1. Introduction
Electroencephalography (EEG) is a widely utilized, non-invasive neurophysiological tool that plays a crucial role in evaluating various neurological conditions, including epilepsy, altered mental status, and encephalopathies. EEG remains the gold standard for detecting epileptiform activity and monitoring brain function in both inpatient and outpatient settings. Despite its diagnostic utility, concerns have been raised regarding its overuse in clinical practice, particularly in cases where its effectiveness in altering clinical management is limited [
1,
2].
The routine EEG (REEG), which involves a standard 30-minute recording, is frequently ordered for patients with a wide range of neurological symptoms, from seizure disorders to non-specific complaints such as dizziness and headache [
3]. However, previous studies have shown that EEG findings do not always correlate with clinical symptoms, leading to unnecessary investigations, increased healthcare costs, and potential misdiagnoses [
4]. Studies conducted in Saudi Arabia suggest that despite the widespread use of EEG, its diagnostic yield varies considerably depending on the indication for the test. A study from King Abdul-Aziz University Hospital in the Western region of Saudi Arabia reported that EEG was often ordered for ICU patients with limited impact on clinical decision-making [
5].
Identifying factors associated with abnormal EEG findings is essential for optimizing its clinical utility. Research has demonstrated that certain demographic and clinical variables, such as age, gender, and the presence of specific neurological symptoms, significantly influence the likelihood of detecting abnormalities [
6]. A study conducted in Saudi Arabia found that males and elderly patients exhibited higher rates of EEG abnormalities compared to younger individuals and females, indicating a potential demographic bias in EEG interpretation and utilization [
2]. Moreover, recent studies have identified specific factors associated with EEG abnormalities. For instance, Gao and colleagues in 2025 demonstrated that patients with prolonged disease duration and higher seizure frequency exhibit markedly increased epileptiform activity, indicating that the severity and chronicity of the condition are strong predictors of EEG abnormalities. Similarly, Dasgupta and colleagues in 2024 found that in pediatric status epilepticus, abnormal EEG patterns—particularly rhythmic and periodic discharges—are significantly correlated with poor clinical outcomes, including prolonged hospital stays and increased mortality. Together, these studies underscore the importance of integrating clinical severity markers and early EEG findings in predicting and managing neurological outcomes. [
7,
8].
Given the ongoing concerns regarding the appropriate utilization of EEG, this study aims to analyze EEG ordering patterns and their diagnostic yield in a secondary hospital in Saudi Arabia. Additionally, it seeks to identify the factors associated with EEG abnormalities and explore their implications for clinical decision-making. By evaluating these trends, this research aims to improve the efficiency and accuracy of EEG use in neurological practice within the Saudi healthcare system.
2. Materials and Methods
2.1. Study Design
This study is a retrospective analysis conducted at Alkharj Military Industries Corporation Hospital (AKMICH), a secondary hospital in Alkharj, Saudi Arabia. The study period extends from May to December 2024.
2.2. Study Population
The study includes all patients who underwent EEG during the specified study period. Inclusion and exclusion criteria were defined to ensure appropriate selection of cases; inpatients and outpatients were included if they underwent EEG for diagnostic purposes from all hospital departments. Exclusion criteria involved patients with incomplete medical records or poor-quality EEG recordings.
2.3. Data Collection
Data was collected from the hospital’s electronic medical records and EEG reports. Variables extracted included patient demographics (age, gender), clinical indications for EEG referral, specialty of referring doctor, and EEG findings. Additional clinical data, such as medical history and prior epilepsy diagnoses, were also recorded where available.
2.4. EEG Indication Categorization
In this study, EEG indications were categorized into distinct clinical groups based on their underlying neurological manifestations. The primary categories included (1) epilepsy evaluation; encompassing conditions such as focal epilepsy, generalized epilepsy, and juvenile myoclonic epilepsy, and (2) seizure evaluation; which included tonic-clonic seizures, absence seizures, nonconvulsive seizures, and status epilepticus. Additionally, (3) sensory changes; tingling, numbness, and paresthesia and (4) motor changes; such as hyperkinetic movements, weakness, facial spasms, nystagmus, dystonia, and tremors were recognized as distinct classifications due to their frequent neurological presentations. (5) Cognitive changes; such as amnesia and dementia, (6) syncope-related EEG requests included cases linked to vasovagal syncope, orthostatic hypotension, and neurocardiogenic syncope, whereas (7) changes in consciousness were classified separately, covering conditions such as dropped or altered mental status, coma, delirium, and acute encephalopathy. (8) Dizziness was a separate category, while (9) post-cardiac arrest evaluations focused on identifying hypoxic-ischemic encephalopathy, and prolonged post-anoxic states. (10) Stroke-related EEG indications were primarily associated with ischemic and hemorrhagic stroke, including brainstem infarctions and intracerebral hemorrhages. Additionally, (11) headache-related EEG assessments addressed conditions such as migraines and thunderclap headaches, often suspected of underlying neurological pathology. Finally, (12) the "Others" category comprised unclassified findings, including pneumonia, acute kidney injury (AKI) or Paroxysmal atrial fibrillation. The systematic grouping of EEG indications enhances diagnostic clarity to facilitate targeted statistical assessments.
2.5. EEG Acquisition and Interpretation
The EEG recordings were performed using standardized protocols. Each enrolled patient undergoes a standard 30-minute EEG obtained with a full set of 19 cup electrodes applied according to the international 10–20 system, with all electrode impedances ≤ 5 kΩ. All EEGs were interpreted by an epilepsy and EEG trained neurologists. The presence of EEG background changes, interictal epileptiform discharges (IEDs), rhythmic and periodic patterns as well as Ictal Interictal continuum (IIC) were documented.
2.6. Statistical Analysis
Descriptive statistics were used to summarize demographic and clinical characteristics. The yield of EEG was analyzed concerning different risk factors, including age, gender, indication for EEG, and referring doctor specialty. Chi-square and logistic regression were employed to assess associations between risk factors and EEG outcomes. A p-value of < 0.05 was considered statistically significant. All analysis and visualizations were illustrated using Python (version 3).
3. Results
3.1. Study Population Characteristics
During the study period, the study included 227 participants, with 135 males (59.5%) and 92 females (40.5%). The average age was 49.4 years (ranging from 14 to 98 years), out of which, 58 (25.6%) were elderly (older than 65 years old). Moreover, 121 (53.3%) were inpatients, while 106 (46.7%) were outpatients. The most common requesting specialty was Neurology (92%), followed by ICU (4.4%). All characteristics are shown in (
Table 1).
3.2. Clinical Indications for EEG
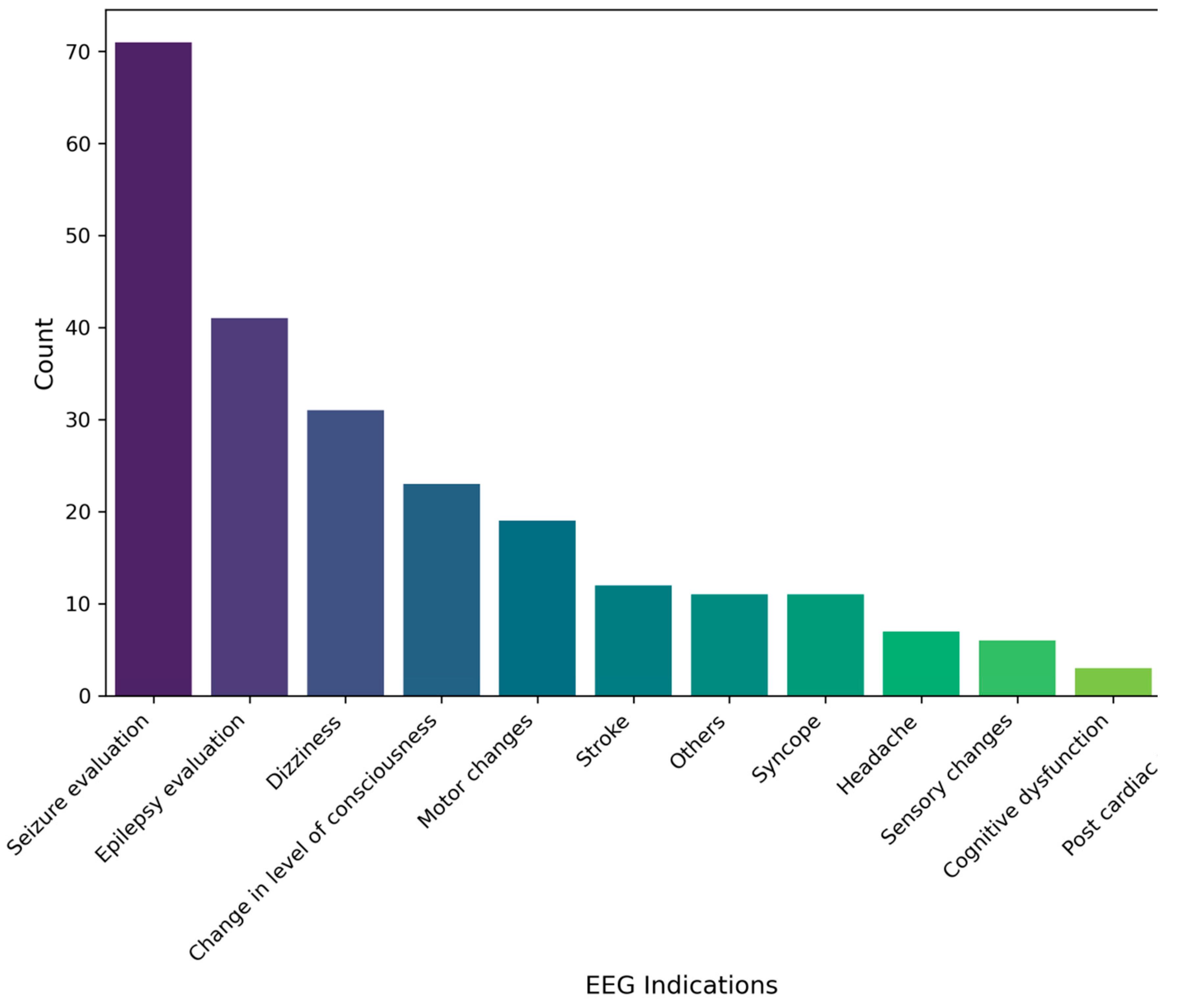
The most common indications for EEG requests among participants were seizure evaluation 71 (31.2%), followed by epilepsy evaluation 41 (18.0%), and dizziness 31 (13.6%). The least indications for requests were post cardiac arrest and cognitive dysfunction as 3 (1.3%) for each. All results are illustrated in (
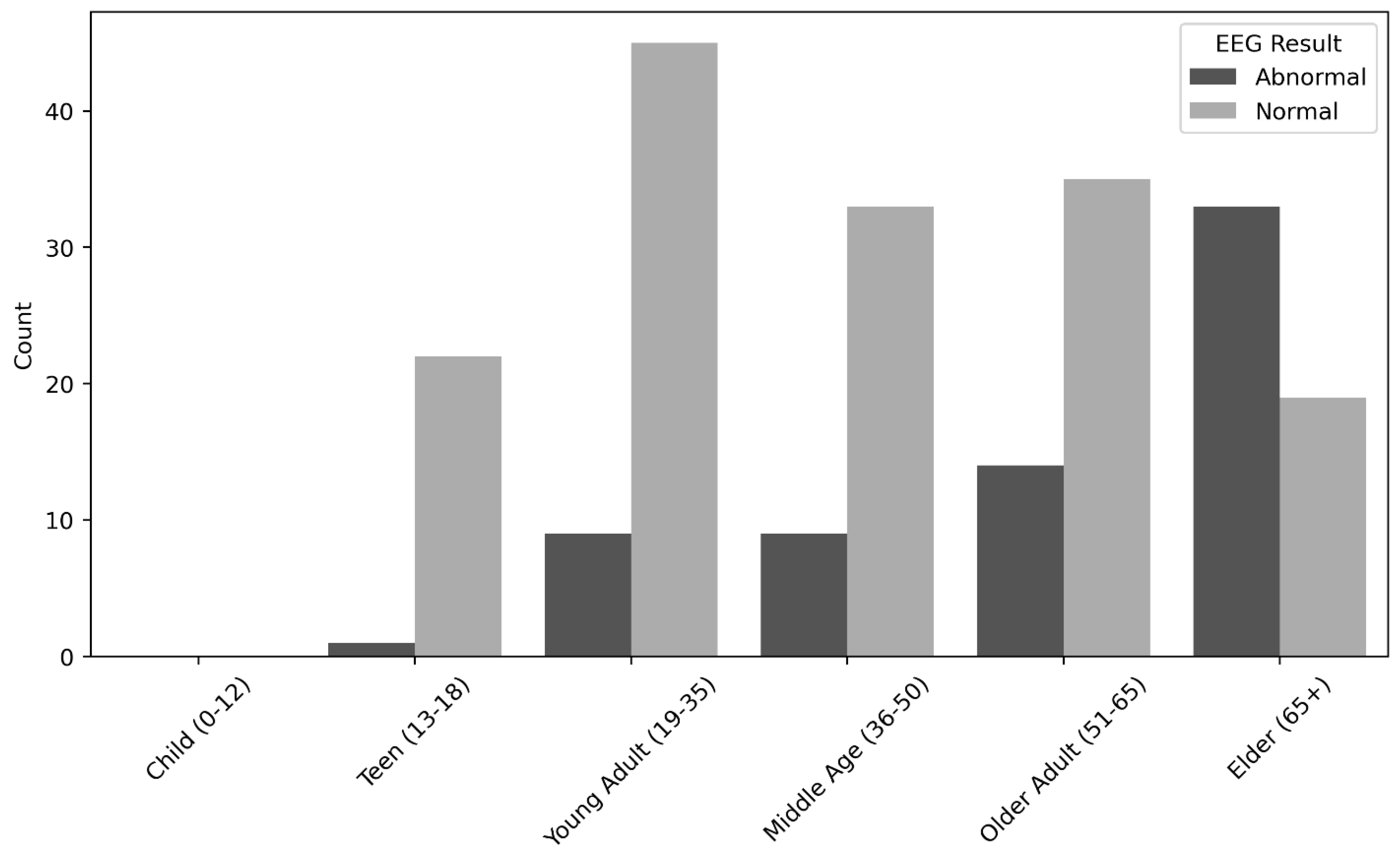
Figure 1). Statistical analysis indicated a significant positive relationship between male gender (P = <0.01), elderly (P = <0.01), patients complained of dizziness (P = <0.01), and patients presented with post cardiac arrest (P = 0.03) and the EEG abnormal findings. On the other hand, a significant negative relationship was detected between teens (P = <0.01) as well as young adults (P = <0.01), female gender (P = <0.01) and the EEG abnormal findings. All results are depicted in (
Table 2 &
Figure 2).
3.3. EEG Findings
Out of all participants (n=227), 7 were excluded due to technical challenges. Among the remaining 220 participants; normal EEG results were found in 154 participants (68%), while 66 participants (29%); 56 inpatients and 10 outpatients had abnormal findings. Among which, 62 (94%) displayed background changes, while 4 (6%) maintained a normal background. Notably, all 4 cases with a normal background exhibited epileptiform discharges.; background were generalized theta slowing in 42 (64%), generalized delta slowing in 10 (7%)and focal slowing in 11 (16%), moreover, the background discontinuous (10 – 49% periods of suppression) in 2 (3%) and burst attenuated in 1 (1%) was observed, furthermore, voltage attenuation was observed in 2 participants (3%), while suppression was noted in 4 participants (6%). Significant associations were detected between background changes and both dizziness (P = <0.01) and altered consciousness (P = 0.04). Moreover, a significant association was found specifically between generalized EEG slowing and patients with dizziness (P = 0.02) or altered consciousness (P <0.01). with<0.01No other significant relationships were detected, as illustrated in (
Table 3).
Moreover, sporadic epileptiform discharges, rhythmic and periodic patterns and electrographic or electroclinical seizures were documented among all abnormal EEG findings (n= 66), epileptiform discharges were observed in 10 (15%); sporadic epileptiform discharges were detected in 7 participants (10%), while rhythmic and periodic patterns was detected in 3 participants (5%), electrographic or electroclinical seizures was not detected among any participants. Significant association was detected between epileptiform discharges and “others” category of EEG indications (p = 0.03)., however, due to low sample size, this finding is unlikely to be relevant. All results are depicted in (
Table 3).
4. Discussion
The current study's demographic analysis highlights a predominance of male participants (59.5%) over female participants (40.5%), with an average age of 49.4 years. A considerable portion (25.6%) were elderly (65+ years), reinforcing the established link between aging and an increased likelihood of neurological conditions necessitating EEG evaluations. A study by Jafari and colleagues in 2025 similarly identified a greater prevalence of EEG abnormalities among the elderly, attributing this trend to age-related neurophysiological changes [
9]. In Saudi Arabia, Aljafen and colleagues found comparable gender trends but reported a slightly lower mean age, likely due to demographic differences in their cohort [
4].
Seizure evaluation (31.3%) was the leading reason for EEG requests, followed by epilepsy assessment (18.0%) and dizziness (13.6%). The least frequent indications included post-cardiac arrest and cognitive dysfunction, both accounting for only 1.3%. The dominance of seizure-related indications confirms the critical role of EEG in diagnosing epilepsy and related disorders.
Chmiel and Stępień-Słodkowska in 2025 also identified epilepsy as the most common reason for EEG referrals [
10]. Moreover, a recent Saudi systematic review by Almuqairsha and colleagues in 2024 reported similar findings, further validating epilepsy as the most frequent EEG indication in the region [
11].
The current statistical analysis revealed a significant association between abnormal EEG findings and male gender, older age, dizziness, and post-cardiac arrest cases. In contrast, younger individuals, females, and teenagers exhibited fewer abnormal EEG patterns. These findings imply that neurological disorders manifest more frequently in elderly males. Supporting this finding; a recent review conducted by Arnold and Young in 2025 observed increased EEG abnormalities among males, suggesting sex-based neurophysiological differences [
12].
Among participants, 68% had normal EEGs while 29% had abnormal findings. Generalized theta slowing was the most prevalent abnormality (64%), followed by focal slowing (16%), and voltage suppression (6%). These findings align with existing literature that examines the prevalence and significance of EEG slowing across various neurological conditions. Kimchi and colleagues noted a high prevalence of generalized slowing as well as theta generalized slowing among participants and concluded a significant association between generalized slowing and poor clinical outcome, nevertheless, it is worth noting that all participants in their study were inpatients of altered mental status [
13]. Moreover, Guo and colleagues in 2024 investigated EEG abnormalities in patients with anti-LGI1 antibody encephalitis, revealing that generalized and focal slow waves were prevalent, particularly in the delta and theta bands, suggesting widespread cortical dysfunction. Similarly, Pirgit and Beniczky in a systematic review published in 2024 analyzed EEG patterns in elderly patients with seizures and cognitive disorders, finding that generalized slowing was more common in dementia, whereas focal slowing was linked to localized vascular damage and epilepsy. Their study underscores EEG slowing as a key diagnostic marker distinguishing neurodegenerative from epileptic conditions. Further, Hadar and colleagues in 2024 examined epileptic patients with sleep disorders, demonstrating that EEG slowing, particularly in the theta and delta bands, was associated with cognitive decline and seizure progression. This suggests that EEG slowing not only serves as a diagnostic tool but may also reflect accelerated brain aging in epilepsy. Collectively, these studies reinforce the clinical relevance of generalized and focal slowing in EEG and highlight their role in diagnosing and monitoring neurological and neurodegenerative disorders [
14,
15,
16].
A statistically significant correlation was found between generalized slowing and individuals presenting with dizziness (P = 0.02) and altered level of consciousness (P = <0.01). However, no substantial associations emerged between EEG abnormalities and other clinical factors such as gender or hospital specialty. Tayeb and colleagues in 2020 conducted a study in Saudi Arabia, they further documented these findings, highlighting the diagnostic value of EEG in patients with altered consciousness [
1].
The current study identified sporadic epileptiform discharges (10%) and rhythmic and periodic patterns (5%). Zhan and colleagues in 2025 reported a higher prevalence of epileptiform discharges (100%), which can be attributed to their study's focus on epilepsy-specific cases [
17]. Electrographic seizures were not observed. This is likely due to the sample size and the duration of the EEG, as the sensitivity for detecting electrographic seizures, particularly in ICU patients, significantly increases with 24 to 48 hours of continuous EEG. This is evident in literature, as Friedman, Claassen, and Hirsch in 2009 reported that in comatose ICU patients, seizure detection increased to 48% with at least 24 hours of continuous EEG (cEEG), emphasizing that longer monitoring durations are essential to capture intermittent seizures. Similarly, Elmer and colleagues in 2020 found that electrographic seizures after cardiac arrest were often transient and episodic, with many cases requiring more than 24 hours of cEEG monitoring to identify ictal activity. This reinforces that a single routine EEG is insufficient in critically ill patients [
18,
19].
5. Conclusions
This study highlights the diagnostic role of EEG in evaluating neurological disorders, particularly in elderly male patients and those presenting with dizziness or altered consciousness. While EEG remains a valuable tool, its diagnostic yield varies based on clinical indications, emphasizing the need for more selective use in certain cases. The findings also suggest that demographic factors, such as age and gender, may influence EEG abnormalities, warranting further investigation. Optimizing EEG utilization through refined clinical guidelines could improve resource efficiency and diagnostic accuracy. Future research should focus on establishing clearer indications for EEG in different patient populations to enhance its clinical utility.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Research and Ethics Committee in Alkharj in AKMICH.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tayeb, H.O.; Yaghmoor, B.E.; Basheikh, M.; Alyoubi, R.A.; Zamzami, A.M.; Almalki, A.A.; et al. The diagnostic yield of the routine EEG at a tertiary care center in Saudi Arabia: A retrospective study. Epilepsy Res. 2020, 166, 106399. [Google Scholar] [CrossRef]
- Aljalal, M.; Aldosari, S.A.; AlSharabi, K.; Alturki, F.A. EEG-Based Detection of Mild Cognitive Impairment Using DWT-Based Features and Optimization Methods. Diagnostics. 2024, 14, 1619. [Google Scholar] [CrossRef]
- Tayeb, H.O. The yield of continuous EEG monitoring in the intensive care unit at a tertiary care hospital in Saudi Arabia: A retrospective study. F1000Res. 2019, 8, 1923. [Google Scholar] [CrossRef] [PubMed]
- Aljafen, B.N.; Alfayez, S.M.; Alanazy, M.H.; Alazwary, N.; Alohali, S.M.; Muayqil, T. Epilepsy monitoring units in Saudi Arabia: Where do we stand compared to developed countries? Neurosciences (Riyadh). 2018, 23, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.A.; Alnajashi, H. The Utility of Electroencephalogram in Intensive Care Unit Patients at King Abdul-Aziz University Hospital. Med Sci. 2014, 6, 19–27. [Google Scholar]
- Owolabi, L.F.; Reda, A.A.; El Sayed, R.; Morsy, D.F.M.; Enwere, O.O.; Mba, U.A.; et al. Study of electroencephalography in people with generalized epilepsy in a Saudi population. J Community Hosp Intern Med Perspect. 2020, 10, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Gu, L.; Zuo, W.; Wang, P. Comprehensive predictors of drug-resistant epilepsy in MELAS: clinical, EEG, imaging, and biochemical factors. BMC Neurol. 2025, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Mukherjee, A.; Guha, G.; Sen, S.; Roy, K. Evaluation of Risk Factors and Prognostic Indicators in Pediatric Status Epilepticus. Eur J Cardiovasc Med. 2024, 14, 685–689. [Google Scholar] [CrossRef]
- Jafari, M.; Tao, X.; Barua, P.; Tan, R.S.; Acharya, U.R. Application of Transfer Learning for Biomedical Signals: A Comprehensive Review of the Last Decade (2014–2024). Inf Fusion. 2025, 118, 102982. [Google Scholar] [CrossRef]
- Chmiel, J.; Stępień-Słodkowska, M. Resting-State EEG Oscillations in Amyotrophic Lateral Sclerosis (ALS): Toward Mechanistic Insights and Clinical Markers. J Clin Med. 2025, 14, 545. [Google Scholar] [CrossRef]
- Almuqairsha, S.A.; Al-Harbi, F.A.; Alaidah, A.M.; Al-Mutairi, T.A.; Al-Oadah, E.K.; Almatham, A.E.; et al. Demographics, Clinical Characteristics, and Management Strategies of Epilepsy in Saudi Arabia: A Systematic Review. Cureus. 2024, 16, e63436. [Google Scholar] [CrossRef] [PubMed]
- Arnold, V.X.; Young, S.D. The Potential of Wearable Sensors for Detecting Cognitive Rumination: A Scoping Review. Sensors (Basel). 2025, 25, 654. [Google Scholar] [CrossRef]
- Kimchi, E.Y.; Neelagiri, A.; Whitt, W.; Sagi, A.R.; Ryan, S.L.; Gadbois, G.; et al. Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology. 2019, 93, e1260–e1271. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, H.; Sun, Y.; Xing, Y.; Guo, X.; Shen, Z.; et al. Clinical Features and Electroencephalogram Analysis of Brain Network Functional Connectivity in Anti-Leucine-Rich Glioma-Inactivated 1 Antibody Encephalitis. J Inflamm Res. 2024, 29, 7881–7891. [Google Scholar] [CrossRef] [PubMed]
- Pirgit, M.L.; Beniczky, S. EEG and semiology in the elderly: A systematic review. Seizure. 2024, 7, S1059-1311(24)00251-6. [Google Scholar] [CrossRef]
- Hadar, P.N.; Westmeijer, M.; Sun, H.; Meulenbrugge, E.J.; Jing, J.; Paixao, L.; et al. Epilepsy is associated with the accelerated aging of brain activity in sleep. Front Physiol. 2024, 28, 1458592. [Google Scholar] [CrossRef]
- Zhan, Y.; Chen, S.; Jin, Z.; Zhou, J.; Zhang, Y.X.; Hou, Q.; et al. Mild malformation of cortical development with oligodendroglial hyperplasia and epilepsy. Neurol Genet. 2025, 11. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Claassen, J.; Hirsch, L.J. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009, 109, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Coppler, P.J.; Solanki, P.; Westover, M.B.; Struck, A.F.; Baldwin, M.E.; et al. Sensitivity of Continuous Electroencephalography to Detect Ictal Activity After Cardiac Arrest. JAMA Netw Open. 2020, 3, e203751. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).