1. Introduction
Since aqueous dispersions of polyurethane are non-toxic, non-flammable and do not pollute the air, they have been forcing their way into the marketplace and widely used in coatings and adhesives for flexible substrates (textile [
1], leathers[
2], papers [
3] and rubber[
4]), wood [
5], glass fibers [
6], electrode material [
7], and inks [
8]. As developed in the past few decades, the production technology and the quality of waterborne polyurethanes (WPU) were improved steadily, consequently, the preparation and industrialization of WPU has been very mature. At present, the mainstream industrial preparation of WPU is still the acetone method [
9,
10], meanwhile a few large multinational companies, such as Dow [
11,
12] and LANXess [
13,
14], had recognized the significance of low energy consumption and environmental protection value of the prepolymer method, and started to conduct in-depth research and industrial exploration of the prepolymer method.
The preparation of WPU by prepolymer method usually includes three stages [
15,
16,
17,
18]. First is the prepolymer stage, polyol, chain extender, hydrophilic chain extender and isocyanate were initially pre-polymerized. Second is the emulsification stage, adding water to disperse the prepolymer with small molecular weight in the water phase to form dispersions; third is the post-extension stage, a large number of amine further react with the residual -NCO carried by the dispersions to achieve molecular weight growth. The prepolymer synthesized by prepolymer method has the characteristics of high NCO% content, small molecular weight and small viscosity of prepolymer, which brings about the following: (i) small molecular weight and low viscosity facilitate smooth emulsification [
19]; (ii) there is still a large amount of unreacted -NCO groups inside and outside the WPU particles after emulsification; (iii) the post-extension reaction is intense and plays a key role in increasing molecular weight [
20]. The post-extension reaction of waterborne polyurethane is carried out in water, and water will compete with chain extension-amines [
21], which is difficult to accurately control, and this stage can be called "a muddle". For the prepolymer method, the post-extension reaction in water may bring about many practical issues, most importantly, the stability of the emulsion in different environments, especially in higher temperature environment.
In practical industrial applications, waterborne polyurethane undergoes upstream storage, transportation, and downstream storage. The conditions of storage and transportation vary with seasonal or environmental changes, which in turn affect the appearance and performance of WPUs after these processes. Therefore, research on the storage stability of WPU under varying temperatures, particularly thermal-storage, is a critical focus within the industry. The existing literature has primarily concentrated on the storage stability of WPUs prepared using the acetone method, with a focus on evaluating stability through monitoring particle size and distribution [
22,
23]. However, there is a lack of research regarding the influencing factors and mechanisms of thermal-storage stability for WPU synthesized
via prepolymer method. In this work, we first investigated in detail the factors that affect the thermal-storage stability of WPUs synthesized based on prepolymer method, including NCO% content of prepolymer, ratio of [NCO] to [H] of WPUs after post-extension, dilution ratio and adding time of post-extension amine, and solid content of WPUs. By decreasing the NCO% content, ratio of [NCO] to [H] and solid content, and increasing dilution ratio and adding time of post-extension amine, the thermal-storage stability was obviously improved. Subsequently, the mechanism for thermal-storage stability using “cage effect” was studied.
2. Experiments
2.1. Materials
Polypropylene glycol (PPG-2000, = 2000) was supplied by Dow Co. Isophorone diisocyanate (IPDI) was supplied by Evonik Co. Dimethylol propionic acid (DMPA), dibutytin dilaurate (DBTDL), 1, 4-butanediol (BDO), triethyl amine (TEA), Hydrazine hydrate (HyHy) were received from Sigma-Aldrich Co. Sodium dodecyl benzene sulfonate (SDBS, 23 wt%) was purchased from Solvay Chemical (Shanghai) Co. PPG was heated to remove water in the vacuum drying flask at 120 °C for 1 h before use. Other materials were used as received. Deionized water was used for all emulsion experiments.
2.2. Preparation of NCO-Terminated PU Prepolymers
Preparation of prepolymer was carried out in a three-neck round-bottom flask equipped with mechanical stirrer, reflux condenser, thermometer and nitrogen gas inlet.
Pre-PU1: PPG-2000 (200 g, 100 mmol) was placed in the flask and was dried in vacuum at 120 °C for 1 h. After the temperature was decreased to 70 °C, IPDI (80 g, 360.36 mmol) was added slowly into the flask, and then the mixture was heated up to 85 °C with continuous stirring. The reaction was performed for 1 h. Then, BDO (8 g, 88.88 mmol), DMPA (5 g, 37.31 mmol), DBTDL (0.2 g) were added and the reaction was kept for another 4 h at 85 °C to obtain an NCO-terminated polyurethane prepolymer until the realization of the theoretical NCO content (3.85 wt%) using a standard di-n-buthylamine back-titration method (ASTM D2572-97).
Pre-PU2: PPG- 2000 (200 g, 100 mmol) was placed in the flask and was dried in vacuum at 120 °C for 1 h. After the temperature was decreased to 70 °C, IPDI (80 g, 360.36 mmol) was added slowly into the flask, and then the mixture was heated up to 85 °C with continuous stirring. The reaction was performed for 1 h. Then, DMPA (5 g, 37.31mmol), DBTDL (0.2 g) were added and the reaction was kept for another 3 h at 85 °C to obtain an NCO-terminated polyurethane prepolymer until the realization of the theoretical NCO content (6.58 wt%) using a standard di-n-buthylamine back-titration method (ASTM D2572-97).
2.3. Dispersion and Amine-Extension
Before dispersion, adding 3.77 g (36.38 mmol) of TEA to the above NCO-terminated prepolymer with stirring for 5min to neutralize the prepolymer. Dispersion of PUs was accomplished by adding the mixture of water and surfactant (SDBS, 2.0 wt% based on prepolymer) to the neutralized prepolymer at agitation rate of 2000 rpm. After 5 min, HyHy solution was added over a period of 1-3 min (for different WPUs) at 800 rpm, finally, dispersions were obtained by continuing to stir for another 30 min at 800 rpm.
For pre-PU1, WPU1-1 was prepared after neutralization, dispersion, and post-extension.
For pre-PU2, by changing the addition amount of HyHy, WPU2-1, WPU2-2, WPU2-3 with different ratio of [NCO] to [H] after post-extension (1.03, 0.95, 0.90) were obtained; by changing dilution ratio of HyHy to deionized water (1:6, 1:10), WPU2-4, WPU2-5 were obtained; by changing the adding time of by changing HyHy solution (2 min, 3 min), WPU2-6, WPU2-7 were obtained; by adding different amounts of acetone (40 wt%, 60 wt%) to dilute the prepolymer before dispersing, WPU2-8, WPU2-9 were obtained; by changing the amount of deionized water for dispersion, WPU2-10, WPU2-11 with different solid content (42 %, 38 %) were obtained. The sample designations of WPUs were shown in
Table S1.
It was notable that for WPU2-8 and WPU2-9 samples acetone remained in the dispersions and was not removed.
2.4. Measurements
Fourier transform infrared spectroscopy (FTIR) experiments of pre-PUs were carried out on a Nicolet iS10 FTIR spectrometer (Nicolet, American). Particle sizes of WPUs were determined using a Malvern Panalytical Zetasizer Nano ZSE light-scattering ultrafine particle analyzer. The sample was diluted to the required concentration with deionized water before measurement. Viscosity measurements of the dispersions were performed using NDJ-9S viscometer, at a shear rate of 100 rpm at 25 °C. Scanning electron microscope (SEM) was employed to observe the morphology of particles with Sigma 300. Thermal-storage experiments were carried out at 50 °C in an oven.
3. Results and Discussion
3.1. Characterization of Prepolymers and WPUs
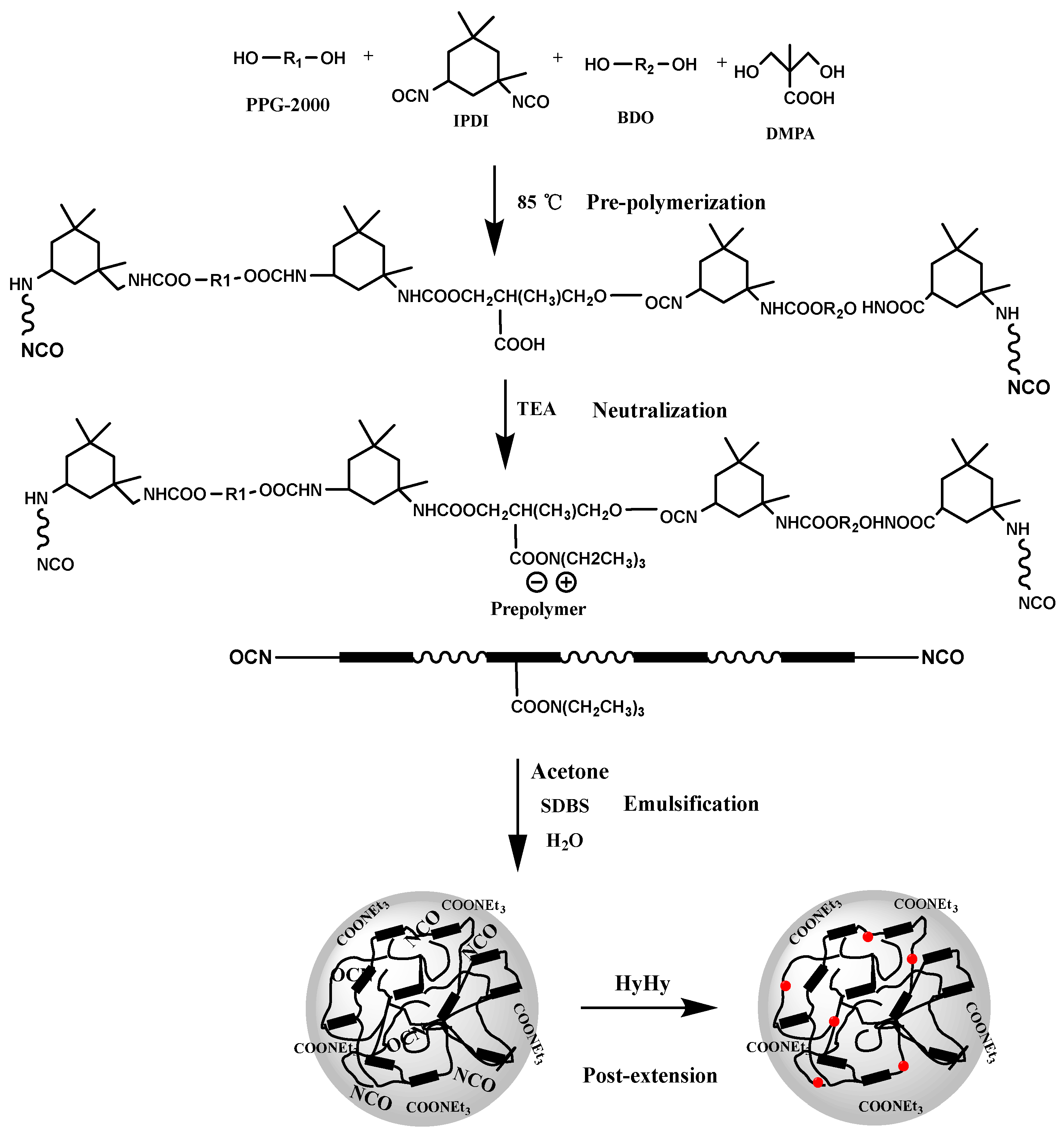
The synthesis route of WPU dispersions is presented in
Scheme 1. For the purpose of comprehensively investigating thermal-storage stability of WPU dispersions, two NCO-terminated PU prepolymers with different NCO% (3.85 % and 6.58 %) were firstly synthesized, PPG-2000 with conventional molecular weight (
=2000) was used as the soft segment, while aliphatic isocyanate (IPDI) was served as the main part of the hard segment. DMPA was introduced into the prepolymer as a carboxylic acid type hydrophilic chain extender, another small molecular chain extender (BDO) was also employed to consume isocyanate to achieve the NCO% of pre-PU1 to 3.85 %. The prepolymer was synthesized by a one-pot process through incubating dehydrated polydiol (PPG-2000), IPDI, BDO and DMPA with DBTDL as catalyst at 85 °C for 4 h. Furthermore, after the carboxylic acid group in the prepolymer was neutralized, the ionized carboxylic acid group tended to be hydrophilic. Then emulsification step of the prepolymers assisted by SDBS, produced polyurethane dispersions to yield a series of WPUs.
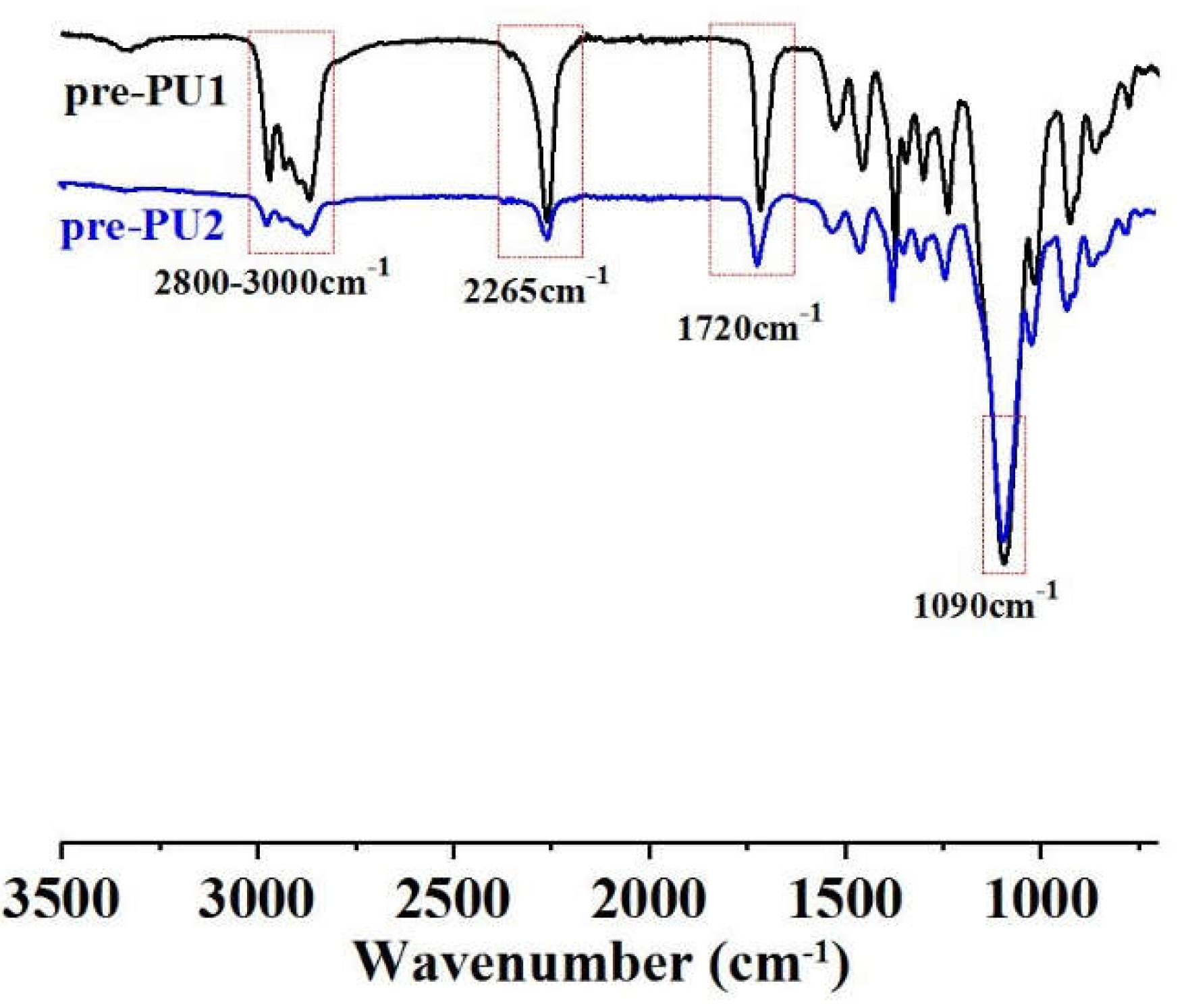
The FTIR spectra of pre-PU1 and pre-PU2 were shown in
Figure 1, and the characteristic peaks of the two curves are quite same because the types of main raw materials used in both are the same. The FTIR spectrum of the prepolymer displayed a strong absorption peak at 1090 cm⁻¹, assigned to the stretching vibration of the -O- group derived from PPG. The peak at 1720 cm⁻¹ was attributed to the C=O stretching vibration in the urethane groups, while the peak at 2265 cm⁻¹ corresponded to the NCO stretching vibration. Additionally, the absorption bands observed in the range of 2800-3000 cm⁻¹ were associated with the C-H stretching vibrations of the soft segment. The particle sizes and distributions of these WPUs were shown in
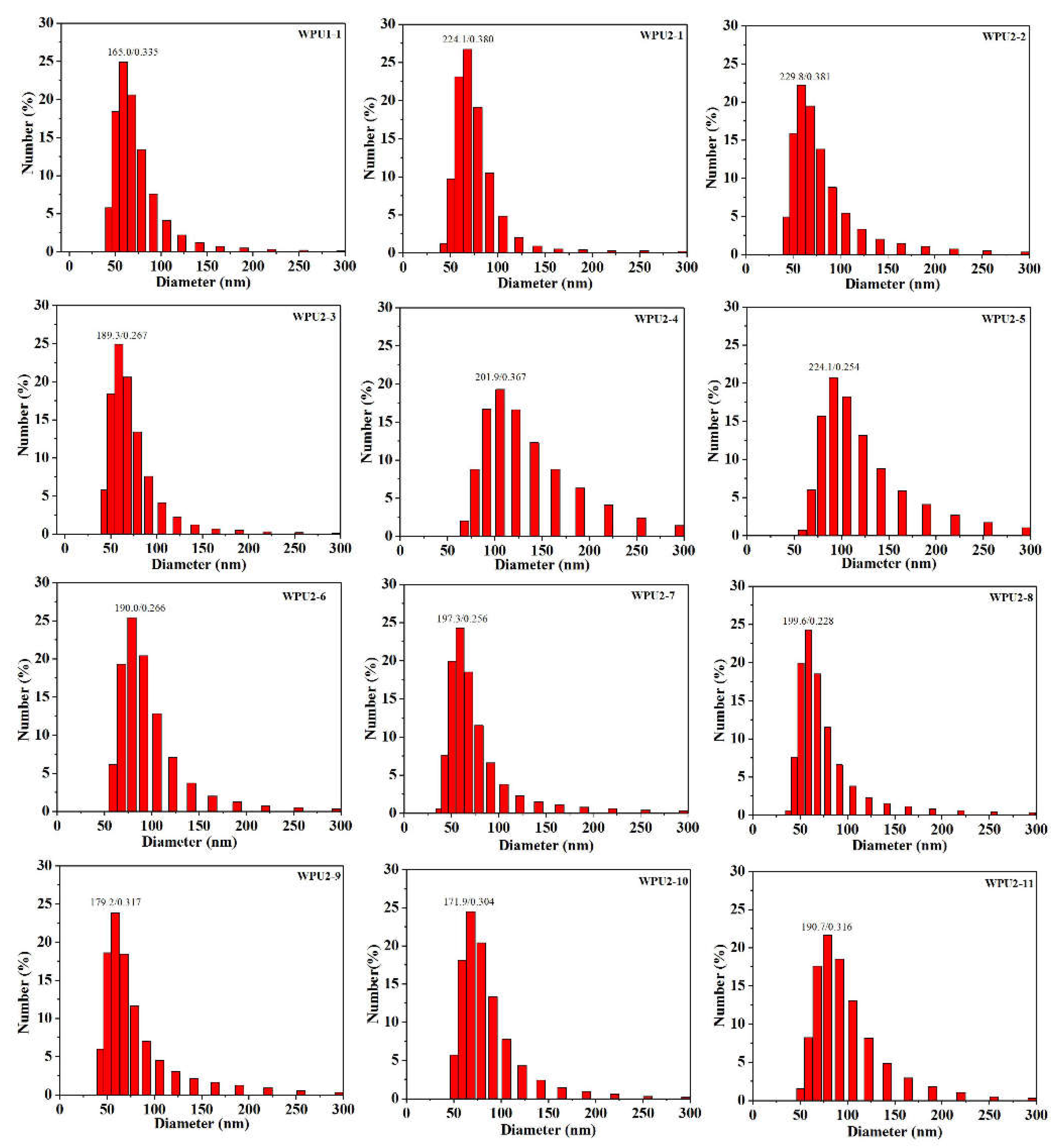
Figure 2 and
Table S2, for pre-PU1, the as-prepared WPU1-1 has an average particle size of 165.0 nm and distribution of 0.335; for pre-PU2, the average particle size of as-prepared WPUs was in a range of 171.9 to 229.8 nm with distribution in a range of 0.228 to 0.381, indicating that both of the two WPU dispersions presented uniform polyurethane particles.
Since the WPU dispersion is a mixed system where polyurethane particles are dispersed and suspended in an aqueous solution, the stability of the system is significantly influenced by the particle structure and their interactions with the solution. Herein, the following section mainly explores the influence of different parameters (
Table 1) to thermal-storage stability, including the NCO% for prepolymer (WPU1-1 and WPU2-1), ratio of [NCO] to [H] of post-extension (WPU2-1, WPU2-2 and WPU2-3), HyHy dilution ratio (WPU2-1, WPU2-4 and WPU2-5) and adding time (WPU2-1, WPU2-6 and WPU2-7), and solid content (SC) of WPUs (WPU2-1, WPU2-8, WPU2-9, WPU2-10 and WPU2-11). To evaluate the difference of thermal-storage stability, viscosity and appearance of WPUs were selected as evaluation indicators in this work.
3.2. Effect of NCO% for Prepolymer and Ratio of [NCO] to [H] for Post-Extension
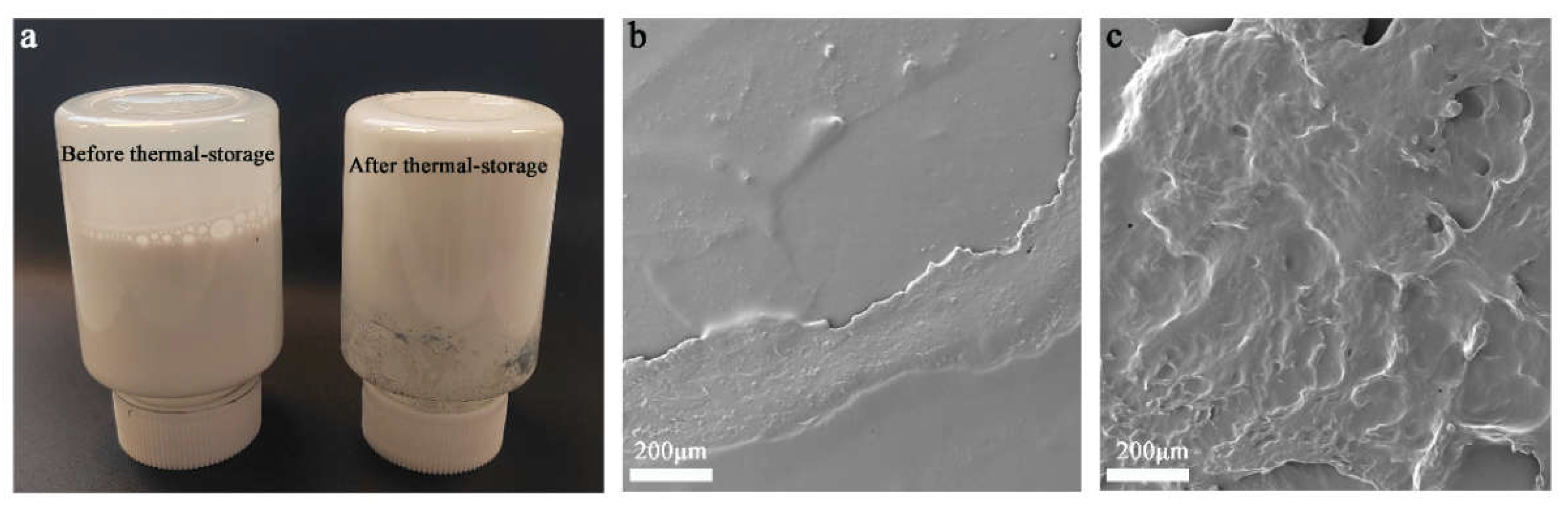
First, the effect of NCO content in the prepolymer on the thermal storage stability of WPUs was investigated. Two prepolymers with different NCO contents were designed: pre-PU1 with an NCO content of 3.85% and pre-PU2 with an NCO content of 6.58%,and then, these prepolymers were used to prepare the corresponding WPU1-1 and WPU2-1 dispersions. The photographs of WPU2-1 before and after thermal-storage (50 °C) for 8 h was shown in
Figure 3a, it can be seen that WPU2-1 showed a good dispersion state before thermal-storage, and after being stored in a 50 °C oven for 8 h, the dispersion became solid state, indicating that WPU2-1 was quite unstable at 50 °C. The changes of WPU2-1 before and after thermal-storage were further observed by SEM images in
Figure 3b, c. Before thermal-storage, WPU2-1 was in a good dispersion state, and SEM image showed uniform film formation; after thermal-storage, the film was uneven and rough.
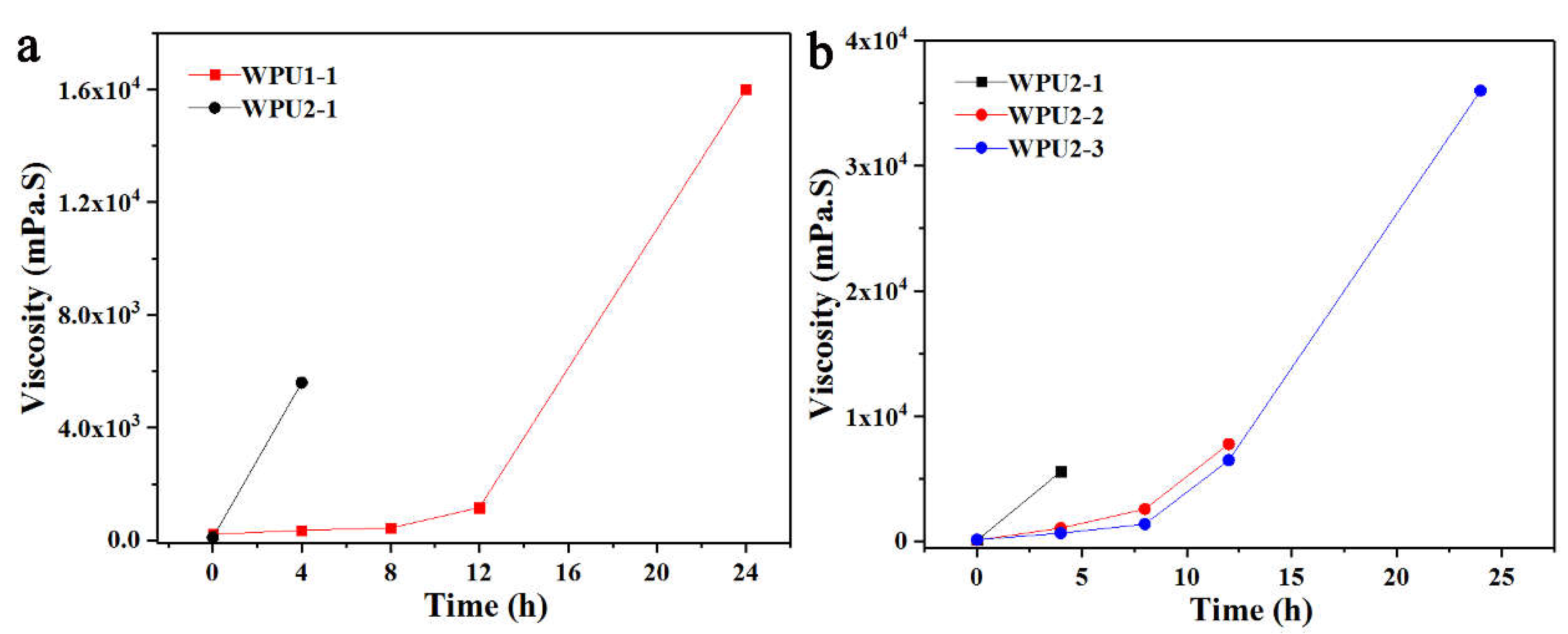
Figure 4a shown the viscosity change curves of WPU1-1 and WPU2-1 stored in an oven at 50 °C for 24 h. From
Figure 4a, the viscosity of WPU2-1 increased rapidly within 4 h, and continued to sharply rise to even a solid state within 8 h; compared with WPU2-1, the viscosity of WPU1-1 increased at a slower rate within 12 h until the viscosity rose to a higher level at 24 h. This indicated that the NCO content of prepolymer was directly related to the stability of thermal-storage, that is, the higher the NCO content was, the worse the stability of thermal-storage was.
Then the ratio of [NCO] to [H] for post-extension was also investigated. Compared with WPU2-1, only WPU2-2 and WPU2-3 had different [NCO] to [H] ratios, which were 1.03, 0.95, and 0.90, respectively. From
Figure 4b, comparing the thermal-storage stability of the three WPUs at 50 °C, WPU2-1 transitioned to a non-flowing state after 4 h, while WPU2-2 solidified after 12 h. However, WPU2-3 maintained a low viscosity until 24 h. These results indicated that the [NCO] to [H] ratio in the post-extension significantly affects the thermal-storage stability of WPUs. Generally, a lower [NCO] to [H] ratio improves stability; however, when the ratio was as low as 0.90, the WPU still remains unstable.
3.3. Effect of HyHy Dilution Ratio and Adding Time
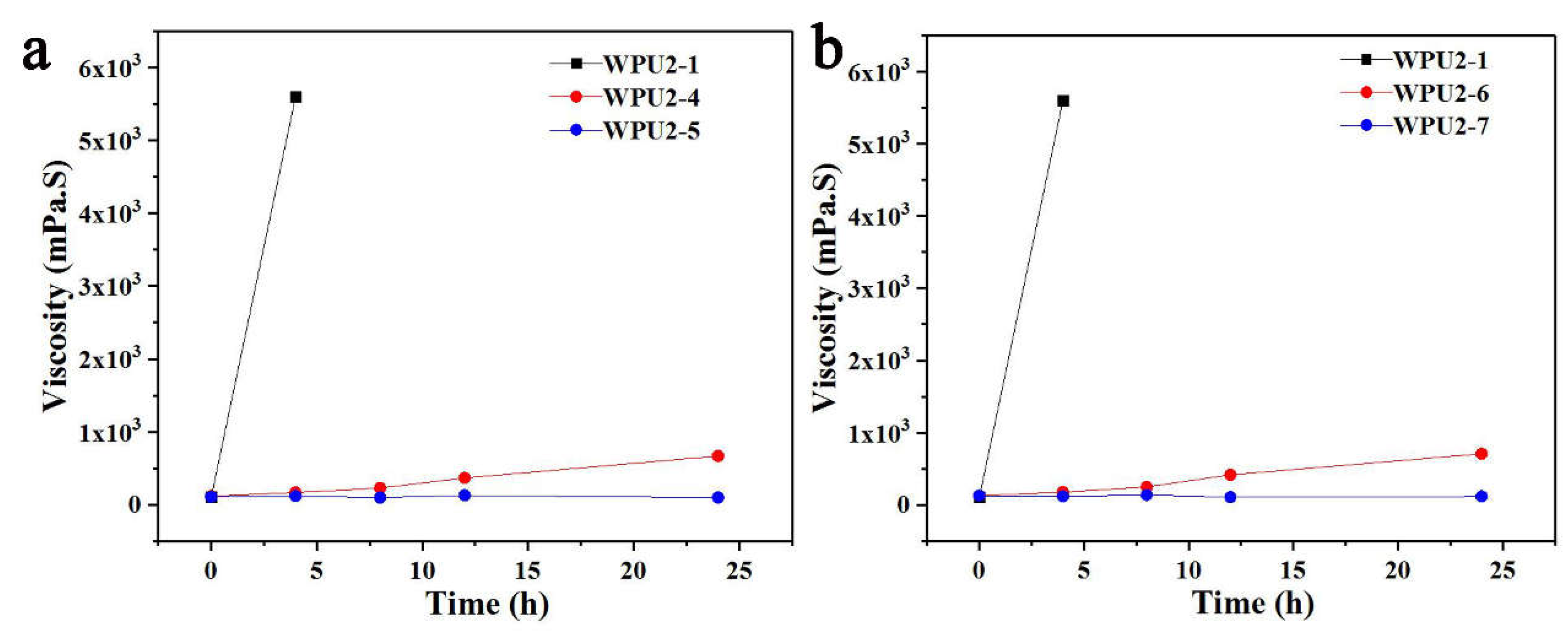
For the prepolymer method, the increase in molecular weight resulting from post-chain extension plays a critical role in determining the performance of WPUs. The influence of the process parameters, including HyHy dilution ratio and adding rate, of the post-extension on the thermal-storage stability was also studied here. Two sets of experiments were designed. One set was designed to investigate the effect of dilution ratio of HyHy, the ratio of HyHy to deionized water for dilution of WPU2-1, WPU2-4 and WPU2-5 was 1:4, 1:6 and 1:10, respectively; the other set was designed to explore the effect of adding rate of HyHy, the corresponding adding time of WPU2-1, WPU2-6 and WPU2-7 was 1, 2, 3 min, respectively. The results for changing the HyHy dilution ratio and adding rate of WPUs were presented in
Figure 5a and 5b. From
Figure 5a, compared with WPU2-1, after 24 h of constant temperature storage at 50 °C, the viscosity of WPU2-4 increased slightly to about 500 mPa·S, while that of WPU2-5 did not increase. With HyHy dilution ratio, the thermal-storage stability of WPUs was significantly improved. In the post-extension stage, the adding rate of HyHy showed similar result. After 24 hours at 50 °C, the viscosity of WPU2-6 increased to approximately 500 mPa·S, while WPU2-7 remained in its initial state (
Figure 5b). By prolonging the addition time of the HyHy aqueous solution while keeping other conditions constant, the thermal-storage stability of the WPUs was significantly improved.
When the dilution ratio of HyHy to deionized water is lower, the local concentration of HyHy becomes higher. This leads to localized reactions between HyHy and -NCO, as HyHy cannot rapidly or uniformly disperse over a broader region. Consequently, the -NCO groups approaching the particle surface cannot be slowly consumed, meanwhile the -NCO groups encapsulated within the particles cannot be effectively released or utilized. In contrast, when the dilution ratio of HyHy is higher, the local concentration of HyHy significantly decreases under the same addition time, facilitating uniform contact between HyHy and -NCO, thereby enabling the gradual consumption of -NCO through a slower and more sufficient reaction, and improving the thermal-storage stability of WPUs. By prolonging the adding time of the HyHy aqueous solution at a fixed dilution ratio with deionized water, HyHy can gradually disperse into the WPUs system. This allows a slow and sufficient reaction of -NCO groups with extender molecules near the particle surface and within the particles, fully consuming -NCO and enhancing the thermal-storage stability of WPUs.
3.4. Effect of Solid Content (SC) on Thermal-Storage Stability
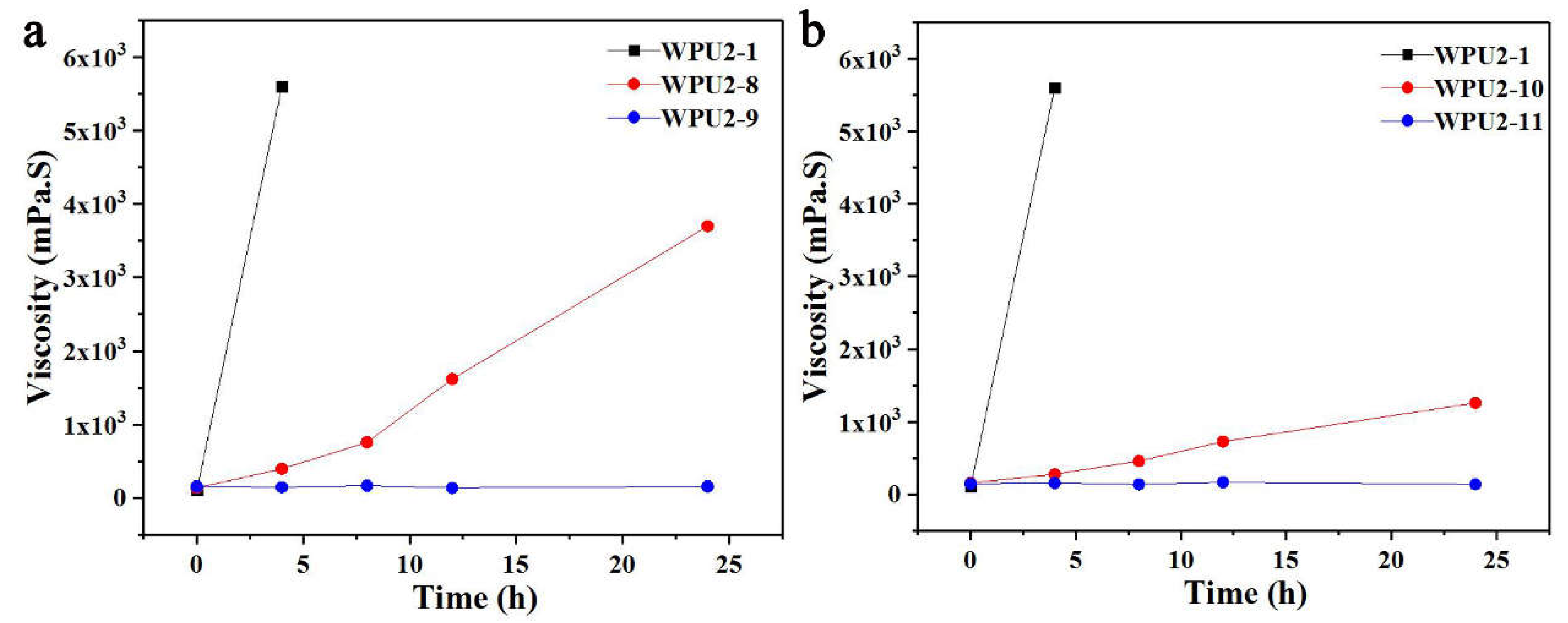
The mature process method for the production and preparation of waterborne polyurethane is acetone method, that is, acetone is added as a dilution solvent in the prepolymer synthesis stage, and acetone is then removed by vacuum distillation to obtain polyurethane dispersions. WPUs prepared by acetone process usually exhibit good thermal-storage stability, therefore, the effect of adding acetone to prepolymer prior to emulsification on thermal-storage stability was investigated here. The effect of acetone dosage by designing the amount of acetone was 0, 40%, 60% of the mass of the prepolymer, corresponding to WPU2-1, WPU2-8 and WPU2-9, was shown in
Figure 6a. It should be noted that for WPUs prepared here, thermal-storage stability experiments were carried out without removing acetone. As the amount of acetone increased, WPUs exhibited varying stability under 50 °C thermal-storage environment, after 24 h, the viscosity of WPU2-8 increased to about 4000 mPa·S, and the viscosity of WPU2-9 did not change significantly, which was consistent with the state before thermal-storage, while WPU2-1 went into the non-flowing state only after 4 h. Surprisingly, it was found that acetone significantly improved the thermal-storage stability of WPUs.
It is confusing whether acetone achieved the effect of improving thermal-storage stability by reducing the solid content of WPUs or diluting the prepolymer to smooth emulsification. Then the experiments were designed to explore the relationship between thermal-storage stability and solid content by increasing the amount of emulsifying water in the emulsification stage, and the results were shown in
Figure 6b. Compared to WPU2-1, both WPU2-10 and WPU2-11 exhibited an increase in the amount of emulsified water while reducing the solid content to 42% and 38%, respectively. The increment in emulsified water for WPU2-10 and WPU2-11 aligns with the acetone usage in WPU2-8 and WPU2-9, respectively. After 24 h of thermal-storage, the viscosity of WPU2-10 increased to about 1500 mPa·S, while that of WPU2-11 remained stable and did not increase, which corresponded to a similar phenomenon as that of WPU2-8 and WPU2-9, respectively. Therefore, it can be concluded that for WPUs prepared by prepolymer method, reducing solid content can effectively improve the thermal-storage stability or eliminate the instability caused by high temperature.
3.5. The Cage Effect of Post-Extension Reaction
Before the phase inversion of the waterborne polyurethane prepolymers, the prepolymers are usually molecular chains with -NCO terminated [
18,
24]. After these molecular chains finished self-assembly under mechanical stirring, these -NCO groups are distributed both near the particle surface and within the WPU particles. It has been reported in the literatures that the "cage effect" may occur in the preparation process of waterborne polyurethane [
25]. “Cage effect” means that the long molecular chains with -NCO groups inside WPUs particles are confined by the internal environment, which brings the low motility for the molecular chains and unable to carry out chain-extension reaction, resulting in the residues of -NCO inside the particles.
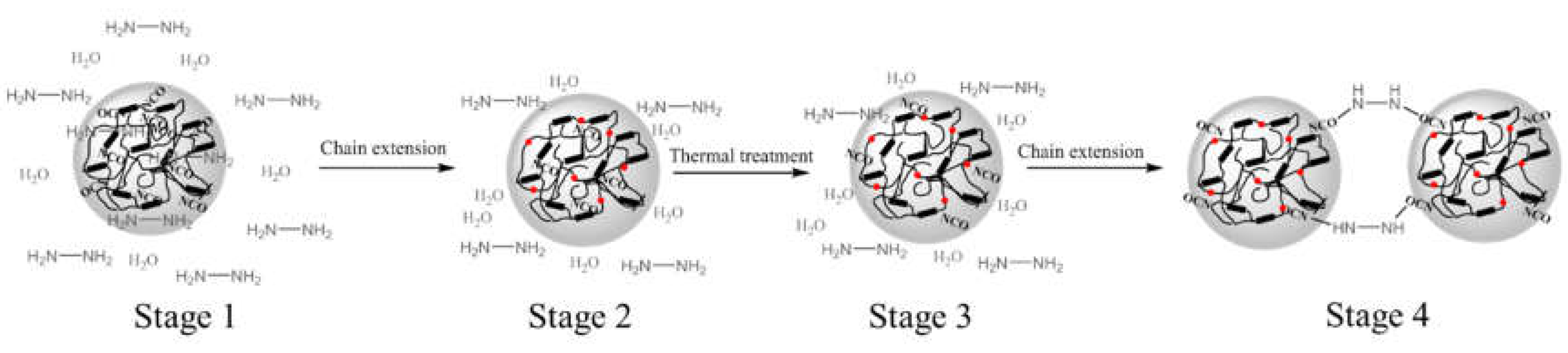
Here, a model based on the “cage effect” was proposed to explain the thermal-storage instability of WPUs observed in this study (
Scheme 2). The prepolymers synthesized by the prepolymer method in this study are molecular chains with small molecular weight and a large number of terminal -NCO groups. Firstly, after phase inversion, these short chain molecules self-assembled into particles with -NCO positioned on the outer surface of the curled molecular chain as well as wrapped within the inner regions of these molecular chains, and then, the particles were exposed to HyHy and H
2O after the addition of chain extender amine (stage 1). Secondly, HyHy, as a small molecular amine with good water solubility and lipophilicity, is easy to diffuse into the particle, reacted with -NCO near the periphery of the particle to form urea bonds to achieve chain exptension, while -NCO wrapped in the interior of the particles did not react with HyHy due to the "cage effect" and remained (stage 2). Thirdly, these particles assembled by short chain molecules were not stable enough, especially in higher temperature environment, such as 50 °C, chain movement and further reassembly occurred, which brings the -NCO originally confined inside the particle to reach relatively close to the surface of particles and re-exposed to HyHy and H
2O (stage 3). Then, the -NCO close to the surface of particles further reacted with HyHy, at this time, if the particle’s movement was insufficient due to no stirring, and then lead to the chain extension of -NCO groups on the surface of two particles with the same HyHy molecule (stage 4). Finally,the bonding of particles was macroscopically manifested as flocculation, hyper viscosity or non-fluidity of WPUs.
According to this model, the higher NCO% content of prepolymer, the more -NCO residues inside the particles due to the cage effect, or the higher ratio of [NCO] to [H] after post-extension, that is, the less the amine is, the less -NCO can be consumed, the more -NCO residues inside the particles, and the higher probability of stage 4 phenomenon will occur. Therefore, compared with WPU1-1, WPU2-1 thermal-storage stability is worse, and compared with WPU2-1, the stability of WPU2-2 and WPU2-3 is better. In the stage of chain extension, increasing the proportion of HyHy diluted by deionized water or prolonging the adding time of HyHy solution will make the chain extension of HyHy with the -NCO near the particle surface and within the WPU particles slower and more effective. At the same time, HyHy has a higher probability of contacting and reacting with the -NCO groups located inside the particles, significantly reducing the residual -NCO content within the particles after chain extension. Therefore, compared with WPU2-1, the thermal-storage stability of WPU2-4, WPU2-5, WPU2-6 and WPU2-7 is also better, and even no phenomenon of increased viscosity occurs. As the solid content of WPUs decreases, the distance between particles increases, consequently, during stage 4, the likelihood of two particles encountering the same HyHy molecule for chain extension is reduced, which is why the thermal-storage stability of WPU2-8, WPU2-9, WPU2-10 and WPU2-11 is better than that of WPU2-1.
4. Conclusions
A systematic study was conducted on the thermal-storage stability of WPUs prepared by the prepolymer method in this work. A series of comparative experiments were designed, the influencing factors studied included the NCO% content of the prepolymer, the ratio of [NCO] to [H] of post-extension, the dilution ratio, additing time of post-extension amine, and the SC of WPUs. The thermal-storage stability of WPUs was characterized by monitoring the appearance and viscosity changes at 50 °C, and the increase of viscosity or the decrease of fluidity was the performance of poor stability. It was demonstrated that the thermal-storage stability of WPUs was effectively improved decreasing -NCO content of the prepolymer, the ratio of [NCO] to [H] of post- extension, the SC of WPUs, and increasing the dilution ratio, prolonging adding time of post- extension amine aqueous solution. Then a model based on the cage effect was proposed to explain the occurrence of thermal-storage instability, that is, the particles obtained from the prepolymer assembly of short molecules were not stable enough, and were further reassembled under high temperature conditions, during the reassembly process, -NCO groups with limited motility inside these particles moved and exposed to the surface of the particles and further underwent chain-extension reaction. When two particles extended with the same amine molecule, an interparticle connection occurred, resulting in larger particles and increased viscosity or loss of fluidity of WPUs. Decreasing the NCO% content of prepolymer, the residual -NCO groups in particles after initial assembly were reduced; decreasing the ratio of [NCO] to [H] of post-extension, more residual -NCO inside particles was consumed. Increasing the dilution ratio of post-extension amine or prolonging the addition time of amine aqueous solution would make the post-extension reaction slower and more fully, and more residual -NCO groups in particles was consumed. All of these effectively improved the thermal-storage stability of WPUs. The foregoing results could provide fundamental theory guide to waterborne polyurethane dispersions with good thermal-storage stability and corresponding performance based on prepolymer method.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
References
- Arshad, N.; Javaid, M. A.; Zia, K. M.; Hussain, M. T.; Arshad, M. M.; Tahir, U. Development of Biocompatible Aqueous Polyurethane Dispersions Using Chitosan and Curcumin to Improve Physicochemical Properties of Textile Surfaces. Int. J. Biol. Macromol. 2023, 251, 126196. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, D. High Solid Content Waterborne Polyurethane with High Foaming Rate to Artificial Leather: Synthesis and Characterization, Prog. Org. Coat. 2024, 189, 108332. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Han, S.; Bai, Y.; Wang, P.; Liu, H.; Zhang, P.; Luo, X. Cellulose Nanocrystal-Modified Bio-Based Aqueous Polyurethane Coating Agent for Kraft Paper Packaging. J. Appl. Polym. Sci. 2024, 141(32), e55745. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Ye, Y.; Ying, X.; Huang, J.; Li, X. Introduction of Aminated Sodium Lignosulfonate as a Chain Extender for Preparation of High-Performance Waterborne Polyurethane, Int. J. Adhes. Adhes. 2023, 125, 103415. [Google Scholar] [CrossRef]
- Alves, T. R.; Heitmann, A. P.; Ayres, E.; Coura, Í. R.; Souza, P. P.; Patricio, P. S. O. Waterborne Polyurethane Dispersions Based on Polypropylene Glycol/Polycarbonate Diol: Evaluation of Their Use as Wood Protective Coatings. J. Appl. Polym. Sci. 2023, 140(40), 1–11. [Google Scholar] [CrossRef]
- Abdu, M. T.; Khattab, T. A.; Abdelrahman, M. S.; Abdelrahman. M. S. Electrospinning of Glass Nanofibers to Roughen a Waterborne Polyurethane Coating toward Smart Applications, Ceram. Int. 2024, 50(7), 11518-1152.
- Wang, F.; Liu, X.; Cao, B.; Wang, X.; Dong, K. Influence of the Dispersion of Carbon Nanotubes on the Electrical Conductivity, Adhesion Strength, and Corrosion Resistance of Waterborne Polyurethane Composites. Coatings 2024, 14, 1108. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Wang, C.; Li, J.; Ren, H.; Zhou, C. Enhancement of the Adhesion Strength of Water-Based Ink Binder Based on Waterborne Polyurethane. Prog. Org. Coat. 2023, 183, 10776. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The Influence of the Ionic Concentration, Concentration of the Polymer, Degree of Neutralization and Chain Extension on Aqueous Polyurethane Dispersions Prepared by the Acetone Process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Llorente, O.; Fernández-Berridi, M.; González, A.; Irusta, L. Study of the Crosslinking Process of Waterborne UV Curable Polyurethane Acrylates. Prog. Org. Coat. 2016, 99, 437–442. [Google Scholar] [CrossRef]
- Malotky, D. L.; Dermody, D. L.; Schmidt, D.; Young, T. J.; Kalinowski, M. Polymer Colloids: Formation, Characterization and Applications, ed. R. Priestley and R. Prud'homme, The Royal Society of Chemistry, 2019, ch. 1, pp. 1-29.
- Liu, X.; Hong, W.; Chen, X. Continuous Production of Water-Borne Polyurethanes: A Review. Polymers 2020, 12, 2875. [Google Scholar] [CrossRef]
- Baysal, G. Effect of Lignin/Water-Borne Polyurethane Composite Coatings on Tensile Strength and UV Protection Properties of PET/PA6 Bicomponent Nonwoven Fabrics. Pamukkale U. J. Eng. Sc. 2024, 31(2), 0. [Google Scholar]
- Baysal, G. Mechanical and UV Protection Performances of Polylactic Acid Spunlace Nonwoven Fabrics Coated by Eco-Friendly Lignin/Water-Borne Polyurethane Composite Coatings. J. Text. I. 2023, 115(11), 2185–2197. [Google Scholar] [CrossRef]
- Pandya, H. Fundamental Insight into Anionic Aqueous Polyurethane Dispersions. Adv. Ind. Eng. Polym. Res. 2020, 3, 102–110. [Google Scholar] [CrossRef]
- Jhon, Y.-K.; Cheong, I.-W.; Kim, J.-H. Chain Extension Study of Aqueous Polyurethane Dispersions. Colloids Surfaces A 2001, 179, 71–78. [Google Scholar] [CrossRef]
- Jang, J.Y.; Jhon, Y.-K.; Cheong, I.W.; Kim, J.H. Effect of Process Variables on Molecular Weight and Mechanical Properties of Water-based Polyurethane Dispersion. Colloids Surfaces A 2002, 196, 135–143. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H. Design, Preparation and Properties of Polyurethane Dispersions via Prepolymer Method. Molecules 2023, 28, 625. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Effect of Ionic Content, Solid Content, Degree of Neutralization, and Chain Extension on Aqueous Polyurethane Dispersions Prepared by Prepolymer Method. J. Appl. Polym. Sci. 2005, 98, 2514–2520. [Google Scholar] [CrossRef]
- Qiao, Z.; Yang, Z.; Liu, W.; Wang, X.; Gao, Y.; Yu, Z.; Zhu, C.; Xu, J.; Xu, J. Molecular weight switchable polyurethanes enable melt processing. Chem. Eng. J. 2020, 384, 123287. [Google Scholar] [CrossRef]
- Mou, J.; Wang, X.; Yu, D.; Wang, S.; Miao, Y.; Liu, Q.; Chen, B. Practical Two-Step Chain Extension Method to Prepare Sulfonated Waterborne Polyurethanes based on Aliphatic Diamine Sulphonate. J. Appl. Polym. Sci. 2020, 138, e50353. [Google Scholar] [CrossRef]
- Chinwanitcharoen, C.; Kanoh, S.; Yamada, T.; Hayashi, S.; Sugano, S. Preparation of Aqueous Dispersible Polyurethane: Effect of Acetone on the Particle Size and Storage Stability of Polyurethane Emulsion. J. Appl. Polym. Sci. 2004, 91, 3455–3461. [Google Scholar] [CrossRef]
- Chinwanitcharoen, C.; Kanoh, S.; Yamada, T.; Tada, K.; Hayashi, S.; Sugano, S. Preparation and Shelf-Life Stability of Aqueous Polyurethane Dispersion. Macromol. Symp. 2004, 216(1), 229–240. [Google Scholar] [CrossRef]
- Nanda, A. K.; Wicks, D. A.; Madbouly, S. A.; Otaigbe, J. U. Effect of Ionic Content, Solid Content, Degree of Neutralization, and Chain Extension on Aqueous Polyurethane Dispersions Prepared by Prepolymer Method. J. Appl. Polym. Sci. 2005, 98, 2514–2520. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W.; Ma, N.; Guo, W.; Yu, X.; Chen,X. Research on the Post-Chain Extension of Aqueous Polyurethane Dispersions, RSC Adv., 2016, 6, 36510.
Scheme 1.
Elementary steps for the preparation of WPU dispersion.
Scheme 1.
Elementary steps for the preparation of WPU dispersion.
Figure 1.
The Fourier transform infrared spectroscopy (FTIR) spectrum of pre-PU1 and pre-PU2.
Figure 1.
The Fourier transform infrared spectroscopy (FTIR) spectrum of pre-PU1 and pre-PU2.
Figure 2.
Histograms of size distribution of WPU1-1, WPU2-1, WPU2-2, WPU2-3, WPU2-4, WPU2-5, WPU2-6, WPU2-7, WPU2-8, WPU2-9, WPU2-10 and WPU2-11, respectively.
Figure 2.
Histograms of size distribution of WPU1-1, WPU2-1, WPU2-2, WPU2-3, WPU2-4, WPU2-5, WPU2-6, WPU2-7, WPU2-8, WPU2-9, WPU2-10 and WPU2-11, respectively.
Figure 3.
(a) Photographs of WPU2-1 before and after thermal-storage at 50 °C for 8 h; (b) and (c) SEM images of dried WPU2-1 before and after thermal-storage at 50 °C for 8 h, respectively.
Figure 3.
(a) Photographs of WPU2-1 before and after thermal-storage at 50 °C for 8 h; (b) and (c) SEM images of dried WPU2-1 before and after thermal-storage at 50 °C for 8 h, respectively.
Figure 4.
Viscosity comparison of (a) WPU1-1 and WPU2-1, and (b) WPU2-1, WPU2-2, and WPU2-3 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Figure 4.
Viscosity comparison of (a) WPU1-1 and WPU2-1, and (b) WPU2-1, WPU2-2, and WPU2-3 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Figure 5.
Viscosity comparison of (a) WPU1-1 WPU2-4 and WPU2-5, and (b) WPU2-1, WPU2-6 and WPU2-7 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Figure 5.
Viscosity comparison of (a) WPU1-1 WPU2-4 and WPU2-5, and (b) WPU2-1, WPU2-6 and WPU2-7 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Figure 6.
Viscosity comparison of (a) WPU2-1, WPU2-8 and WPU2-9, and (b) WPU2-1, WPU2-10 and WPU2-11 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Figure 6.
Viscosity comparison of (a) WPU2-1, WPU2-8 and WPU2-9, and (b) WPU2-1, WPU2-10 and WPU2-11 at 50 °C over 24 h. Note that, the absence of viscosity data indicates that the WPUs have transitioned to a non-flowing state.
Scheme 2.
Schematic illustration for thermal-storage instability of WPUs due to cage effect.
Scheme 2.
Schematic illustration for thermal-storage instability of WPUs due to cage effect.
Table 1.
Parameters of WPU dispersions.
Table 1.
Parameters of WPU dispersions.
| |
NCO % of prepolymer
(%)
|
Acetone
Content
(%) a
|
Ratio of [NCO] to [H] |
Solid content
(%)
|
Ratio of HyHy : H2O |
Time of adding HyHy solution (min) |
| WPU1-1 |
3.85 |
/ |
1.03 |
50 |
1:4 |
1 |
| WPU2-1 |
6.58 |
/ |
1.03 |
50 |
1:4 |
1 |
| WPU2-2 |
6.58 |
/ |
0.95 |
50 |
1:4 |
1 |
| WPU2-3 |
6.58 |
/ |
0.90 |
50 |
1:4 |
1 |
| WPU2-4 |
6.58 |
/ |
1.03 |
50 |
1:6 |
1 |
| WPU2-5 |
6.58 |
/ |
1.03 |
50 |
1:10 |
1 |
| WPU2-6 |
6.58 |
/ |
1.03 |
50 |
1:4 |
2 |
| WPU2-7 |
6.58 |
/ |
1.03 |
50 |
1:4 |
3 |
| WPU2-8 |
6.58 |
40 |
1.03 |
50 |
1:4 |
1 |
| WPU2-9 |
6.58 |
60 |
1.03 |
50 |
1:4 |
1 |
| WPU2-10 |
6.58 |
/ |
1.03 |
42 |
1:4 |
1 |
| WPU2-11 |
6.58 |
/ |
1.03 |
38 |
1:4 |
1 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).