Submitted:
01 April 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
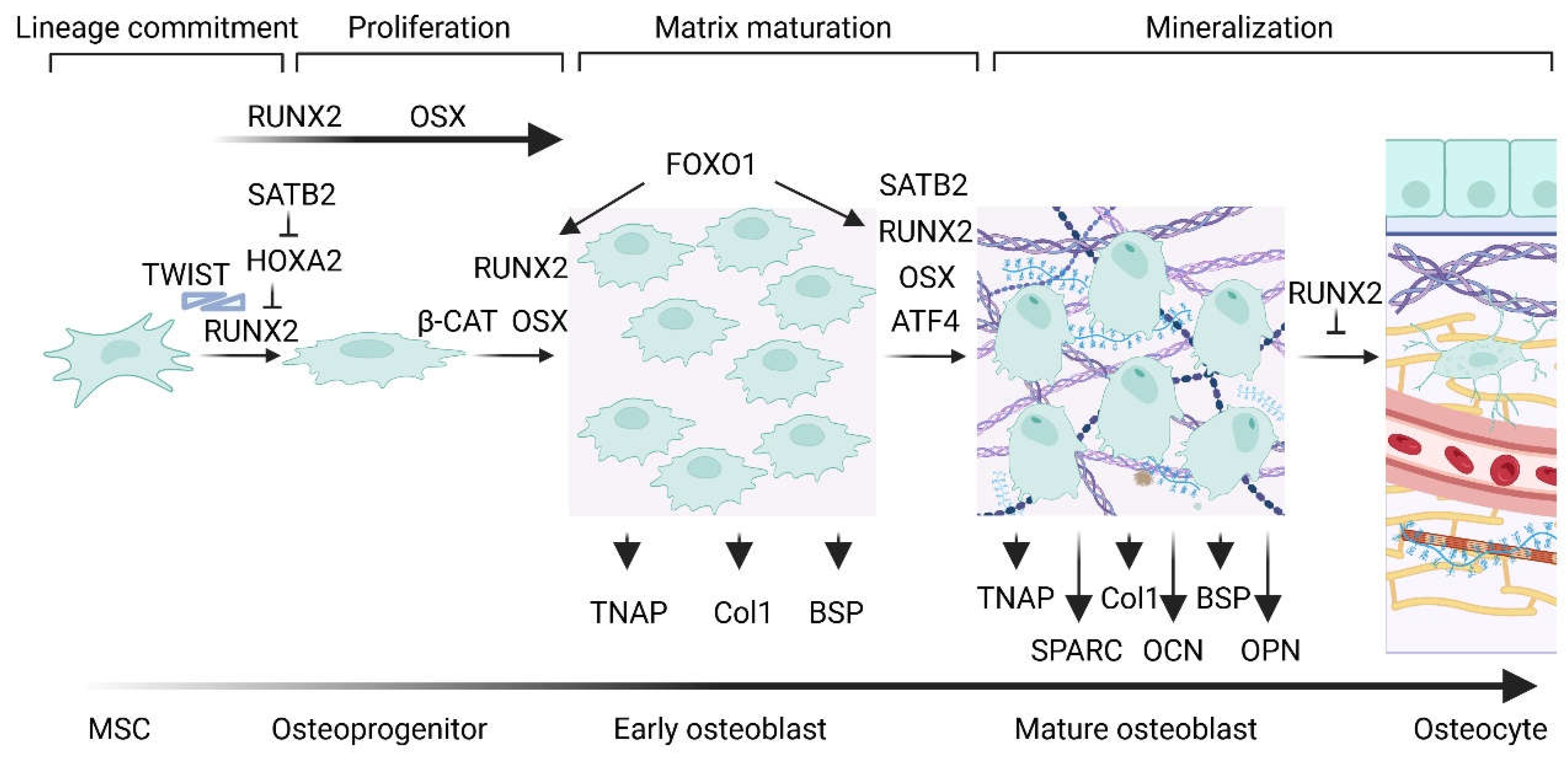
1. Cellular, Molecular and Genetic Aspects of Bone Formation
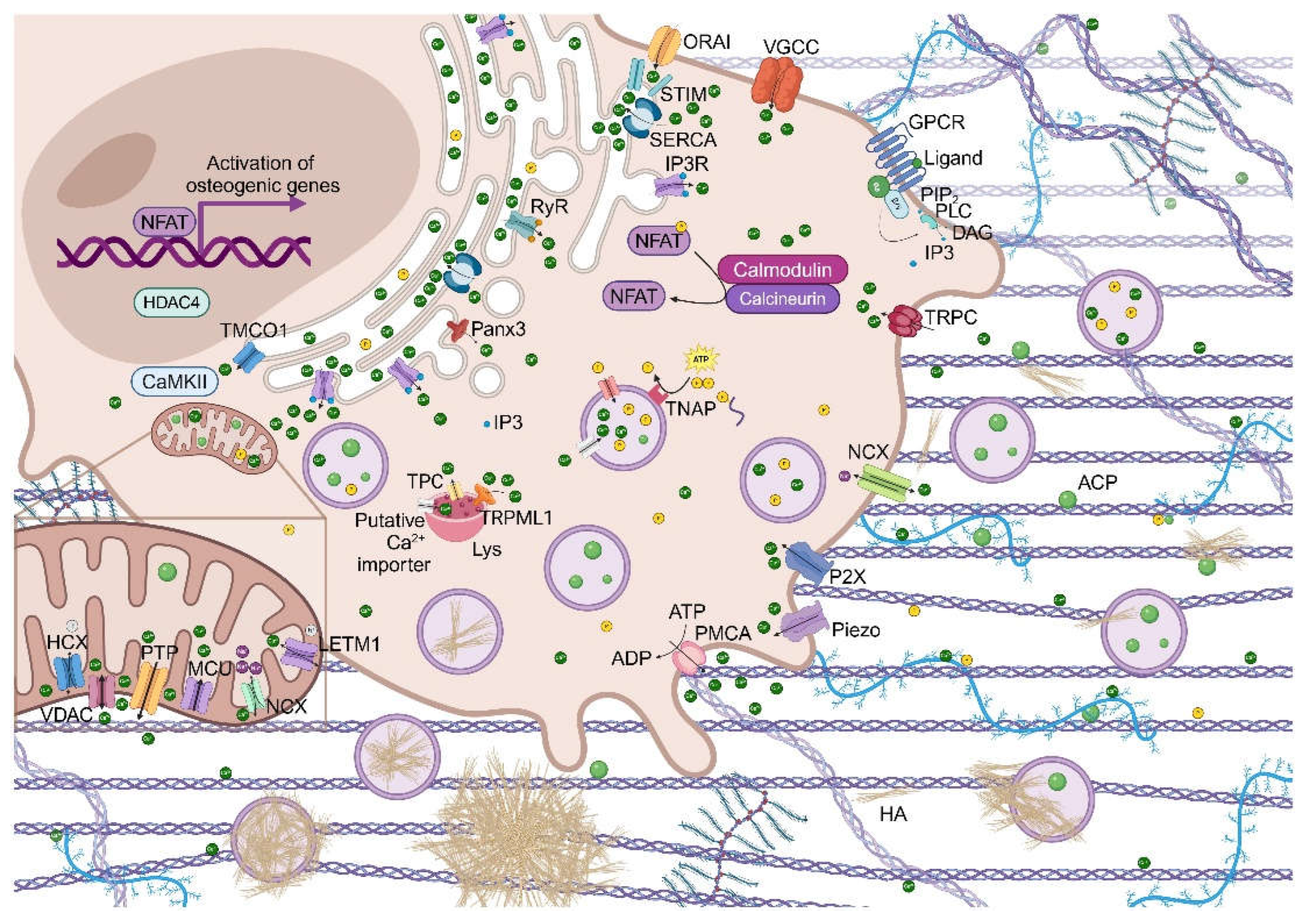
2. Calcium Signaling and Cellular Transcription Activity
3. Regulation of Calcium Homeostasis
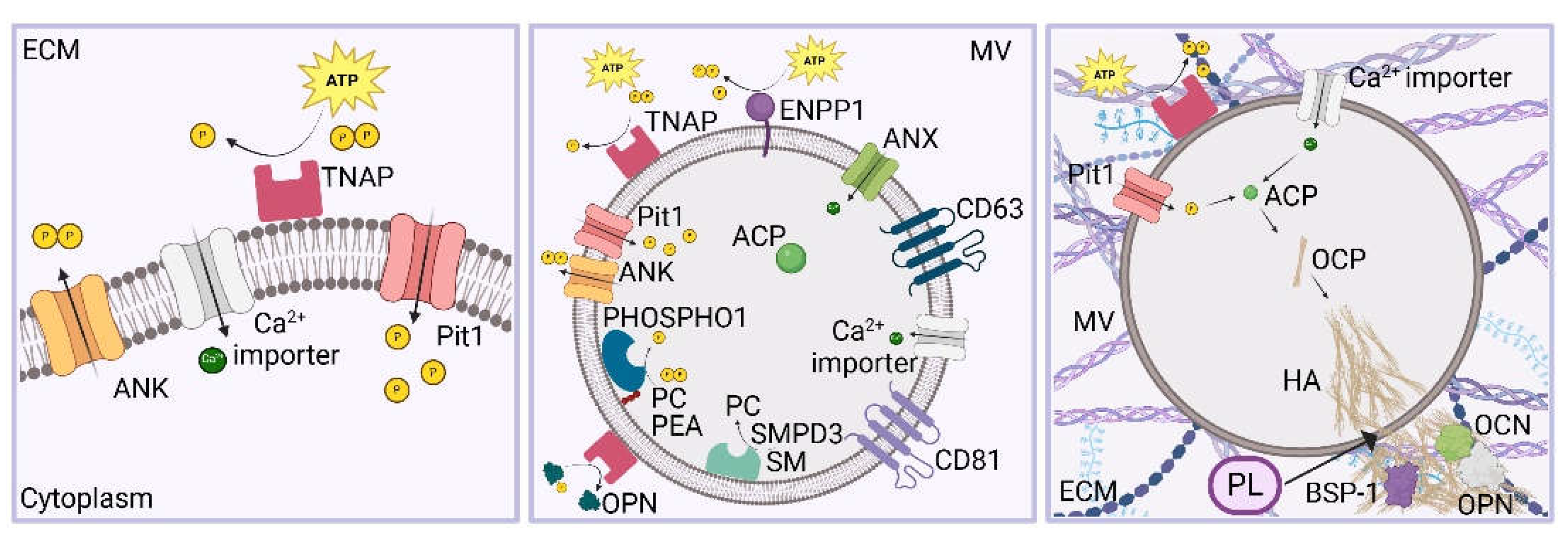
4. Formation of the Prebone and Bone Matrix
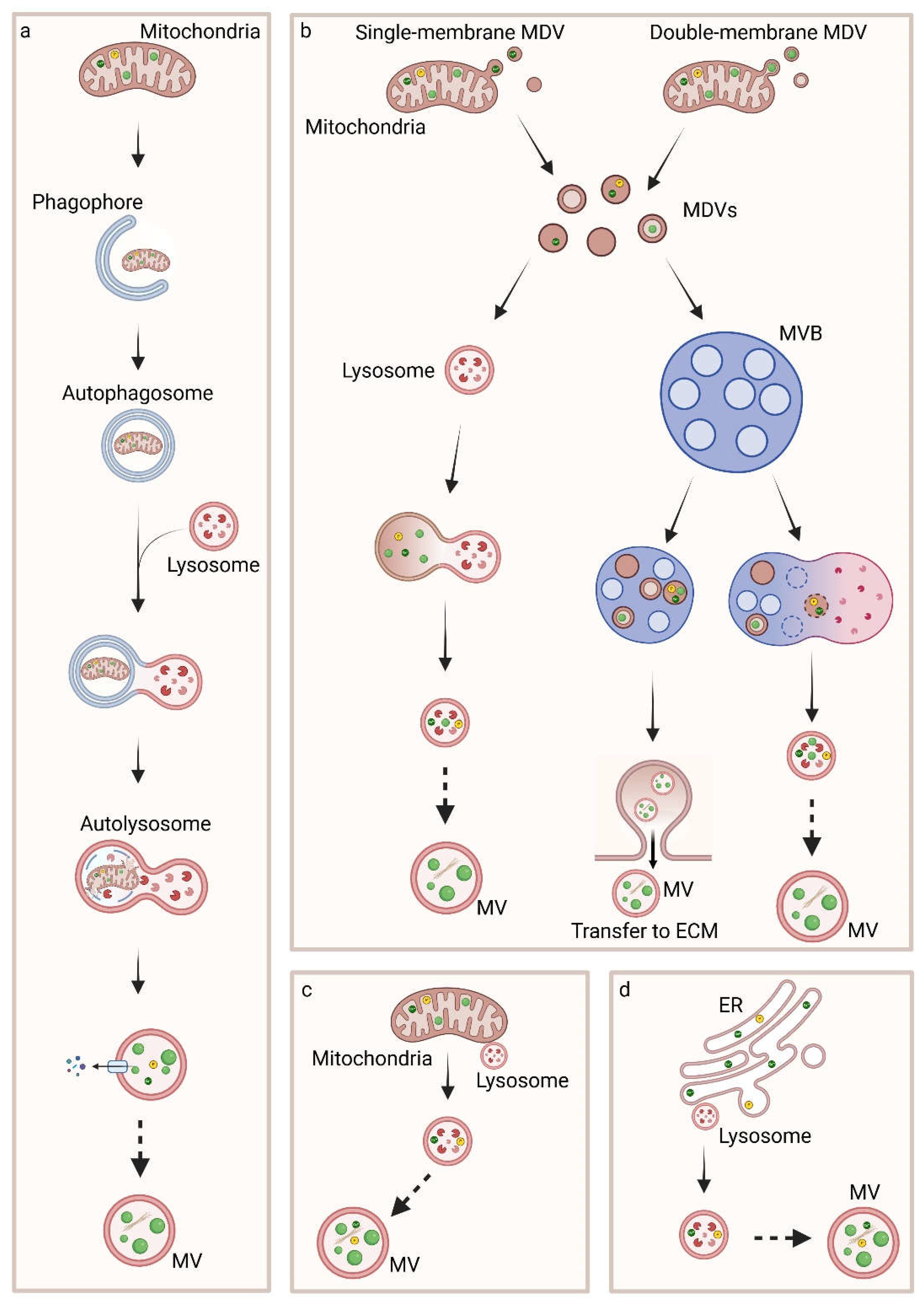
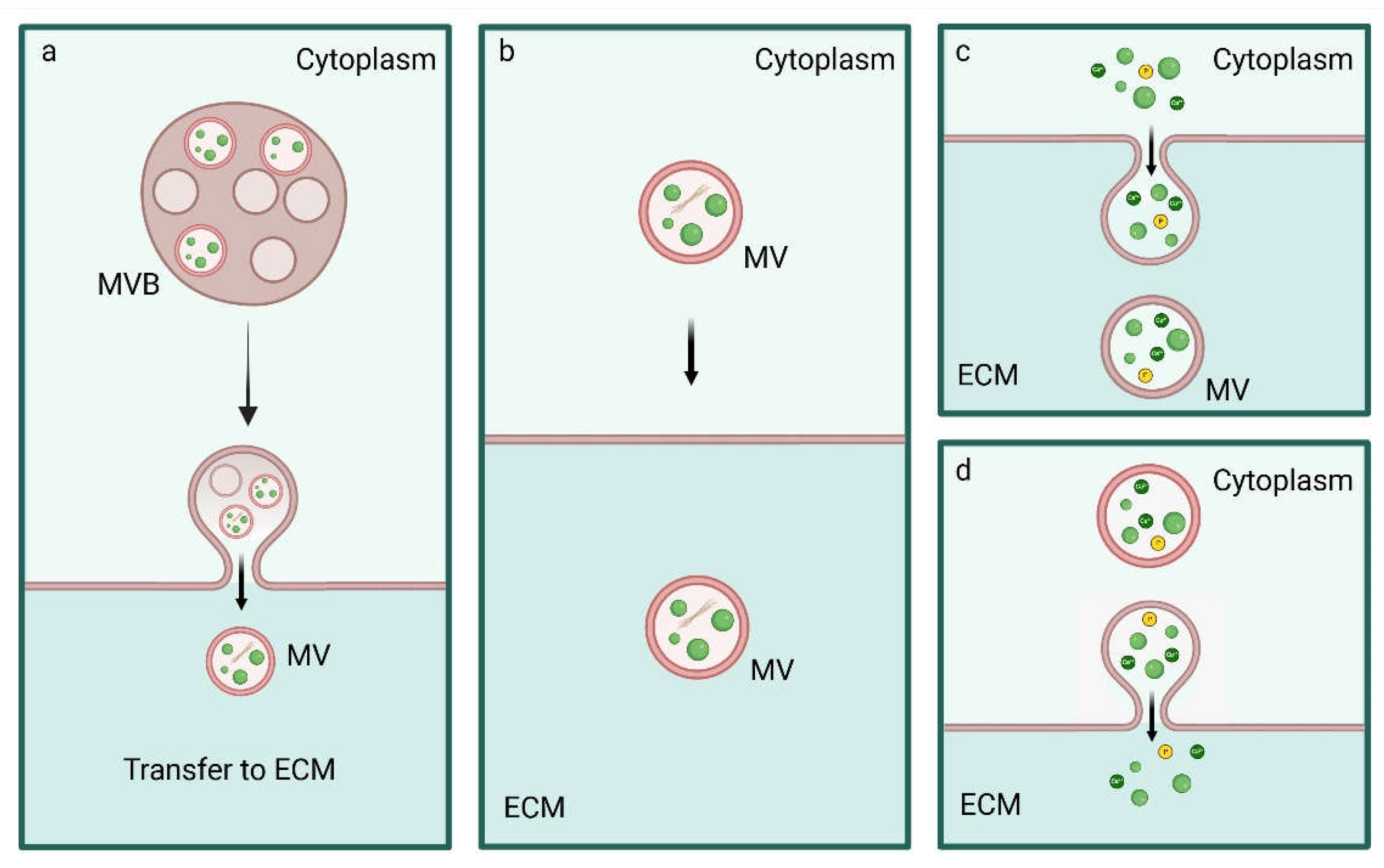
5. Biogenesis, Trafficking and Release of MVs
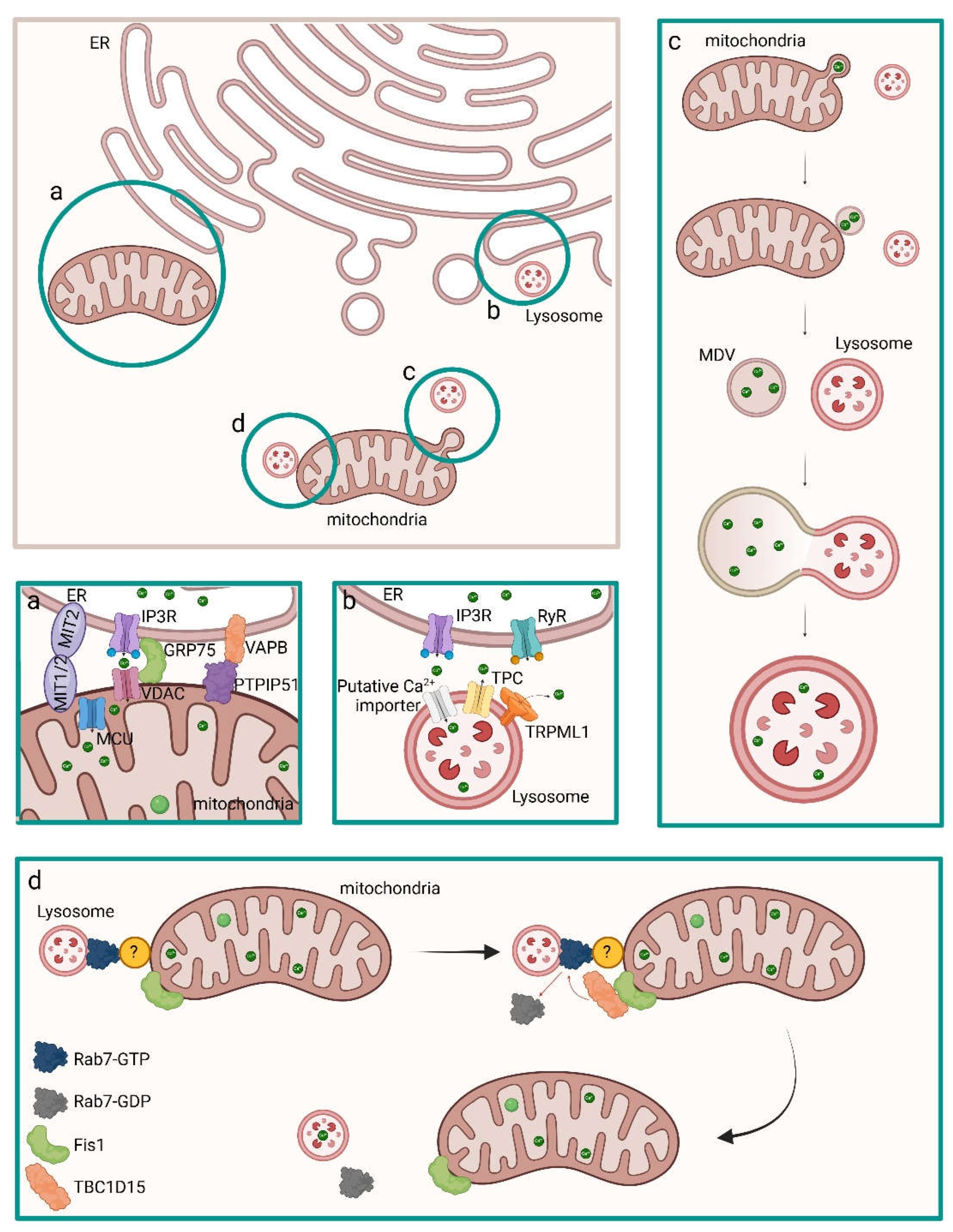
5.1. Inter-Organelle Communication and Physicochemical Structure Development of CaP
5.2. Regulation of MVs Biogenesis and Intracellular Trafficking
6. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, R.; Guo, J.; Chen, Y.; Zhang, Y.; Chen, L.; Xiong, W. The role of Ca. Cell Prolif 2021, 54 (11), e13122. DOI: 10.1111/cpr.13122. [CrossRef]
- Bartold, M.; Gronthos, S.; Haynes, D.; Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J Clin Periodontol 2019, 46 Suppl 21, 12-32. DOI: 10.1111/jcpe.13053. [CrossRef]
- Ariffin, N. S. RUNX1 as a Novel Molecular Target for Breast Cancer. Clin Breast Cancer 2022, 22 (6), 499-506. DOI: 10.1016/j.clbc.2022.04.006. [CrossRef]
- Krasnova, O.; Neganova, I. Assembling the Puzzle Pieces. Insights for in Vitro Bone Remodeling. Stem Cell Rev Rep 2023, 19 (6), 1635-1658. DOI: 10.1007/s12015-023-10558-6. [CrossRef]
- Chan, W. C. W.; Tan, Z.; To, M. K. T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int J Mol Sci 2021, 22 (11). DOI: 10.3390/ijms22115445. [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int J Mol Sci 2019, 20 (7). DOI: 10.3390/ijms20071694. [CrossRef]
- Teixeira, C. C.; Liu, Y.; Thant, L. M.; Pang, J.; Palmer, G.; Alikhani, M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem 2010, 285 (40), 31055-31065. DOI: 10.1074/jbc.M109.079962. [CrossRef]
- Khotib, J.; Marhaeny, H. D.; Miatmoko, A.; Budiatin, A. S.; Ardianto, C.; Rahmadi, M.; Pratama, Y. A.; Tahir, M. Differentiation of osteoblasts: the links between essential transcription factors. J Biomol Struct Dyn 2023, 41 (19), 10257-10276. DOI: 10.1080/07391102.2022.2148749. [CrossRef]
- Chen, G.; Deng, C.; Li, Y. P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012, 8 (2), 272-288. DOI: 10.7150/ijbs.2929. [CrossRef]
- Otto, F.; Thornell, A. P.; Crompton, T.; Denzel, A.; Gilmour, K. C.; Rosewell, I. R.; Stamp, G. W.; Beddington, R. S.; Mundlos, S.; Olsen, B. R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89 (5), 765-771. DOI: 10.1016/s0092-8674(00)80259-7. [CrossRef]
- Yoshida, C. A.; Furuichi, T.; Fujita, T.; Fukuyama, R.; Kanatani, N.; Kobayashi, S.; Satake, M.; Takada, K.; Komori, T. Core-binding factor beta interacts with Runx2 and is required for skeletal development. Nat Genet 2002, 32 (4), 633-638. DOI: 10.1038/ng1015. [CrossRef]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 2010, 339 (1), 189-195. DOI: 10.1007/s00441-009-0832-8. [CrossRef]
- Meyer, M. B.; Benkusky, N. A.; Pike, J. W. The RUNX2 cistrome in osteoblasts: characterization, down-regulation following differentiation, and relationship to gene expression. J Biol Chem 2014, 289 (23), 16016-16031. DOI: 10.1074/jbc.M114.552216. [CrossRef]
- Wu, H.; Whitfield, T. W.; Gordon, J. A.; Dobson, J. R.; Tai, P. W.; van Wijnen, A. J.; Stein, J. L.; Stein, G. S.; Lian, J. B. Genomic occupancy of Runx2 with global expression profiling identifies a novel dimension to control of osteoblastogenesis. Genome Biol 2014, 15 (3), R52. DOI: 10.1186/gb-2014-15-3-r52. [CrossRef]
- Weng, J. J.; Su, Y. Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochim Biophys Acta 2013, 1830 (3), 2839-2852. DOI: 10.1016/j.bbagen.2012.12.021. [CrossRef]
- Hojo, H.; Saito, T.; He, X.; Guo, Q.; Onodera, S.; Azuma, T.; Koebis, M.; Nakao, K.; Aiba, A.; Seki, M.; et al. Runx2 regulates chromatin accessibility to direct the osteoblast program at neonatal stages. Cell Rep 2022, 40 (10), 111315. DOI: 10.1016/j.celrep.2022.111315. [CrossRef]
- Schroeder, T. M.; Jensen, E. D.; Westendorf, J. J. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 2005, 75 (3), 213-225. DOI: 10.1002/bdrc.20043. [CrossRef]
- Franco, H. L.; Casasnovas, J.; Rodríguez-Medina, J. R.; Cadilla, C. L. Redundant or separate entities?--roles of Twist1 and Twist2 as molecular switches during gene transcription. Nucleic Acids Res 2011, 39 (4), 1177-1186. DOI: 10.1093/nar/gkq890. [CrossRef]
- Makowski, A. J.; Uppuganti, S.; Wadeer, S. A.; Whitehead, J. M.; Rowland, B. J.; Granke, M.; Mahadevan-Jansen, A.; Yang, X.; Nyman, J. S. The loss of activating transcription factor 4 (ATF4) reduces bone toughness and fracture toughness. Bone 2014, 62, 1-9. DOI: 10.1016/j.bone.2014.01.021. [CrossRef]
- Xiao, G.; Jiang, D.; Ge, C.; Zhao, Z.; Lai, Y.; Boules, H.; Phimphilai, M.; Yang, X.; Karsenty, G.; Franceschi, R. T. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem 2005, 280 (35), 30689-30696. DOI: 10.1074/jbc.M500750200. [CrossRef]
- Yang, X.; Matsuda, K.; Bialek, P.; Jacquot, S.; Masuoka, H. C.; Schinke, T.; Li, L.; Brancorsini, S.; Sassone-Corsi, P.; Townes, T. M.; et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 2004, 117 (3), 387-398. DOI: 10.1016/s0092-8674(04)00344-7. [CrossRef]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci 2020, 245, 117389. DOI: 10.1016/j.lfs.2020.117389. [CrossRef]
- Hojo, H. Emerging RUNX2-Mediated Gene Regulatory Mechanisms Consisting of Multi-Layered Regulatory Networks in Skeletal Development. Int J Mol Sci 2023, 24 (3). DOI: 10.3390/ijms24032979. [CrossRef]
- Li, J.; Liu, C.; Li, Y.; Zheng, Q.; Xu, Y.; Liu, B.; Sun, W.; Ji, S.; Liu, M.; Zhang, J.; et al. TMCO1-mediated Ca. Nat Commun 2019, 10 (1), 1589. DOI: 10.1038/s41467-019-09653-5. [CrossRef]
- Adhami, M.; Ghori-Javed, F. Y.; Chen, H.; Gutierrez, S. E.; Javed, A. Runx2 regulates the gene network associated with insulin signaling and energy homeostasis. Cells Tissues Organs 2011, 194 (2-4), 232-237. DOI: 10.1159/000324763. [CrossRef]
- Wei, J.; Shimazu, J.; Makinistoglu, M. P.; Maurizi, A.; Kajimura, D.; Zong, H.; Takarada, T.; Lezaki, T.; Pessin, J. E.; Hinoi, E.; et al. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell 2015, 161 (7), 1576-1591. DOI: 10.1016/j.cell.2015.05.029. [CrossRef]
- Liu, L.; Xie, H.; Zhao, S.; Huang, X. The GLUT1-mTORC1 axis affects odontogenic differentiation of human dental pulp stem cells. Tissue Cell 2022, 76, 101766. DOI: 10.1016/j.tice.2022.101766. [CrossRef]
- Masuda, T.; Kubota, H.; Sakuramoto, N.; Hada, A.; Horiuchi, A.; Sasaki, A.; Takeda, K.; Takeda, M.; Matsuo, H.; Sugiyama, H.; et al. RUNX-NFAT Axis As a Novel Therapeutic Target for AML and T Cell Immunity. Blood 2020, 136 (Supplement 1), 25-26. DOI: 10.1182/blood-2020-143458 (accessed 2/6/2025). [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front Cell Dev Biol 2020, 8, 601224. DOI: 10.3389/fcell.2020.601224. [CrossRef]
- Koga, T.; Matsui, Y.; Asagiri, M.; Kodama, T.; de Crombrugghe, B.; Nakashima, K.; Takayanagi, H. NFAT and Osterix cooperatively regulate bone formation. Nat Med 2005, 11 (8), 880-885. DOI: 10.1038/nm1270. [CrossRef]
- Canalis, E.; Schilling, L.; Eller, T.; Yu, J. Nuclear factor of activated T cells 1 and 2 are required for vertebral homeostasis. J Cell Physiol 2020, 235 (11), 8520-8532. DOI: 10.1002/jcp.29696. [CrossRef]
- Amarasekara, D. S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int J Mol Sci 2021, 22 (6). DOI: 10.3390/ijms22062851. [CrossRef]
- Chen, R.; Hao, Z.; Wang, Y.; Zhu, H.; Hu, Y.; Chen, T.; Zhang, P.; Li, J. Mesenchymal Stem Cell-Immune Cell Interaction and Related Modulations for Bone Tissue Engineering. Stem Cells Int 2022, 2022, 7153584. DOI: 10.1155/2022/7153584. [CrossRef]
- Hao, Y.; Yang, N.; Sun, M.; Yang, S.; Chen, X. The role of calcium channels in osteoporosis and their therapeutic potential. Front Endocrinol (Lausanne) 2024, 15, 1450328. DOI: 10.3389/fendo.2024.1450328. [CrossRef]
- Kito, H.; Ohya, S. Role of K. Int J Mol Sci 2021, 22 (19). DOI: 10.3390/ijms221910459. [CrossRef]
- S, C.; M, P. NFAT Signaling and Bone Homeostasis. J Hematol Thromb Dis: 2013; Vol. 1, p 102.
- Lee, H. L.; Bae, O. Y.; Baek, K. H.; Kwon, A.; Hwang, H. R.; Qadir, A. S.; Park, H. J.; Woo, K. M.; Ryoo, H. M.; Baek, J. H. High extracellular calcium-induced NFATc3 regulates the expression of receptor activator of NF-κB ligand in osteoblasts. Bone 2011, 49 (2), 242-249. DOI: 10.1016/j.bone.2011.04.006. [CrossRef]
- Hu, F.; Pan, L.; Zhang, K.; Xing, F.; Wang, X.; Lee, I.; Zhang, X.; Xu, J. Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: a pathway contributes to the proliferation of osteoblasts. PLoS One 2014, 9 (9), e107217. DOI: 10.1371/journal.pone.0107217. [CrossRef]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem 2006, 97 (1), 56-70. DOI: 10.1002/jcb.20675. [CrossRef]
- Shaw, J. P.; Utz, P. J.; Durand, D. B.; Toole, J. J.; Emmel, E. A.; Crabtree, G. R. Identification of a putative regulator of early T cell activation genes. Science 1988, 241 (4862), 202-205. DOI: 10.1126/science.3260404. [CrossRef]
- Winslow, M. M.; Pan, M.; Starbuck, M.; Gallo, E. M.; Deng, L.; Karsenty, G.; Crabtree, G. R. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell 2006, 10 (6), 771-782. DOI: 10.1016/j.devcel.2006.04.006. [CrossRef]
- Fromigué, O.; Haÿ, E.; Barbara, A.; Marie, P. J. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem 2010, 285 (33), 25251-25258. DOI: 10.1074/jbc.M110.110502. [CrossRef]
- Choo, M. K.; Yeo, H.; Zayzafoon, M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone 2009, 45 (3), 579-589. DOI: 10.1016/j.bone.2009.05.009. [CrossRef]
- Chen, W.; Zhang, X.; Siu, R. K.; Chen, F.; Shen, J.; Zara, J. N.; Culiat, C. T.; Tetradis, S.; Ting, K.; Soo, C. Nfatc2 is a primary response gene of Nell-1 regulating chondrogenesis in ATDC5 cells. J Bone Miner Res 2011, 26 (6), 1230-1241. DOI: 10.1002/jbmr.314. [CrossRef]
- Chen, S.; Pan, M. NFAT Signaling and Bone Homeostasis. Journal of Hematology & o Thromboembolic Diseases: 2013; Vol. 1.
- Pan, M. G.; Xiong, Y.; Chen, F. NFAT gene family in inflammation and cancer. Curr Mol Med 2013, 13 (4), 543-554. DOI: 10.2174/1566524011313040007. [CrossRef]
- Matsuo, K.; Galson, D. L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K. Z.; Bachler, M. A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 2004, 279 (25), 26475-26480. DOI: 10.1074/jbc.M313973200. [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E. F.; Mak, T. W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 2005, 202 (9), 1261-1269. DOI: 10.1084/jem.20051150. [CrossRef]
- Cao, X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res 2018, 6, 35. DOI: 10.1038/s41413-018-0040-9. [CrossRef]
- Gao, Y.; Wu, X.; Terauchi, M.; Li, J. Y.; Grassi, F.; Galley, S.; Yang, X.; Weitzmann, M. N.; Pacifici, R. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab 2008, 8 (2), 132-145. DOI: 10.1016/j.cmet.2008.07.001. [CrossRef]
- Sarode, A. Y.; Jha, M. K.; Zutshi, S.; Ghosh, S. K.; Mahor, H.; Sarma, U.; Saha, B. Residue-Specific Message Encoding in CD40-Ligand. iScience 2020, 23 (9), 101441. DOI: 10.1016/j.isci.2020.101441. [CrossRef]
- Bai, H.; Zhu, H.; Yan, Q.; Shen, X.; Lu, X.; Wang, J.; Li, J.; Chen, L. TRPV2-induced Ca. Cell Commun Signal 2018, 16 (1), 68. DOI: 10.1186/s12964-018-0280-8. [CrossRef]
- Park, H. J.; Baek, K.; Baek, J. H.; Kim, H. R. TNFα Increases RANKL Expression via PGE₂-Induced Activation of NFATc1. Int J Mol Sci 2017, 18 (3). DOI: 10.3390/ijms18030495. [CrossRef]
- Sitara, D.; Aliprantis, A. O. Transcriptional regulation of bone and joint remodeling by NFAT. Immunol Rev 2010, 233 (1), 286-300. DOI: 10.1111/j.0105-2896.2009.00849.x. [CrossRef]
- Okada, H.; Okabe, K.; Tanaka, S. Finely-Tuned Calcium Oscillations in Osteoclast Differentiation and Bone Resorption. Int J Mol Sci 2020, 22 (1). DOI: 10.3390/ijms22010180. [CrossRef]
- Parekh, A. B.; Putney, J. W. Store-operated calcium channels. Physiol Rev 2005, 85 (2), 757-810. DOI: 10.1152/physrev.00057.2003. [CrossRef]
- Ishikawa, M.; Williams, G.; Forcinito, P.; Petrie, R. J.; Saito, K.; Fukumoto, S.; Yamada, Y. Pannexin 3 ER Ca. Sci Rep 2019, 9 (1), 18759. DOI: 10.1038/s41598-019-55371-9. [CrossRef]
- Jin, X.; Zhang, Y.; Alharbi, A.; Hanbashi, A.; Alhoshani, A.; Parrington, J. Targeting Two-Pore Channels: Current Progress and Future Challenges. Trends Pharmacol Sci 2020, 41 (8), 582-594. DOI: 10.1016/j.tips.2020.06.002. [CrossRef]
- Wang, X.; Zhang, X.; Dong, X. P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151 (2), 372-383. DOI: 10.1016/j.cell.2012.08.036. [CrossRef]
- Boyman, L.; Williams, G. S.; Khananshvili, D.; Sekler, I.; Lederer, W. J. NCLX: the mitochondrial sodium calcium exchanger. J Mol Cell Cardiol 2013, 59, 205-213. DOI: 10.1016/j.yjmcc.2013.03.012. [CrossRef]
- Carraro, M.; Bernardi, P. The mitochondrial permeability transition pore in Ca. Cell Calcium 2023, 111, 102719. DOI: 10.1016/j.ceca.2023.102719. [CrossRef]
- Bischof, H.; Burgstaller, S.; Waldeck-Weiermair, M.; Rauter, T.; Schinagl, M.; Ramadani-Muja, J.; Graier, W. F.; Malli, R. Live-Cell Imaging of Physiologically Relevant Metal Ions Using Genetically Encoded FRET-Based Probes. Cells 2019, 8 (5). DOI: 10.3390/cells8050492. [CrossRef]
- García-Sancho, J. The coupling of plasma membrane calcium entry to calcium uptake by endoplasmic reticulum and mitochondria. J Physiol 2014, 592 (2), 261-268. DOI: 10.1113/jphysiol.2013.255661. [CrossRef]
- Pei, D. D.; Sun, J. L.; Zhu, C. H.; Tian, F. C.; Jiao, K.; Anderson, M. R.; Yiu, C.; Huang, C.; Jin, C. X.; Bergeron, B. E.; et al. Contribution of Mitophagy to Cell-Mediated Mineralization: Revisiting a 50-Year-Old Conundrum. Adv Sci (Weinh) 2018, 5 (10), 1800873. DOI: 10.1002/advs.201800873. [CrossRef]
- Reith, E. J. The binding of calcium within the Golgi saccules of the rat odontoblast. Am J Anat 1976, 147 (3), 267-270. DOI: 10.1002/aja.1001470302. [CrossRef]
- Jiang, D.; Zhao, L.; Clapham, D. E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 2009, 326 (5949), 144-147. DOI: 10.1126/science.1175145. [CrossRef]
- Báthori, G.; Csordás, G.; Garcia-Perez, C.; Davies, E.; Hajnóczky, G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem 2006, 281 (25), 17347-17358. DOI: 10.1074/jbc.M600906200. [CrossRef]
- de Brito, O. M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456 (7222), 605-610. DOI: 10.1038/nature07534. [CrossRef]
- Stenzinger, A.; Schreiner, D.; Koch, P.; Hofer, H. W.; Wimmer, M. Cell and molecular biology of the novel protein tyrosine-phosphatase-interacting protein 51. Int Rev Cell Mol Biol 2009, 275, 183-246. DOI: 10.1016/S1937-6448(09)75006-3. [CrossRef]
- De Vos, K. J.; Mórotz, G. M.; Stoica, R.; Tudor, E. L.; Lau, K. F.; Ackerley, S.; Warley, A.; Shaw, C. E.; Miller, C. C. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet 2012, 21 (6), 1299-1311. DOI: 10.1093/hmg/ddr559. [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M. R.; Cavagna, D.; Nagy, A. I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 2006, 175 (6), 901-911. DOI: 10.1083/jcb.200608073. [CrossRef]
- Vandecaetsbeek, I.; Vangheluwe, P.; Raeymaekers, L.; Wuytack, F.; Vanoevelen, J. The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 2011, 3 (5). DOI: 10.1101/cshperspect.a004184. [CrossRef]
- Garrity, A. G.; Wang, W.; Collier, C. M.; Levey, S. A.; Gao, Q.; Xu, H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 2016, 5. DOI: 10.7554/eLife.15887. [CrossRef]
- Lloyd-Evans, E.; Waller-Evans, H. Lysosomal Ca. Cold Spring Harb Perspect Biol 2020, 12 (6). DOI: 10.1101/cshperspect.a035311. [CrossRef]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol 2023, 33 (4), 312-323. DOI: 10.1016/j.tcb.2022.07.004. [CrossRef]
- Ferrucci, L.; Guerra, F.; Bucci, C.; Marzetti, E.; Picca, A. Mitochondria break free: Mitochondria-derived vesicles in aging and associated conditions. Ageing Res Rev 2024, 102, 102549. DOI: 10.1016/j.arr.2024.102549. [CrossRef]
- Iwayama, T.; Bhongsatiern, P.; Takedachi, M.; Murakami, S. Matrix Vesicle-Mediated Mineralization and Potential Applications. J Dent Res 2022, 101 (13), 1554-1562. DOI: 10.1177/00220345221103145. [CrossRef]
- Iwayama, T.; Okada, T.; Ueda, T.; Tomita, K.; Matsumoto, S.; Takedachi, M.; Wakisaka, S.; Noda, T.; Ogura, T.; Okano, T.; et al. Osteoblastic lysosome plays a central role in mineralization. Sci Adv 2019, 5 (7), eaax0672. DOI: 10.1126/sciadv.aax0672. [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N. D.; McComb, D. W.; Porter, A. E.; Stevens, M. M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A 2012, 109 (35), 14170-14175. DOI: 10.1073/pnas.1208916109. [CrossRef]
- Wong, Y. C.; Kim, S.; Peng, W.; Krainc, D. Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell Biol 2019, 29 (6), 500-513. DOI: 10.1016/j.tcb.2019.02.004. [CrossRef]
- Kirsch, T.; Harrison, G.; Golub, E. E.; Nah, H. D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J Biol Chem 2000, 275 (45), 35577-35583. DOI: 10.1074/jbc.M005648200. [CrossRef]
- Shapiro, I. M.; Landis, W. J.; Risbud, M. V. Matrix vesicles: Are they anchored exosomes? Bone 2015, 79, 29-36. DOI: 10.1016/j.bone.2015.05.013. [CrossRef]
- Blair, H. C.; Larrouture, Q. C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R. S.; Robinson, L. J.; Schlesinger, P. H.; Nelson, D. J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng Part B Rev 2017, 23 (3), 268-280. DOI: 10.1089/ten.TEB.2016.0454. [CrossRef]
- Cui, J.; Dean, D.; Hornicek, F. J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and metastasis. J Exp Clin Cancer Res 2020, 39 (1), 178. DOI: 10.1186/s13046-020-01685-w. [CrossRef]
- Bhadada, S. K.; Rao, S. D. Role of Phosphate in Biomineralization. Calcif Tissue Int 2021, 108 (1), 32-40. DOI: 10.1007/s00223-020-00729-9. [CrossRef]
- The Chemical Dynamics of Bone Mineral., William F. Newman and Margaret W. Newman. Chicago, The University of Chicago Press, 1958. xi + 209 pp. $5.00. Arthritis & Rheumatism 1958, 1 (5), 473-474. DOI: https://doi.org/10.1002/art.1780010510. [CrossRef]
- Yan, X.; Zhang, Q.; Ma, X.; Zhong, Y.; Tang, H.; Mai, S. The mechanism of biomineralization: Progress in mineralization from intracellular generation to extracellular deposition. Jpn Dent Sci Rev 2023, 59, 181-190. DOI: 10.1016/j.jdsr.2023.06.005. [CrossRef]
- Merametdjian, L.; Beck-Cormier, S.; Bon, N.; Couasnay, G.; Sourice, S.; Guicheux, J.; Gaucher, C.; Beck, L. Expression of Phosphate Transporters during Dental Mineralization. J Dent Res 2018, 97 (2), 209-217. DOI: 10.1177/0022034517729811. [CrossRef]
- Adams, C. S.; Mansfield, K.; Perlot, R. L.; Shapiro, I. M. Matrix regulation of skeletal cell apoptosis. Role of calcium and phosphate ions. J Biol Chem 2001, 276 (23), 20316-20322. DOI: 10.1074/jbc.M006492200. [CrossRef]
- Hu, P.; Lacruz, R. S.; Smith, C. E.; Smith, S. M.; Kurtz, I.; Paine, M. L. Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs 2012, 196 (6), 501-509. DOI: 10.1159/000337493. [CrossRef]
- Parry, D. A.; Poulter, J. A.; Logan, C. V.; Brookes, S. J.; Jafri, H.; Ferguson, C. H.; Anwari, B. M.; Rashid, Y.; Zhao, H.; Johnson, C. A.; et al. Identification of mutations in SLC24A4, encoding a potassium-dependent sodium/calcium exchanger, as a cause of amelogenesis imperfecta. Am J Hum Genet 2013, 92 (2), 307-312. DOI: 10.1016/j.ajhg.2013.01.003. [CrossRef]
- Goretti Penido, M.; Alon, U. S. Phosphate homeostasis and its role in bone health. Pediatr Nephrol 2012, 27 (11), 2039-2048. DOI: 10.1007/s00467-012-2175-z. [CrossRef]
- Landis, W. J.; Glimcher, M. J. Electron optical and analytical observations of rat growth plate cartilage prepared by ultracryomicrotomy: the failure to detect a mineral phase in matrix vesicles and the identification of heterodispersed particles as the initial solid phase of calcium phosphate deposited in the extracellular matrix. J Ultrastruct Res 1982, 78 (3), 227-268. DOI: 10.1016/s0022-5320(82)80001-4. [CrossRef]
- Appleton, J.; Lyon, R.; Swindin, K. J.; Chesters, J. Ultrastructure and energy-dispersive x-ray microanalysis of cartilage after rapid freezing, low temperature freeze drying, and embedding in Spurr's resin. J Histochem Cytochem 1985, 33 (10), 1073-1079. DOI: 10.1177/33.10.3900194. [CrossRef]
- Hsu, H. H.; Morris, D. C.; Davis, L.; Moylan, P.; Anderson, C. H. In vitro Ca deposition by rat matrix vesicles: is the membrane association of alkaline phosphatase essential for matrix vesicle-mediated calcium deposition? Int J Biochem 1993, 25 (12), 1737-1742. DOI: 10.1016/0020-711x(88)90301-1. [CrossRef]
- Mackie, E. J. Osteoblasts: novel roles in orchestration of skeletal architecture. Int J Biochem Cell Biol 2003, 35 (9), 1301-1305. DOI: 10.1016/s1357-2725(03)00107-9. [CrossRef]
- Mahamid, J.; Aichmayer, B.; Shimoni, E.; Ziblat, R.; Li, C.; Siegel, S.; Paris, O.; Fratzl, P.; Weiner, S.; Addadi, L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci U S A 2010, 107 (14), 6316-6321. DOI: 10.1073/pnas.0914218107. [CrossRef]
- Boraldi, F.; Lofaro, F. D.; Quaglino, D. Apoptosis in the Extraosseous Calcification Process. Cells 2021, 10 (1). DOI: 10.3390/cells10010131. [CrossRef]
- Brookes, P. S.; Yoon, Y.; Robotham, J. L.; Anders, M. W.; Sheu, S. S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004, 287 (4), C817-833. DOI: 10.1152/ajpcell.00139.2004. [CrossRef]
- Rohde, M.; Mayer, H. Exocytotic process as a novel model for mineralization by osteoblasts in vitro and in vivo determined by electron microscopic analysis. Calcif Tissue Int 2007, 80 (5), 323-336. DOI: 10.1007/s00223-007-9013-5. [CrossRef]
- Tang, C.; Wei, Y.; Gu, L.; Zhang, Q.; Li, M.; Yuan, G.; He, Y.; Huang, L.; Liu, Y.; Zhang, Y. Biomineral Precursor Formation Is Initiated by Transporting Calcium and Phosphorus Clusters from the Endoplasmic Reticulum to Mitochondria. Adv Sci (Weinh) 2020, 7 (8), 1902536. DOI: 10.1002/advs.201902536. [CrossRef]
- Akisaka, T.; Kawaguchi, H.; Subita, G. P.; Shigenaga, Y.; Gay, C. V. Ultrastructure of matrix vesicles in chick growth plate as revealed by quick freezing and freeze substitution. Calcif Tissue Int 1988, 42 (6), 383-393. DOI: 10.1007/BF02556357. [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int J Mol Sci 2020, 21 (7). DOI: 10.3390/ijms21072576. [CrossRef]
- GREENAWALT, J. W.; ROSSI, C. S.; LEHNINGER, A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol 1964, 23 (1), 21-38. DOI: 10.1083/jcb.23.1.21. [CrossRef]
- Anderson, H. C. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 1967, 35 (1), 81-101. DOI: 10.1083/jcb.35.1.81. [CrossRef]
- Bonucci, E. Fine structure of early cartilage calcification. J Ultrastruct Res 1967, 20 (1), 33-50. DOI: 10.1016/s0022-5320(67)80034-0. [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D. W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct Target Ther 2023, 8 (1), 304. DOI: 10.1038/s41392-023-01503-7. [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H. J.; Landi, F.; Bucci, C.; Marzetti, E. Mitochondrial-Derived Vesicles: The Good, the Bad, and the Ugly. Int J Mol Sci 2023, 24 (18). DOI: 10.3390/ijms241813835. [CrossRef]
- Docampo, R. The origin and evolution of the acidocalcisome and its interactions with other organelles. Mol Biochem Parasitol 2016, 209 (1-2), 3-9. DOI: 10.1016/j.molbiopara.2015.10.003. [CrossRef]
- Liou, W.; Geuze, H. J.; Geelen, M. J.; Slot, J. W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol 1997, 136 (1), 61-70. DOI: 10.1083/jcb.136.1.61. [CrossRef]
- Mahamid, J.; Sharir, A.; Gur, D.; Zelzer, E.; Addadi, L.; Weiner, S. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: a cryo-electron microscopy study. J Struct Biol 2011, 174 (3), 527-535. DOI: 10.1016/j.jsb.2011.03.014. [CrossRef]
- Ponpuak, M.; Mandell, M. A.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory autophagy. Curr Opin Cell Biol 2015, 35, 106-116. DOI: 10.1016/j.ceb.2015.04.016. [CrossRef]
- Somogyi, E.; Petersson, U.; Hultenby, K.; Wendel, M. Calreticulin--an endoplasmic reticulum protein with calcium-binding activity is also found in the extracellular matrix. Matrix Biol 2003, 22 (2), 179-191. DOI: 10.1016/s0945-053x(02)00117-8. [CrossRef]
- Petersson, U.; Somogyi, E.; Reinholt, F. P.; Karlsson, T.; Sugars, R. V.; Wendel, M. Nucleobindin is produced by bone cells and secreted into the osteoid, with a potential role as a modulator of matrix maturation. Bone 2004, 34 (6), 949-960. DOI: 10.1016/j.bone.2004.01.019. [CrossRef]
- Lavoie, C.; Meerloo, T.; Lin, P.; Farquhar, M. G. Calnuc, an EF-hand Ca(2+)-binding protein, is stored and processed in the Golgi and secreted by the constitutive-like pathway in AtT20 cells. Mol Endocrinol 2002, 16 (11), 2462-2474. DOI: 10.1210/me.2002-0079. [CrossRef]
- Bonucci, E. Fine structure and histochemistry of "calcifying globules" in epiphyseal cartilage. Z Zellforsch Mikrosk Anat 1970, 103 (2), 192-217. DOI: 10.1007/BF00337312. [CrossRef]
- Ali, S. Y.; Sajdera, S. W.; Anderson, H. C. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A 1970, 67 (3), 1513-1520. DOI: 10.1073/pnas.67.3.1513. [CrossRef]
- Anderson, H. C.; Matsuzawa, T.; Sajdera, S. W.; Ali, S. Y. Membranous particles in calcifying cartilage matrix. Trans N Y Acad Sci 1970, 32 (5), 619-630. DOI: 10.1111/j.2164-0947.1970.tb02737.x. [CrossRef]
- Anderson, H. C. Molecular biology of matrix vesicles. Clin Orthop Relat Res 1995, (314), 266-280.
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J 2021, 288 (1), 36-55. DOI: 10.1111/febs.15453. [CrossRef]
- Han, J.; Pluhackova, K.; Böckmann, R. A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front Physiol 2017, 8, 5. DOI: 10.3389/fphys.2017.00005. [CrossRef]
- Rosenthal, A. K.; Gohr, C. M.; Ninomiya, J.; Wakim, B. T. Proteomic analysis of articular cartilage vesicles from normal and osteoarthritic cartilage. Arthritis Rheum 2011, 63 (2), 401-411. DOI: 10.1002/art.30120. [CrossRef]
- Goettsch, C.; Hutcheson, J. D.; Aikawa, M.; Iwata, H.; Pham, T.; Nykjaer, A.; Kjolby, M.; Rogers, M.; Michel, T.; Shibasaki, M.; et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest 2016, 126 (4), 1323-1336. DOI: 10.1172/JCI80851. [CrossRef]
- Escrevente, C.; Bento-Lopes, L.; Ramalho, J. S.; Barral, D. C. Rab11 is required for lysosome exocytosis through the interaction with Rab3a, Sec15 and GRAB. J Cell Sci 2021, 134 (11). DOI: 10.1242/jcs.246694. [CrossRef]
- Krols, M.; van Isterdael, G.; Asselbergh, B.; Kremer, A.; Lippens, S.; Timmerman, V.; Janssens, S. Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol 2016, 131 (4), 505-523. DOI: 10.1007/s00401-015-1528-7. [CrossRef]
- Komarova, S. V.; Ataullakhanov, F. I.; Globus, R. K. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol 2000, 279 (4), C1220-1229. DOI: 10.1152/ajpcell.2000.279.4.C1220. [CrossRef]
- Vidavsky, N.; Masic, A.; Schertel, A.; Weiner, S.; Addadi, L. Mineral-bearing vesicle transport in sea urchin embryos. J Struct Biol 2015, 192 (3), 358-365. DOI: 10.1016/j.jsb.2015.09.017. [CrossRef]
- Marsh, M. E. Biomineralization in coccolithophores. Gravit Space Biol Bull 1999, 12 (2), 5-14.
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem Cell Biol 2018, 149 (4), 289-304. DOI: 10.1007/s00418-018-1646-0. [CrossRef]
- Wuthier, R. E.; Lipscomb, G. F. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci (Landmark Ed) 2011, 16 (8), 2812-2902. DOI: 10.2741/3887. [CrossRef]
- Skelton, A. M.; Cohen, D. J.; Boyan, B. D.; Schwartz, Z. Osteoblast-Derived Matrix Vesicles Exhibit Exosomal Traits and a Unique Subset of microRNA: Their Caveolae-Dependent Endocytosis Results in Reduced Osteogenic Differentiation. Int J Mol Sci 2023, 24 (16). DOI: 10.3390/ijms241612770. [CrossRef]
- De Rooij, J. F.; Heughebaert, J. C.; Nancollas, G. H. A ph study of calcium phosphate seeded precipitation. Journal of Colloid and Interface Science 1984, 100 (2), 350-358. DOI: https://doi.org/10.1016/0021-9797(84)90440-5. [CrossRef]
- Casey, J. R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 2010, 11 (1), 50-61. DOI: 10.1038/nrm2820. [CrossRef]
- Kerschnitzki, M.; Akiva, A.; Ben Shoham, A.; Asscher, Y.; Wagermaier, W.; Fratzl, P.; Addadi, L.; Weiner, S. Bone mineralization pathways during the rapid growth of embryonic chicken long bones. J Struct Biol 2016, 195 (1), 82-92. DOI: 10.1016/j.jsb.2016.04.011. [CrossRef]
- Liu, F.; Fang, F.; Yuan, H.; Yang, D.; Chen, Y.; Williams, L.; Goldstein, S. A.; Krebsbach, P. H.; Guan, J. L. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res 2013, 28 (11), 2414-2430. DOI: 10.1002/jbmr.1971. [CrossRef]
- Nollet, M.; Santucci-Darmanin, S.; Breuil, V.; Al-Sahlanee, R.; Cros, C.; Topi, M.; Momier, D.; Samson, M.; Pagnotta, S.; Cailleteau, L.; et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 2014, 10 (11), 1965-1977. DOI: 10.4161/auto.36182. [CrossRef]
- Yan, J.; Shen, M.; Sui, B.; Lu, W.; Han, X.; Wan, Q.; Liu, Y.; Kang, J.; Qin, W.; Zhang, Z.; et al. Autophagic LC3. Sci Adv 2022, 8 (19), eabn1556. DOI: 10.1126/sciadv.abn1556. [CrossRef]
- Hale, J. E.; Wuthier, R. E. The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem 1987, 262 (4), 1916-1925.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



