Submitted:
27 March 2025
Posted:
28 March 2025
You are already at the latest version
Abstract
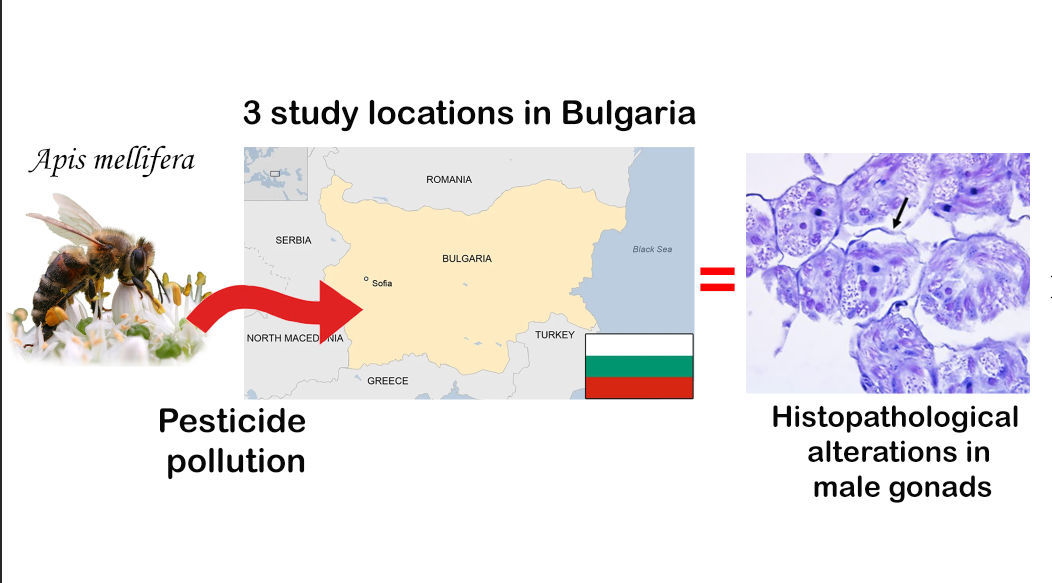
Keywords:
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Chemical Analysis
2.3. Histopathological Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. International COLOSS Questionnaire
3.2. Chemical Levels
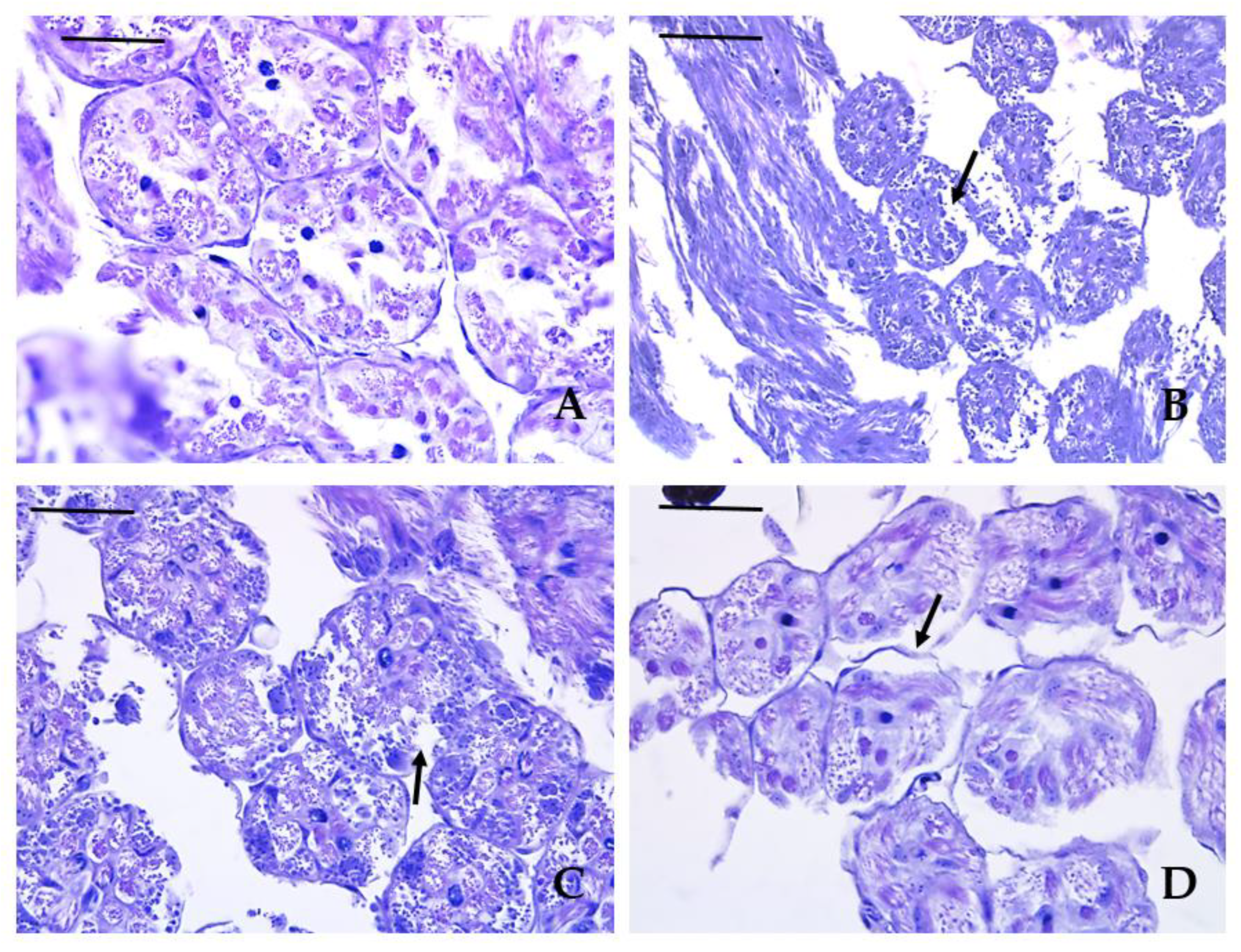
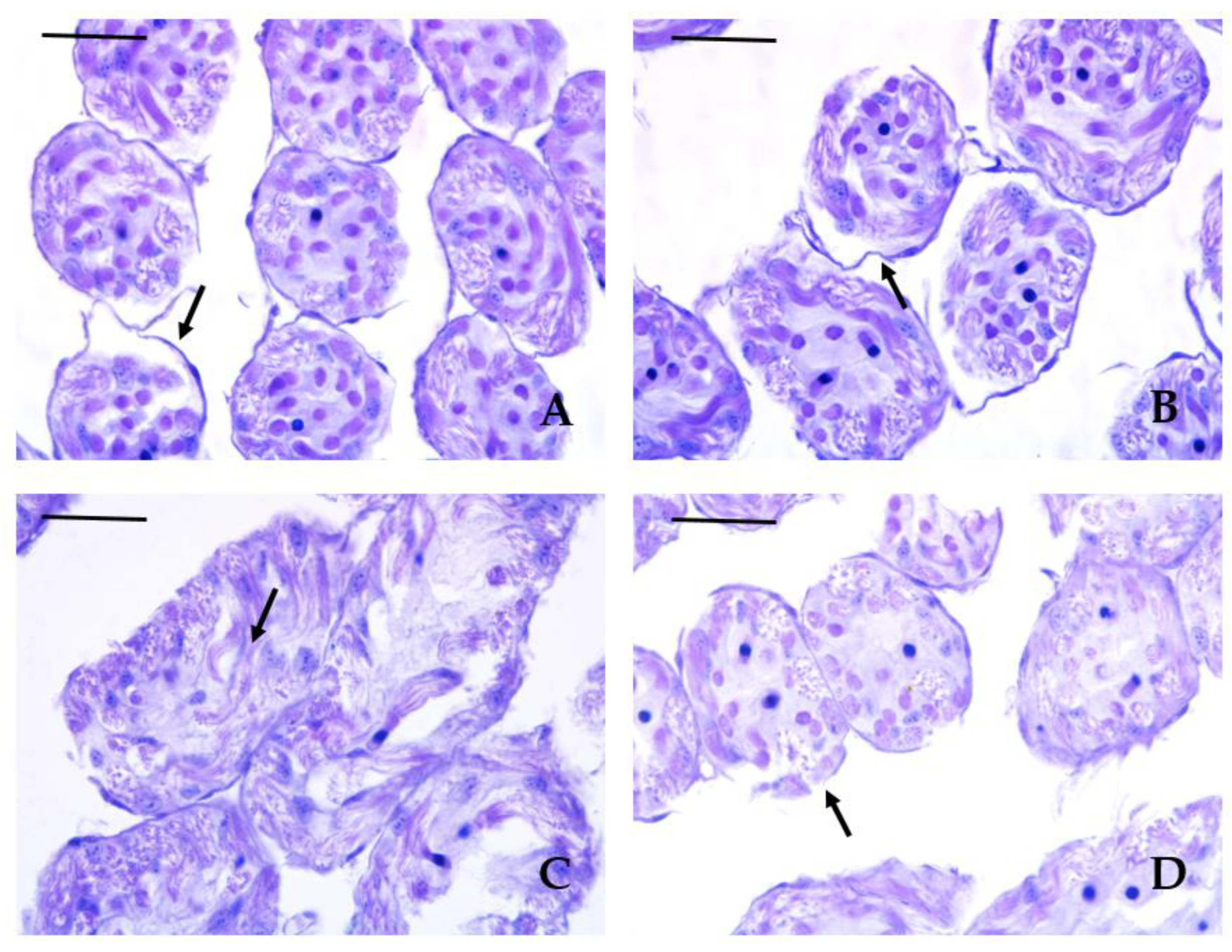
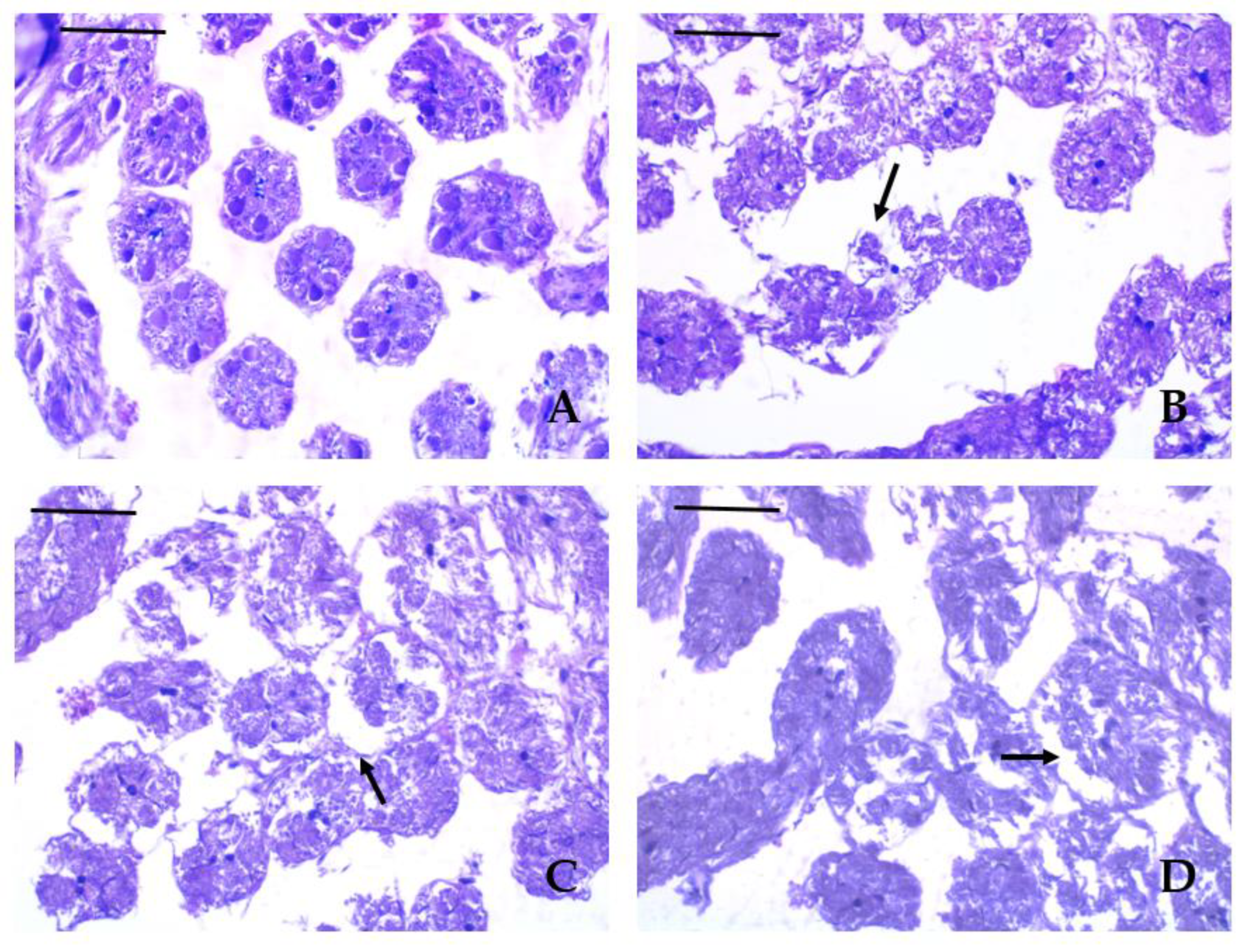
3.3. Histopathological Lesions
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Raven, P.H.; Wagner; D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA. 2021, 118(2), e2002548117. [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K. et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [CrossRef]
- Kaila, L.; Ketola, J.; Toivonen, M.; Loukola, O.; Hakala, K.; Raiskio, S.; Hurme, T.; Jalli, M. Pesticide residues in honeybee-collected pollen: does the EU regulation protect honeybees from pesticides? Environ. Sci. Pollut. Res. 2022, 29, 18225–18244. [CrossRef]
- Martinello, M.; Manzinello, C.; Dainese, N.; Giuliato, I.; Gallina, A.; Mutinelli, F. The Honey Bee: An Active Biosampler of Environmental Pollution and a Possible Warning Biomarker for Human Health. Appl. Sci. 2021, 11, 6481. [CrossRef]
- Klein C.D.; Kozii I.V.; Wood S.C.; Koziy R.V.; Zabrodski M.W.; Dvylyuk I.; Medici de Mattos I.; Moshynskyy I.; Honaramooz A.; Simko E. Testicular changes of honey bee drones, Apis mellifera (Hymenoptera: Apidae), during sexual maturation. J. Insect Sci. 2021, 21(6), 3. [CrossRef]
- Papa, G.; Maier, R.; Durazzo, A.; Lucarini, M.; Karabagias, I.K.; Plutino, M.; Bianchetto, E.; Aromolo, R.; Pignatti, G.; Ambrogio, A.; Pellecchia, M.; Negri, I. The Honey bee Apis mellifera: an insect at the interface between human and ecosystem health. Biology 2022, 11(2), 233. [CrossRef]
- Jiang, J.; Ma, D.; Zou, N.; Yu, X.; Zhang, Z.; Liu, F.; Mu, W. Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere 2018, 201, 159-167. [CrossRef]
- Chiesa, L.M.; Labella, G.F.; Giorgi, A.; Panseri, S.; Pavlovic, R.; Bonacci, S.; Arioli, F. The occurrence of pesticides and persistent organic pollutants in Italian organic honeys from different productive areas in relation to potential environmental pollution. Chemosphere 2016, 154, 482-490. [CrossRef]
- Eissa, F.; Taha, E.K.A. Contaminants in honey: an analysis of EU RASFF notifications from 2002 to 2022. J. Consum. Prot. Food Saf. 2023. [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLOS One 2013, 8(1), e54092. [CrossRef]
- Margaoan, R.; Papa, G., Nicolescu, A.; Cornea-Cipcigan, M.; Kösoğlu, M.; Topal, E.; Negri, I. Environmental pollution effect on honey bees and their derived products: a comprehensive analysis. Environ. Sci. Pollut. Res. 2024. [CrossRef]
- Kunat-Budzyńska, M.; Łabuć, E.; Ptaszyńska, A.A. Seasonal detection of pathogens in honeybees kept in natural and laboratory conditions. Parasitol. Int. 2025, 104, 102978. [CrossRef]
- Kasiotis, K.M.; Anagnostopoulos, C.; Anastasiadou, P.; Machera, K. Pesticide residues in honeybees, honey and bee pollen by LC–MS/MS screening: reported death incidents in honeybees. Sci. Total Environ. 2014, 485, 633-642. [CrossRef]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.M. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemophere 2015, 120, 457-461. [CrossRef]
- Catalano, P.; Della Sala, F.; Cavaliere, M.; Caputo, C.; Pecoraro, D.; Crispino, G.; Lettera, S.; Caioni, G.; Esposito, M.; Verre, A.; et al. Use of honey bees and hive products as bioindicators to assess environmental contamination in targeted areas of the Campania region (Italy). Animals 2024, 14, 1446. [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.; Mommaerts, V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicol. 2012, 21(4), 973-992. [CrossRef]
- van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.-M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5(3-4), 293-305. [CrossRef]
- De Jong, D.; Lester, P.J. The global challenge of improving bee protection and health. Front. Bee Sci. 2023, 1. [CrossRef]
- Cresswell, J.E.; Desneux, N.; van Engelsdorp, D. Dietary traces of neonicotinoid pesticides as a cause of population declines in honey bees: an evaluation by Hill's epidemiological criteria. Pest. Manag. Sci. 2012, 68(6), 819-827. [CrossRef]
- Gill, R.J.; Ramos-Rodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491(7422), 105-108. [CrossRef]
- Whitehorn, P.R.; O'Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336(6079), 351-352. [CrossRef]
- Gusso-Choueri P.K.; Choueri R.B.; de Araújo G.S.; et al. Univariate or multivariate approaches for histopathological biomarkers in the context of environmental quality assessments? Mar. Pollut. Bull. 2022, 181, 113828. [CrossRef]
- Tanabe, P.; Schlenk, D.; Forsgren, K.L.; Pampanin, D.M. Using digital pathology to standardize and automate histological evaluations of environmental samples. Environ. Toxicol. Chem. 2025, 44(2), 306-317. [CrossRef]
- Gray, A.; Adjlane, N.; Arab, A.; Ballis, A.; Brusbardis, V.; Bugeja Douglas, A.; et al. Honey bee colony loss rates in 37 countries using the COLOSS survey for winter 2019–2020: the combined effects of operation size, migration and queen replacement. J. Apic. Res. 2022, 62(2), 204-210. [CrossRef]
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological stains: a literature review and case study. Glob. J. Health Sci. 2015, 8(3), 72-79.
- Power, K.; Martano, M.; Altamura, G.; Maiolino, P. Histopathological findings in testes from apparently healthy drones of Apis mellifera ligustica. Vet. Sci. 2020, 7, 124. [CrossRef]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50(6), 1007-1015. [CrossRef]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A histology-based fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375-381. [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T. E., Haberland, M., Reddy, T., Cournapeau, D.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17(3), 261-272. [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with Python. Proceed. 9th Python in Science Conference (SciPy2010) 2010, 92-96. [CrossRef]
- European Food Safety Authority (EFSA). Scientific services to support EFSA systematic reviews: Lot 5 Systematic literature review on the neonicotinoids (namely active substances clothianidin, thiamethoxam and imidacloprid) and the risks to bees. EFSA supporting publication 2015: EN-756, 2013. Available online: http://www.efsa.europa.eu/en/supporting/doc/756e.pdf.
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; van Engelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58(5), 613-620. [CrossRef]
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R., Mullin, C.A.; Frazier, J.L.; Frazier, M. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the eastern United States. Sci. Rep. 2016, 6, 33207. [CrossRef]
- Mullins, C.A.; Frazier, M.; Frazier, J.L.; Ashcroft, S.; Simonds, R.; van Engelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 2010, 5, e9754. [CrossRef]
- Rinderer, T.E.; De Guzman, L.I.; Lancaster, V.A.; Delatte, G.T.; Seltzer, J.A. Varroa in the mating yard: I. The effects of Varroa jacobsoni and Apistan® on drone honey bees. Am. Bee J. 1999, 134–139.
- Benito-Murcia, M.; Botías, C.; Martín-Hernández, R.; Higes, M.; Soler, F.; Pérez-López, M.; Míguez-Santiyán, M.P.; Martínez-Morcillo, S. 2024. Biomarker responses and lethal dietary doses of tau-fluvalinate and coumaphos in honey bees: Implications for chronic acaricide toxicity. Environ. Toxicol. Pharmacol. 2024, 105, 104330. [CrossRef]
- Ilyasov, R.A.; Lim, S., Lee, L.M.L; Kwon, H.W.; Nikolenko, A.G. Effect of miticides amitraz and fluvalinate on reproduction and productivity of honey bee Apis mellifera (Akarisit Amitraz ve Fluvalinat'ın Bal Arısı Apis mellifera'nın Üreme ve Verimliliğine Etkisi). U. Arı D./U. Bee J. 2021, 21, 21-30. [CrossRef]
- Bishop, G.H. Fertilization in the honey-bee. I. The male sexual organs: their histological structure and physiological functioning. J. Exp. Zool. 1920, 31, 224-265.
- Elhamalawy, O.; Bakr, A.; Eissa, F. Impact of pesticides on non-target invertebrates in agricultural ecosystems. Pest. Biochem. Physiol. 2024, 202, 105974. [CrossRef]
- Ebrahimi, M.; Taherianfard, M. The effects of heavy metals exposure on reproductive system of cyprinid fish from Kor River. Iran. J. Fish. Sci. 2011, 10, 13-26.
- Babazadeh, M.; Najafi, G. Effect of chlorpyrifos on sperm characteristics and testicular tissue changes in adult male rats. Vet. Res. Forum. 2017, 8, 319-326.
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239-247. [CrossRef]
- Sandroc, C.; Tanadini, L.G.; Pettis, J.S.; Biesmeijer, J.C.; Potts, S.G.; Neumann, P. Sublethal neonicotinoid insecticide exposure reduces solitary bee reproductive success. Agric. For. Entomol. 2014, 16, 119-128.
- Baines, D.; Wilton, E.; Pawluk, A.; de Gorter, M.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 10979.
- Basak, M.; Choudhury, R.A.; Goswami, P.; Dey, B.K.; Laskar, M.A. A review on non-target toxicity of deltamethrin and piperonyl butoxide. J. Pharm. Res. Int. 2021, 33(51B), 85-89. [CrossRef]
- Elhamalawy, O.; Bakr, A.; Eissa, F. Impact of pesticides on non-target invertebrates in agricultural ecosystems. Pest. Biochem. Physiol. 2024, 202, 105974. [CrossRef]
| Apiaries | Queen problem losses % | Losses due to bee mortality % | Total loss % | Reported treatment | Risky vegetation |
| Krasnovo | 0 | 0 | 0 | Oxalic acid | No |
| Dimovtsi | 0 | 19.74 | 19.74 | Oxalic acid Amitraz |
Orchards |
| Plovdiv | 8.43 | 32.53 | 40.96 | No treatment | Orchards, rapeseed, sunflower, maize |
| Chemical | Dimovtsi | Krasnovo | Plovdiv | ||||||
| dead honey bees | alive honey bees | food stocks | dead honey bees | alive honey bees | food stocks | dead honey bees | alive honey bees | food stocks | |
| Amitraz | BDL | BDL | BDL | BDL | BDL | BDL | 0.0039 ± 0.0003 | BDL | BDL |
| Carbendazim | BDL | BDL | BDL | BDL | BDL | BDL | 0.0011 ± 0.0001 | BDL | BDL |
| Clothianidin | 0.0203 ± 0.0015A | BDL | BDL | BDL | BDL | BDL | 0.0030 ± 0.0002B | BDL | BDL |
| Coumaphos | BDL | 0.0433 ± 0.0012A | 0.3133 ± 0.0208B | BDL | BDL | BDL | BDL | 0.0617 ± 0.0021A | 0.0603 ± 0.0015A |
| Cypermethrin | 0.0103 ± 0.0006 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| DEET (N,N-diethyl-M-toluamide) |
BDL | BDL | BDL | BDL | BDL | BDL | BDL | 0.0387 ± 0.0015 | BDL |
| Dimethoate | BDL | BDL | BDL | BDL | BDL | BDL | 0.0011 ± 0.0002 | BDL | BDL |
| Fenamidone | BDL | BDL | BDL | BDL | BDL | BDL | 0.0013 ± 0.0002 | BDL | BDL |
| Fenbutatin oxide | BDL | BDL | BDL | BDL | BDL | BDL | 0.0032 ± 0.0002 | BDL | BDL |
| Fenoxycarb | BDL | BDL | BDL | BDL | BDL | BDL | 0.0018 ± 0.0001 | BDL | BDL |
| Flonicamid | BDL | BDL | BDL | BDL | BDL | BDL | 0.0052 ± 0.0002 | BDL | BDL |
| Fluvalinate | BDL | BDL | BDL | BDL | BDL | 0.0057 ± 0.0015A | BDL | BDL | 0.0237 ± 0.0015B |
| Fluxapyroxad | 0.0620 ± 0.0020 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Hexythiazox | BDL | BDL | BDL | BDL | BDL | BDL | 0.0015 ± 0.0002 | BDL | BDL |
| Linuron | BDL | BDL | BDL | BDL | BDL | BDL | 0.0023 ± 0.0002 | BDL | BDL |
| Piperonyl-butoxide | BDL | BDL | BDL | BDL | BDL | 0.0073 ± 0.0021 | BDL | BDL | BDL |
| Prosulfocarb | BDL | BDL | BDL | BDL | BDL | BDL | 0.0035 ± 0.0002 | BDL | BDL |
| Pyraclostrobin | 0.1433 ± 0.0208 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Tebuconazole | BDL | BDL | BDL | BDL | BDL | BDL | 0.0026 ± 0.0004 | BDL | BDL |
| Thiamethoxam | 0.0071 ± 0.0052 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Thiophanate-methyl | BDL | BDL | BDL | BDL | BDL | BDL | 0.1367 ± 0.0153 | BDL | BDL |
| Histopathological lesion | Krasnovo (control) | Dimovtsi | Plovdiv | |||
| mature drones | immature drones | mature drones | immature drones | mature drones | immature drones | |
| Thinning of the covering epithelium of the seminiferous tubules | 0A | 0A | 1.90 ± 0.74B | 1.00 ± 0.67C | 0.80 ± 0.63C | 0.90 ± 0.57C |
| Detachment of the basement membrane of the seminiferous tubule | 0A | 0A | 2.20 ± 0.79B | 1.30 ± 0.48C | 1.00 ± 0.67C | 1.00 ± 0.67C |
| Necrotic areas | 0A | 0A | 0.70 ± 0.67A | 0.60 ± 0.52A | 2.10 ± 0.74B | 2.00 ± 0.94B |
| Lack of spermatozoa (only in mature drones) |
0A | 0A | 0.20 ± 0.42A | - | 0A | - |
| Аbsence of lumen (only in mature drones) | 0A | 0A | 1.00 ± 0.67B | - | 0A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





