steiner, K.K.; Needle, G.A.; Hatem, Z.; et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 2024, 630, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kerzel, T.; Giacca, G.; Beretta, S.; Bresesti, C.; Notaro, M.; Scotti, G.M.; Balestrieri, C.; Canu, T.; Redegalli, M.; Pedica, F.; et al. In vivo macrophage engineering reshapes the tumor microenvironment leading to eradication of liver metastases. Cancer Cell 2023, 41, 1892–1910.e10. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Khafaga, D.S.; El-Morsy, M.T.; Farhan, M.; Aatif, M.; Hosney, M. Targeting tumor-associated macrophages with nanocarrier-based treatment for breast cancer: A step toward developing innovative anti-cancer therapeutics. Heliyon 2024, 10, e37217. [Google Scholar] [CrossRef]
- Coënon, L.; Geindreau, M.; Ghiringhelli, F.; Villalba, M.; Bruchard, M. Natural Killer cells at the frontline in the fight against cancer. Cell Death Dis. 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization. Signal Transduct. Target. Ther. 2024, 9, 1–39. [Google Scholar] [CrossRef]
- Vidal-Manrique, M.; Nieuwenstein, T.; Hooijmaijers, L.; de Jonge, P.; Djojoatmo, M.; Jansen, J.; van der Waart, A.; Brock, R.; Dolstra, H. IL-15 transpresentation by ovarian cancer cells improves CD34 + progenitor-derived NK cell's anti-tumor functionality. OncoImmunology 2025, 14, 2465010. [Google Scholar] [CrossRef]
- Jin, P.; Bai, M.; Li, J.; Jia, W.; Yu, J.; Meng, X. Synergistic enhancement of radio-immunotherapy efficacy by IL-15 via macrophage activation and memory T cell response. Cancer Lett. 2025, 613, 217511. [Google Scholar] [CrossRef]
- Horta, A.L.; Gigley, J.; Boutet, M.; Lavau, G.; Weiss, L.M.; Huang, H. Memory-like NK Cells Are a Critical Component of Vaccine-Induced Immunity to Trypanosoma cruzi Infection. J. Immunol. 2024, 212, 617–631. [Google Scholar] [CrossRef]
- Guo, R.; Wang, R.; Zhang, W.; Li, Y.; Wang, Y.; Wang, H.; Li, X.; Song, J. Macrophage Polarisation in the Tumour Microenvironment: Recent Research Advances and Therapeutic Potential of Different Macrophage Reprogramming. Cancer Control. 2025, 32. [Google Scholar] [CrossRef]
- Chiba, S.; Ikushima, H.; Ueki, H.; Yanai, H.; Kimura, Y.; Hangai, S.; Nishio, J.; Negishi, H.; Tamura, T.; Saijo, S.; et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. eLife 2014, 3, e04177. [Google Scholar] [CrossRef] [PubMed]
- Aftabi, S. , et al., Therapeutic targeting of TGF-β in lung cancer. The FEBS Journal. n/a(n/a).
- Mathews, J.A.; Borovsky, D.T.; Reid, K.T.; Murphy, J.M.; Colpitts, S.J.; Carreira, A.S.; Moya, T.A.; Chung, D.C.; Novitzky-Basso, I.; Mattsson, J.; et al. Single cell profiling of hematopoietic stem cell transplant recipients reveals TGF-β1 and IL-2 confer immunoregulatory functions to NK cells. iScience 2024, 27, 111416. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-Y.; Zhu, Y.; Xie, S.-Z.; Qin, L.-X. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: current status and prospectives. J. Hematol. Oncol. 2024, 17, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.; Krzywinska, E.; Castells, M.; Gotthardt, D.; Putz, E.M.; Kantari-Mimoun, C.; Chikdene, N.; Meinecke, A.-K.; Schrödter, K.; Helfrich, I.; et al. Targeting VEGF-A in myeloid cells enhances natural killer cell responses to chemotherapy and ameliorates cachexia. Nat. Commun. 2016, 7, 12528. [Google Scholar] [CrossRef]
- Jiang, P.; Jing, S.; Sheng, G.; Jia, F. The basic biology of NK cells and its application in tumor immunotherapy. Front. Immunol. 2024, 15, 1420205. [Google Scholar] [CrossRef]
- Lin, X., et al., Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer 2024, 23, 108. [CrossRef]
- Fanijavadi, S.; Hansen, T.F.; Zedan, A.H. NK Cell-Microbiota Interaction Biomarker Strategy: Advancing Prostate Cancer Management. Biomolecules 2025, 15, 273. [Google Scholar] [CrossRef]
- Tredicine, M.; Mucci, M.; Recchiuti, A.; Mattoscio, D. Immunoregulatory mechanisms of the arachidonic acid pathway in cancer. FEBS Lett. 2025. [CrossRef]
- Ji, S.; Shi, Y.; Yin, B. Macrophage barrier in the tumor microenvironment and potential clinical applications. Cell Commun. Signal. 2024, 22, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Chai, D.; Dang, Y.; Zheng, J.; Li, H. iNKT: A new avenue for CAR-based cancer immunotherapy. Transl. Oncol. 2022, 17, 101342. [Google Scholar] [CrossRef]
- Díaz-Basabe, A.; Strati, F.; Facciotti, F. License to Kill: When iNKT Cells Are Granted the Use of Lethal Cytotoxicity. Int. J. Mol. Sci. 2020, 21, 3909. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.M.; Sholevar, C.J.; Judge, S.J.; Darrow, M.A.; Iranpur, K.R.; Farley, L.E.; Lammers, M.; Razmara, A.M.; Dunai, C.; Gingrich, A.A.; et al. Intratumoral NKp46+ natural killer cells are spatially distanced from T and MHC-I+ cells with prognostic implications in soft tissue sarcoma. Front. Immunol. 2023, 14, 1230534. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Micke, P.; Elebro, J.; Heby, M.; Hrynchyk, I.; Nodin, B.; Leandersson, K.; Mezheyeuski, A.; Jirström, K. Topographical Distribution and Spatial Interactions of Innate and Semi-Innate Immune Cells in Pancreatic and Other Periampullary Adenocarcinoma. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Delfanti, G.; Dellabona, P.; Casorati, G.; Fedeli, M. Adoptive Immunotherapy With Engineered iNKT Cells to Target Cancer Cells and the Suppressive Microenvironment. Front. Med. 2022, 9, 897750. [Google Scholar] [CrossRef]
- Janakiram, N.B., et al., Loss of natural killer T cells promotes pancreatic cancer in LSL-Kras(G12D/+) mice. Immunology 2017, 152, 36–51. [CrossRef]
- Cruz, M.S.; Loureiro, J.P.; Oliveira, M.J.; Macedo, M.F. The iNKT Cell–Macrophage Axis in Homeostasis and Disease. Int. J. Mol. Sci. 2022, 23, 1640. [Google Scholar] [CrossRef]
- Li, Y.-R.; Zhou, Y.; Yu, J.; Zhu, Y.; Lee, D.; Zhu, E.; Li, Z.; Kim, Y.J.; Zhou, K.; Fang, Y.; et al. Engineering allorejection-resistant CAR-NKT cells from hematopoietic stem cells for off-the-shelf cancer immunotherapy. Mol. Ther. 2024, 32, 1849–1874. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Dou, Z.; Delfanti, G.; Tsahouridis, O.; Pellegry, C.M.; Zingarelli, M.; Atassi, G.; Woodcock, M.G.; Casorati, G.; et al. CAR-redirected natural killer T cells demonstrate superior antitumor activity to CAR-T cells through multimodal CD1d-dependent mechanisms. Nat. Cancer 2024, 5, 1607–1621. [Google Scholar] [CrossRef]
- Kostic, M., et al., Dissecting the immune response of CD4+ T cells in Alzheimer’s disease. Reviews in the Neurosciences 2025, 36, 139–168. [CrossRef]
- Khalaf, K.; Chamieh, M.; Welc, N.; Singh, C.; Kaouk, J.L.; Kaouk, A.; Mackiewicz, A.; Kaczmarek, M.; Perek, B. Cellular aspects of immunity involved in the development of atherosclerosis. Front. Immunol. 2025, 16, 1461535. [Google Scholar] [CrossRef]
- Martinenaite, E.; Lecoq, I.; Aaboe-Jørgensen, M.; Ahmad, S.M.; Perez-Penco, M.; Glöckner, H.J.; Chapellier, M.; de la Torre, L.L.; Olsen, L.R.; Rømer, A.M.A.; et al. Arginase-1-specific T cells target and modulate tumor-associated macrophages. J. Immunother. Cancer 2025, 13, e009930. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Nelde, A.; Ramirez, B.C.; Hatin, I.; Arbes, H.; François, P.; Demais, S.; Labaronne, E.; Decimo, D.; Guiguettaz, L.; et al. Unveiling conserved HIV-1 open reading frames encoding T cell antigens using ribosome profiling. Nat. Commun. 2025, 16, 1–18. [Google Scholar] [CrossRef]
- Chandra, D.J.; Alber, B.; Saultz, J.N. The Immune Resistance Signature of Acute Myeloid Leukemia and Current Immunotherapy Strategies. Cancers 2024, 16, 2615. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Sun, L.; Lin, H.; Liao, Y.; Yang, H.; Mao, Y. Harnessing innate immune pathways for therapeutic advancement in cancer. Signal Transduct. Target. Ther. 2024, 9, 1–59. [Google Scholar] [CrossRef]
- Tsomidis, I.; Voumvouraki, A.; Kouroumalis, E. Immune Checkpoints and the Immunology of Liver Fibrosis. Livers 2025, 5, 5. [Google Scholar] [CrossRef]
- Xu, H., et al., Immunoglobulin-like transcript 5 polarizes M2-like tumor-associated macrophages for immunosuppression in non-small cell lung cancer. International Journal of Cancer. n/a(n/a).
- Ge, Y., et al., Utilizing Nanoparticles to Overcome Anti-PD-1/PD-L1 Immunotherapy Resistance in Non-Small Cell Lung cancer: A Potential Strategy. Int J Nanomedicine 2025, 20: p. 2371-2394.
- Xia, Y.; Huang, C.; Zhong, M.; Zhong, H.; Ruan, R.; Xiong, J.; Yao, Y.; Zhou, J.; Deng, J. Targeting HGF/c-MET signaling to regulate the tumor microenvironment: Implications for counteracting tumor immune evasion. Cell Commun. Signal. 2025, 23, 1–18. [Google Scholar] [CrossRef]
- Bos, J.; Schooten, T.G.-V.; Brugman, C.; Jamaludin, F.; van Laarhoven, H.; Derks, S. The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review. Cancer Treat. Rev. 2024, 127, 102737. [Google Scholar] [CrossRef]
- Jumaniyazova, E.; Lokhonina, A.; Dzhalilova, D.; Miroshnichenko, E.; Kosyreva, A.; Fatkhudinov, T. The Role of Macrophages in Various Types of Tumors and the Possibility of Their Use as Targets for Antitumor Therapy. Cancers 2025, 17, 342. [Google Scholar] [CrossRef]
- Vilbois, S.; Xu, Y.; Ho, P.-C. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer 2023, 10, 242–255. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef]
- Wang, H.; Franco, F.; Tsui, Y.-C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernández-García, J.; Tsai, C.-H.; Schulze, I.; et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.T.; Jo, Y.; Chiale, C.; Macal, M.; Fang, Z.; Khatri, F.S.; Codrington, A.L.; Kazane, K.R.; Akbulut, E.; Swaminathan, S.; et al. Metabolic deficiencies underlie reduced plasmacytoid dendritic cell IFN-I production following viral infection. Nat. Commun. 2025, 16, 1–19. [Google Scholar] [CrossRef]
- Niemetz, L.; Bodmer, B.S.; Olal, C.; Escudero-Pérez, B.; Hoehn, K.; Bencsik, A.; A Vickers, M.; Rodríguez, E.; Oestereich, L.; Hoenen, T.; et al. Ebola Virus Infection of Flt3-Dependent, Conventional Dendritic Cells and Antigen Cross-presentation Leads to High Levels of T-Cell Activation. J. Infect. Dis. 2024, 231, 501–511. [Google Scholar] [CrossRef]
- Vafaeian, A.; Rajabi, F.; Rezaei, N. Toll-like receptors in atopic dermatitis: pathogenesis and therapeutic implications. Heliyon 2025, 11, e42226. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zhang, F.; Goedegebuure, S.P.; Gillanders, W.E. Dendritic cell subsets and implications for cancer immunotherapy. Front. Immunol. 2024, 15, 1393451. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, J.; Ruan, S.; Zhang, B.; Zhu, H.; Que, Y.; Ying, S.; Li, X.; Hu, Y.; Song, Z. Overcoming immunotherapy resistance in gastric cancer: insights into mechanisms and emerging strategies. Cell Death Dis. 2025, 16, 1–18. [Google Scholar] [CrossRef]
- Niveau, C.; Cettour-Cave, M.; Mouret, S.; Cuevas, E.S.; Pezet, M.; Roubinet, B.; Gil, H.; De Fraipont, F.; Landemarre, L.; Charles, J.; et al. MCT1 lactate transporter blockade re-invigorates anti-tumor immunity through metabolic rewiring of dendritic cells in melanoma. Nat. Commun. 2025, 16, 1–24. [Google Scholar] [CrossRef]
- Guo, F.; Kong, W.; Li, D.; Zhao, G.; Anwar, M.; Xia, F.; Zhang, Y.; Ma, C.; Ma, X. M2-type tumor-associated macrophages upregulated PD-L1 expression in cervical cancer via the PI3K/AKT pathway. Eur. J. Med Res. 2024, 29, 357. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Z.; Wei, X.; Zhao, G.; Han, D.; Zhang, T.; Chen, X.; Cao, F.; Dong, J.; Zhao, L.; et al. Spatial Distribution and Predictive Significance of Dendritic Cells and Macrophages in Esophageal Cancer Treated With Combined Chemoradiotherapy and PD-1 Blockade. Front. Immunol. 2022, 12, 786429. [Google Scholar] [CrossRef]
- Xie, D.; Lu, G.; Mai, G.; Guo, Q.; Xu, G. Tissue-resident memory T cells in diseases and therapeutic strategies. Medcomm 2025, 6, e70053. [Google Scholar] [CrossRef]
- Li, Y., et al., DNMT1 inhibition improves the activity of memory-like natural killer cells by enhancing the level of autophagy. Mol Biol Rep 2024, 52, 68.
- Li, L.; Xu, T.; Qi, X. Balanced regulation of ROS production and inflammasome activation in preventing early development of colorectal cancer. Immunol. Rev. 2024, 329. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Tu, C.; Xiong, H.; Xu, Y.; Shi, X.; Zhang, X.; Yang, R.; Zhang, N.; Lin, B.; Liu, M.; et al. GITRL enhances cytotoxicity and persistence of CAR-T cells in cancer therapy. Mol. Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Luyang, H.; Zeng, F.; Lei, Y.; He, Q.; Zhou, Y.; Xu, J. Bidirectional role of neutrophils in tumor development. Mol. Cancer 2025, 24, 1–16. [Google Scholar] [CrossRef]
- Schmitt, H., M.F. Neurath, and R. Atreya, Role of the IL23/IL17 Pathway in Crohn's Disease. Front Immunol 2021, 12: p. 622934.
- Zhao, J.; Lu, Q.; Liu, Y.; Shi, Z.; Hu, L.; Zeng, Z.; Tu, Y.; Xiao, Z.; Xu, Q. Th17 Cells in Inflammatory Bowel Disease: Cytokines, Plasticity, and Therapies. J. Immunol. Res. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Surman, M.; Przybyło, M.; Wilczak, M. Melanoma-derived extracellular vesicles transfer proangiogenic factors. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2025, 33, 245–262. [Google Scholar] [CrossRef]
- Mehdikhani, F.; Hajimehdipoor, H.; Tansaz, M.; Maresca, M.; Rajabi, S. Sesquiterpene Lactones as Promising Phytochemicals to Cease Metastatic Propagation of Cancer. Biomolecules 2025, 15, 268. [Google Scholar] [CrossRef]
- García-Navas, R.; Gajate, C.; Mollinedo, F. Neutrophils drive endoplasmic reticulum stress-mediated apoptosis in cancer cells through arginase-1 release. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Yang, Q.; Cui, M.; Wang, J.; Zhao, Y.; Yin, W.; Liao, Z.; Liang, Y.; Jiang, Z.; Li, Y.; Guo, J.; et al. Circulating mitochondrial DNA promotes M2 polarization of tumor associated macrophages and HCC resistance to sorafenib. Cell Death Dis. 2025, 16, 1–14. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, P.; Sun, R.; Li, J.; Hu, Z.; Xin, H.; Luo, C.; Zhou, J.; Fan, J.; Zhou, S. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J. Immunother. Cancer 2021, 9, e001946. [Google Scholar] [CrossRef]
- Raggi, C.; Correnti, M.; Sica, A.; Andersen, J.B.; Cardinale, V.; Alvaro, D.; Chiorino, G.; Forti, E.; Glaser, S.; Alpini, G.; et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017, 66, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, A.; Sudarskikh, T.; Kovalev, O.; Kzhyshkowska, J.; Larionova, I. Interaction of tumor-associated macrophages with stromal and immune components in solid tumors: Research progress (Review). Int. J. Oncol. 2023, 62, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Rao, A.S.; Stadanlick, J.; Bruns, K.; Sullivan, N.T.; Bermudez, A.; Honig-Frand, A.; Krouse, R.; Arambepola, S.; Guo, E.; et al. Human Tumor–Associated Macrophages and Neutrophils Regulate Antitumor Antibody Efficacy through Lethal and Sublethal Trogocytosis. Cancer Res. 2024, 84, 1029–1047. [Google Scholar] [CrossRef]
- Lei, Q.; Zhen, S.; Zhang, L.; Zhao, Q.; Yang, L.; Zhang, Y. A2AR-mediated CXCL5 upregulation on macrophages promotes NSCLC progression via NETosis. Cancer Immunol. Immunother. 2024, 73, 1–16. [Google Scholar] [CrossRef]
- Schmidt, E.; Distel, L.; Erber, R.; Büttner-Herold, M.; Rosahl, M.-C.; Ott, O.J.; Strnad, V.; Hack, C.C.; Hartmann, A.; Hecht, M.; et al. Tumor-Associated Neutrophils Are a Negative Prognostic Factor in Early Luminal Breast Cancers Lacking Immunosuppressive Macrophage Recruitment. Cancers 2024, 16, 3160. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, J.; Xu, T.; Cai, A.; Han, B.; Li, Y.; Fang, Z.; Yu, D.; Wang, S.; Zhou, J.; et al. VSIG4+ tumor-associated macrophages mediate neutrophil infiltration and impair antigen-specific immunity in aggressive cancers through epigenetic regulation of SPP1. J. Exp. Clin. Cancer Res. 2025, 44, 1–19. [Google Scholar] [CrossRef]
- Chu, X.; Tian, Y.; Lv, C. Decoding the spatiotemporal heterogeneity of tumor-associated macrophages. Mol. Cancer 2024, 23, 1–24. [Google Scholar] [CrossRef]
- Werner, W.; Kuzminskaya, M.; Lurje, I.; Tacke, F.; Hammerich, L. Overcoming Resistance to Immune Checkpoint Blockade in Liver Cancer with Combination Therapy: Stronger Together? Semin. Liver Dis. 2024, 44, 159–179. [Google Scholar] [CrossRef]
- Yin, Y.; Feng, W.; Chen, J.; Chen, X.; Wang, G.; Wang, S.; Xu, X.; Nie, Y.; Fan, D.; Wu, K.; et al. Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside. Exp. Hematol. Oncol. 2024, 13, 1–37. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, X.; Sheng, J.; Lu, S.; Yu, X.; Wu, J.D. Soluble NKG2D ligand promotes MDSC expansion and skews macrophage to the alternatively activated phenotype. J. Hematol. Oncol. 2015, 8, 13–13. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Kumar, A.; Amdare, N.P.; Pathak, R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology 2024, 13, 307. [Google Scholar] [CrossRef]
- Kumar, V.; Cheng, P.; Condamine, T.; Mony, S.; Languino, L.R.; McCaffrey, J.C.; Hockstein, N.; Guarino, M.; Masters, G.; Penman, E.; et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity 2016, 44, 303–315. [Google Scholar] [CrossRef]
- Tang, X.; Gao, L.; Jiang, X.; Hou, Z.; Wang, Y.; Hou, S.; Qu, H. Single-cell profiling reveals altered immune landscape and impaired NK cell function in gastric cancer liver metastasis. Oncogene 2024, 43, 2635–2646. [Google Scholar] [CrossRef]
- Tabachnick-Cherny, S.; Pulliam, T.; Rodriguez, H.J.; Fan, X.; Hippe, D.S.; Jones, D.C.; Moshiri, A.S.; Smythe, K.S.; Kulikauskas, R.M.; Zaba, L.C.; et al. Characterization of Immunosuppressive Myeloid Cells in Merkel Cell Carcinoma: Correlation with Resistance to PD-1 Pathway Blockade. Clin. Cancer Res. 2023, 30, 1189–1199. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Gu, Y.; Liu, T.; Zhao, X.; Cheng, S.; Duan, L.; Huang, C.; Wu, S.; Gao, S. Complement C3 of tumor-derived extracellular vesicles promotes metastasis of RCC via recruitment of immunosuppressive myeloid cells. Proc. Natl. Acad. Sci. 2025, 122. [Google Scholar] [CrossRef]
- Guo, F.; Song, Y.; Dong, S.; Wei, J.; Li, B.; Xu, T.; Wang, H. Characterization and anti-tuberculosis effects of γδ T cells expanded and activated by Mycobacterium tuberculosis heat-resistant antigen. Virulence 2025, 16, 2462092. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, Y.; Niu, S.; Lyu, Z.; Tian, Y.; Shen, X.; Li, Y.-R.; Yang, L. Biological functions and therapeutic applications of human mucosal-associated invariant T cells. J. Biomed. Sci. 2025, 32, 1–17. [Google Scholar] [CrossRef]
- Hu, Y., et al., γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther 2023, 8, 434. [CrossRef]
- Revesz, I.A., et al., Effective γδ T-cell clinical therapies: current limitations and future perspectives for cancer immunotherapy. Clin Transl Immunology 2024, 13, e1492. [CrossRef]
- Petruk, N.; Sousa, S.; Croset, M.; Polari, L.; Zlatev, H.; Selander, K.; Mönkkönen, J.; Clézardin, P.; Määttä, J. Liposome-encapsulated zoledronate increases inflammatory macrophage population in TNBC tumours. Eur. J. Pharm. Sci. 2023, 190, 106571. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, X.; Cui, X. Effect of Liposome-Encapsulated Zoledronic Acid on Microenvironment of Hepatocellular Carcinoma May Depend on the Ratio Between M1 and M2 Polarized Macrophages. Bull. Exp. Biol. Med. 2020, 170, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wendong, Y.; Hengwu, X.; Yanhong, C.; Yingying, X.; Feng, Z.; Zeng, W.; Xinjun, C. Mannose modified co-loaded zoledronic liposomes deplete M2-tumor-associated macrophages to enhance anti-tumor effect of doxorubicin on TNBC. J. Drug Deliv. Sci. Technol. 2022, 74. [Google Scholar] [CrossRef]
- Man, F.; Lim, L.; Volpe, A.; Gabizon, A.; Shmeeda, H.; Draper, B.; Parente-Pereira, A.C.; Maher, J.; Blower, P.J.; Fruhwirth, G.O.; et al. In Vivo PET Tracking of 89Zr-Labeled Vγ9Vδ2 T Cells to Mouse Xenograft Breast Tumors Activated with Liposomal Alendronate. Mol. Ther. 2019, 27, 219–229. [Google Scholar] [CrossRef]
- Parente-Pereira, A.C., et al., Adoptive immunotherapy of epithelial ovarian cancer with Vγ9Vδ2 T cells, potentiated by liposomal alendronic acid. J Immunol 2014, 193, 5557–66. [CrossRef]
- Gao, Z.; Bai, Y.; Lin, A.; Jiang, A.; Zhou, C.; Cheng, Q.; Liu, Z.; Chen, X.; Zhang, J.; Luo, P. Gamma delta T-cell-based immune checkpoint therapy: attractive candidate for antitumor treatment. Mol. Cancer 2023, 22, 31. [Google Scholar] [CrossRef]
- Shiravand, Y., et al., Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022, 29, 3044–3060. [CrossRef]
- Liu, Y.; Tan, H.; Dai, J.; Lin, J.; Zhao, K.; Hu, H.; Zhong, C. Targeting macrophages in cancer immunotherapy: Frontiers and challenges. J. Adv. Res. 2025. [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
- Brahmer, J.R., et al., Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J Clin Oncol 2023, 41, 715–723. [CrossRef]
- Li, J.-W.; Shi, D.; Wan, X.-C.; Hu, J.; Su, Y.-F.; Zeng, Y.-P.; Hu, Z.-J.; Yu, B.-H.; Zhang, Q.-L.; Wei, P.; et al. Universal extracellular vesicles and PD-L1+ extracellular vesicles detected by single molecule array technology as circulating biomarkers for diffuse large B cell lymphoma. OncoImmunology 2021, 10, 1995166. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H., et al., Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer 2023, 22, 58. [CrossRef] [PubMed]
- Qi, J.; Sun, H.; Zhang, Y.; Wang, Z.; Xun, Z.; Li, Z.; Ding, X.; Bao, R.; Hong, L.; Jia, W.; et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 2022, 13, 1–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1578–1593.e8. [Google Scholar] [CrossRef]
- Ning, J.; Hou, X.; Hao, J.; Zhang, W.; Shi, Y.; Huang, Y.; Ruan, X.; Zheng, X.; Gao, M. METTL3 inhibition induced by M2 macrophage-derived extracellular vesicles drives anti-PD-1 therapy resistance via M6A-CD70-mediated immune suppression in thyroid cancer. Cell Death Differ. 2023, 30, 2265–2279. [Google Scholar] [CrossRef]
- You, Q.; Wang, F.; Du, R.; Pi, J.; Wang, H.; Huo, Y.; Liu, J.; Wang, C.; Yu, J.; Yang, Y.; et al. m6A Reader YTHDF1-Targeting Engineered Small Extracellular Vesicles for Gastric Cancer Therapy via Epigenetic and Immune Regulation. Adv. Mater. 2022, 35, e2204910. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Hu, H.; Qin, G.; Wu, X.; Bai, F.; Zhang, J.; Cai, Y.; Huang, Y.; Wang, C.; et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell 2023, 41, 1152–1169.e7. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Xia, H.; Yan, Y.; Zhu, X.; Sun, F.; Sun, L.; Li, S.; Li, D.; Wang, J.; et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Med. 2023, 15, 1–25. [Google Scholar] [CrossRef]
- Song, C.-H.; Kim, N.; Nam, R.H.; Choi, S.I.; Jang, J.Y.; Kim, J.W.; Na, H.Y.; Lee, H.-N. Combination treatment with 17β-estradiol and anti-PD-L1 suppresses MC38 tumor growth by reducing PD-L1 expression and enhancing M1 macrophage population in MC38 colon tumor model. Cancer Lett. 2022, 543, 215780. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Kluger, H.; George, S.; Tykodi, S.S.; Kuzel, T.M.; Perets, R.; Nair, S.; Procopio, G.; A Carducci, M.; Castonguay, V.; et al. FRACTION-RCC: nivolumab plus ipilimumab for advanced renal cell carcinoma after progression on immuno-oncology therapy. J. Immunother. Cancer 2022, 10, e005780. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M., et al., Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016, 17, 651–62.
- Haag, G.M.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschäbitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer – The PICCASSO phase I trial. Eur. J. Cancer 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.; Le Tourneau, C.; Toulmonde, M.; D'Angelo, S.P.; Weber, K.; Loirat, D.; Jacob, W.; Jegg, A.-M.; et al. Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour. Eur. J. Cancer 2020, 141, 162–170. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Cassier, P.; Zamarin, D.; Machiels, J.-P.; Gracia, J.L.P.; Hodi, F.S.; Taus, A.; Garcia, M.M.; Boni, V.; Eder, J.P.; et al. Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naïve or experienced for immune checkpoint blockade. J. Immunother. Cancer 2022, 10, e004076. [Google Scholar] [CrossRef]
- Machiels, J.-P.; Gomez-Roca, C.; Michot, J.-M.; Zamarin, D.; Mitchell, T.; Catala, G.; Eberst, L.; Jacob, W.; Jegg, A.-M.; A Cannarile, M.; et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J. Immunother. Cancer 2020, 8, e001153. [Google Scholar] [CrossRef]
- Razak, A.R.; Cleary, J.M.; Moreno, V.; Boyer, M.; Aller, E.C.; Edenfield, W.; Tie, J.; Harvey, R.D.; Rutten, A.; A Shah, M.; et al. Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1 receptor antibody, in combination with pembrolizumab in adults with advanced solid tumors. J. Immunother. Cancer 2020, 8, e001006. [Google Scholar] [CrossRef]
- Johnson, M.; Dudek, A.Z.; Sukari, A.; Call, J.; Kunk, P.R.; Lewis, K.; Gainor, J.F.; Sarantopoulos, J.; Lee, P.; Golden, A.; et al. ARRY-382 in Combination with Pembrolizumab in Patients with Advanced Solid Tumors: Results from a Phase 1b/2 Study. Clin. Cancer Res. 2022, 28, 2517–2526. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Burg, J.M.; Mick, R.; Trosko, J.A.; Li, D.; Shaik, M.N.; Tolcher, A.W.; Hamid, O. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. OncoImmunology 2013, 2, e23033. [Google Scholar] [CrossRef]
- Bauer, C.; Kühnemuth, B.; Duewell, P.; Ormanns, S.; Gress, T.; Schnurr, M. Prevailing over T cell exhaustion: New developments in the immunotherapy of pancreatic cancer. Cancer Lett. 2016, 381, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, X.; Dong, S.; Guo, Y.; Luo, Z.; Zhuang, S.-M.; Liu, J.; Liu, T.; Liao, J.; Wen, W. Modulating tumor-associated macrophages through CSF1R inhibition: a potential therapeutic strategy for HNSCC. J. Transl. Med. 2025, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Reddy, R.; Sharma, A.; Li, J.; Chang, M.; Sharma, R.; Salazar, S.; Kannan, S.; Kannan, R.M. Targeted systemic dendrimer delivery of CSF-1R inhibitor to tumor-associated macrophages improves outcomes in orthotopic glioblastoma. Bioeng. Transl. Med. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- A Siddiqui, B.; Chapin, B.F.; Jindal, S.; Duan, F.; Basu, S.; Yadav, S.S.; Gu, A.-D.; Espejo, A.B.; Kinder, M.; A Pettaway, C.; et al. Immune and pathologic responses in patients with localized prostate cancer who received daratumumab (anti-CD38) or edicotinib (CSF-1R inhibitor). J. Immunother. Cancer 2023, 11, e006262. [Google Scholar] [CrossRef]
- Wiehagen, K.R.; Girgis, N.M.; Yamada, D.H.; Smith, A.A.; Chan, S.R.; Grewal, I.S.; Quigley, M.; Verona, R.I. Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol. Res. 2017, 5, 1109–1121. [Google Scholar] [CrossRef]
- Omstead, A.N.; Paskewicz, M.; Gorbunova, A.; Zheng, P.; Salvitti, M.S.; Mansoor, R.; Reed, P.; Ballengee, S.; Wagner, P.L.; A Jobe, B.; et al. CSF-1R inhibitor, pexidartinib, sensitizes esophageal adenocarcinoma to PD-1 immune checkpoint blockade in a rat model. Carcinog. 2022, 43, 842–850. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Xu, M.; Wang, L.; Chen, X.; Feng, Y.; Li, Y.; Zhang, X.; Cui, W.; Jia, X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer 2020, 19, 41. [Google Scholar] [CrossRef]
- Kim, D.; An, L.; Moon, J.; Maymi, V.I.; McGurk, A.I.; Rudd, B.D.; Fowell, D.J.; White, A.C. Ccr2+ Monocyte-Derived Macrophages Influence Trajectories of Acquired Therapy Resistance in Braf-Mutant Melanoma. Cancer Res. 2023, 83, 2328–2344. [Google Scholar] [CrossRef]
- Miyamoto, T.; Murakami, R.; Hamanishi, J.; Tanigaki, K.; Hosoe, Y.; Mise, N.; Takamatsu, S.; Mise, Y.; Ukita, M.; Taki, M.; et al. B7-H3 Suppresses Antitumor Immunity via the CCL2–CCR2–M2 Macrophage Axis and Contributes to Ovarian Cancer Progression. Cancer Immunol. Res. 2021, 10, 56–69. [Google Scholar] [CrossRef]
- Trac, N.; Chen, L.-Y.; Zhang, A.; Liao, C.-P.; Poon, C.; Wang, J.; Ando, Y.; Joo, J.; Garri, C.; Shen, K.; et al. CCR2-targeted micelles for anti-cancer peptide delivery and immune stimulation. J. Control. Release 2020, 329, 614–623. [Google Scholar] [CrossRef]
- Wang, D. , et al., Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett 2020, 474: p. 36-52.
- Kocher, F.; Puccini, A.; Untergasser, G.; Martowicz, A.; Zimmer, K.; Pircher, A.; Baca, Y.; Xiu, J.; Haybaeck, J.; Tymoszuk, P.; et al. Multi-omic Characterization of Pancreatic Ductal Adenocarcinoma RelatesCXCR4mRNA Expression Levels to Potential Clinical Targets. Clin. Cancer Res. 2022, 28, 4957–4967. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Dong, C.; Jia, W.; Ma, B. Exosomal miR-655-3p inhibits growth, and invasion and macrophage M2 polarization through targeting CXCR4 in papillary thyroid carcinoma. Acta Biochim. Pol. 2022, 69, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Kaur, G.; Kumar, P.; Singh, T.G. Therapeutic targeting of angiopoietins in tumor angiogenesis and cancer development. Biochem. Biophys. Res. Commun. 2023, 687, 149130. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Li, J.; Zhang, P.; Li, H.; Chen, G.; Zhang, W.; Wang, T.; Frazer, I.; Ni, G. Caerin 1.1/1.9 Enhances Antitumour Immunity by Activating the IFN-α Response Signalling Pathway of Tumour Macrophages. Cancers 2022, 14, 5785. [Google Scholar] [CrossRef]
- Guerriero, J.L.; Sotayo, A.; Ponichtera, H.E.; Castrillon, J.A.; Pourzia, A.L.; Schad, S.; Johnson, S.F.; Carrasco, R.D.; Lazo, S.; Bronson, R.T.; et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017, 543, 428–432. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Repolarizing macrophages improves breast cancer therapy. Cell Res. 2017, 27, 963–964. [Google Scholar] [CrossRef]
- Goulielmaki, E.; Bermudez-Brito, M.; Andreou, M.; Tzenaki, N.; Tzardi, M.; de Bree, E.; Tsentelierou, E.; Makrigiannakis, A.; Papakonstanti, E.A. Pharmacological inactivation of the PI3K p110δ prevents breast tumour progression by targeting cancer cells and macrophages. Cell Death Dis. 2018, 9, 678. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, F.; Ren, X.; Sun, R.; Guo, X.; Liu, W.; Wang, B.; Yang, Y.; Zhang, X.; Xu, Y.; et al. Phosphoinositide-Binding Protein TIPE1 Promotes Alternative Activation of Macrophages and Tumor Progression via PIP3/Akt/TGFβ Axis. Cancer Res. 2022, 82, 1603–1616. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, L.; Zhang, H.; Chen, S.; Xiao, Y. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front. Immunol. 2022, 13, 927153. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.-C. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Tichet, M.; Wullschleger, S.; Chryplewicz, A.; Fournier, N.; Marcone, R.; Kauzlaric, A.; Homicsko, K.; Deak, L.C.; Umaña, P.; Klein, C.; et al. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8+ T cells and reprogramming macrophages. Immunity 2023, 56, 162–179.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N., et al., Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes antitumor immune response. J Immunother Cancer 2023, 11.
- Zhou, C.; Weng, J.; Liu, C.; Liu, S.; Hu, Z.; Xie, X.; Gao, D.; Zhou, Q.; Sun, J.; Xu, R.; et al. Disruption of SLFN11 Deficiency–Induced CCL2 Signaling and Macrophage M2 Polarization Potentiates Anti–PD-1 Therapy Efficacy in Hepatocellular Carcinoma. Gastroenterology 2023, 164, 1261–1278. [Google Scholar] [CrossRef]
| Drug | Phase | Cancer type | Combination therapy | NCT identifier |
|---|---|---|---|---|
| Chemokine inhibitors | ||||
| Carlumab (anti-CCL2 antibodies; Centocor) | Phase II (completed) | Prostate cancer | NA | NCT00992186 |
| BMS-813160 (CCR2/CCR5 antagonist; Bristol Myers Squibb) | Phase II (completed) | Renal cell carcinoma | Nivolumab (OPDIVO) in conjunction with ipilimumab (Yervoy) | NCT02996110 |
| Phase I/II (completed) | Pancreatic cancer, colorectal cancer, non-small cell lung cancer | Nab-paclitaxel with nivolumab | NCT03184870 | |
| Phase II (ongoing) | Hepatocellular carcinoma | Nivolumab | NCT04123379 | |
| PF-4136309 (CCR2 antagonist; Pfizer) | Phase II (completed) | PDAC | Nab-paclitaxel, gemcitabine | NCT01413022 |
| CSF1R inhibitors | ||||
| PLX3397 (Plexxikon) | Phase I/II (ongoing) | Tumors of the nerve sheath and sarcoma | Sirolimus (Rapamune) | NCT02584647 |
| Phase I/II (Terminated) | Melanoma and solid tumors, both at advanced stages | Pembrolizumab (Keytruda) | NCT02452424 | |
| Phase I/II (Completed) | Breast carcinoma | Eribulin (Halaven) | NCT01596751 | |
| Phase I/II (completed) | Glioblastoma | Radiotherapy, temozolomide (TMZ) | NCT01790503 | |
| BLZ945 (Novartis) | Phase I/II (Terminated) | Solid tumors | PDR001 (anti- PD1) | NCT02829723 |
| Antibodies targeting CSF1R | ||||
| LY3022855 (Eli Lilly's IMC-C S4) | Phase I/II (completed) | Melanoma | MEK/BRAF inhibitors | NCT03101254 |
| Emactuzumab (RO5509554/RG7155; Roche) | Phase II (Terminated) | Gynecological neoplasms and ovarian cancer | Gynecological neoplasms and ovarian cancer | NCT02923739 |
| Phase I/II (ongoing) | PDAC | Nab- paclitaxel, gemcitabine | NCT03193190 | |
| AMG820 (Amgen) | Phase I/II (completed) | Pancreatic cancer, CRC, NSCLC | Pembrolizumab | NCT02713529 |
| ARRAY-382 (Pfizer) | Phase I/II (terminated) | Solid tumors | Solid tumors | NCT02880371 |
| Agonist anti-CD40 antibodies (cont.) | ||||
| APX005M (Apexigen) | Phase II (completed) | Oesophageal cancer | Radiation, paclitaxel, carboplatin | NCT03214250 |
| Phase I/II (completed) | Pancreatic cancer | Nab- paclitaxel, gemcitabine, nivolumab | NCT03214250 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




