Submitted:
26 March 2025
Posted:
27 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Expert Working Group
2.2. Preparation, Convergence, & Collaboration
2.3. Reporting
3. Results and Discussion
3.1. Clinical Questions
- Clinical Question 1: Which patients should be offered germline genetic testing for breast cancer?
- Clinical Question 2: What approaches should be used to offer germline genetic testing for breast cancer, and which genes should be tested?
- Clinical Question 3: How should patients with breast cancer who are considering genetic testing be counselled in the pre- and post-test setting?
- Clinical Question 4: What challenges exist, and what steps are necessary to implement these recommendations equitably across Canada?
3.2. Overview of Recommendations
- Acknowledgement of the clinical value of testing patients with ductal carcinoma in situ (DCIS).
- Inclusion of concurrent or asynchronous bilateral breast cancers among the testing criteria for patients aged >65 years.
- Increased emphasis on the importance of breast cancer prevention and early detection in addition to individualized treatment of affected individuals.
- Standardized use of multigene panel testing rather than a primary focus on BRCA1/BRCA2, as well as specification of additional genes that should be tested at minimum (with recognition that those recommended may evolve as new evidence becomes available).
- Increased reflection of Canadian approaches to germline genetic testing in terms of patient flow and non-genetics-provider-initiated testing practices (i.e., mainstreaming).
- Considerations related to genetic counselling within the Canadian health care system, both in general and with increased use of mainstreaming.
- Discussion of specific challenges encountered in Canada related to germline testing and guidance on solutions that may support broader and more efficient implementation.
3.3. Recommendations
- Clinical Question 1: Which patients with breast cancer should be offered germline genetic testing?
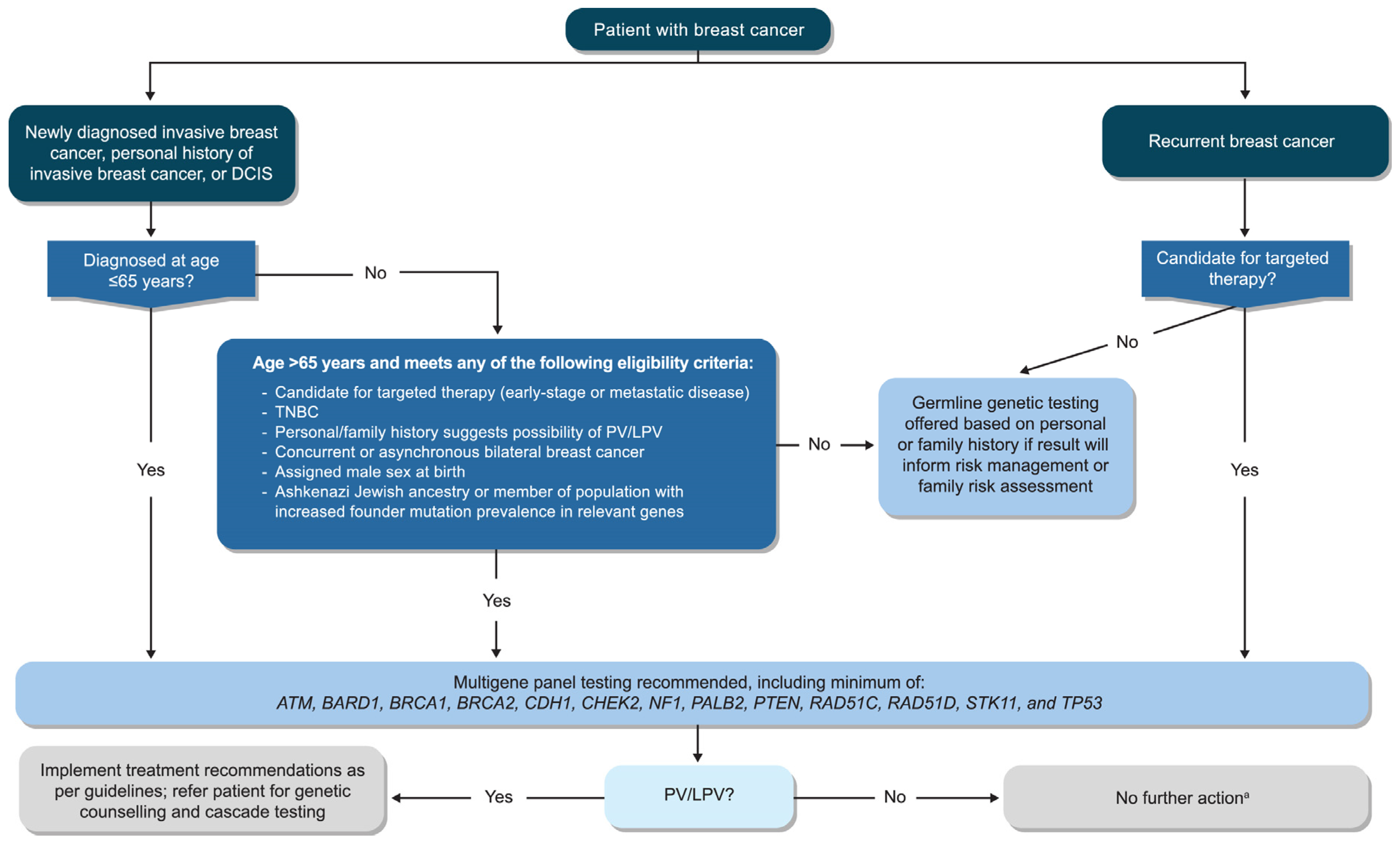
- Recommendation 1.1: All patients with newly diagnosed invasive breast cancer, a personal history of invasive breast cancer, or DCIS who are aged ≤65 years at diagnosis should be offered germline genetic testing.
- Recommendation 1.2: All patients with newly diagnosed invasive breast cancer, a personal history of invasive breast cancer, or DCIS who are aged >65 years at diagnosis should be offered germline genetic testing if:
- they are candidates for targeted therapies indicated for the presence of germline PVs in early-stage or metastatic disease (e.g., poly[ADP-ribose] polymerase inhibitors [PARPi])
- they have triple-negative breast cancer
- their personal or family history suggests the possibility of a PV/LPV (e.g., multiple primary cancers in the individual or family member[s])
- they have bilateral breast cancer, either concurrent or asynchronous
- they were assigned male sex at birth
- they are of Ashkenazi Jewish ancestry or are members of a population with an increased prevalence of founder mutations in relevant genes
- Recommendation 1.3: All patients with recurrent breast cancer (local or metastatic) who are candidates for targeted therapies indicated for germline PVs should be offered germline genetic testing.
Patients Eligible for Targeted Therapies
- Clinical Question 2: What approaches should be used to offer germline genetic testing for breast cancer, and which genes should be tested?
- Recommendation 2.1: Germline genetic testing should be included in the initial assessment of patients via mainstreaming or other modalities that ensure a timely and efficient approach.
- Recommendation 2.2: Germline genetic testing should use next-generation sequencing with a multigene panel that includes, but is not limited to, the following genes: ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11, and TP53.
- Clinical Question 3: How should patients with breast cancer who are considering germline genetic testing be counselled in the pre- and post-test setting?
- Recommendation 3.1: All patients who are candidates for germline genetic testing should be given sufficient information before testing to support their informed consent.
- Recommendation 3.2: All patients with a PV/LPV should be provided with individualized post-test genetic counselling and offered a referral to a provider experienced in clinical cancer genetics.
- Recommendation 3.3: Identification of a VUS typically should not alter management. Patients should be made aware that although most VUS are eventually reclassified as non-disease causing or benign, such variants may occasionally be reclassified as pathogenic. As VUS reassessment and reporting of reclassification are not standardized across laboratories, ordering providers should be aware of their local testing laboratory’s practices. Consultation with a provider experienced in clinical cancer genetics can be helpful and should be made available, especially if a patient’s personal or family history is suspicious for a hereditary cancer syndrome or they have ongoing concerns regarding the impact of a VUS despite explanation.
- Recommendation 3.4: Patients without a PV on germline genetic testing may benefit from counselling if there is a significant personal or family history of cancer. Referral to a provider experienced in clinical cancer genetics is especially recommended if the patient’s personal or family history is suspicious for a hereditary cancer syndrome, regardless of the patient’s negative test result. Consultation between the HCP and the cancer genetics service and referral to a provider experienced in clinical cancer genetics can be helpful when there is uncertainty.
- Clinical Question 4: What challenges exist, and what steps are necessary to implement these recommendations equitably across Canada?
- Recommendation 4.1: Policy changes and frameworks are needed to support expanded education, testing, counselling, and clinical follow-up needs related to germline genetic testing of breast cancer in Canada.
- Recommendation 4.2: A national guideline for genetic testing should be developed to improve consistency and uptake across the country and to provide a foundation for funding in each province/territory.
- Recommendation 4.3: A national working group of experts in cancer and genetics should be established to provide expertise to the provinces/territories and to ensure national standards are communicated and maintained as genetic testing evolves.
Key Challenges
Proposed Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menko, F.H.; Monkhorst, K.; Hogervorst, F.B.L.; Rosenberg, E.H.; Adank, M.A.; Ruijs, M.W.G.; Bleiker, E.M.A.; Sonke, G.S.; Russell, N.S.; Oldenburg, H.S.A.; van der Kolk, L.E. Challenges in breast cancer genetic testing. A call for novel forms of multidisciplinary care and long-term evaluation. Crit Rev Oncol Hematol 2022, 176, 103642. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, P.; Jackisch, C.; Im, S.A.; Hunt, K.K.; Li, C.F.; Unger, S.; Paluch-Shimon, S. BRCA genetic testing and counseling in breast cancer: how do we meet our patients' needs? NPJ Breast Cancer 2024, 10, 77. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; Roche, H.; Im, Y.H.; Quek, R.G.W.; Markova, D.; Tudor, I.C.; Hannah, A.L.; Eiermann, W.; Blum, J.L. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; Wu, W.; Goessl, C.; Runswick, S.; Conte, P. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; Armstrong, A.C.; Arnedos, M.; Balmana, J.; Bergh, J.; Bliss, J.; Delaloge, S.; Domchek, S.M.; Eisen, A.; Elsafy, F.; Fein, L.E.; Fielding, A.; Ford, J.M.; Friedman, S.; Gelmon, K.A.; Gianni, L.; Gnant, M.; Hollingsworth, S.J.; Im, S.A.; Jager, A.; Johannsson, O.; Lakhani, S.R.; Janni, W.; Linderholm, B.; Liu, T.W.; Loman, N.; Korde, L.; Loibl, S.; Lucas, P.C.; Marme, F.; Martinez de Duenas, E.; McConnell, R.; Phillips, K.A.; Piccart, M.; Rossi, G.; Schmutzler, R.; Senkus, E.; Shao, Z.; Sharma, P.; Singer, C.F.; Spanic, T.; Stickeler, E.; Toi, M.; Traina, T.A.; Viale, G.; Zoppoli, G.; Park, Y.H.; Yerushalmi, R.; Yang, H.; Pang, D.; Jung, K.H.; Mailliez, A.; Fan, Z.; Tennevet, I.; Zhang, J.; Nagy, T.; Sonke, G.S.; Sun, Q.; Parton, M.; Colleoni, M.A.; Schmidt, M.; Brufsky, A.M.; Razaq, W.; Kaufman, B.; Cameron, D.; Campbell, C.; Tutt, A.N.J.; Committee, O.C.T.S.; Investigators. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef]
- Albertsen, H.; Plaetke, R.; Ballard, L.; Fujimoto, E.; Connolly, J.; Lawrence, E.; Rodriguez, P.; Robertson, M.; Bradley, P.; Milner, B.; et al. Genetic mapping of the BRCA1 region on chromosome 17q21. Am J Hum Genet 1994, 54, 516–525. [Google Scholar]
- Tung, N.; Lin, N.U.; Kidd, J.; Allen, B.A.; Singh, N.; Wenstrup, R.J.; Hartman, A.R.; Winer, E.P.; Garber, J.E. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol 2016, 34, 1460–1468. [Google Scholar] [CrossRef]
- Beitsch, P.D.; Whitworth, P.W.; Hughes, K.; Patel, R.; Rosen, B.; Compagnoni, G.; Baron, P.; Simmons, R.; Smith, L.A.; Grady, I.; Kinney, M.; Coomer, C.; Barbosa, K.; Holmes, D.R.; Brown, E.; Gold, L.; Clark, P.; Riley, L.; Lyons, S.; Ruiz, A.; Kahn, S.; MacDonald, H.; Curcio, L.; Hardwick, M.K.; Yang, S.; Esplin, E.D.; Nussbaum, R.L. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol 2019, 37, 453–460. [Google Scholar] [CrossRef]
- O'Shaughnessy, J.; Brezden-Masley, C.; Cazzaniga, M.; Dalvi, T.; Walker, G.; Bennett, J.; Ohsumi, S. Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study. Breast Cancer Res 2020, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Yadav, S.; Hu, C.; Wunderle, M.; Haberle, L.; Hart, S.N.; Rubner, M.; Polley, E.C.; Lee, K.Y.; Gnanaolivu, R.D.; Hadji, P.; Hubner, H.; Tesch, H.; Ettl, J.; Overkamp, F.; Lux, M.P.; Ekici, A.B.; Volz, B.; Uhrig, S.; Luftner, D.; Wallwiener, M.; Muller, V.; Belleville, E.; Untch, M.; Kolberg, H.C.; Beckmann, M.W.; Reis, A.; Hartmann, A.; Janni, W.; Wimberger, P.; Taran, F.A.; Fehm, T.N.; Wallwiener, D.; Brucker, S.Y.; Schneeweiss, A.; Hartkopf, A.D.; Couch, F.J. Mutations in BRCA1/2 and other panel genes in patients with metastatic breast cancer-association with patient and disease characteristics and effect on prognosis. J Clin Oncol 2021, 39, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Burcher, S.; Meiser, B.; Mitchell, G.; Saunders, C.; Rahman, B.; Tucker, K.; Barlow-Stewart, K.; Watts, K.; Gleeson, M.; Kirk, J. Oncology health professionals' attitudes toward treatment-focused genetic testing for women newly diagnosed with breast cancer. Per Med 2013, 10, 431–440. [Google Scholar] [CrossRef]
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus guidelines on genetic testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol 2019, 26, 3025–3031. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Huang, T.; Zhou, J. Genetic testing enhances the precision diagnosis and treatment of breast cancer. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Scheinberg, T.; Young, A.; Woo, H.; Goodwin, A.; Mahon, K.L.; Horvath, L.G. Mainstream consent programs for genetic counseling in cancer patients: A systematic review. Asia Pac J Clin Oncol 2021, 17, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, I.; Somerfield, M.R.; Achatz, M.I.; Boughey, J.C.; Curigliano, G.; Friedman, S.; Kohlmann, W.K.; Kurian, A.W.; Laronga, C.; Lynce, F.; Norquist, B.S.; Plichta, J.K.; Rodriguez, P.; Shah, P.D.; Tischkowitz, M.; Wood, M.; Yadav, S.; Yao, K.; Robson, M.E. Germline testing in patients with breast cancer: ASCO-Society of Surgical Oncology guideline. J Clin Oncol 2024, 42, 584–604. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, and Prostate V.3.2025 (C) National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 13, 2025. To view the most recent and complete version of the guideline, go to NCCN.org.
- McDevitt, T.; Durkie, M.; Arnold, N.; Burghel, G.J.; Butler, S.; Claes, K.B.M.; Logan, P.; Robinson, R.; Sheils, K.; Wolstenholme, N.; Hanson, H.; Turnbull, C.; Hume, S. EMQN best practice guidelines for genetic testing in hereditary breast and ovarian cancer. Eur J Human Genetics 2024, 32, 479–488. [Google Scholar] [CrossRef]
- Pujol, P.; Barberis, M.; Beer, P.; Friedman, E.; Piulats, J.M.; Capoluongo, E.D.; Garcia Foncillas, J.; Ray-Coquard, I.; Penault-Llorca, F.; Foulkes, W.D.; Turnbull, C.; Hanson, H.; Narod, S.; Arun, B.K.; Aapro, M.S.; Mandel, J.-L.; Normanno, N.; Lambrechts, D.; Vergote, I.; Anahory, M.; Baertschi, B.; Baudry, K.; Bignon, Y.-J.; Bollet, M.; Corsini, C.; Cussenot, O.; De la Motte Rouge, T.; Duboys de Labarre, M.; Duchamp, F.; Duriez, C.; Fizazi, K.; Galibert, V.; Gladieff, L.; Gligorov, J.; Hammel, P.; Imbert-Bouteille, M.; Jacot, W.; Kogut-Kubiak, T.; Lamy, P.-J.; Nambot, S.; Neuzillet, Y.; Olschwang, S.; Rebillard, X.; Rey, J.-M.; Rideau, C.; Spano, J.-P.; Thomas, F.; Treilleux, I.; Vandromme, M.; Vendrell, J.; Vintraud, M.; Zarca, D.; Hughes, K.S.; Alés Martínez, J.E. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer 2021, 146, 30–47. [Google Scholar] [CrossRef]
- Sessa, C.; Balmaña, J.; Bober, S.L.; Cardoso, M.J.; Colombo, N.; Curigliano, G.; Domchek, S.M.; Evans, D.G.; Fischerova, D.; Harbeck, N.; Kuhl, C.; Lemley, B.; Levy-Lahad, E.; Lambertini, M.; Ledermann, J.A.; Loibl, S.; Phillips, K.A.; Paluch-Shimon, S. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol 2023, 34, 33–47. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. Clinical guideline [CG164]. Last updated: 14 Nov 2023. Available online: https://www.nice.org.uk/guidance/cg164/chapter/Recommendations#care-of-people-in-secondary-care-and-specialist-genetic-clinics (accessed on 24 February 2025).
- Cancer Care Ontario. Hereditary Cancer Testing Eligibility Criteria: Version 3.1. October 2024. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/70161 (accessed on 28 January 2025).
- Sharma, P.; Klemp, J.R.; Kimler, B.F.; Mahnken, J.D.; Geier, L.J.; Khan, Q.J.; Elia, M.; Connor, C.S.; McGinness, M.K.; Mammen, J.M.; Wagner, J.L.; Ward, C.; Ranallo, L.; Knight, C.J.; Stecklein, S.R.; Jensen, R.A.; Fabian, C.J.; Godwin, A.K. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat 2014, 145, 707–714. [Google Scholar] [CrossRef]
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; Slettedahl, S.; Hallberg, E.; Guidugli, L.; Davila, J.I.; Beckmann, M.W.; Janni, W.; Rack, B.; Ekici, A.B.; Slamon, D.J.; Konstantopoulou, I.; Fostira, F.; Vratimos, A.; Fountzilas, G.; Pelttari, L.M.; Tapper, W.J.; Durcan, L.; Cross, S.S.; Pilarski, R.; Shapiro, C.L.; Klemp, J.; Yao, S.; Garber, J.; Cox, A.; Brauch, H.; Ambrosone, C.; Nevanlinna, H.; Yannoukakos, D.; Slager, S.L.; Vachon, C.M.; Eccles, D.M.; Fasching, P.A. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015, 33, 304–311. [Google Scholar] [CrossRef]
- Yadav, S.; Hu, C.; Hart, S.N.; Boddicker, N.; Polley, E.C.; Na, J.; Gnanaolivu, R.; Lee, K.Y.; Lindstrom, T.; Armasu, S.; Fitz-Gibbon, P.; Ghosh, K.; Stan, D.L.; Pruthi, S.; Neal, L.; Sandhu, N.; Rhodes, D.J.; Klassen, C.; Peethambaram, P.P.; Haddad, T.C.; Olson, J.E.; Hoskin, T.L.; Goetz, M.P.; Domchek, S.M.; Boughey, J.C.; Ruddy, K.J.; Couch, F.J. Evaluation of germline genetic testing criteria in a hospital-based series of women with breast cancer. J Clin Oncol 2020, 38, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.W.; Bernhisel, R.; Larson, K.; Caswell-Jin, J.L.; Shadyab, A.H.; Ochs-Balcom, H.; Stefanick, M.L. Prevalence of pathogenic variants in cancer susceptibility genes among women with postmenopausal breast cancer. JAMA 2020, 323, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Emborgo, T.S.; Saporito, D.; Muse, K.I.; Barrera, A.M.G.; Litton, J.K.; Lu, K.H.; Arun, B.K. Prospective evaluation of universal BRCA testing for women with triple-negative breast cancer. JNCI Cancer Spectr 2020, 4, pkaa002. [Google Scholar] [CrossRef]
- Desai, S.; Jena, A.B. Do celebrity endorsements matter? Observational study of BRCA gene testing and mastectomy rates after Angelina Jolie’s New York Times editorial. BMJ 2016, 355, i6357. [Google Scholar] [CrossRef]
- Smith, K.L.; Adank, M.; Kauff, N.; Lafaro, K.; Boyd, J.; Lee, J.B.; Hudis, C.; Offit, K.; Robson, M. BRCA mutations in women with ductal carcinoma in situ. Clin Cancer Res 2007, 13, 4306–4310. [Google Scholar] [CrossRef]
- Petridis, C.; Arora, I.; Shah, V.; Megalios, A.; Moss, C.; Mera, A.; Clifford, A.; Gillett, C.; Pinder, S.E.; Tomlinson, I.; Roylance, R.; Simpson, M.A.; Sawyer, E.J. Frequency of pathogenic germline variants in BRCA1, BRCA2, PALB2, CHEK2 and TP53 in ductal carcinoma in situ diagnosed in women under the age of 50 years. Breast Cancer Res 2019, 21, 58. [Google Scholar] [CrossRef]
- Claus, E.B.; Petruzella, S.; Matloff, E.; Carter, D. Prevalence of BRCA1 and BRCA2 mutations in women diagnosed with ductal carcinoma in situ. JAMA 2005, 293, 964–969. [Google Scholar] [CrossRef]
- Huang, H.; Couch, R.E.; Karam, R.; Hu, C.; Boddicker, N.; Polley, E.C.; Na, J.; Ambrosone, C.B.; Yao, S.; Trentham-Dietz, A.; Eliassen, A.H.; Penney, K.; Brantley, K.; Bodelon, C.; Teras, L.R.; Hodge, J.; Patel, A.; Haiman, C.A.; John, E.M.; Neuhausen, S.L.; Martinez, E.; Lacey, J.V.; O'Brien, K.M.; Sandler, D.P.; Weinberg, C.R.; Palmer, J.R.; Bertrand, K.A.; Vachon, C.M.; Olson, J.E.; Ruddy, K.E.; Anton-Culver, H.; Ziogas, A.; Goldgar, D.E.; Nathanson, K.L.; Domchek, S.M.; Weitzel, J.N.; Kraft, P.; Dolinsky, J.S.; Pesaran, T.; Richardson, M.E.; Yadav, S.; Couch, F.J. Pathogenic variants in cancer susceptibility genes predispose to ductal carcinoma in situ of the breast. Clin Cancer Res 2025, 31, 130–138. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; Domchek, S.M.; Gelmon, K.A.; Hollingsworth, S.J.; Korde, L.A.; Linderholm, B.; Bandos, H.; Senkus, E.; Suga, J.M.; Shao, Z.; Pippas, A.W.; Nowecki, Z.; Huzarski, T.; Ganz, P.A.; Lucas, P.C.; Baker, N.; Loibl, S.; McConnell, R.; Piccart, M.; Schmutzler, R.; Steger, G.G.; Costantino, J.P.; Arahmani, A.; Wolmark, N.; McFadden, E.; Karantza, V.; Lakhani, S.R.; Yothers, G.; Campbell, C.; Geyer, C.E., Jr. OlympiA Clinical Trial Steering Committee Investigators. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- AstraZeneca Canada. Product monograph including patient medication information. LYNPARZA (olaparib capsules). Date of revision: 27 Sep 2024. Available online: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/lynparza-product-monograph-en.pdf (accessed on 21 January 2025).
- Canadian Agency for Drugs and Technologies in Health. CADTH Reimbursement Recommendation: Olaparib (Lynparza). Can J Health Tech 2023, 3, 1–23. [Google Scholar]
- Pfizer Canada. Product monograph including patient medication information. TALZENNA (talazoparib capsules). Date of revision: 2 Feb 2022. Available online: https://www.pfizer.ca/en/our-products/talzenna-talazoparib (accessed on 21 January 2025).
- Robson, M.E.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Tung, N.; Armstrong, A.; Dymond, M.; Fielding, A.; Allen, A.; Conte, P. OlympiAD extended follow-up for overall survival and safety: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Eur J Cancer 2023, 184, 39–47. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; Wu, W.; Goessl, C.; Runswick, S.; Domchek, S.M. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; Roché, H.; Im, Y.H.; Eiermann, W.; Quek, R.G.W.; Usari, T.; Lanzalone, S.; Czibere, A.; Blum, J.L.; Martin, M.; Ettl, J. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Canada's Drug Agency. Reimbursement review: Talazoparib. Last updated: 24 July 2024. Available online: https://www.cda-amc.ca/talazoparib (accessed on 21 January 2025).
- Canada's Drug Agency. Reimbursement review: Olaparib. Last updated: 24 July 2024. Available online: https://www.cda-amc.ca/olaparib-1 (accessed on 21 January 2025).
- Robson, M.; Ruddy, K.J.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Li, W.; Tung, N.; Armstrong, A.; Delaloge, S.; Bannister, W.; Goessl, C.; Degboe, A.; Hettle, R.; Conte, P. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer 2019, 120, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ettl, J.; Quek, R.G.W.; Lee, K.H.; Rugo, H.S.; Hurvitz, S.; Gonçalves, A.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; Roché, H.; Im, Y.H.; Markova, D.; Bhattacharyya, H.; Hannah, A.L.; Eiermann, W.; Blum, J.L.; Litton, J.K. Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol 2018, 29, 1939–1947. [Google Scholar] [CrossRef]
- Gelmon, K.A.; Fasching, P.A.; Couch, F.J.; Balmaña, J.; Delaloge, S.; Labidi-Galy, I.; Bennett, J.; McCutcheon, S.; Walker, G.; O'Shaughnessy, J. Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis. Eur J Cancer 2021, 152, 68–77. [Google Scholar] [CrossRef]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; Melisko, M.; Brufsky, A.; DeMeo, M.; Jenkins, C.; Domchek, S.; D’Andrea, A.; Lin, N.U.; Hughes, M.E.; Carey, L.A.; Wagle, N.; Wulf, G.M.; Krop, I.E.; Wolff, A.C.; Winer, E.P.; Garber, J.E. TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 2020, 38, 4274–4282. [Google Scholar] [CrossRef]
- Gruber, J.J.; Afghahi, A.; Timms, K.; DeWees, A.; Gross, W.; Aushev, V.N.; Wu, H.T.; Balcioglu, M.; Sethi, H.; Scott, D.; Foran, J.; McMillan, A.; Ford, J.M.; Telli, M.L. A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes. Nat Cancer 2022, 3, 1181–1191. [Google Scholar] [CrossRef]
- Cheng, J.M.; Canzoniero, J.; Lee, S.; Soni, S.; Mangini, N.; Santa-Maria, C.A. Exceptional responses to PARP inhibitors in patients with metastatic breast cancer in oncologic crisis. Breast Cancer Res Treat 2023, 199, 389–397. [Google Scholar] [CrossRef]
- Kuemmel, S.; Harrach, H.; Schmutzler, R.K.; Kostara, A.; Ziegler-Löhr, K.; Dyson, M.H.; Chiari, O.; Reinisch, M. Olaparib for metastatic breast cancer in a patient with a germline PALB2 variant. NPJ Breast Cancer 2020, 6, 31. [Google Scholar] [CrossRef]
- Batalini, F.; Madison, R.; Pavlick, D.C.; Sokol, E.; Snow, T.; Sondhi, A.; Frampton, G.M.; Jenkins, C.; Garber, J.E.; Wulf, G.M.; Venstrom, J.M.; Tung, N.M.; Castellanos, E.; Schrock, A.B.; McGregor, K. Analysis of real-world (RW) data for metastatic breast cancer (mBC) patients (pts) with somatic BRCA1/2 (sBRCA) or other homologous recombination (HR)-pathway gene mutations (muts) treated with PARP inhibitors (PARPi). J Clin Oncol 2021, 39, 10512–10512. [Google Scholar] [CrossRef]
- Jahan, N.; Taraba, J.; Boddicker, N.J.; Giridhar, K.V.; Leon-Ferre, R.A.; Tevaarwerk, A.J.; Cathcart-Rake, E.; O'Sullivan, C.C.; Peethambaram, P.P.; Hobday, T.J.; Mina, L.A.; Batalini, F.; Advani, P.; Sideras, K.; Haddad, T.C.; Ruddy, K.J.; Goetz, M.P.; Couch, F.J.; Yadav, S. Real-world evidence on prescribing patterns and clinical outcomes of metastatic breast cancer patients treated with PARP inhibitors: The Mayo Clinic experience. Clin Breast Cancer 2025, 25, e211–e219.e212. [Google Scholar] [CrossRef]
- Kemp, Z.; Turnbull, A.; Yost, S.; Seal, S.; Mahamdallie, S.; Poyastro-Pearson, E.; Warren-Perry, M.; Eccleston, A.; Tan, M.M.; Teo, S.H.; Turner, N.; Strydom, A.; George, A.; Rahman, N. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open 2019, 2, e194428. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Foulkes, W.D. Moving breast cancer susceptibility gene testing into the mainstream. Med J Aus 2023, 218, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Cremin, C.; Bedard, A.C.; Hong, Q.; Mung, S.W.; Nuk, J.; Wong, A.; Akbar, H.; Cheung, E.; Renouf, D.; Schaeffer, D.; Sun, S.; Schrader, K.A. Improving access to hereditary testing in pancreatic ductal carcinoma. JCO Precis Oncol 2024, 8, e2400167. [Google Scholar] [CrossRef]
- Strømsvik, N.; Olsson, P.; Gravdehaug, B.; Luras, H.; Schlichting, E.; Jorgensen, K.; Wangensteen, T.; Vamre, T.; Heramb, C.; Maehle, L.; Grindedal, E.M. "It was an important part of my treatment": a qualitative study of Norwegian breast Cancer patients' experiences with mainstreamed genetic testing. Hered Cancer Clin Pract 2022, 20, 6. [Google Scholar] [CrossRef]
- Lambert, D.M.; Patrinos, D.; Knoppers, B.M.; Zawati, M.H. Genetic counselors and legal recognition: A made-for-Canada approach. J Genet Couns 2022, 31, 49–58. [Google Scholar] [CrossRef]
- Rezoug, Z.; Totten, S.P.; Szlachtycz, D.; Atayan, A.; Mohler, K.; Albert, S.; Feng, L.; Lemieux Anglin, B.; Shen, Z.; Jimenez, D.; Hamel, N.; Meti, N.; Esfahani, K.; Boileau, J.F.; Prakash, I.; Basik, M.; Meterissian, S.; Tremblay, F.; Fleiszer, D.; Anderson, D.; Chong, G.; Wong, S.M.; Foulkes, W.D. Universal genetic testing for newly diagnosed invasive breast cancer. JAMA Netw Open 2024, 7, e2431427. [Google Scholar] [CrossRef]
- Thompson, E.R.; Rowley, S.M.; Li, N.; McInerny, S.; Devereux, L.; Wong-Brown, M.W.; Trainer, A.H.; Mitchell, G.; Scott, R.J.; James, P.A.; Campbell, I.G. Panel testing for familial breast cancer: Calibrating the tension between research and clinical care. J Clin Oncol 2016, 34, 1455–1459. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Wubbenhorst, B.; D'Andrea, K.; Garman, B.; Long, J.M.; Powers, J.; Rathbun, K.; Stopfer, J.E.; Zhu, J.; Bradbury, A.R.; Simon, M.S.; DeMichele, A.; Domchek, S.M.; Nathanson, K.L. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med 2015, 17, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Association, C.; Dorling, L.; Carvalho, S.; Allen, J.; Gonzalez-Neira, A.; Luccarini, C.; Wahlstrom, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q.; Bolla, M.K.; Dennis, J.; Keeman, R.; Alonso, M.R.; Alvarez, N.; Herraez, B.; Fernandez, V.; Nunez-Torres, R.; Osorio, A.; Valcich, J.; Li, M.; Torngren, T.; Harrington, P.A.; Baynes, C.; Conroy, D.M.; Decker, B.; Fachal, L.; Mavaddat, N.; Ahearn, T.; Aittomaki, K.; Antonenkova, N.N.; Arnold, N.; Arveux, P.; Ausems, M.; Auvinen, P.; Becher, H.; Beckmann, M.W.; Behrens, S.; Bermisheva, M.; Bialkowska, K.; Blomqvist, C.; Bogdanova, N.V.; Bogdanova-Markov, N.; Bojesen, S.E.; Bonanni, B.; Borresen-Dale, A.L.; Brauch, H.; Bremer, M.; Briceno, I.; Bruning, T.; Burwinkel, B.; Cameron, D.A.; Camp, N.J.; Campbell, A.; Carracedo, A.; Castelao, J.E.; Cessna, M.H.; Chanock, S.J.; Christiansen, H.; Collee, J.M.; Cordina-Duverger, E.; Cornelissen, S.; Czene, K.; Dork, T.; Ekici, A.B.; Engel, C.; Eriksson, M.; Fasching, P.A.; Figueroa, J.; Flyger, H.; Forsti, A.; Gabrielson, M.; Gago-Dominguez, M.; Georgoulias, V.; Gil, F.; Giles, G.G.; Glendon, G.; Garcia, E.B.G.; Alnaes, G.I.G.; Guenel, P.; Hadjisavvas, A.; Haeberle, L.; Hahnen, E.; Hall, P.; Hamann, U.; Harkness, E.F.; Hartikainen, J.M.; Hartman, M.; He, W.; Heemskerk-Gerritsen, B.A.M.; Hillemanns, P.; Hogervorst, F.B.L.; Hollestelle, A.; Ho, W.K.; Hooning, M.J.; Howell, A.; Humphreys, K.; Idris, F.; Jakubowska, A.; Jung, A.; Kapoor, P.M.; Kerin, M.J.; Khusnutdinova, E.; Kim, S.W.; Ko, Y.D.; Kosma, V.M.; Kristensen, V.N.; Kyriacou, K.; Lakeman, I.M.M.; Lee, J.W.; Lee, M.H.; Li, J.; Lindblom, A.; Lo, W.Y.; Loizidou, M.A.; Lophatananon, A.; Lubinski, J.; MacInnis, R.J.; Madsen, M.J.; Mannermaa, A.; Manoochehri, M.; Manoukian, S.; Margolin, S.; Martinez, M.E.; Maurer, T.; Mavroudis, D.; McLean, C.; Meindl, A.; Mensenkamp, A.R.; Michailidou, K.; Miller, N.; Mohd Taib, N.A.; Muir, K.; Mulligan, A.M.; Nevanlinna, H.; Newman, W.G.; Nordestgaard, B.G.; Ng, P.S.; Oosterwijk, J.C.; Park, S.K.; Park-Simon, T.W.; Perez, J.I.A.; Peterlongo, P.; Porteous, D.J.; Prajzendanc, K.; Prokofyeva, D.; Radice, P.; Rashid, M.U.; Rhenius, V.; Rookus, M.A.; Rudiger, T.; Saloustros, E.; Sawyer, E.J.; Schmutzler, R.K.; Schneeweiss, A.; Schurmann, P.; Shah, M.; Sohn, C.; Southey, M.C.; Surowy, H.; Suvanto, M.; Thanasitthichai, S.; Tomlinson, I.; Torres, D.; Truong, T.; Tzardi, M.; Valova, Y.; van Asperen, C.J.; Van Dam, R.M.; van den Ouweland, A.M.W.; van der Kolk, L.E.; van Veen, E.M.; Wendt, C.; Williams, J.A.; Yang, X.R.; Yoon, S.Y.; Zamora, M.P.; Evans, D.G.; de la Hoya, M.; Simard, J.; Antoniou, A.C.; Borg, A.; Andrulis, I.L.; Chang-Claude, J.; Garcia-Closas, M.; Chenevix-Trench, G.; Milne, R.L.; Pharoah, P.D.P.; Schmidt, M.K.; Spurdle, A.B.; Vreeswijk, M.P.G.; Benitez, J.; Dunning, A.M.; Kvist, A.; Teo, S.H.; Devilee, P.; Easton, D.F. Breast cancer risk genes - association analysis in more than 113,000 women. N Engl J Med 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Gnanaolivu, R.; Huang, H.; Lee, K.Y.; Na, J.; Gao, C.; Lilyquist, J.; Yadav, S.; Boddicker, N.J.; Samara, R.; Klebba, J.; Ambrosone, C.B.; Anton-Culver, H.; Auer, P.; Bandera, E.V.; Bernstein, L.; Bertrand, K.A.; Burnside, E.S.; Carter, B.D.; Eliassen, H.; Gapstur, S.M.; Gaudet, M.; Haiman, C.; Hodge, J.M.; Hunter, D.J.; Jacobs, E.J.; John, E.M.; Kooperberg, C.; Kurian, A.W.; Le Marchand, L.; Lindstroem, S.; Lindstrom, T.; Ma, H.; Neuhausen, S.; Newcomb, P.A.; O'Brien, K.M.; Olson, J.E.; Ong, I.M.; Pal, T.; Palmer, J.R.; Patel, A.V.; Reid, S.; Rosenberg, L.; Sandler, D.P.; Scott, C.; Tamimi, R.; Taylor, J.A.; Trentham-Dietz, A.; Vachon, C.M.; Weinberg, C.; Yao, S.; Ziogas, A.; Weitzel, J.N.; Goldgar, D.E.; Domchek, S.M.; Nathanson, K.L.; Kraft, P.; Polley, E.C.; Couch, F.J. A population-based study of genes previously implicated in breast cancer. N Engl J Med 2021, 384, 440–451. [Google Scholar] [CrossRef]
- Sunnybrook Hospital. Cancer Genetics & High Risk Program: Genetic Counselling. Available online: https://www.youtube.com/watch?v=lE0MVNGAeow (accessed on 19 February 2025).
- Sunnybrook Health Sciences Centre. What to expect if your Sunnybrook doctor ordered genetic testing, and resources to help guide you through the process. Available online: https://sunnybrook.ca/content/?page=occ-cancer-genetics-testing-resources (accessed on 19 February 2025).
- NHS England. National Genomics Education Programme. GeNotes. Genomics in Action: Mainstreaming cancer susceptibility gene testing. Available online: https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/mainstreaming-cancer-susceptibility-gene-testing/?utm_source=chatgpt.com (accessed on 19 February 2025).
- Canadian Association of Genetic Counsellors. Hereditary cancer mainstreaming. Available online: https://www.cagc-accg.ca/index.php?page=470&id= (accessed on 19 February 2025).
- Hallowell, N.; Wright, S.; Stirling, D.; Gourley, C.; Young, O.; Porteous, M. Moving into the mainstream: healthcare professionals' views of implementing treatment focussed genetic testing in breast cancer care. Fam Cancer 2019, 18, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.S.; Kagedan, B.L.; Graham, G.E.; Heim-Myers, B.; Bombard, Y. Health care implications of the Genetic Non-Discrimination Act: Protection for Canadians' genetic information. Can Fam Physician 2022, 68, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership Against Cancer. Available online: https://www.partnershipagainstcancer.ca/ (accessed on 31 January 2025).
- Canadian Task Force on Preventive Health Care. Breast Cancer (Update) Draft Recommendations 2024. Available online: https://canadiantaskforce.ca/guidelines/published-guidelines/breast-cancer-update-2024/ (accessed on 31 January 2025).
- Vos, J.R.; Fakkert, I.E.; de Hullu, J.A.; van Altena, A.M.; Sie, A.S.; Ouchene, H.; Willems, R.W.; Nagtegaal, I.D.; Jongmans, M.C.J.; Mensenkamp, A.R.; Woldringh, G.H.; Bulten, J.; Leter, E.M.; Kets, C.M.; Simons, M.; Ligtenberg, M.J.L.; Hoogerbrugge, N. Universal tumor DNA BRCA1/2 testing of ovarian cancer: prescreening PARPi treatment and genetic predisposition. J Natl Cancer Inst 2020, 112, 161–169. [Google Scholar] [CrossRef]
- Balmana, J.; Fasching, P.A.; Couch, F.J.; Delaloge, S.; Labidi-Galy, I.; O'Shaughnessy, J.; Park, Y.H.; Eisen, A.F.; You, B.; Bourgeois, H.; Goncalves, A.; Kemp, Z.; Swampillai, A.; Jankowski, T.; Sohn, J.H.; Poddubskaya, E.; Mukhametshina, G.; Aksoy, S.; Timcheva, C.V.; Park-Simon, T.W.; Anton-Torres, A.; John, E.; Baria, K.; Gibson, I.; Gelmon, K.A.; investigators, L. Clinical effectiveness and safety of olaparib in BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: final analysis of LUCY. Breast Cancer Res Treat 2024, 204, 237–248. [Google Scholar] [CrossRef]
- Nakamura, S.; Kojima, Y.; Takeuchi, S. Causative genes of homologous recombination deficiency (HRD)-related breast cancer and specific strategies at present. Curr Oncol 2025, 32, 90. [Google Scholar] [CrossRef]
|
Guideline Question |
Which Canadian patients should be offered germline genetic testing for PVs in breast cancer susceptibility genes? |
| Target Population | Patients with breast cancer and their families |
| Target Audience | Medical oncologists, radiation oncologists, surgical oncologists, medical geneticists, genetic counsellors, oncology nurses, oncology advanced practice providers, primary care practitioners, patients, caregivers |
| Methods | A pan-Canadian EWG convened to review current germline genetic testing guidelines for breast cancer and develop revised questions and recommendations reflecting approaches, needs, and considerations relevant to the Canadian setting. |
| Clinical Question 1: Which patients with breast cancer should be offered germline genetic testing? | |
|
Recommendation 1.1: All patients with newly diagnosed invasive breast cancer, a personal history of invasive breast cancer or DCIS who are aged ≤65 years at diagnosis should be offered germline genetic testing. Recommendation 1.2: All patients with newly diagnosed invasive breast cancer, a personal history of invasive breast cancer, or DCIS who are aged >65 years at diagnosis should be offered germline genetic testing if:
| |
| Clinical Question 2: What approaches should be used to offer germline genetic testing for breast cancer and which genes should be tested? | |
|
Recommendation 2.1: Germline genetic testing should be included in the initial assessment of patients via mainstreaming or other modalities that ensure a timely and efficient approach. Recommendation 2.2: Germline genetic testing should use next-generation sequencing with a multigene panel that includes, but is not limited to, the following genes: ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11, and TP53. | |
| Clinical Question 3: How should patients with breast cancer who are considering germline genetic testing be counselled in the pre- and post-test setting? | |
|
Recommendation 3.1: All patients who are candidates for germline genetic testing should be given sufficient information before testing to support their informed consent. Recommendation 3.2: All patients with a PV/LPV should be provided with individualized post-test genetic counselling and offered a referral to a provider experienced in clinical cancer genetics. Recommendation 3.3: Identification of a VUS typically should not alter management. Patients should be made aware that although most VUS are eventually reclassified as non-disease causing or benign, such variants may occasionally be reclassified as pathogenic. As VUS reassessment and reporting of reclassifications are not standardized across laboratories, ordering providers should be aware of their local testing laboratory’s practices. Consultation with a provider experienced in clinical cancer genetics can be helpful and should be made available, especially if a patient’s personal or family history is suspicious for a hereditary cancer syndrome or they have ongoing concerns regarding the impact of a VUS despite explanation. Recommendation 3.4: Patients without a PV on germline genetic testing may still benefit from counselling if there is a significant personal or family history of cancer. Referral to a provider experienced in clinical cancer genetics is especially recommended if the patient’s personal or family history is suspicious for a hereditary cancer syndrome, regardless of the patient’s negative test result. Consultation between the HCP and the cancer genetics service and referral to a provider experienced in clinical cancer genetics can be helpful when there is uncertainty. | |
| Clinical Question 4: What challenges exist, and what steps are necessary to implement these recommendations equitably across Canada? | |
|
Recommendation 4.1: Policy changes and frameworks are needed to support expanded education, testing, counselling, and clinical follow-up needs related to germline genetic testing of breast cancer in Canada. Recommendation 4.2: A national guideline for genetic testing should be developed to improve consistency and uptake across the country and to provide a foundation for funding in each province/territory. Recommendation 4.3: A national working group of experts in breast cancer and genetics should be established to provide ongoing expertise to the provinces/territories and to ensure national standards are communicated and maintained as genetic testing evolves. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





