1. Introduction
In recent decades, an immense research attention has been focused on the development of novel methods of fabrication of multifunctional nanostructured materials with unusual physical, electrical and optical properties originated from the strong quantum confinement effect or surface defects. The successful development of the multifunctional nanostructured materials opens a broad spectrum of new opportunities for their applications in sensor -basedchip devices, electromagnetic interference shielding, energy generation and storage, chemical- bio- and electro-sensing, fuel cells, multimodal imaging and photothermal therapy, solar and photovoltaic cells and photocatalytic water splitting [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10].
The nanostructured materials with high surface area show improved chemical reactivity, magnetic moment and polarizability and enhanced photoactivities under UV/Vis light irradiation, in comparison to larger particles or bulk materials, due to increasing number of the photo-generated electron–hole pairs on the surface [
11,
12].
Among all nanostructured materials, the bismuth-based compounds have been extensively studied in the last decade due to their broad spectrum of potential applications. Among all bismuth-based compounds, publications based on bismuth orthovanadate (BiVO
4) have seen an exponential increase in number over the last years. These compounds have many interesting and unique properties originating from the electronic and/or steric influences of the 6s
2 lone pair of Bi
3+ that has a strong role in determining the site occupancy of the Bi
3+ ions. The bismuth-vanadate (BiVO
4) is widely applied as a yellow pigment [
13], photoelectroanalytical sensor [
14], ferroelectric material [
15], antibacterial agent [
16], luminescent material and a host for rare-earth ions [
17,
18], as well as a photocatalytic material [
19].
BiVO
4 exists in nature in three crystalline forms: orthorhombic pucherite, tetragonal dreyerite (tz-BiVO
4, zircon-type structure, space group I41/amd), and monoclinic clinobisvanite (ms-BiVO
4, distorted scheelite-type structure, space group I2/b) [
20]. Up to date, large number of methods has been adopted for the preparation of ms-BiVO
4, such as: hydrothermal method with and without using surfactant or template [
21,
22,
23], microwave-assisted hydrothermal [
24,
25], solvothermal [
26], and co-precipitation method [
27]. Several methods have also been utilized for the synthesis of tz-BiVO
4: co-precipitation method [
28,
29,
30,
31], hydrothermal method [
32,
33,
34,
35,
36], rapid microwave assisted method [
37] and epitaxial growth on FTO substrate [
38]. An enhanced photocatalytic activity of un-doped or no hybrid tetragonal zircon-type nanostructured BiVO
4 semiconductor is reported in dozen published papers [
39]. Recently, MOF- derived tetragonal BiVO
4 and rare-earth doped BiVO
4 systems [
40,
41,
42,
43] with enhanced photocatalytic properties for water splitting were studied, where the presence of RE
3+ could induce the progressive stabilization of the tetragonal phase [
44].
This work was motivated by recent evidence that tetragonal zircon-type BiVO
4, tz-BiVO
4, one of three commonly found polymorphs of BiVO
4, is moderately photocatalytically active [
45]. Only 30 or so relevant papers can be found in the literature: thus, tz-BiVO
4 has not been extensively studied and, in particular, its photocatalytic properties are insufficiently examined, new synthetic approaches to tz-BiVO
4 are most needed [
46,
47]. Interestingly, synthesis of tz-BiVO
4 in a non-aqueous medium is an obvious way to avoid hydrolysis and precipitation of side products.
Herein, aiming at producing nanocrystalline tz-BiVO4 a new and non-conventional way of synthesis was attempted through a novel straightforward room-temperature non-aqueous preparation method. The as-prepared colloids and the obtained nanostructured particles were examined with a view of quantum size effects on their optical properties and their suitability in photocatalytic applications. The ultra-small tetragonal zircon-type BiVO4 nanostructures, with size range from 2 to 8 nm, were prepared by ethylene glycol-assisted colloidal route and characterized using optical, structural and microscopic techniques. These size-quantized nanoparticles present a remarkable synthetic achievement. Adsorption behaviors and mechanisms of methyl orange on the size-quantized tetragonal BiVO4 nanoparticles were studied in details, as well as photocatalytic activities. Good optical performances and enhanced photocatalytic activity in comparison to titania photocatalyst Degussa P25, give to the size-quantized tetragonal BiVO4 nanoparticles the potential for different applications such as degradation of methyl orange (MO) and other organic dye-pollutants.
2. Materials and Methods
2.1. Materials and Chemicals Used
All chemicals were of high purity and were used without further purification. These included: bismuth(III) nitrate pentahydrate (Bi(NO3)3x5H2O, Sigma-Aldrich, 97%), ammonium metavanadate (NH4VO3, Alfa Aesar, 99.999%), trisodium citrate dihydrate (Na3C6H5O7x2H2O, ≥99%, Sigma–Aldrich), ethylene glycol (C2H6O2, Sigma-Aldrich, 97%), polyethylene glycol 200 (PEG-200, Alfa Aesar), nitric acid, HNO3 (J.T. Baker, 65%) distilled water, methyl orange (C14H14N3NaO3S, Merck) and titanium(IV) oxide nanopowder, Degussa P25 (Sigma-Aldrich, > 99.0%).
2.2. Synthesis of Colloidal Tetragonal BiVO4
Colloidal BiVO
4 samples were synthesized by modified ethylene glycol-assisted colloidal route at room temperature [
48]. Here, NH
4VO
3 and Bi(NO
3)
3x5H
2O were used as precursors and ethylene glycol was utilized as a solvent for precursors, in order to avoid the hydrolysis of Bi(NO
3)
3 and precipitation of side products like bismuth(III)-hydroxonitrate, a reaction medium for a precipitation and a capping agent (to limit a particle growth and prohibit agglomeration). Ethylene glycol is a dihydroxy alcohol (HO–CH
2–CH
2–OH) that is liquid at room temperature; it is more viscous than water, biodegradable and boils at 197 °C. There are also additional advantages of an ethylene glycol-mediated synthesis; this is one of the most general and powerful methods for preparation of high-quality nanomaterials, is used for conventional glassware and the synthesis is simple, easily scalable, “green”, versatile and low-cost [
49]. PEG-200 has role as a structure-directing agent to synthesize vanadate nanoparticles, as an organic additive and as a surface modifier.
The appropriate amounts of NH4VO3, Bi(NO3)3x5H2O were separately dissolved in ethylene glycol to prepare different concentrations of bismuth and vanadium solutions precursors (0.075M, 0.050M and 0.025M). The same concentration of solution of trisodium citrate together with HNO3 was added dropwise to a solution at a stoichiometric ratio of Bi3+ ions at room temperature. A white precipitate consisting of a Bi3+-Cit3- complex was formed. The PEG-200 in ethylene glycol solution (with the same concetrations as precursors) was slowly, drop wise, added into the NH4VO3 containing solution and the resulting mixture was left under vigorous stirring for 1 hour. Afterwards, the ethylene glycol mixture solution of precursor of Bi3+ was slowly added into the mixture solution of NH4VO3 and PEG-200 under vigorous stirring for 2 hours and orange-yellow transparent colloids of BiVO4 were obtained. It is important to emphasize that this preparation procedure proved to be fully reproducible over multiple trials, and, in all the as-prepared colloids, no evidence of precipitation has been noticed over a period of more than one year, thus indicating superior colloid stability of BiVO4 nanoparticles in ethylene glycol solution. These colloids, corresponding to concentrations of precursor 0.075M, 0.050M and 0.025M, are hereafter referred to as samples A, B, C, respectively. In order to obtain the powders of BiVO4, following the synthesis of colloidal BiVO4, as-prepared colloids were additionally treated, centrifugated, washed with water several times and dried in an oven at 110°C for 24h. In the following, powders prepared using the colloids (A, B and C) are denoted as A-tz, B-tz and C-tz.
2.3. Characterization Methods and Instrumentation
Absorption measurements were performed by the UV-vis spectrophotometer (LLG-uniSPEC 2 UV/VIS-Spectrometer 190-1100 nm) in a range of wavelengths from 300 to 500 nm with 1 nm step. X-ray photoelectron spectroscopy (XPS) data were collected using a PHI Versa Probe III-XPS-spectrometer equipped with a monochromatic Al-Kα X-ray source and a hemispherical analyzer. Phase and purity of the powder samples were examined by powder X-ray diffraction (XRD) measurements on a Rigaku SmartLab diffractometer using Cu-Kα radiation (λ = 0.15405 nm). Diffraction data were collected with a step size of 0.02◦ and a counting time of 0.7◦/min over the angular range 2θ from 15◦ to 70◦. The photoluminescence (PL) measurements were recorded at room temperature on a Fluorolog-3 spectro-fluorimeter in the range of wavelengths from 450 nm to 600 nm. HRTEM measurements were performed using a FEI Tecnai F20 at 200 kV electron acceleration voltages after drop-casting of sample material on lacey carbon TEM grids.
2.4. Photocatalytic Experiment
Methyl orange (MO), anionic and water soluble azo dye, was widely used material in the dye industry and is commonly selected as a model organic pollutant to evaluate the behavior of a material for the removal of organic pollutants from its aqueous solutions. The removal of MO in aqueous solutions was carried out in double-walls cylindrical photochemical reactor. Temperature was maintained at 18° C by continuous flow of water through the reactor walls during the adsorption and photocatalytic experiments. Adsorption experiments were carried out in the dark before subsequent (photodegradation) experiments with visible light illumination. The suspension of BiVO4 powder and solution of MO was than exposed to visible light from a LED lamp (Xled E27-15W, 220 V, 3000 K, 1350Lm) placed 15 cm above the reactor.
In a typical experiment, MO was dissolved in 200 ml of deionized water to obtain a 5 mgL-1 solution while optimal photocatalyst concentration was found to be 1 mgmL-1. Prior to irradiation, the adsorption−desorption equilibrium of the dye on the photocatalysts surface was achieved through the vigorous stirring for 90 minutes in the dark to ensure adsorption/desorption equilibrium before lighting. During the irradiation procedure, the reaction sample was collected and centrifuged to remove photocatalyst particles. The change in dye concentration over time was monitored by measuring the absorbance of MO at wavelength of 464 nm using the UV-Vis spectrophotometer. At given time intervals, samples were collected from the reaction mixture during 240 minutes of irradiation.
3. Results and Discussion
3.1. Optical Properties of Colloidal BiVO4 Nanoparticles
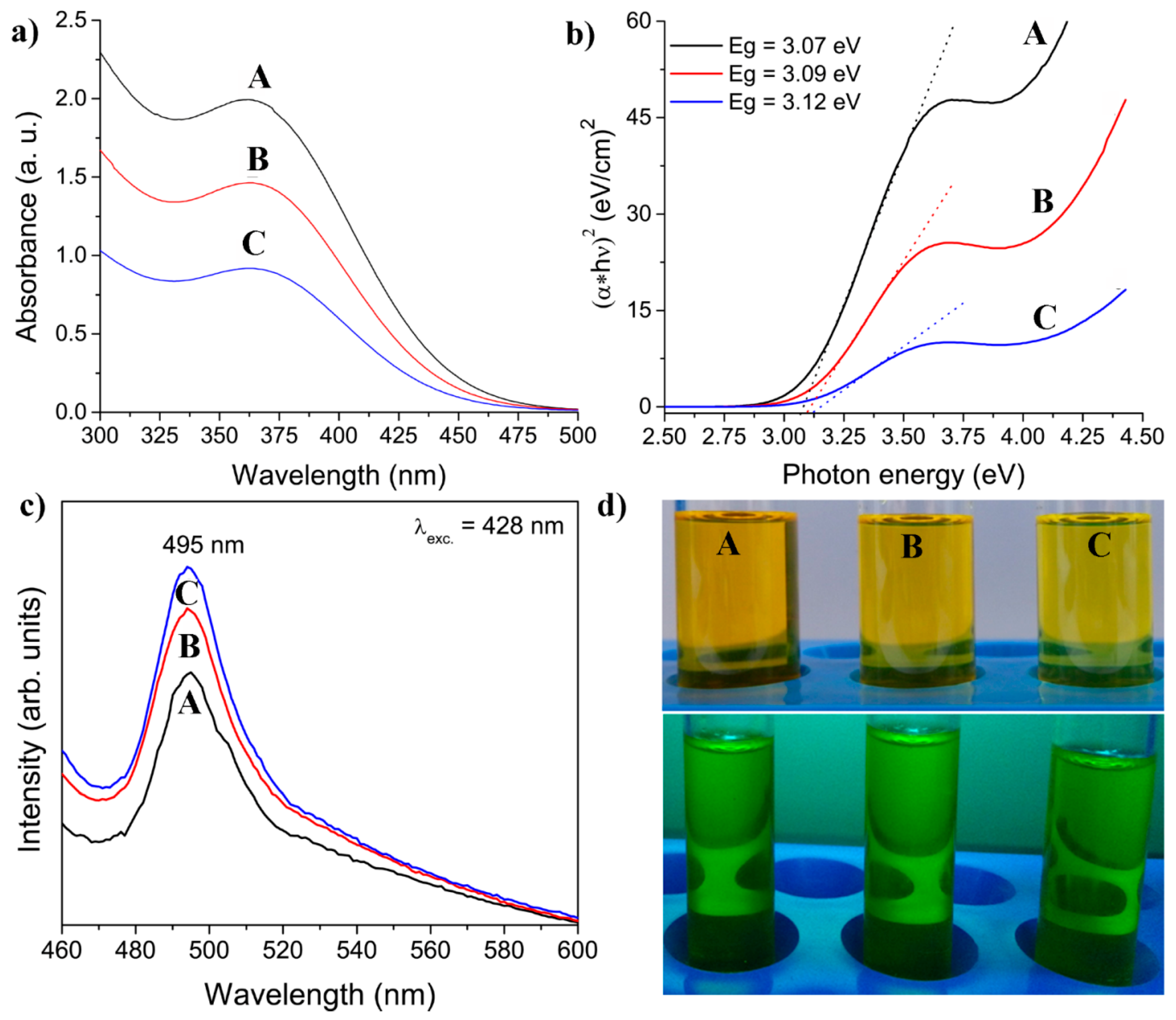
3.1.1. UV-Vis Absorption and Photoluminescent Spectra
It is a well-known fact that the optical properties of semiconducting nanoparticles are essentially determined by their energy band gap (Eg). BiVO
4 is a semiconductor with a direct optical band gap with energy of 2.9 eV for tetragonal structure, measured at room temperature [
50]. The optical UV-Vis absorption spectra of dispersions of colloidal tz-BiVO
4 nanoparticles in ethylene-glycol are given in
Figure 1a. It is noteworthy that there are very few reports in the literature on absorption spectra of solutions of any polymorph of BiVO
4 [
51,
52,
53]; UV-Vis diffuse reflection spectra, however, are more common [
28,
54,
55]. It was observed that all the prepared samples exhibited absorption bands in the near UV and in the blue-violet regions. The absorption, centered at around 362 nm can be readily explained by ligand-to-metal charge transfer transitions localized within tetrahedral vanadate VO
43-groups; electrons from filled oxygen 2p levels are excited into vacant vanadium 3d levels [
56,
57].
The extended absorption tail in UV-Vis spectrum is assigned to subgap absorption due to defect states or intra-band absorption [
57].
In general, the band gap energies of semiconducting materials can be extracted from their absorption spectra by using Tauc’s plots, i.e., by plotting (αhν)
1/n versus incident photon energy hν. The determination of optical band gap is obtained by Tauc’s Equation (1):
where A is a proportionality constant, α is the measured optical absorption coefficient and Eg is the band gap energy of the material. The exponent n depends on the type of the transition and the values of n = 1/2, 2, and 3/2, are for direct allowed transitions, indirect allowed and direct forbidden transitions, respectively. To determine the type of transitions, (αhν)
1/n versus hν was plotted; the band tail constant A is obtained through the slope of the linear region of the graphs, while the corresponding optical band gaps were estimated by extrapolating to zero absorption in the linear part of the graph at (αhν)
1/n = 0 Tauc’s plots (with n = 1/2) of the three as-prepared samples of colloidal BiVO
4 are given in
Figure 1b. The estimated band-gap values of 3.07, 3.09 and 3.12 eV are higher than the value of 2.9 eV reported for tz-BiVO
4 [
31]. The calculated values of band gaps are shifted to larger values than those for bulk materials due to quantum confinement. Obtained Eg values are in agreement with reported band gap values by other authors for tz-BiVO
4 nanoparticles. [
57,
58]
The radius (r) of the nanoparticles were estimated using Brus equation [
59]:
where E
g(nano) is the values of the energy gap determined as the x-intercept of the linear portion of the absorbance as a function of wavelength for nanoparticles with unknown radius (r); E
0(bulk) is the energy gap for bulk material (E
0(bulk) = 2.90 eV, for tetragonal BiVO
4) [
60], me* and mh* are the effective masses of electrons and holes (m
e* = 17.322×m
e, m
h* = 1.210 × m
e) for tetragonal BiVO
4, respectively [
61], m
0 is the mass of electron (m
0 = 9.110x10
-31 kg), ε
0 is the permittivity of vacuum (ε
0 = 8.854187817×10
−12 F×m
−1), ε is the dielectric constant for the tetragonal-BiVO
4 (ε = 68) [
62], h is the Planck constant (h = 6.62607004×10-34 m
2kgs
-1) and e is the charge of an electron (e = 1.60217662×10
-19 C). The second term in equation (2), which dominates when r is small, corresponds to the confinement energies for an electron–hole pair in a spherical nanoparticles, while the third term accounts for the Coulomb interaction between an electron and hole modified by the screening of charges by the crystal. After multiplying by r
2, rearranging, and using the quadratic formula:
one obtains the radii (r) of the nanoparticles equal to 3.24, 3.20 and 3.12 nm, for A, B, and C samples, respectively. These values are consistent with average nanoparticles size determined from TEM images.
Photoluminescent spectra, PL, with excitation wavelength at 428 nm of dispersions of tz-BiVO
4 nanoparticles are shown in
Figure 1c. A vanadate group, VO
43-, where the central vanadium ion is coordinated by four oxygen ions in a tetrahedral (Td) symmetry, is known to be an efficient luminescent center. Hence, strong emission band centered at 495 nm could be attributed to the charge-transfer transitions (generated upon photo-excitation) between vanadium 3d and oxygen 2p orbitals in VO
43-. The results presented in this paper are similar to those of Bajaj et al. [
28]. Photographs of colloidal solutions under day light and UV-lamp (253 nm) are presented in the
Figure 1d.
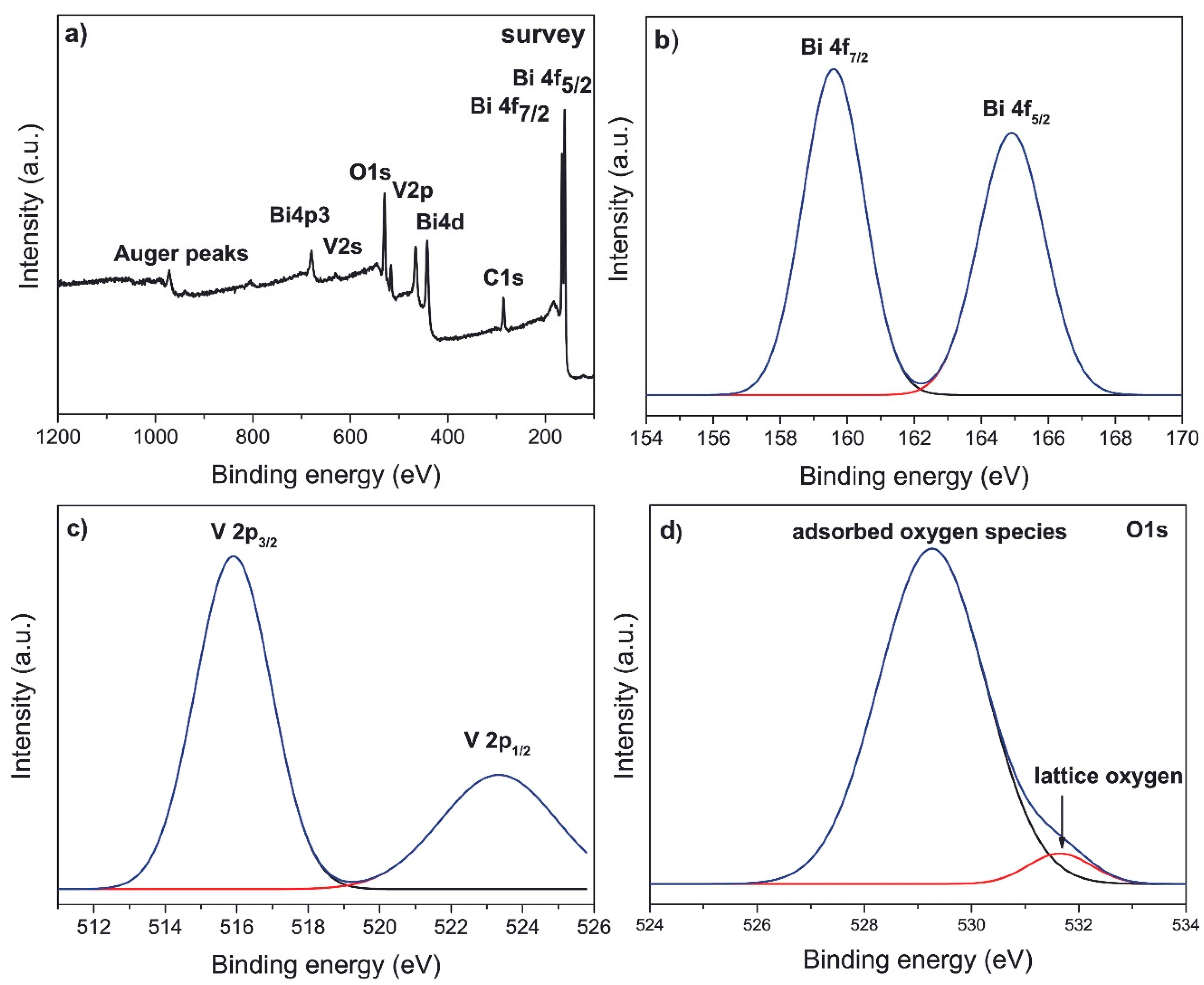
3.1.2. XPS Spectra
To explore the chemical states of surface Bi and V sites in tetragonal nanostructured BiVO
4 semiconductor, the X-ray photoelectron spectroscopy (XPS) technique was used.
Figure 2a presents the wide energy range 0-1200 eV XPS survey spectrum of BiVO
4 nanoparticles with tetragonal structure.
The binding energy levels of Bi, V and O, along with the Auger peaks, identified from the XPS survey spectrum, indicate that no other impurity elements or secondary phases were found in obtained BiVO
4 nanoparticles. The C1s peak arises from the reference. The chemical binding energy of C 1s at 284.54 eV was used for calibration to adjust the binding energies of the other elements. As it can be seen from
Figure 2b, the Bi 4f orbital of tz-BiVO
4 can be well reproduced by two peaks with binding energies of 159.60 eV and 164.91 eV, which can be assigned to the Bi 4f
7/2 and Bi 4f
5/2 orbitals of Bi
3+ indicating the absence of the metallic state of Bi
0.The Bi4f photoelectron core level spectra point out a spin orbit splitting of about 5.31 eV between Bi 4f
7/2 and Bi 4f
5/2 peaks and corresponds to the binding energy of the Bi
3+ state. For the V2p region (
Figure 2c), the peaks located at binding energies of 515.91 eV and 523.37 eV are assigned to V 2p
3/2 and V 2p
1/2 of V
5+, respectively.
Figure 2d presents the high-resolution O1s spectra of the BiVO
4 photocatalyst, which were fitted into two components at binding energy values of 529.28 and 531.71 eV, which can be assigned to the lattice oxygen and oxygen of surface hydration of the nanostructured tz-BiVO
4 [
63]. The obtained values for binding energies of Bi4f, V2p and O1s are in accordance with literature [
64,
65,
66].
3.1.3. Photocatalytic Performance
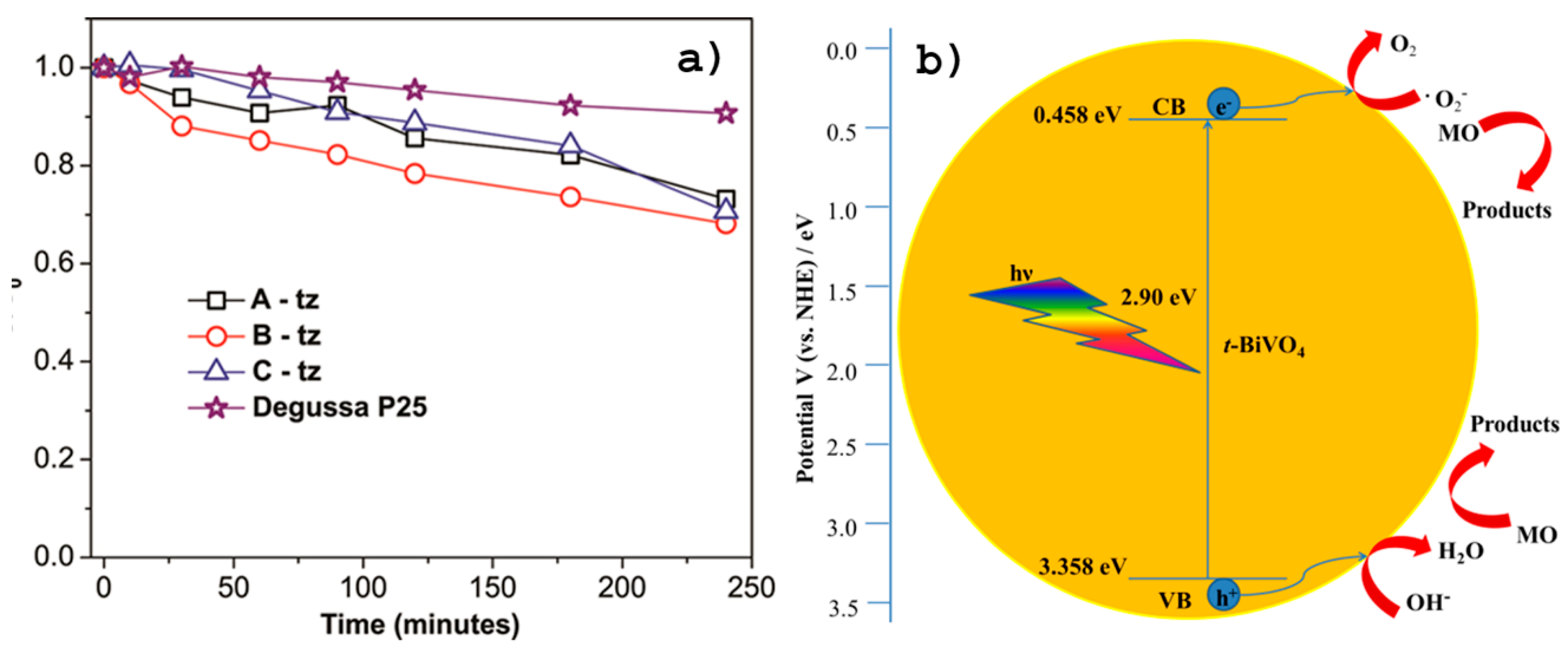
The photocatalytic activities of the tz-BiVO4 samples were evaluated for the photodegradation of MO, which was used as a model of organic pollutants. The powders A-tz, B-tz and C-tz were evaluated for photocatalytic performance, and compared with performance of powdered Degussa P25, a standard commercially available photocatalyst, by photocatalytic degradation of MO, under simulated solar light.
Upon reaching the equilibrium (under no illumination) the initial time was chosen and the dye solution with photocatalyst was exposed to simulated solar light for 240 minutes. As is customary, a relative concentration (C/C
0) of MO versus a contact time t describes kinetics of the dye removal, C
0 and C being respectively the concentration of MO before illumination (t = 0) and the concentration of MO after illumination for t min, while the decolorization efficiency is defined as percentage of decreasing absorbance intensity according to the following equation:
where A
0 is the absorbance at the maximum absorption wavelength (464 nm) of initial methyl orange solution, and A is the maximum absorption wavelength at 464 nm of the same solution mixed with ultra-small tz-BiVO
4 nanoparticles after the treatment. The efficiency of commercial TiO
2 P25 in removing the MO dye was found to be about 10%, while efficiencies of about 26%, 32% and 29% were observed for the samples A-tz, B-tz and C-tz, respectively, as presented in
Figure 3a. The proposed mechanisms of the photocatalytic oxidation of MO in presence of tz-BiVO
4 photocatalyst are drawn in
Figure 3b. Although still preliminary, this is a remarkably good performance of the system for MO removal and it would be interesting to examine it further for use in environmental purification [
67]. This result suggests that ultrasmall tetragonal zircon-type BiVO
4 nanoparticles have a good photocatalytic performance and could be used in water and wastewater treatments, due to their large surface area and good efficiency under UV/Vis and solar light. Before doing any of the experiments, stability of methyl orange was tested under the proposed photocatalytic conditions. MO showed great stability under the conditions (lamp, temperature, etc.) used for all photocatalytic experiments confirming that the decreasing absorbance intensity should be attributed exclusively to the presence of BiVO
4 nanoparticles. The absorbance band at 464 nm corresponds to the conjugated structure that was associated with the azo bond (–N=N–) under the strong influence of the electron-donating dimethylamino group. Decreasing of absorbance intensity at 464 nm for A-tz, B-tz and C-tz Degussa P25 is presented in
Table 1.
The possible mechanism of the photocatalytic oxidation of MO over tz-BiVO
4 can be represented by the following equations:
Under irradiation with visible-light tz-BiVO
4 nanoparticles can be excited to electron–hole pairs and the electron would transform the valence band to the conduction band of tz-BiVO
4. At the same time, the photo-generated electrons are transferred to the excited state (MO*) of the MO owing to the intramolecular π–π* transition. The photogenerated electrons of MO* are immediately injected into the CB of BiVO
4, leaving
.MO+ radicals [
68,
69].
3.2. Structural and Microstructural Properties
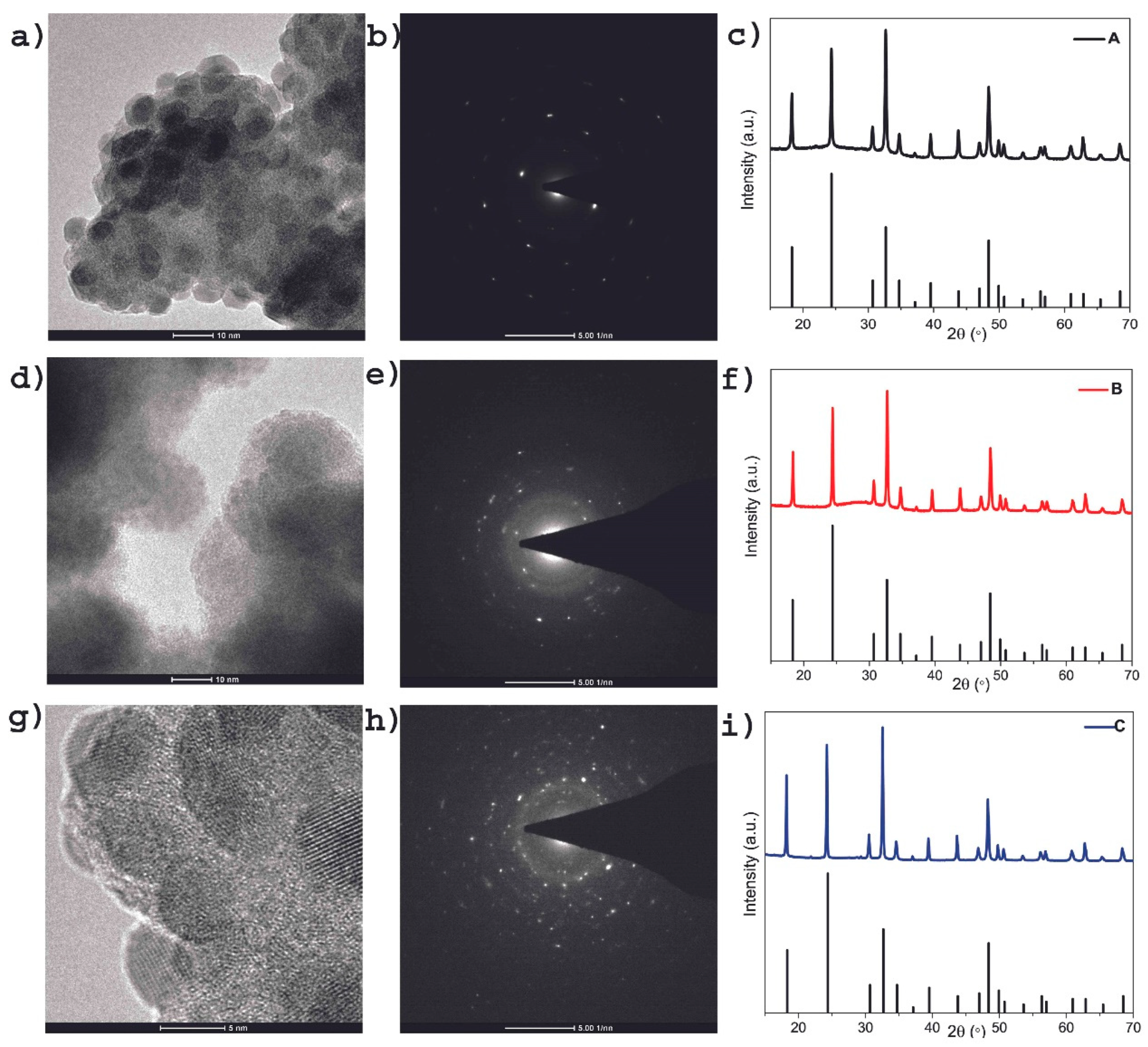
Representative high-resolution TEM (HRTEM) images of BiVO
4 particles of the as-synthesized colloidal dispersions A, B and C are depicted in
Figure 4 (see
Figure 4a, 4d and 4g). All TEM specimens were prepared by evaporating a drop of a colloidal dispersion of BiVO
4 in ethylene glycol on a carbon-coated specimen grid. Well-defined, non-agglomerated highly crystalline nanoparticles together with the diffractogram patterns produced from the digitally-recorded HRTEM images of the samples by means of the two-dimensional Fast Fourier Transformation (FFT) (see
Figure 4b, 4e and 4h) can be seen in the TEM micrographs. The nanoparticle with size of 6-8 nm, 4-6 nm and 2-4 nm were found in the samples A, B and C, respectively.
From these figures it could be concluded that the diameter of colloidal tetragonal zircon-type nanostructured BiVO
4 particles decreases as the concentration of precursor decreases. These findings from the HRTEM micrographs of the colloids A, B and C were fully supported by X-ray analysis of the powders A-tz, B-tz and C-tz obtained from the dispersions [
70,
71]. Moreover, the average crystallite sizes of 4–9.4 nm estimated from the XRD diffraction peaks by the Halder–Wagner method were consistent with the sizes evaluated from the TEM images. In particular, similar values of the crystalline domain size and microscopically estimated average particle size of the nanostructured BiVO
4 imply that each particle consists of a single crystallite. It is in accordance with theoretically estimated radii using Brus equation.
The obtained BiVO
4 particles crystallized in tetragonal zircon-type structure (space group I41/amd, a = b = 7.300 Å and c = 6.457 Å, JCPDS card no. 00-014-0133) and corresponding XRD patterns are given in
Figure 4c, 4f and 4i. All patterns clearly show the presence of a single tetragonal zircon type phase of BiVO
4. The relatively intense reflection peaks suggest that the as-synthesized nanostructured BiVO
4 are highly crystalline. The effect of size-dependent lattice expansion in nanoparticles is observed. The size dependence can be explained naturally from the increasing surface-to-volume ratio and the sensitivity of the surface stress to environmental conditions. Also, point defects may cause lattice expansion in special cases, where the particles are not in thermodynamic equilibrium or where special effects modify the thermodynamic conditions [
72].
4. Conclusions
In summary, the colloidal dispersions of highly nanocrystalline tetragonal zircon-structured BiVO4 particles with 2-8 nm in size were successfully prepared through a facile inexpensive room-temperature precipitation method using ethylene glycol simultaneously as a solvent, a reaction medium and a capping agent. The preparation procedure has proved to be fully reproducible over multiple runs and the as-prepared colloids have been stable and homogeneously colored over longer periods of time. The optical, structural and microstructural properties were examined in detail. The obtained band-gap values, using Tauc’s plot, of 3.07, 3.09 and 3.12 eV are higher than the values for tz-BiVO4 reported in literature before. X-ray photoelectron spectroscopy technique confirmed the occurrence of Bi, V, and O elements and also found that Bi and V exist in +3 and +5 oxidation states, respectively. The radius (r) of the nanoparticles was estimated theoretically using Brus equation. The findings from the HRTEM micrographs of colloidal dispersions were fully supported by X-ray analysis of the powders obtained from the dispersions. Similar values of the crystalline domain size and microscopically estimated average particle size of the nanostructured BiVO4 imply that each particle consists of a single crystallite. It is in accordance with theoretically estimated radii using Brus equation. Interesting experimental findings in photodegradation experiments with a suspension of tz-BiVO4 catalyst powders and MO dye under simulated solar light were encouraging and deserve further, more elaborate investigation of poorly studied tz-BiVO4. It was found that adsorption was the dominant mechanism by which the MO solution was purified, whereas photocatalysis played a minor role under indoor lighting conditions. The interactions between the p-electrons of the MO ring and the surface (-OH) groups dominated the mechanism according to which adsorption occurred. This study demonstrated that the ultrasmall BiVO4 nanoparticles with tetragonal structure could be excellent candidate for wastewater treatment via their highly efficient adsorption and photocatalytic properties.
Author Contributions
Conceptualization, D.M. and M.F.; methodology, D.M.; software, G.C.R. and M.F.; validation, D.M. G.C.R., and M.F.; resources, D.M. and M.F.; writing—original draft preparation, D.M..; writing—review and editing, D.M. G:C:R. and M.F; funding acquisition, D.M. and M.F. All authors have read and agreed to the published version of the manuscript.”
Funding
D.M. thanks to the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grant number 451-03-136/2025-03/ 200017).
Data Availability Statement
Data, Graphics and Figures that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The Authors thankful to Dr. Krisjanis Smits and Dr. Tanja Barudžija for providing TEM and XRD measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liao, J.; Qi, T.; Chu, B.; Peng, J.; Luo, F.; Qian, Z. Multifunctional nanostructured materials for multimodal cancer imaging and therapy. J. Nanosci. Nanotechnol. 2014, 14, 175–189. [Google Scholar] [CrossRef]
- Chiappini, A.; Armellini, C.; Piccolo, V.; Zur, L.; Ristic, D.; Jovanovic, D.J.; Vaccari, A.; Zonta, D.; Righini, G.C.; Ferrari, M. Colloidal crystals based portable chromatic sensor for butanol isomers and water mixtures detection. Opt. Mater. 2019, 90, 152–158. [Google Scholar] [CrossRef]
- Jovanović, D.J.; Chiappini, A.; Zur, L.; Gavrilović, T.V.; Tran, T.N.L.M; Chiasera, A.; Lukowiak, A.; Smits, K.; Dramićanin, M.D; Ferrari, M. Synthesis, structure and spectroscopic properties of luminescent GdVO4:Dy3+ and DyVO4 particles. Opt. Mater. 2018, 76, 308–316. [Google Scholar] [CrossRef]
- del Rosal, B.; Pérez-Delgado, A.; Carrasco, E.; Jovanović, D.J.; Dramićanin, M.D.; Dražić, G.; de la Fuente, Á.J.; Sanz-Rodriguez, F.; Jaque, D. Neodymium-based stoichiometric ultrasmall nanoparticles for multifunctional deep-tissue photothermal therapy. Adv. Opt. Mater. 2016, 4, 782–789. [Google Scholar] [CrossRef]
- Periša, J.; Antić, Ž.; Ma, C.-G.; Papan, J.; Jovanović, D.; Dramićanin, M.D. Pesticide-induced photoluminescence quenching of ultra-small Eu3+-activated phosphate and vanadate nanoparticles. J. Mater. Sci. Technol. 2020, 38, 197–204. [Google Scholar] [CrossRef]
- Ye, M.; Liu, X.; Iocozzia, J.; Liu, X.; Lin, Zhiqun, Chapter 1: Nanostructured materials for high efficiency perovskite solar cells. In Nanomaterials for Sustainable Energy, Book NanoScience and Technology, 1st Edition, Springer International Publishing, Cham, Switzerland, 2016, pp. 1-39. [CrossRef]
- Cheng, B.; Lou, H.; Zeng, Z.; Liu, Y.; Zeng, Q. Structural phase transition in BiVO4 nanosheets under high pressure. J. Phys. Chem. C 2024, 128, 12267–12273. [Google Scholar] [CrossRef]
-
Nanostructured and Advanced Materials for Fuel Cells, 1st Edition, Jiang, S.P.; Shen, P.K. CRC Press, Taylor and Francis Group, Boca Raton, 2013. [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting-the untamed dream: A review of recent advances, Molecules 2016, 21, 900. [CrossRef]
- Ghosh, S. Chapter 12: Promising inorganic nanomaterials for future generation. In Applications of Multifunctional Nanomaterials, A volume in Micro and Nano Technologies, Thomas, S.; Kalarikkal N.; Abraham, A.R. Elsevier Inc. 1000 AE Amsterdam, Netherlands, Oxford OX5 1GB, United Kingdom, Cambridge, MA 02139, United States, 2023, pp. 247-263. [CrossRef]
- Cox, D.M. Chapter 4: High surface area materials. In R&D Status and Trends in Nanoparticles, Nanostructured Materials and Nanodevices, Siegel, R.W.; Hu, E.; Cox, D.M.; Goronkin, H.; Jelinski, L.: Koch, C.C.; Mendel, J.; Roco, M.C.; Shaw, D.T. Springer Dordrecht, 1999. [CrossRef]
- Cao, X.; Gu, Y.; Tian, H.; Fang, Y.; Johnson, D.; Ren, Z.; Chen, C.; Huang, Y. Microemulsion synthesis of ms/tz-BiVO4 composites: The effect of pH on crystal structure and photocatalytic performance. Ceram. Int. 2020, 46, 20788–20797. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, H.; Zhu, W.; Liu, L.; Chen, K.; Yan, J. The transition of tetragonal to monoclinic phase in BiVO4 coupled with peroxymonosulfate for photocatalytic degradation of tetracycline hydrochloride. Environ. Res. 2025, 267, 120631. [Google Scholar] [CrossRef]
- Ribeiro, F.W.P.; Moraes, F.C.; Pereira, E.C.; Marken, F.; Mascaro, L.H. New application for the BiVO4 photoanode: A photoelectroanalytical sensor for nitrite. Electrochem. Commun. 2015, 61, 1–4. [Google Scholar] [CrossRef]
- David, W.I.F. Ferroelastic phase transition in BiVO4: III. Thermodynamics. J. Phys. C: Solid State Phys. 1983, 16, 5093. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, P.; Yang, X.; Wang, F.; Zhang, W.; Fei, C. Microstructure and characteristic of BiVO4 prepared under different pH values: Photocatalytic efficiency and antibacterial activity. Materials 2016, 9, 129. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Heterostructured Er3+ doped BiVO4 with exceptional photocatalytic performance by cooperative electronic and luminescence sensitization mechanism. Appl. Catal. B: Environ. 2014, 158–159, 242–249. [Google Scholar] [CrossRef]
- Dragomir, M.; Arčon, I.; Gardonio, S.; Valant, M. Phase relations and optoelectronic characteristics in the NdVO4-BiVO4 system. Acta Mater. 2013, 61, 1126–1135. [Google Scholar] [CrossRef]
- Polo, A.; Dozzi, M.V.; Marra, G.; Sivula, K.; Selli, E. Improving the photoelectrocatalytic efficiency of CuWO4 through molybdenum for tungsten substitution and coupling with BiVO4. Sustain. Energy Fuels 2024, 8, 3182–3191. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.- S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Xue, L.; Li-Li, Y.; Li-Na, Y.; Qing-Feng, G.; Yong-Sheng, Y.; Han, Z. Controllable synthesis and photocatalytic activity of spherical, flowerlike and threadlike bismuth vanadates. Acta Phys. Chim. Sin. 2013, 29, 1771–1777. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, M.; Ma, Di.; Jing, D. Effect of preparation parameters on photoactivity of BiVO4 by hydrothermal method. J. Nanomater. 2012, 2012, 621254. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Uchida, A.; Tominaga, Y.; Fujii, Y.; Yadav, A.A.; Kang, S.-W.; Suzuki, N.; Shitanda, I.; Kondo, T.; Itagaki, M.; Yuasa, M.; Gosavi, S.; Fujishima, A.; Terashima, C. Visible light-assisted photocatalysis using spherical-shaped BiVO4 photocatalyst. Catalysts 2021, 11, 460. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhai, Y.; Yu, Y.; Guo, Y.; Hao, S. An optimization strategy for photo-Fenton-like catalysts: Based on crystal plane engineering of BiVO4 and electron transfer properties of 0D CQDs. Environ. Res. 2023, 222, 115347. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, L.; Ren, H.; Wei, S.; Huang, J.; Xia, A. Effects of pH on the hierarchical structures and photocatalytic performance of BiVO4 powders prepared via the microwave hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 5186–5193. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Wang, J.; Chang, L.; Song, N.; Yan, Z.; Wan, X. Additive-free solvothermal preparation, characterization, and photocatalytic activity of 3D butterfly-like BiVO4. Res. Chem. Intermed. 2015, 41, 2465–2477. [Google Scholar] [CrossRef]
- Saison, T. Chemin, N.; Chanéac, C.; Durupthy, O.; Mariey, L.; Maugé, F.; Brezová, V.; Jolivet, J.-P. New insights into BiVO4 properties as visible light photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Tavakoli, A.H.; Navrotsky, A. Energetics of bismuth vanadate. J. Solid State Chem. 2015, 225, 187–192. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Cao, V.D.; Nguyen, V.H.; Nong, L.X.; Luu, T.D.; Vo, D.-V.N.; Do, S.T.; Lam, T.D. Synthesized BiVO4 was by the co-precipitation method for Rhodamine B degradation under visible light. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 542, 012058. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Mallick, K.K.; Hartridge, A. Phase transition in BiVO4. Mater. Lett. 1997, 30, 7–13. [Google Scholar] [CrossRef]
- Li, G.; Bai, Y.; Zhang, W.F. Difference in valence band top of BiVO4 with different crystal structure. Mater. Chem. Phys. 2012, 136, 930–934. [Google Scholar] [CrossRef]
- Fan, H.; Jiang, T.; Li, H.; Wang, D.; Wang, L.; Zhai, J.; He, D.; Wang, P.; Xie, T. Effect of BiVO4 crystalline phases on the photoinduced carriers behavior and photocatalytic activity. J. Phys. Chem. C 2012, 116, 2425–2430. [Google Scholar] [CrossRef]
- Fu, L.; Li, Z.; Shang, X. Recent surficial modification strategies on BiVO4 based photoanodes for photoelectrochemical water splitting enhancement. Int. J. Hydrogen Energy 2024, 55, 611–624. [Google Scholar] [CrossRef]
- Patial, B.; Bansal, A.; Gupta, R.; Mittal, S.K. BiVO4-based heterojunction nanophotocatalysts for water splitting and organic pollutant degradation: a comprehensive review of photocatalytic innovation. Rev. Inorg. Chem. 2024, 44, 495–519. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Mu, L.; Li, C. Significance of crystal morphology controlling in semiconductor-based photocatalysis: A case study on BiVO4 photocatalyst. Cryst. Growth Des. 2017, 17, 2923–2928. [Google Scholar] [CrossRef]
- Satyavir, T.; Praneetha, D.; Arumugam, S.; Murugan, V. Tetragonal to monoclinic crystalline phases change of BiVO4 via microwave-hydrothermal reaction: in correlation with visible-light-driven photocatalytic performance. Inorg. Chem. 2019, 58, 5096–5110. [Google Scholar] [CrossRef]
- Zhang, H.M.; Liu, J.B.; Wang, H.; Zhang, W. X.; Yan, H. Rapid microwave-assisted synthesis of phase controlled BiVO4 nanocrystals and research on photocatalytic properties under visible light irradiation. J. Nanopart Res. 2008, 10, 767–774. [Google Scholar] [CrossRef]
- Dai, D.; Liang, X.; Zhang, B.; Wang, Y.; Wu, Q.; Bao, X.; Wang, Z.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B.; Wang, P. Strain adjustment realizes the photocatalytic overall water splitting on tetragonal zircon BiVO4. Adv. Sci. 2022, 9, 2105299. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, Y.; Guo, W.; Liu, J.; Jiang, Z.; Shanggun, W. Enhanced photocatalytic overall water splitting via MOF-derived tetragonal BiVO4-based solid solution. J. Chem. Eng. 2021, 414, 128911. [Google Scholar] [CrossRef]
- Obregón, S.; Lee, S.W.; Colón, G. Exalted photocatalytic activity of tetragonal BiVO4 by Er3+ doping through a luminescence cooperative mechanism. Dalton Trans. 2014, 43, 311–316. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, G.; Dong, G.; Ren, H.; Xia, A. A comprehensive investigation of tetragonal Gd-doped BiVO4 with enhanced photocatalytic performance under sun-light. Appl. Surf. Sci. 2016, 364, 156–165. [Google Scholar] [CrossRef]
- Usai, S.; Obregón, S.; Becerro, A.I.; Colón, G. Monoclinic-tetragonal heterostructured BiVO4 by yttrium doping with improved photocatalytic activity. J. Phys. Chem. C 2013, 117, 24479–24484. [Google Scholar] [CrossRef]
- Huang, J.; Tan, G.; Zhang, L.; Ren, H.; Xia, A.; Zhao, C. Enhanced photocatalytic activity of tetragonal BiVO4: Influenced by rare earth ion Yb3+. Mater. Lett. 2014, 133, 20–23. [Google Scholar] [CrossRef]
- Gomes, L.E.; Nogueira, A.C.; da Silva, M.F.; Plaça, L.F.; Maia, L.J.Q.; Gonçalves, R.V.; Ullah, S.; Khan, S.; Wender, H. Enhanced photocatalytic activity of BiVO4/Pt/PtOx photocatalyst: The role of Pt oxidation state. Appl. Surf. Sci. 2021, 567, 150773. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chanéac, C.; Durupthy, O.; Mariey, L.; Maugé, F.; Brezová, V.; Jolivet, J.-P. New insights into BiVO4 properties as visible light photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Mallick, K.K.; Hartridge, A. Phase transition in BiVO4. Mater. Lett. 1997, 30, 7–13. [Google Scholar] [CrossRef]
- Glasser, F.P.; Woods, P. Preparation and properties of pigmentary grade BiVO4 precipitated from aqueous solution. Ceram. Int. 2004, 30, 875–882. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Smits, K.; Babić, B.; Marinović-Cincović, M.; Porobić, S.; Dramićanin, M.D. A comparative study of photocatalytically active nanocrystalline tetragonal zyrcon-type and monoclinic scheelite-type bismuth vanadate. Ceram. Int. 2018, 44, 17953–17961. [Google Scholar] [CrossRef]
- Dong, L.; Guo, S.; Zhu, S.; Xu, D.; Zhang, L.; Huo, M.; Yang, X. Sunlight responsive BiVO4 photocatalyst: Effects of pH on L-cysteine-assisted hydrothermal treatment and enhanced degradation of ofloxacin. Catal. Commun. 2011, 16, 250–254. [Google Scholar] [CrossRef]
- Tokunaga, S. Hideki Kato, Akihiko Kudo, Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Guisheng, L.; Dieqing, Z.; Jimmy, C.Y. Ordered mesoporous BiVO4 through nanocasting: A superior visible light-driven photocatalyst. Chem. Mater. 2008, 20, 3983–3992. [Google Scholar] [CrossRef]
- Sarkar, S.; Garain, S.; Mandal, D.; Chattopadhyay, K.K. Electro-active phase formation in PVDF–BiVO4 flexible nanocomposite films for high energy density storage application. RSC Adv. 2014, 4, 48220–48227. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Lin, X.; Yu, L.; Yan, L.; Li, H.; Yan, Y.; Liu, C.; Zhai, H. Visible light photocatalytic activity of BiVO4 particles with different morphologies. Solid State Sci. 2014, 32, 61–66. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Pei, Y.; Han, W. Enhancing photoelectrocatalytic efficiency of BiVO4 photoanodes by crystal orientation control. Nanomaterials 2024, 14, 1870. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Nikama, S.; Joshi, S. Irreversible phase transition in BiVO4 nanostructures synthesized by a polyol method and enhancement in photo degradation of methylene blue. RSC Adv. 2016, 6, 107463–107474. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Dupuisc, M.; Li, C. The nature of photogenerated charge separation among different crystal facets of BiVO4 studied by density functional theory. Phys. Chem. Chem. Phys. 2015, 17, 23503–23510. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Song, X.; Chen, X.; Sha, X.; Tang, J.; Yang, Z.; Lv, Y.; Jiang, C. In situ photoelectrochemical-induced surface reconstruction of BiVO4 photoanodes for solar fuel production. RRL Solar 2024, 8, 2400523. [Google Scholar] [CrossRef]
- Gu, F.-f.; Chen, G.-h.; Kang, X.-l.; Li, X.; Zhou, C.-r.; Yuan, C.-l.; Yun, Y.; Yang, T. A new BiVO4/Li0.5Sm0.5WO4 ultra-low firing high-k microwave dielectric ceramic. J. Mater. Sci. 2015, 50, 1295–1299. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Qian, F.; Tong, Y.; Zhang, J.Z.; Lordi, V.; Rocha Leao, C.; Li, Y. Computational and photoelectrochemical study of hydrogenated bismuth vanadate. J. Phys. Chem. C 2013, 117, 10957–10964. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Uchida, A.; Tominaga, Y.; Fujii, Y.; Yadav, A.A.; Kang, S.-W.; Suzuki, N.; Shitanda, I.; Kondo, T.; Itagaki, M.; Yuasa, M.; Gosavi, S.; Fujishima, A.; Terashima, C. Visible light-assisted photocatalysis using spherical-shaped BiVO4 photocatalyst. Catalysts 2021, 11, 460. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Tong, F.; Liu, X.; Wang, C.; Wang, M.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Dai, Y.; Huang, B. Bias-free solar water splitting by tetragonal zircon BiVO4 nanocrystal photocathode and monoclinic scheelite BiVO4 nanoporous photoanode. Adv. Funct. Mater. 2021, 31, 2008656. [Google Scholar] [CrossRef]
- Bulut, D.T. Exploring the dual role of BiVO4 nanoparticles: unveiling enhanced antimicrobial efficacy and photocatalytic performance. J. Sol-Gel Sci. Technol. 2025; In Press. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, L.; Li, J.; Du, J.; Zhang, Y.; Zhou, J. Synthesis and photocatalytic behavior of BiVO4 with decahedral structure. Ceram. Int. 2013, 39, 7461–7465. [Google Scholar] [CrossRef]
- Aghakhaninejad, S.; Rahimi, R.; Zargari, S. Application of BiVO4 nanocomposite for photodegradation of methyl orange. Proceedings 2019, 9, 52. [Google Scholar] [CrossRef]
- Lei, B.-X.; Zhang, P.; Wang, S.-N.; Li, Y.; Huang, G.-L.; Sun, Z.-F. Additive-free hydrothermal synthesis of novel bismuth vanadium oxide dendritic structures as highly efficient visible-light photocatalysts. Mater. Sci. Semicond. Process. 2015, 30, 429–434. [Google Scholar] [CrossRef]
- Lu, Y.; Shang, H.; Shi, F.; Chao, C.; Zhang, X.; Zhang, B. Preparation and efficient visible light-induced photocatalytic activity of m-BiVO4 with different morphologies. J. Phys. Chem. Sol. 2015, 85, 44–50. [Google Scholar] [CrossRef]
- Ravidhas, C.; Josephine, A.J.; Sudhagar, P.; Devadoss, A.; Terashima, C.; Nakata, K.; Fujishima, A.; Raj, A.M.E.; Sanjeeviraja, C. Facile synthesis of nanostructured monoclinic bismuth vanadate by a co-precipitation method: Structural, optical and photocatalytic properties. Mater. Sci. Semicond. Process. 2015, 30, 343–351. [Google Scholar] [CrossRef]
- Diehm, P.M.; Goston, P.; Albe, K. Size-dependent lattice expansion in nanoparticles: reality or anomaly? Chem. Phys. Chem. 2012, 13, 2443–2454. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).