Submitted:
31 March 2025
Posted:
02 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Notch Signal Transduction System
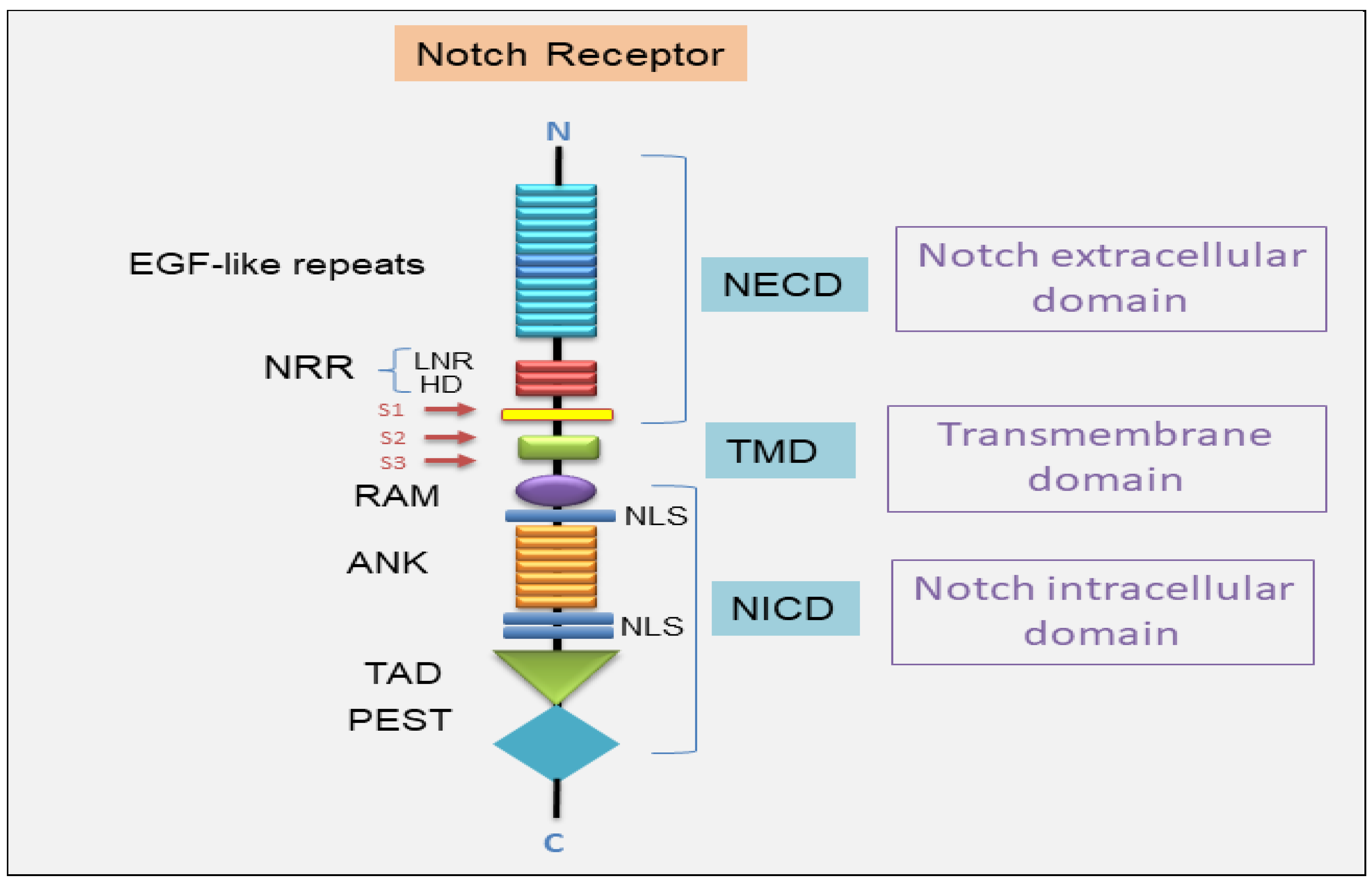
2.1. The Canonical Notch Signaling Pathway
2.2. The Non-Canonical Notch Signaling Pathway
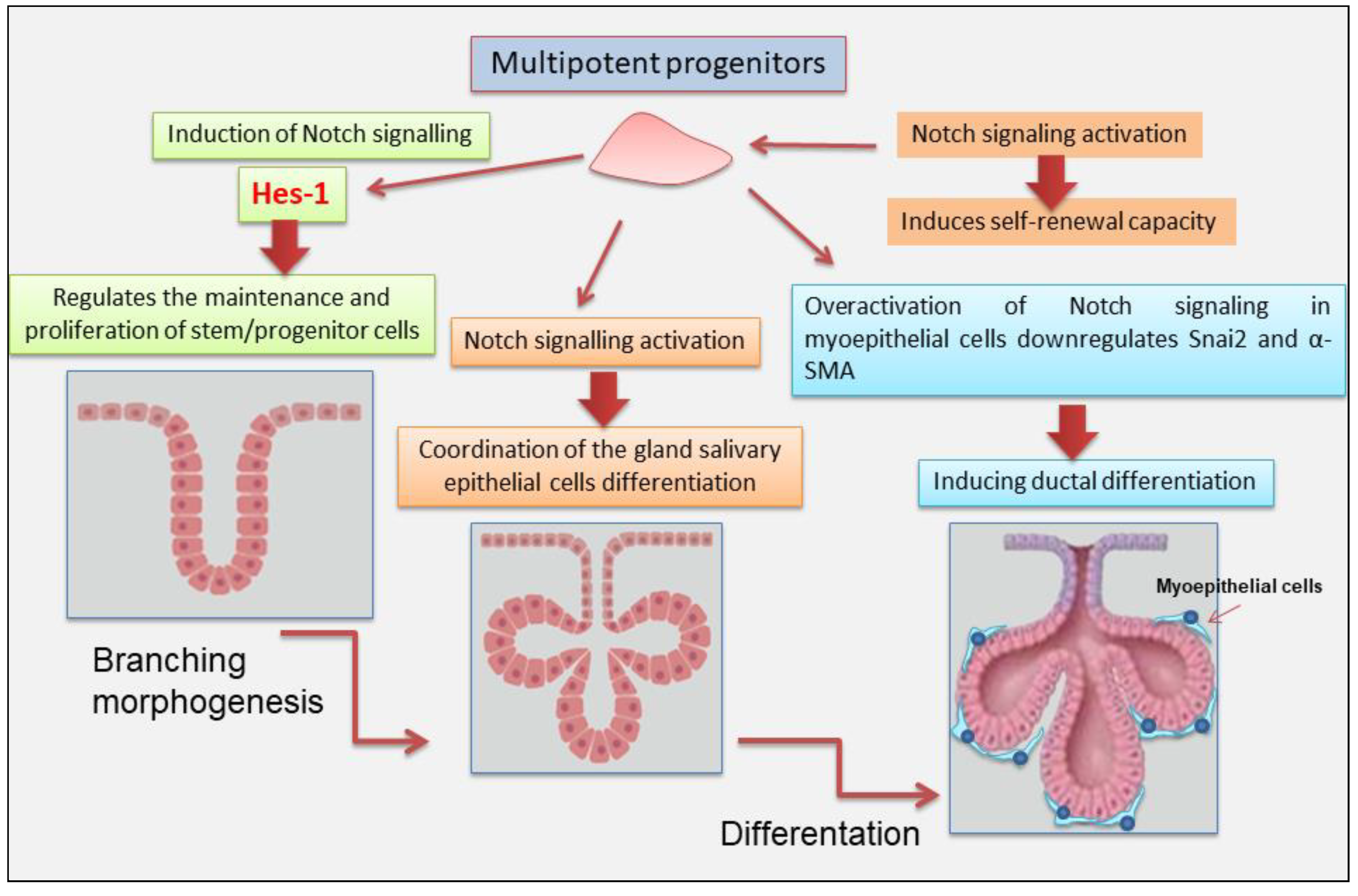
3. Notch Signaling in Salivary Gland Development
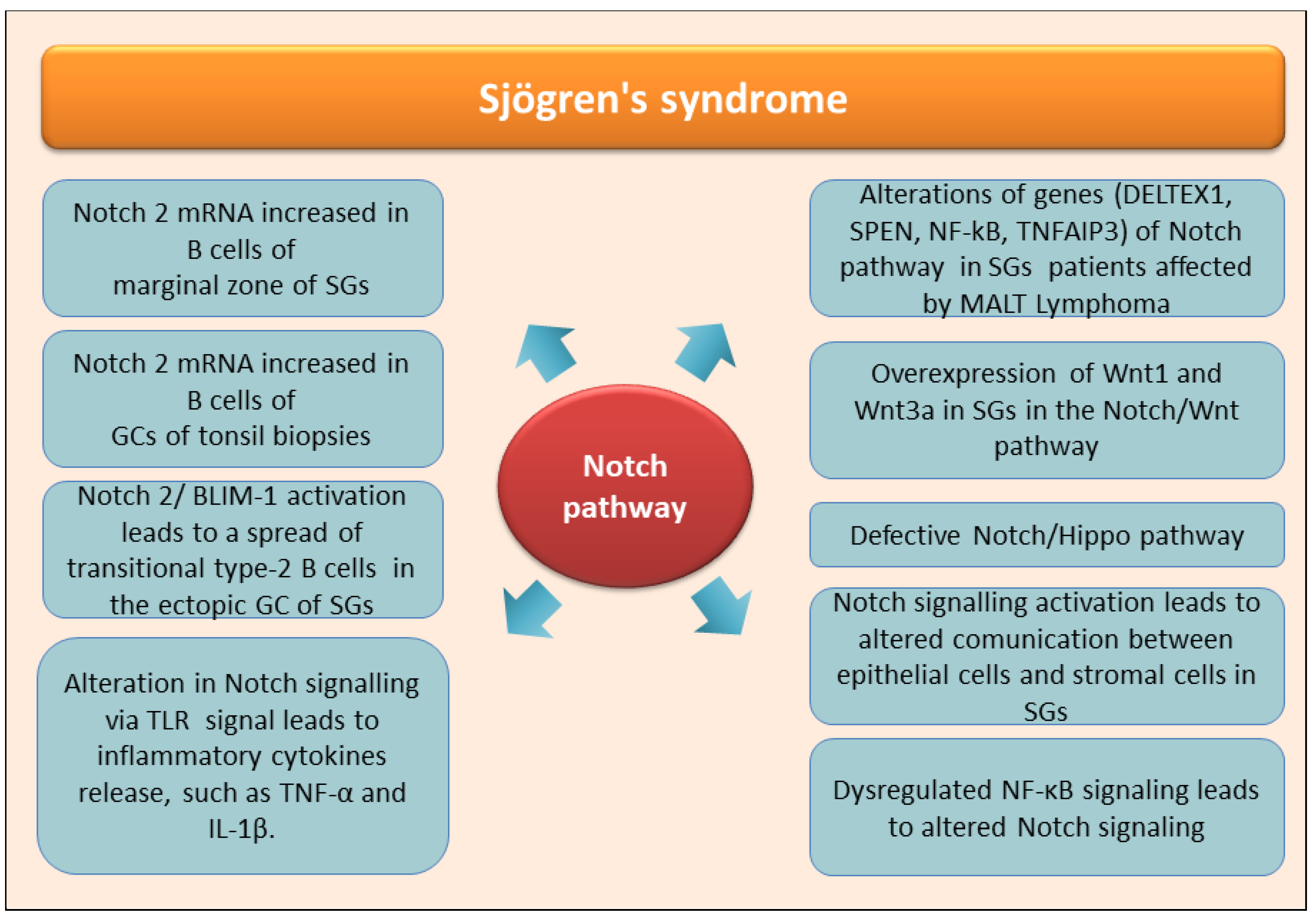
4. The Role of Notch in the Autoimmune Disease Sjӧgren’s Syndrome
5. Knowledge Regards Notch Pathways Activation in Non-Autoimmune SGs Diseases
6. Notch-Targeted Therapies in SGs Diseases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parambath:, S.; Selvraj, N.R.; Venugopal, P.; Aradhya, R. Notch Signaling: An Emerging Paradigm in the Pathogenesis of Reproductive Disorders and Diverse Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 5423. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Sharma, V.; Mutsuddi, M.; Mukherjee, A. Notch signalling: Multifaceted role in development and disease. FEBS J. 2024, 291, 3030–3059. [Google Scholar] [CrossRef]
- Condorelli, A.G.; El Hachem, M.; Zambruno, G. Nystrom, A.; Candi, E.; Castiglia, D. Notching up knowledge on molecular mechanisms of skin fibrosis: Focus on the multifaceted Notch signalling pathway. J. Biomed. Sci. 2021, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Bray, S. Notch signalling: A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Cinat, D.; Maturi, R.; Gunawan J., P.; Jellema-de Bruin, A. L.; Kracht, L.; Serrano Martinez, P.; Wu, Y.; Soto-Gamez, A.; van Goethem, M. J.; Holtman, I. R.; Pringle, S.; Barazzuol, L.; Coppes, R.P. Notch Signaling Drives Pro-Regenerative and Migratory Traits in Glandular 2 Stem/Progenitor cells. Biorixv 2025, in press. [Google Scholar]
- Bray, S.J.; Bigas, A. Modes of Notch signalling in development and disease. Nat. Rev. Mol. Cell Biol. 2025, in press. [Google Scholar] [CrossRef]
- Purow, B.W.; Haque, R.M.; Noel, M.W.; Su, Q.; Burdick, M.J.; Lee, J.; Sundaresan, T.; Pastorino, S.; Park, J.K.; Mikolaenko, I.; Maric, D.; Eberhart, C.G.; Fine, H.A. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005, 65, 2353–2363. [Google Scholar] [CrossRef]
- Brou, C.; Logeat, F.; Gupta, N.; Bessia, C.; LeBail, O.; Doedens, J.R.; Cumano, A.; Roux, P.; Black, R.A.; Israël, A. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol. Cell. 2000, 5, 207–216. [Google Scholar] [CrossRef]
- Gazdik, T.R.; Crow, J.J.; Lawton, T.; Munroe, C.J.; Theriault, H.; Wood, T.M.; Albig, A.R. Notch intracellular domains form transcriptionally active heterodimeric complexes on sequence-paired sites. Sci. Rep. 2024, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, K.; Varnum-Finney, B.; Bernstein, I.D. The notch pathway: Modulation of cell fate decisions in hematopoiesis. Int. J. Hematol. 2002, 75, 449–459. [Google Scholar] [CrossRef]
- Le Gall, M.; De Mattei, C.; Giniger, E. Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 2008, 313, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Sanalkumar, R.; Dhanesh, S.B.; James, J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol. Life Sci. 2010, 67, 2957–2968. [Google Scholar] [CrossRef]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev. Cell. 2017, 41, 2. [Google Scholar] [CrossRef]
- Henrique, D.; Schweisguth, F. Mechanisms of Notch signaling: A simple logic deployed in time and space. Development. 2019, 146, dev172148. [Google Scholar] [CrossRef]
- Sprinzak, D.; Blacklow, S.C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021, 50, 157–189. [Google Scholar] [CrossRef]
- Seib, E.; Klein, T. The role of ligand endocytosis in notch signalling. Biol. Cell. 2021, 113, 401–418. [Google Scholar] [CrossRef]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X. , Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Sig. Transduc.t Target Ther. 2024, 9, 128. [Google Scholar]
- Varshney, S.; Stanley, P. Multiple roles for O-glycans in Notch signalling. FEBS Lett. 2018, 592, 3819–3834. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Luther, K. B.; Haltiwanger, R. S. Diseases related to Notch glycosylation. Mol. Asp. Med. 2021, 79, 100938. [Google Scholar] [CrossRef] [PubMed]
- Logeat, F.; Bessia, C.; Brou, C.; LeBail, O.; Jarriault, S.; Seidah, N.G.; Israël, A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. U S A. 1998, 95, 8108–8112. [Google Scholar] [CrossRef]
- Lieber, T.; Kidd, S.; Young, M. W. Kuzbanian-mediated cleavage of Drosophila Notch. Genes Dev. 2002, 16, 209–221. [Google Scholar] [CrossRef]
- Zolkiewska, A. ADAM proteases: Ligand processing and modulation of the Notch pathway. Cell Mol. Life Sci. 2008, 65, 2056–2068. [Google Scholar] [CrossRef]
- Deatherage, C. L.; Lu, Z.; Kim, J. H.; Sanders, C. R. Notch transmembrane domain: Secondary structure and topology. Biochemistry, 2015, 54, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Crabtree, J.S.; Golde, T.E.; Minter, L.M.; Osborne, B.A.; Miele, L. Targeting notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021, 20, 125–144. [Google Scholar] [CrossRef]
- Kim, G. S.; Park, H. S.; Lee, Y. C. OPTHiS identifies the molecular basis of the direct interaction between CSL and SMRT corepressor. Mol. Cells 2018, 41, 842–852. [Google Scholar]
- Kwon, C.; Cheng, P.; King, I.N.; Andersen, P.; Shenje, L.; Nigam, V.; Srivastava, D. Notch post-translationally regulates β-catenin protein in stem and progenitor cells. Nat. Cell Biol. 2011, 13, 1244–1251. [Google Scholar] [CrossRef]
- Ayaz, F.; Osborne, B.A. Non-canonical notch signaling in cancer and immunity. Front. Oncol. 2014, 4, 345. [Google Scholar] [CrossRef]
- 31. Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; Zhu, X.; Li, R.; Yan, J.; Wei, Y.; Zhao, Y.; Wang, W.; Ren, Y.; Yuan, P.; Yan, Z.; Hu, B.; Guo, F.; Wen, L.; Tang, F.; Qiao, J. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell. 2017, 20, 858–873.e4. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, J.; Osborne, B.A. Noncanonical Notch Signaling. In Targeting Notch in Cancer; Miele, L., Artavanis-Tsakonas, S., Eds.; Springer: New York, NY, 2018. [Google Scholar]
- Jiang, N.; Hu, Y.; Wang, M.; Zhao, Z.; Li, M. The Notch Signaling Pathway Contributes to Angiogenesis and Tumor Immunity in Breast Cancer. Breast Cancer (Dove Med Press). 2022, 14, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Uosaki, H.; Shenje, L.T.; Kwon, C. Non-canonical Notch signaling: Emerging role and mechanism. Trends Cell Biol. 2012, 22, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hurlbut, G. D.; Kankel, M. W.; Lake, R. J.; Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 2007, 19, 166–175. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Zhao, S.; Zhao, X.Y.; Min, P.X.; Ma, Y.D.; Wang, Y.Y.; Chen, Y.; Tang, S.J.; Zhang, Y.J.; Du, J.; Gu, L. Non-canonical notch signaling regulates actin remodeling in cell migration by activating PI3K/AKT/Cdc42 pathway. Front Pharm. 2019, 10, 370. [Google Scholar] [CrossRef]
- Bhat, V.; Sun, Y. J.; Weger, S.; Raouf, A. Notch-induced expression of FZD7 requires noncanonical NOTCH3 signaling in human breast epithelial cells. Stem. Cells Dev. 2016, 25, 522–529. [Google Scholar] [CrossRef]
- 38. Jin, S.; Mutvei, A.P.; Chivukula, I.V.; Andersson, E.R.; Ramsköld, D.; Sandberg, R.; Lee, K.L.; Kronqvist, P.; Mamaeva, V.; Ostling, P.; Mpindi, J.P.; Kallioniemi, O.; Screpanti, I.; Poellinger, L.; Sahlgren, C.; Lendahl, U. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene 2013, 32, 4892–4902. [Google Scholar] [CrossRef]
- Hu, X.; li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Sig Transduct Target Ther 2021, 6, 402. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 2017, 8, 709. [Google Scholar] [CrossRef]
- Lee, K. S.; Wu, Z.; Song, Y.; Mitra, S.S.; Feroze, A.H.; Cheshier, S.H.; Lu, B. Roles of PINK1, mTORC2, and mitochondria in preserving brain tumor-forming stem cells in a noncanonical Notch signaling pathway. Genes Dev. 2013, 27, 2642–2647. [Google Scholar] [CrossRef]
- Perumalsamy, L. R.; Nagala, M.; Banerjee, P.; Sarin, A. A hierarchical cascade activated by non-canonical Notch signaling and the mTOR-Rictor complex regulates neglect-induced death in mammalian cells. Cell Death Differ. 2009, 16, 879–889. [Google Scholar] [CrossRef]
- Lin, S.; Negulescu, A.; Bulusu, S.; Gibert, B.; Delcros, J.G.; Ducarouge, B.; Rama, N.; Gadot, N.; Treilleux, I.; Saintigny, P.; Meurette, O.; Mehlen, P. Non-canonical NOTCH3 signalling limits tumour angiogenesis. Nat Commun. 2017, 8, 16074. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tang, X.; Wong, P.; Jacobs, B.; Borden, E.C.; Bedogni, B. Noncanonical activation of Notch1 protein by membrane type 1 matrix metalloproteinase (MT1-MMP) controls melanoma cell proliferation. J. Biol. Chem. 2014, 289, 8442–8449. [Google Scholar] [CrossRef] [PubMed]
- Lammel, U.; Meadows, L.; Saumweber, H. Analysis of Drosophila salivary gland, epidermis and CNS development suggests an additional function of brinker in anterior-posterior cell fate specification. Mech. Dev. 2000, 92, 179–191. [Google Scholar] [CrossRef]
- Morgan, T. H. The theory of the gene. Am. Nat. 1917, 51, 513–544. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science. 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Bin Jang, S.; Lim, N.; Yeo, H.C.; Kwak, Y.; Lee, S.H.; Shin, H.J.; Cho, S.G. Advances in lacrimal gland organoid development: Techniques and therapeutic applications. Biomed Pharmacother. 2025, 183, 117870. [Google Scholar] [CrossRef]
- Šale, S.; Lafkas, D.; Artavanis-Tsakonas, S. Notch2 genetic fate mapping reveals two previously unrecognized mammary epithelial lineages. Nat. Cell Biol. 2013, 15, 451–460. [Google Scholar] [CrossRef]
- Dang, H.; Lin, A.L.; Zhang, B.; Zhang, H.M.; Katz, M.S.; Yeh, C.K. Role for Notch signaling in salivary acinar cell growth and differentiation. Dev Dyn. 2009, 238, 724–731. [Google Scholar] [CrossRef]
- Chatzeli, L.; Bordeu, I.; Han, S.; Bisetto, S.; Waheed, Z.; Koo, B.K.; Alcolea, M.P.; Simons, B.D. A cellular hierarchy of Notch and Kras signaling controls cell fate specification in the developing mouse salivary gland. Dev. Cell. 2023, 58, 94–109. [Google Scholar] [CrossRef]
- Yasuhara, R.; Kang, S.; Irié, T.; Mabuchi, Y.; Kujiraoka, S.; Yukimori, A.; Ishida, S.; Tanaka, J.; Mishima, K. Role of Snai2 and Notch signaling in salivary gland myoepithelial cell fate. Lab. Invest. 2022, 102, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Qian, J.; Liang, W.; Xiang, H.; Ding, P.; Jin, M.; Lin, Z.; Chen, Y.; Wang, Z.; Huang, X.; Sun, Z.; Pan, B.; Zhao, Z. Establishment of human minor salivary gland organoids in laminin-GelMA hydrogel from healthy individuals and Sjögren’s disease patients. Chem. Eng. J. 2025, 503, 158257. [Google Scholar] [CrossRef]
- Gorodetskiy, V.R.; Probatova, N.A.; Vasilyev, V.I. Characteristics of diffuse large B-cell lymphoma in patients with primary Sjögren's syndrome. Int. J. Rheum. Dis. 2020, 23, 540–548. [Google Scholar] [CrossRef]
- Mohammadnezhad, L.; Shekarkar Azgomi, M.; La Manna, M.P.; Guggino, G.; Botta, C.; Dieli, F.; Caccamo, N. B. Cell Receptor Signaling Is Thought to Be a Bridge between Primary Sjogren Syndrome and Diffuse Large B-Cell Lymphoma. Int. J. Mol. Sci. 2023, 24, 8385. [Google Scholar] [CrossRef]
- Le Pottier, L.; Devauchelle, V.; Fautrel, A.; Daridon, C.; Saraux, A.; Youinou, P.; Pers, J.O. Ectopic germinal centers are rare in Sjogren's syndrome salivary glands and do not exclude autoreactive B cells. J. Immunol. 2009, 182, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, T.; Le Pottier, L.; Devauchelle, V.; Pers, J.O.; Jamin, C.; Youinou, P. Role of Toll-like receptors in primary Sjögren's syndrome with a special emphasis on B-cell maturation within exocrine tissues. J. Autoimmun. 2012, 39, 69–76. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Han, J.; Yang, M.; Zhu, J.; Jin, T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020, 18, 131. [Google Scholar] [CrossRef]
- Titsinides, S.; Nikitakis, N.; Piperi, E.; Sklavounou, A. MALT Lymphoma of Minor Salivary Glands in a Sjögren's Syndrome Patient: A Case Report and Review of Literature. J. Oral Maxillofac. Res. 2017, 8, e5. [Google Scholar] [CrossRef]
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Famà, R.; Ciardullo, C.; Greco, M.; Cresta, S.; Piranda, D.; Holmes, A.; Fabbri, G.; Messina, M.; Rinaldi, A.; Wang, J.; Agostinelli, C.; Piccaluga, P.P.; Lucioni, M.; Tabbò, F.; Serra, R.; Franceschetti, S.; Deambrogi, C.; Daniele, G.; Gattei, V.; Marasca, R.; Facchetti, F.; Arcaini, L.; Inghirami, G.; Bertoni, F.; Pileri, S.A.; Deaglio, S.; Foà, R.; Dalla-Favera, R.; Pasqualucci, L.; Rabadan, R.; Gaidano, G. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551. [Google Scholar] [CrossRef]
- Bertoni, F.; Zucca, E. Delving deeper into MALT lymphoma biology. J. Clin. Invest. 2006, 116, 22–26. [Google Scholar] [CrossRef]
- Chanudet, E.; Huang, Y.; Zeng, N.; Streubel, B.; Chott, A.; Raderer, M.; Du, M.Q. TNFAIP3 abnormalities in MALT lymphoma with autoimmunity. Br. J. Haematol. 2011, 154, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.; Escudero-Ibarz, L.; Wang, M.; Clipson, A.; Ochoa Ruiz, E.; Dunn-Walters, D.; Xue, X.; Zeng, N.; Robson, A.; Chuang, S.S.; Cogliatti, S.; Liu, H.; Goodlad, J.; Ashton-Key, M.; Raderer, M.; Bi, Y.; Du, M.Q. Significant association between TNFAIP3 inactivation and biased immunoglobulin heavy chain variable region 4-34 usage in mucosa-associated lymphoid tissue lymphoma. J. Pathol. 2017, 243, 3–8. [Google Scholar] [CrossRef]
- Kuksin, C.A.; Minter, L.M. The Link between Autoimmunity and Lymphoma: Does NOTCH Signaling Play a Contributing Role? Front. Oncol. 2015, 5, 51. [Google Scholar] [CrossRef]
- Hou, J.; Feng, Y.; Yang, Z.; Ding, Y.; Cheng, D.; Shi, Z.; Li, R.; Xue, L. Primary Sjögren’s syndrome: New perspectives on salivary gland epithelial cells. Eur. J. Med. Res. 2024, 29, 371. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Zhu, Z.; Wang, J.; Zhou, J.; Yu, S.; Zhou, X.; Zhao, Z.; Xie, A.; Lu, L.; Yang, J. Maintenance of adult stem cells from human minor salivary glands via the Wnt signaling pathway. Stem. Cell Res. Ther. 2023, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, L.; Zhao, L.; Su, Y. The interaction of Notch and Wnt signaling pathways in vertebrate regeneration. Cell Regen. 2021, 10, 11. [Google Scholar] [CrossRef]
- Varelas, X.; Wrana, J.L. Coordinating developmental signaling: Novel roles for the Hippo pathway. Trends Cell Biol. 2012, 22, 88–96. [Google Scholar] [CrossRef]
- Enger, T.B.; Samad-Zadeh, A.; Bouchie, M.P.; Skarstein, K.; Galtung, H.K.; Mera, T.; Walker, J.; Menko, A.S.; Varelas, X.; Faustman, D.L.; Jensen, J.L.; Kukuruzinska, M.A. The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren's syndrome. Lab. Invest. 2013, 93, 1203–1218. [Google Scholar] [CrossRef]
- Peng, B.; Ling, J.; Lee, A.J.; Wang, Z.; Chang, Z.; Jin, W.; Kang, Y.; Zhang, R.; Shim, D.; Wang, H.; Fleming, J.B.; Zheng, H.; Sun, S.C.; Chiao, P.J. Defective feedback regulation of NF-kappaB underlies Sjӧgren’s syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc. Natl. Acad. Sci. USA. 2010, 107, 15193–8. [Google Scholar] [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. Understanding the Complexity of Sjögren's Syndrome: Remarkable Progress in Elucidating NF-κB Mechanisms. J. Clin. Med. 2020, 9, 2821. [Google Scholar] [CrossRef]
- Huang, J.; Tang, J.; Zhang, C.; Liu, T.; Deng, Z.; Liu, L. Single-cell transcriptomic analysis uncovers heterogeneity in the labial gland microenvironment of primary Sjögren's syndrome. J. Transl. Autoimmun. 2024, 9, 100248. [Google Scholar] [CrossRef]
- Vitali, C.; Dolcino, M.; Del Papa, N.; Minniti, A.; Pignataro, F.; Maglione, W.; Lunardi, C.; Puccetti, A. Gene expression profiles in primary Sjögren's Syndrome with and without systemic manifestations. ACR Open Rheumatol. 2019, 1, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Jr Glisson, B.S.; Wick, M.J.; Kapoun, A.M.; Patnaik, A.; Eckhardt, G.; Munster, P.; Faoro, L.; Dupont, J.; Lee, J.J.; Futreal, A.; El-Naggar, A.K.; Heymach, J.V. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, M.; Näsman, A. , Berglund, A.; Dalianis, T.; Friesland, S. Adenoid Cystic Carcinoma (AdCC): A Clinical Survey of a Large Patient Cohort. Cancers (Basel). 2023, 15, 1499. [Google Scholar] [CrossRef] [PubMed]
- Tasoulas, J.; Schrank, T.P.; Bharambe, H.; Mehta, J.; Johnson, S.; Divaris, K.; Hackman, T.G.; Sheth, S.; Kirtane, K.; Hernandez-Prera, J.C.; Chung, C.H.; Yarbrough, W.G.; Ferrarotto, R.; Issaeva, N.; Theocharis, S.; Amelio, A.L. Molecular characterization of the salivary adenoid cystic carcinoma immune landscape by anatomic subsites. Sci. Rep. 2024, 14, 15821. [Google Scholar] [CrossRef]
- Morita, N.; Murase, T.; Ueda, K.; Nagao, T.; Kusafuka, K.; Nakaguro, M.; Urano, M.; Taguchi, K.I.; Yamamoto, H.; Kano, S.; Tada, Y.; Tsukahara, K.; Okami, K.; Onitsuka, T.; Fujimoto, Y.; Kawakita, D.; Sakurai, K.; Nagao, T.; Hanai, N.; Kawata, R.; Hato, N.; Otsuki, N.; Nibu, K.I.; Inagaki, H. Pathological evaluation of tumor grade for salivary adenoid cystic carcinoma: A proposal of an objective grading system. Cancer Sci. 2021, 112, 1184–1195. [Google Scholar] [CrossRef]
- Bell, D.; Roberts, D.; Kies, M.; Rao, P.; Weber, R.S.; El-Naggar, A.K. Cell type-dependent biomarker expression in adenoid cystic carcinoma: Biologic and therapeutic implications. Cancer. 2010, 116, 5749–5756. [Google Scholar] [CrossRef]
- Barsky, S.H.; Karlin, N.J. Myoepithelial cells: Autocrine and paracrine suppressors of breast cancer progression. J Mammary gland neoplasm Biol. 2005, 10, 249–260. [Google Scholar] [CrossRef]
- Hong, S.D.; Katuwal, N.B.; Kang, M.S.; Ghosh, M.; Park, S.M.; Kim, T.H.; Baek, Y.S.; Lee, S.R.; Moon, Y.W. Trastuzumab-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) Enhances Natural Killer Cell Cytotoxicity in HER2-Overexpressing Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 11733. [Google Scholar] [CrossRef]
- Bell, D.; Hanna, E.Y.; Miele, L.; Roberts, D.; Weber, R.S.; El-Naggar, A.K. Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann. Diagn. Pathol. 2014, 18, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Ma, S.R.; Wang, W.M.; Huang, C.F.; Yu, G.T.; Wu, T.F.; Bu, L.L.; Wang, Y.F.; Zhao, Y.F.; Zhang, W.F.; Sun, Z.J. Notch signaling induces epithelial-mesenchymal transition to promote invasion and metastasis in adenoid cystic carcinoma. Am. J. Transl. Res. 2015, 7, 162–174. [Google Scholar]
- Luo, J.; Wang, P.; Wang, R.; Wang, J.; Liu, M.; Xiong, S.; Li, Y.; Cheng, B. The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget. 2016, 7, 9525–9537. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Sig. Transduct Target Ther. 2024, 9, 170. [Google Scholar]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Aval, S.F.; Lotfi, H.; Sheervalilou, R.; Zarghami, N. Tuning of major signaling networks (TGF-β, Wnt, Notch and Hedgehog) by miRNAs in human stem cells commitment to different lineages: Possible clinical application. Biomed Pharmacother. 2017, 91, 849–860. [Google Scholar] [CrossRef]
- Manni, W.; Min, W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. Med. Comm 2020, 3, e176. [Google Scholar] [CrossRef]
- Olsauskas-Kuprys, R.; Zlobin, A.; Osipo, C. Gamma secretase inhibitors of Notch signaling. Onco Targets Ther. 2013, 6, 943–955. [Google Scholar]
- McCaw, T.R.; Inga, E.; Chen, H.; Jaskula-Sztul, R.; Dudeja, V.; Bibb, J.A.; Ren, B.; Rose, J.B. Gamma Secretase Inhibitors in Cancer: A Current Perspective on Clinical Performance. Oncologist. 2021, 26, e608–e621. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Xu, C.; Gao, M.; Li, N.; Geng, Q. The critical role of γ-secretase and its inhibitors in cancer and cancer therapeutics. Int. J. Biol. Sci. 2023, 19, 5089–5103. [Google Scholar] [CrossRef]
- Fujita, S.; Ikeda, T. Cancer stem-like cells in adenoid cystic carcinoma of salivary glands: Relationship with morphogenesis of histological variants. J. Oral. Pathol. Med. 2012, 41, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Saffar, H.; Taheri, P.; Yazdani, F.; Etebarian, A. Prognostic significance of cancer stem cell markers in patients with salivary gland carcinomas. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 284–290. [Google Scholar] [CrossRef]
- Porcheri, C. ; Meisel, CT; Mitsiadis, T. Multifactorial contribution of notch signaling in head and neck squamous cell carcinoma. internal J. Mol. Sci. 2019, 20, 1520. [Google Scholar]
- Feeney, L.; Hapuarachi, B.; Adderley, H.; Rack, S.; Morgan., D.; Walker., R.; Rauch, R.; Herz, E.; Kaye, J.; Harrington, K.; Metcalf, R. Clinical disease course and survival outcomes following disease recurrence in adenoid cystic carcinoma with and without NOTCH signaling pathway activation. Oral Oncol. 2022, 133, 106028. [Google Scholar] [CrossRef]
- Li, R.; Hu, Z.; Qiao, Q.; Zhou, D.; Sun, M. Anti-NOTCH1 therapy with OMP-52 M51 inhibits salivary adenoid cystic carcinoma by depressing epithelial-mesenchymal transition (EMT) process and inducing ferroptosis. Toxicol. Appl. Pharmacol. 2024, 484, 116825. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, R. Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells. J. Clin. Med. 2019, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Khan, J.; Waheed, A.; Karki, N.R.; Goodbee, M.; Yasinzai, A.Q.K.; Tareen, B.; Wali, A.; Khan, K.A.; Zarak, M.S.; Khan, I.; Garcia, A.A.; Khan, A.; Khan, M.; Jogezai, S.; Ahmad, J.; Zarate, L.V.; Patel, N.; Karim, N.A.; Heneidi, S. Mucoepidermoid Carcinoma of the Salivary Gland: Demographics and Comparative Analysis in U.S. Children and Adults with Future Perspective of Management. Cancers (Basel). 2022, 15, 250. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, S.; Li, J.L.; Ni, W.; Guo, R.; Lu, J.; Kaye, F.J.; Wu, L. CRTC1-MAML2 fusion-induced lncRNA LINC00473 expression maintains the growth and survival of human mucoepidermoid carcinoma cells. Oncogene. 2018, 37, 1885–1895. [Google Scholar] [CrossRef]
- Tonon, G.; Modi, S.; Wu, L.; Kubo, A.; Coxon, A.B.; Komiya, T.; O'Neil, K.; Stover, K.; El-Naggar, A.; Griffin, J.D.; Kirsch, I.R.; Kaye, F.J. t(11;19) (q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003, 33, 208–213. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Gu, Y.; Hu, C.; Li, J.L.; Lin, S.; Shen, H.; Cao, C.; Gao, R.; Li, J.; Ha, P.K.; Kaye, F.J.; Griffin, J.D.; Wu, L. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene. 2014, 33, 3869–3877. [Google Scholar] [CrossRef]
- Ni, W.; Chen, Z.; Zhou, X.; Yang, R.; Yu, M.; Lu, J.; Kaye, F.J.; Wu, L. Targeting Notch and EGFR signaling in human mucoepidermoid carcinoma. Sig. Transduct. Target Ther. 2021, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E. R.; Lendahl, U. Therapeutic modulation of Notch signalling-are we there yet? Nat. Rev. Drug Disco. 2014, 13, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; O'Sullivan Coyne, G.; Do, K.T.; Turkbey, B.; Meltzer, P.S.; Polley, E.; Choyke, P.L.; Meehan, R.; Vilimas, R.; Horneffer, Y.; Juwara, L.; Lih, A.; Choudhary, A.; Mitchell, S.A.; Helman, L.J.; Doroshow, J.H.; Chen, A.P. Clinical Activity of the γ-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis). J. Clin. Oncol. 2017, 35, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Ileana Dumbrava, E.E.; Mills, G.B.; Yap, T.A. Targeting gamma secretase: Has progress moved up a Notch? Ann. Oncol. 2018, 29, 1889–1891. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Desai, J.; Iyer, S.P.; Gadgeel, S.M.; Ramalingam, S.S.; Horn, L.; et al. A phase I study of AL101, a pan-NOTCH inhibitor, in patients (pts) with locally advanced or metastatic solid tumors. J. Clin. Oncol. 2018, 36, 2515. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Metcalf, R.; Rodriguez, C.P.; Muzaffar, J.; Even, C.; Perez, C.A.; Van Herpen, C.M.L.-; Oliva, M.; Xia, B.; Bowles, D.W.; et al. Results of ACCURACY: A phase 2 trial of AL101, a selective gamma secretase inhibitor, in subjects with recurrent/metastatic (R/M) adenoid cystic carcinoma (ACC) harboring Notch activating mutations (Notchmut). J. Clin. Oncol. 2022, 40, 6046. [Google Scholar] [CrossRef]
- Massard, C.; Azaro, A.; Soria, J.C.; Lassen, U.; Le Tourneau, C.; Sarker, D.; Smith, C.; Ohnmacht, U.; Oakley, G.; Patel, B.K.R.; Yuen, E.S.M.; Benhadji, K.A.; Rodon, J. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann. Oncol. 2018, 29, 1911–1917. [Google Scholar] [CrossRef]
- Even, C.; Lassen, U.; Merchan, J.; Le Tourneau, C.; Soria, J.C.; Ferte, C.; Ricci, F.; Diener, J.T.; Yuen, E.; Smith, C.; Oakley, G.J.; 3rd Benhadji, K.A.; Massard, C. Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Invest. New Drugs. 2020, 38, 402–409. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Chen, Z.; Ni, W.; Li, J.L.; Lin, S.; Zhou, X.; Sun, Y.; Li, J.W.; Leon, M.E.; Hurtado, M.D.; Zolotukhin, S.; Liu, C.; Lu, J.; Griffin, J.D.; Kaye, F.J.; Wu, L. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight. 2021, 6, e139497. [Google Scholar]
- Sundaram, M.V. The love-hate relationship between Ras and Notch. Dev. Genes. 2005, 19, 1825–1839. [Google Scholar] [CrossRef]
- Arasada, R.R.; Amann, J.M.; Rahman, M.A.; Huppert, S.S.; Carbone, D.P. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res. 2014, 74, 5572–5584. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fu, W.; Li, T.; Yuan, Q.; Wang, F.; Lv, G.; Lv, Y.; Fan, X.; Shen, Y.; Lin, F.; Tang, Y.; Ye, X.; Yang, Y.; Lei, C. Antagonism of EGFR and Notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci. Transl. Med 2017, 9, eaag0339. [Google Scholar] [CrossRef] [PubMed]
- Turtoi, A.; Cañamero, M.; Wong, K.K.; Yarden, Y.; Casanova, E.; Soria, J.C.; Colinge, J.; Siebel, C.W.; Mazieres, J.; Favre, G.; Paz-Ares, L.; Maraver, A. Notch inhibition overcomes resistance to tyrosine kinase inhibitors in EGFR-driven lung adenocarcinoma. J. Clin. Invest. 2020, 130, 612–624. [Google Scholar]
- Lopez Miranda, E.; Stathis, A.; Hess, D.; Racca, F.; Quon, D.; Rodon, J.; Saavedra Santa Gadea, O.; Perez Garcia, J.M.; Nuciforo, P.; Vivancos, A.; et al. Phase 1 study of CB-103, a novel first-in-class inhibitor of the CSL-NICD gene transcription factor complex in human cancers. J. Clin. Oncol. 2021, 39, 3020. [Google Scholar] [CrossRef]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting cancer stemness in the clinic: From hype to hope. Cell Stem Cell. 2019, 24, 25–40. [Google Scholar] [CrossRef]

| DRUG | TARGET | MODE OF ACTION | EFFECT | REF |
|---|---|---|---|---|
| AL101 | γ-secretase inhibitor | Notch pathway inhibition during the cleavage process in the intracellular domain | Anti tumor effect in patients with metastatic solid tumors | 106 |
| Crenigacestat | γ-secretase inhibitor | Inhibition of the release of the Notch intracellular domain by suppression of γ-secretase complex | Anti tumor effect in patients with advanced or metastatic disease as patients with ADCC | 108 |
| DBZ dibenzazepine | γ-secretase inhibitor | The block of the cleavage of Notch into its active signalling effector, Notch intracellular domain | Anti tumor efficacy with strong anti-stem cell effect in human mucoepidermoid carcinoma of the salivary gland | 102 |
| Erlotinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti tumor effect in human mucoepidermoid carcinoma | 102 |
| Gefitinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti tumor effect in lung adenocarcinoma | 114 |
| Osimertinib | EGFR inhibitor | Inhibition of the intracellular phosphorylation of tyrosine kinase | Anti tumor effect in lung adenocarcinoma | 114 |
| Brontictuzumab | Notch 1 inhibitor | Humanized monoclonal antibody against the Notch1 protein | Repression of the proliferation and EMT inhibiting salivary adenoid cystic carcinoma | 96 |
| CB-103 | Notch 1 inhibitor | Inhibits the CSL–NICD | Downregulation in Notch signalling | 115 |
| Amcasertib | Notch inhibitor | Cancer stemness kinase inhibitor | Impairment of cancer stem cell survival deregulating Notch signalling in AdCC | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





