Submitted:
31 March 2025
Posted:
01 April 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Material & Methods
2.1. General Information
2.2. Synthetic Methods
2.2.1. General Procedure A: Claisen-Schmidt Condensation
2.2.2. General Procedure B: Oxidative Cyclization
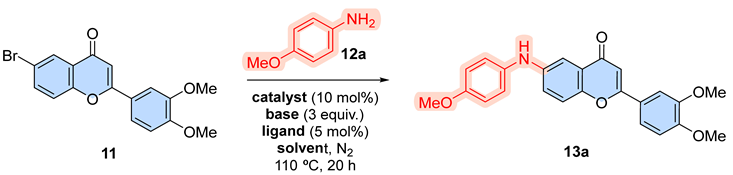
2.2.3. General Procedure C: Buchwald-Hartwig Amination
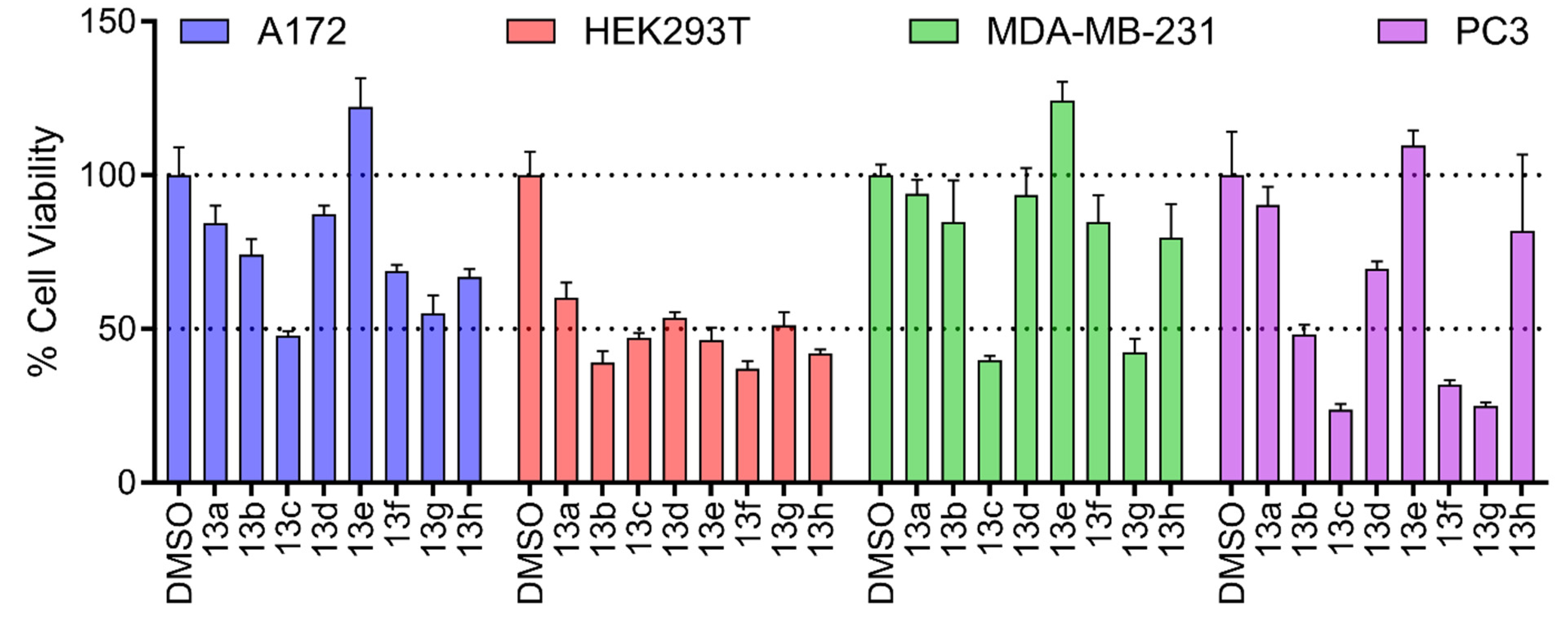
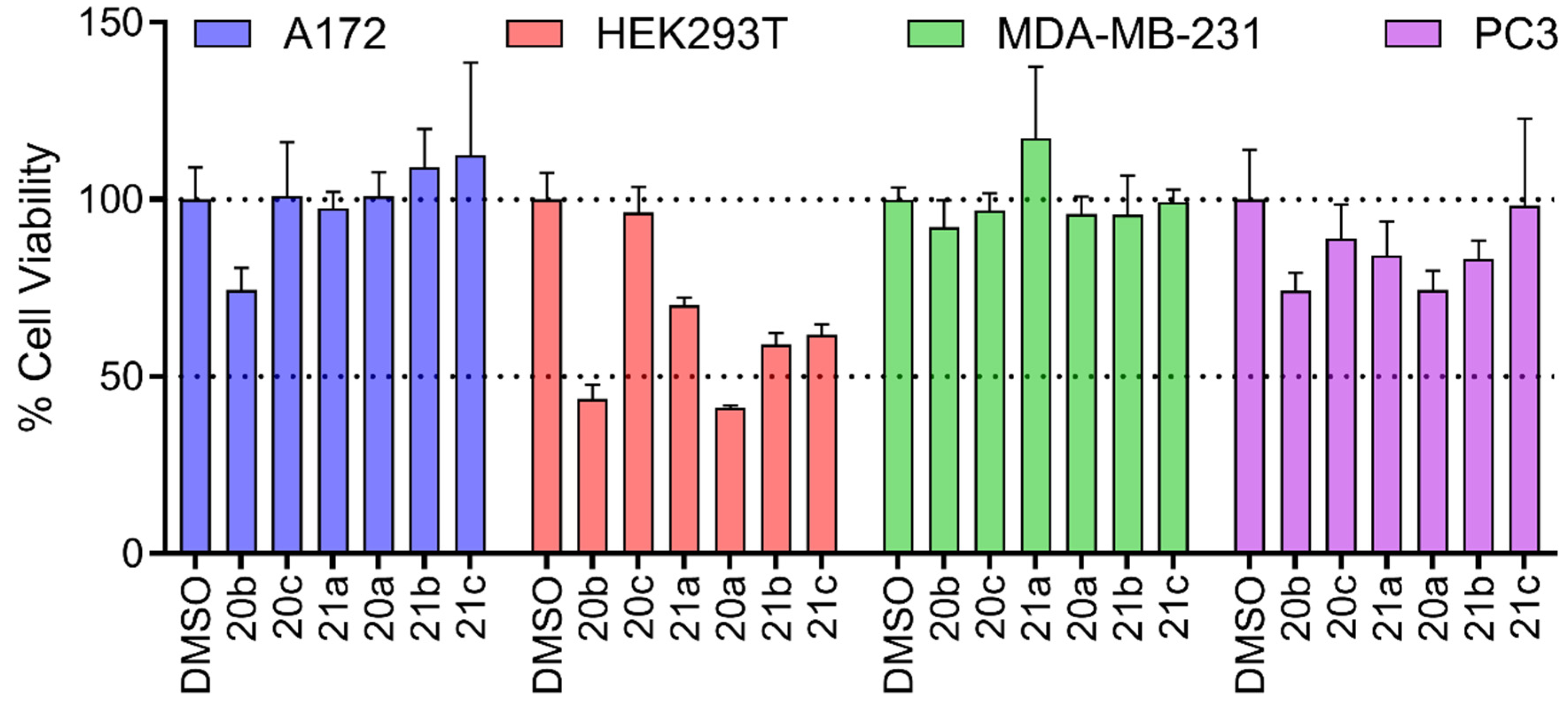
2.3. Cytotoxicity Assays
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Beecher, G. R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. The Journal of Nutrition 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P. K.; Elizabeth, F. I. Flavones as a Privileged Scaffold in Drug Discovery: Current Developments. COS 2019, 16, 968–1001. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An Important Scaffold for Medicinal Chemistry. European Journal of Medicinal Chemistry 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure–Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef] [PubMed]
- Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. Novel 5-Aminoflavone Derivatives as Specific Antitumor Agents in Breast Cancer. J. Med. Chem. 1996, 39, 3461–3469. [Google Scholar] [CrossRef]
- Akama, T.; Ishida, H.; Shida, Y.; Kimura, U.; Gomi, K.; Saito, H.; Fuse, E.; Kobayashi, S.; Yoda, N.; Kasai, M. Design and Synthesis of Potent Antitumor 5,4‘-Diaminoflavone Derivatives Based on Metabolic Considerations. J. Med. Chem. 1997, 40, 1894–1900. [Google Scholar] [CrossRef]
- Callero, M. A.; Rodriguez, C. E.; Sólimo, A.; Bal De Kier Joffé, E.; Loaiza Perez, A. I. The Immune System As a New Possible Cell Target for AFP 464 in a Spontaneous Mammary Cancer Mouse Model: AFP 464 T ARGETS T HE I MMUNE S YSTEM. J. Cell. Biochem. 2017, 118, 2841–2849. [Google Scholar] [CrossRef]
- Luzzani, G. A.; Callero, M. A.; Kuruppu, A. I.; Trapani, V.; Flumian, C.; Todaro, L.; Bradshaw, T. D.; Loaiza Perez, A. I. In Vitro Antitumor Effects of AHR Ligands Aminoflavone (AFP 464) and Benzothiazole (5F 203) in Human Renal Carcinoma Cells. J of Cellular Biochemistry 2017, 118, 4526–4535. [Google Scholar] [CrossRef]
- Goetz, M. P.; Reid, J. M.; Qi, Y.; Chen, A.; McGovern, R. M.; Kuffel, M. J.; Scanlon, P. D.; Erlichman, C.; Ames, M. M. A Phase I Study of Once-Weekly Aminoflavone Prodrug (AFP464) in Solid Tumor Patients. JCO 2011, 29, 2546–2546. [Google Scholar] [CrossRef]
- Dauzonne, D.; Folléas, B.; Martinez, L.; Chabot, G. Synthesis and in Vitro Cytotoxicity of a Series of 3-Aminoflavones. European Journal of Medicinal Chemistry 1997, 32, 71–82. [Google Scholar] [CrossRef]
- Alessi, D. R.; Cuenda, A.; Cohen, P.; Dudley, D. T.; Saltiel, A. R. PD 098059 Is a Specific Inhibitor of the Activation of Mitogen-Activated Protein Kinase Kinase in Vitro and in Vivo. Journal of Biological Chemistry 1995, 270, 27489–27494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ge, C.-C.; Wang, J.; Wu, X.-X.; Li, X.-M.; Li, W.; Wang, S.-S.; Liu, T.; Hou, J.-Z.; Sun, H.; Fang, D.; Xie, S.-Q. MEK Inhibitor, PD98059, Promotes Breast Cancer Cell Migration by Inducing β-Catenin Nuclear Accumulation. Oncology Reports 2017, 38, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N. M.; Sarkate, A. P.; Lokwani, D. K.; Tiwari, S. V.; Azad, R.; Thopate, S. R. N-Benzylation of 6-Aminoflavone by Reductive Amination and Efficient Access to Some Novel Anticancer Agents via Topoisomerase II Inhibition. Mol Divers 2021, 25, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N. A. A.; Hrichi, H.; Alolayan, R. A.; Derafa, W.; Zahou, F. M.; Bakr, R. B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Torres Ribón, D. J.; Roa De La Fuente, L. F.; Ceronio, N. R.; Abreu, O. H.; Rivera, M. R.; Juárez, J. R.; Torralba, R.; Ximello, M. V.; Sauret, Q. T.; Sánchez, C. A. Iodine Promoted One-Pot Synthesis of Flavones. Results in Chemistry 2025, 13, 101968. [Google Scholar] [CrossRef]
- Leonte, D.; Ungureanu, D.; Zaharia, V. Flavones and Related Compounds: Synthesis and Biological Activity. Molecules 2023, 28, 6528. [Google Scholar] [CrossRef]
- Dorel, R.; Grugel, C. P.; Haydl, A. M. The Buchwald–Hartwig Amination After 25 Years. Angew Chem Int Ed 2019, 58, 17118–17129. [Google Scholar] [CrossRef]
- Fitzner, M.; Wuitschik, G.; Koller, R. J.; Adam, J.-M.; Schindler, T.; Reymond, J.-L. What Can Reaction Databases Teach Us about Buchwald–Hartwig Cross-Couplings? Chem. Sci. 2020, 11, 13085–13093. [Google Scholar] [CrossRef]
- Clarke, G. E.; Firth, J. D.; Ledingham, L. A.; Horbaczewskyj, C. S.; Bourne, R. A.; Bray, J. T. W.; Martin, P. L.; Eastwood, J. B.; Campbell, R.; Pagett, A.; MacQuarrie, D. J.; Slattery, J. M.; Lynam, J. M.; Whitwood, A. C.; Milani, J.; Hart, S.; Wilson, J.; Fairlamb, I. J. S. Deciphering Complexity in Pd–Catalyzed Cross-Couplings. Nat Commun 2024, 15, 3968. [Google Scholar] [CrossRef]
- Sunesson, Y.; Limé, E.; Nilsson Lill, S. O.; Meadows, R. E.; Norrby, P.-O. Role of the Base in Buchwald–Hartwig Amination. J. Org. Chem. 2014, 79, 11961–11969. [Google Scholar] [CrossRef]
- Surry, D. S.; Buchwald, S. L. Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew Chem Int Ed 2008, 47, 6338–6361. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Clark, J. H.; Fairlamb, I. J. S.; Slattery, J. M. Solvent Effects in Palladium Catalysed Cross-Coupling Reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- Tian, J.; Wang, G.; Qi, Z.-H.; Ma, J. Ligand Effects of BrettPhos and RuPhos on Rate-Limiting Steps in Buchwald–Hartwig Amination Reaction Due to the Modulation of Steric Hindrance and Electronic Structure. ACS Omega 2020, 5, 21385–21391. [Google Scholar] [CrossRef] [PubMed]
- Seifinoferest, B.; Tanbakouchian, A.; Larijani, B.; Mahdavi, M. Ullmann-Goldberg and Buchwald-Hartwig C−N Cross Couplings: Synthetic Methods to Pharmaceutically Potential N-Heterocycles. Asian J Org Chem 2021, 10, 1319–1344. [Google Scholar] [CrossRef]
- Stepanenko, A. A.; Dmitrenko, V. V. HEK293 in Cell Biology and Cancer Research: Phenotype, Karyotype, Tumorigenicity, and Stress-Induced Genome-Phenotype Evolution. Gene 2015, 569, 182–190. [Google Scholar] [CrossRef]


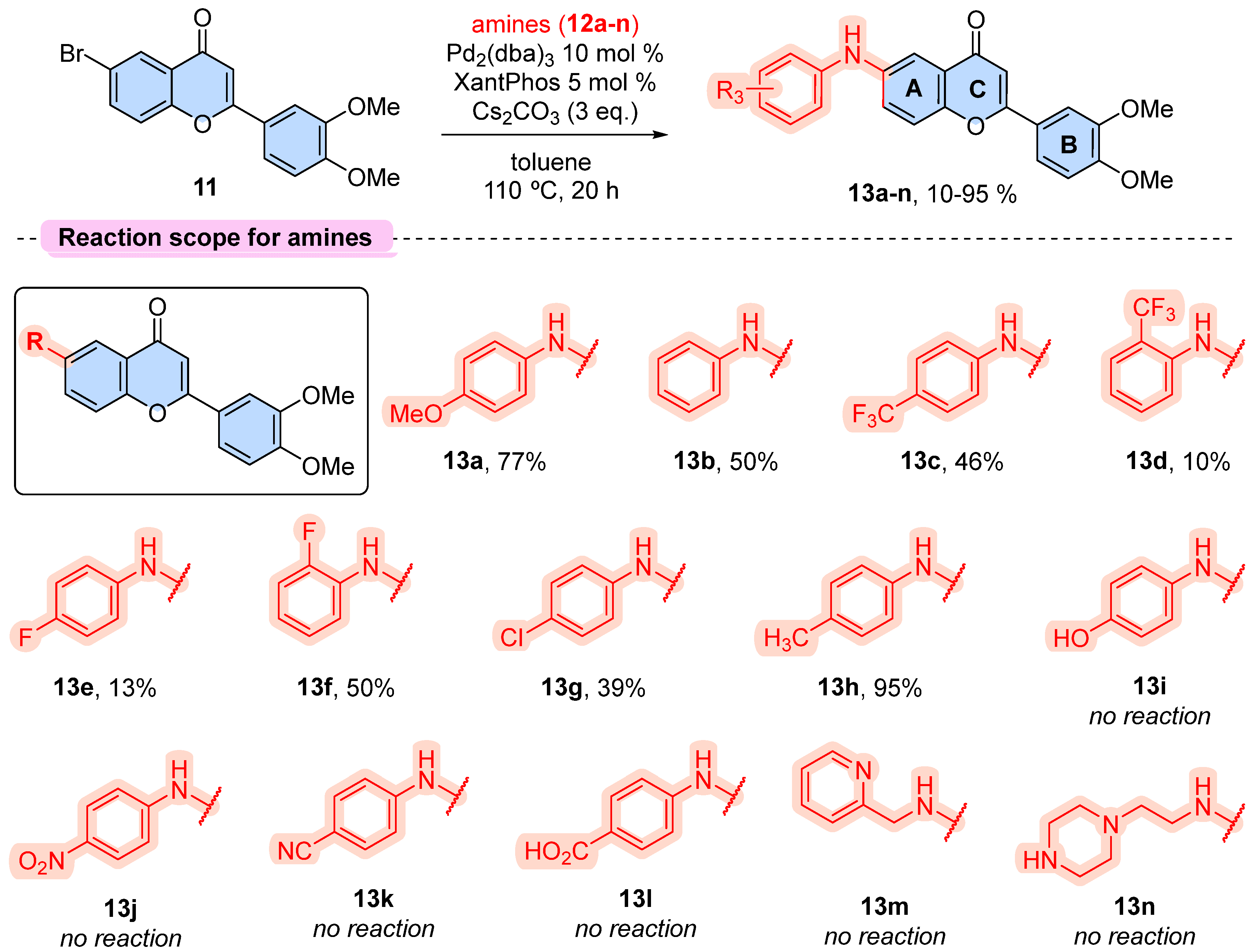

 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | Base | Solvent | Yield (%) |
| 1 | Pd2(dba)3 | XantPhos | CsF | toluene | No reaction |
| 2 | Pd2(dba)3 | XantPhos | NaOtBu | toluene | trace |
| 3 | Pd2(dba)3 | XantPhos | K2CO3 | toluene | 32 |
| 4 | Pd2(dba)3 | XantPhos | Cs2CO3 | toluene | 77 |
| 5 | Pd2(dba)3 | DavePhos | Cs2CO3 | toluene | 75 |
| 6 | PdCl2(PPh3)2 | XantPhos | Cs2CO3 | toluene | 20 |
| 7 | Pd2(dba)3 | XantPhos | Cs2CO3 | THF | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





