Submitted:
22 March 2025
Posted:
25 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Conventional Methods for HM Removal
3. Microalgae as Sentinel Species for HM Pollution
4. The Role of Microalgae in HM Removal
4.1. Microalgae for HM Removal: Current Technologies, Mechanisms and Advantages
4.2. The Influence of Abiotic Factors on HM Removal
4.3. The Influence of Biotic Factors on HM Removal
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure and Applied Chemistry 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’— proposal of a comprehensive definition. Toxicological & Environmental Chemistry 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Global Ecology and Conservation 2020, 22, e00925. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: a review. Environmental Chemistry Letters 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC advances 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. Journal of Chemistry 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environmental Technology & Innovation 2021, 22, 101504. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC advances 2023, 13, 17595–17610. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.S.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; López-Díaz, J.A. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. International Journal of Hydrogen Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-g.; Zeng, G.-m.; Jiang, M.; Yang, Z.-z.; Cui, F.; Zhu, M.-y.; Shen, L.-q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environment International 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Muhammad, S.; Khan, B.; Khan, H. Investigating the levels of selected heavy metals in surface water of Shah Alam River (A tributary of River Kabul, Khyber Pakhtunkhwa). Journal of Himalayan Earth Sciences 2011, 44, 71–79. [Google Scholar]
- Asiminicesei, D.-M.; Vasilachi, I.C.; Gavrilescu, M. Heavy Metal Contamination of Medicinal Plants and Potential Implications on Human Health. Revista de Chimie 2020, 71, 16–36. [Google Scholar] [CrossRef]
- Verde, R.; Vigliotti, M.; Prevedello, L.; Sprovieri, M.; Ruberti, D. An integrated approach to environmental quality assessment in a coastal setting in Campania (Southern Italy). Environmental Earth Sciences 2013, 70, 407–424. [Google Scholar] [CrossRef]
- Lu, R.; Rong, S.; Wu, J.; Yue, W.; Li, Q. Pollution Assessment and SSD-Based Ecological Assessment of Heavy Metals in Multimedia in the Coast of Southeast China. International Journal of Environmental Research and Public Health 2022, 19, 16022. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Cocozza di Montanara, A.; Corinaldesi, C.; Dell’Anno, A.; Illuminati, S.; Willis, T.J.; Gambi, C. Bioaccumulation and biomagnification of heavy metals in marine micro-predators. Commun Biol 2023, 6, 1206. [Google Scholar] [CrossRef]
- Ahlf, W.; Drost, W.; Heise, S. Incorporation of metal bioavailability into regulatory frameworks—metal exposure in water and sediment. Journal of Soils and Sediments 2009, 9, 411–419. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.; Rahman, H.; Ashekuzzaman, S.; Islam, M.M.; Chowdhury, S.R.; Hossain, M.S. Heavy metals accumulation in coastal sediments. Environmental remediation technologies for metal-contaminated soils.
- Saddik, M.; Fadili, A.; Makan, A. Assessment of heavy metal contamination in surface sediments along the Mediterranean coast of Morocco. Environmental Monitoring and Assessment 2019, 191. [Google Scholar] [CrossRef]
- Ozmen, S. Ecological assesment of Akkuyu nuclear power plant site marine sediments in terms of radionuclide and metal accumulation. Journal of Radioanalytical and Nuclear Chemistry 2020, 325, 133–145. [Google Scholar] [CrossRef]
- Abdallah, M.; Badr-ElDin, A. Ecological risk assessment of surficial sediment by heavy metals from a submerged archaeology harbor, South Mediterranean Sea, Egypt. Acta Geochimica 2020, 39, 226–235. [Google Scholar] [CrossRef]
- Ben Amor, R.; Yahyaoui, A.; Abidi, M.; Chouba, L.; Gueddari, M. Bioavailability and Assessment of Metal Contamination in Surface Sediments of Rades-Hamam Lif Coast, around Meliane River (Gulf of Tunis, Tunisia, Mediterranean Sea). Journal of Chemistry 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Gambi, C.; Dell’Anno, A.; Corinaldesi, C.; Lo Martire, M.; Musco, L.; Da Ros, Z.; Armiento, G.; Danovaro, R. Impact of historical contamination on meiofaunal assemblages: The case study of the Bagnoli-Coroglio Bay (southern Tyrrhenian Sea). Marine Environmental Research 2020, 104907. [Google Scholar] [CrossRef] [PubMed]
- Surricchio, G.; Pompilio, L.; Novelli, A.A.; Scamosci, E.; Marinangeli, L.; Tonucci, L.; d’Alessandro, N.; Tangari, A.C. Evaluation of heavy metals background in the Adriatic Sea sediments of Abruzzo region, Italy. Science of the Total Environment 2019, 684, 445–457. [Google Scholar] [CrossRef]
- Pitacco, V.; Mistri, M.; Granata, T.; Moruzzi, L.; Meloni, M.L.; Massara, F.; Sfriso, A.; Sfriso, A.A.; Munari, C. Sediment contamination by heavy metals and PAH in the Piombino channel (Tyrrhenian Sea). Water 2021, 13, 1487. [Google Scholar] [CrossRef]
- Piazzolla, D.; Scanu, S.; Frattarelli, F.M.; Mancini, E.; Tiralongo, F.; Brundo, M.V.; Tibullo, D.; Pecoraro, R.; Copat, C.; Ferrante, M. Trace-metal enrichment and pollution in coastal sediments in the Northern Tyrrhenian Sea, Italy. Archives of environmental contamination and toxicology 2015, 69, 470–481. [Google Scholar] [CrossRef]
- Sprovieri, M.; Feo, M.L.; Prevedello, L.; Manta, D.S.; Sammartino, S.; Tamburrino, S.; Marsella, E. Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (southern Italy). Chemosphere 2007, 67, 998–1009. [Google Scholar] [CrossRef]
- De Pippo, T.; Donadio, C.; Guida, M.; Petrosino, C. The Case of Sarno River (Southern Italy). Effects of geomorphology on the environmental impacts (8 pp). Environmental Science and Pollution Research—International 2006, 13, 184–191. [Google Scholar] [CrossRef]
- Montuori, P.; Lama, P.; Aurino, S.; Naviglio, D.; Triassi, M. Metals loads into the Mediterranean Sea: estimate of Sarno River inputs and ecological risk. Ecotoxicology 2013, 22, 295–307. [Google Scholar] [CrossRef]
- Breuer, E.; Stevenson, A.G.; Howe, J.A.; Carroll, J.; Shimmield, G.B. Drill cutting accumulations in the Northern and Central North Sea: a review of environmental interactions and chemical fate. Marine Pollution Bulletin 2004, 48, 12–25. [Google Scholar] [CrossRef]
- Fichet, D.; Radenac, G.; Miramand, P. Experimental studies of impacts of harbour sediments resuspension to marine invertebrates larvae: Bioavailability of Cd, Cu, Pb and Zn and toxicity. Marine Pollution Bulletin 1998, 36, 509–518. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W. Modeling sediment resuspension and transport induced by storm wind in Apalachicola Bay, USA. Environmental Modelling & Software 2009, 24, 1302–1313. [Google Scholar]
- Rial, D.; Beiras, R. Prospective ecological risk assessment of sediment resuspension in an estuary. Journal of Environmental Monitoring 2012, 14, 2137–2144. [Google Scholar] [CrossRef]
- Webster, T.; Lemckert, C. Sediment resuspension within a microtidal estuary/embayment and the implication to channel management. Journal of Coastal Research 2002, 753–759. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management 1995, 19, 81–97. [Google Scholar] [CrossRef]
- De Mora, S.; Fowler, S.W.; Wyse, E.; Azemard, S. Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and Gulf of Oman. Mar Pollut Bull 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Mejdoub, Z.; Zaid, Y.; Hmimid, F.; Kabine, M. Assessment of metals bioaccumulation and bioavailability in mussels Mytilus galloprovincialis exposed to outfalls pollution in coastal areas of Casablanca. J Trace Elem Med Biol 2018, 48, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, D.; Castelli, A.; Perošević, A.; Djurović, D.; Stanković, S. Determination of trace metals in Mytilus galloprovincialis along the Boka Kotorska Bay, Montenegrin coast. Journal of Trace Elements in Medicine and Biology 2018, 50, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Capelli, R.; Contardi, V.; Fassone, B.; Zanicchi, G. Heavy metals in mussels (Mytilus galloprovincialis) from the gulf of la spezia and from the promontory of Portofino, Italy. Marine Chemistry 1978, 6, 179–185. [Google Scholar] [CrossRef]
- Türk Çulha, S.; Çulha, M.; Karayücel, İ.; Çelik, M.Y.; Işler, Y. Heavy metals in Mytilus galloprovincialis, suspended particulate matter and sediment from offshore submerged longline system, Black Sea. International Journal of Environmental Science and Technology 2017, 14, 385–396. [Google Scholar] [CrossRef]
- Ndiaye, B.; Ndiaye, M.; Cid, B.P.; Diop, A.; Diagne, I.; Cissé, D.; Dione, C.T.; Hanne, M. Trace Metals in Mussels Mytilus galloprovincialis from Dakar Coast (Senegal). American Journal of Analytical Chemistry 2020, 11, 137–145. [Google Scholar] [CrossRef]
- Appenroth, K.J. Definition of “Heavy Metals” and Their Role in Biological Systems. 2010.
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int J Prev Med 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace elements in soils and plants; CRC press: 2000.
- Kalaivanan, D.; Ganeshamurthy, A. Mechanisms of Heavy Metal Toxicity in Plants. 2015; pp. 85-102.
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General aspects of metal toxicity. Curr Med Chem 2014, 21, 3721–3740. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J Cancer Prev 2015, 20, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Sharma, M.; Kant, R.; Sharma, A.; Sharma, A. Exploring the impact of heavy metals toxicity in the aquatic ecosystem. International Journal of Energy and Water Resources 2024. [Google Scholar] [CrossRef]
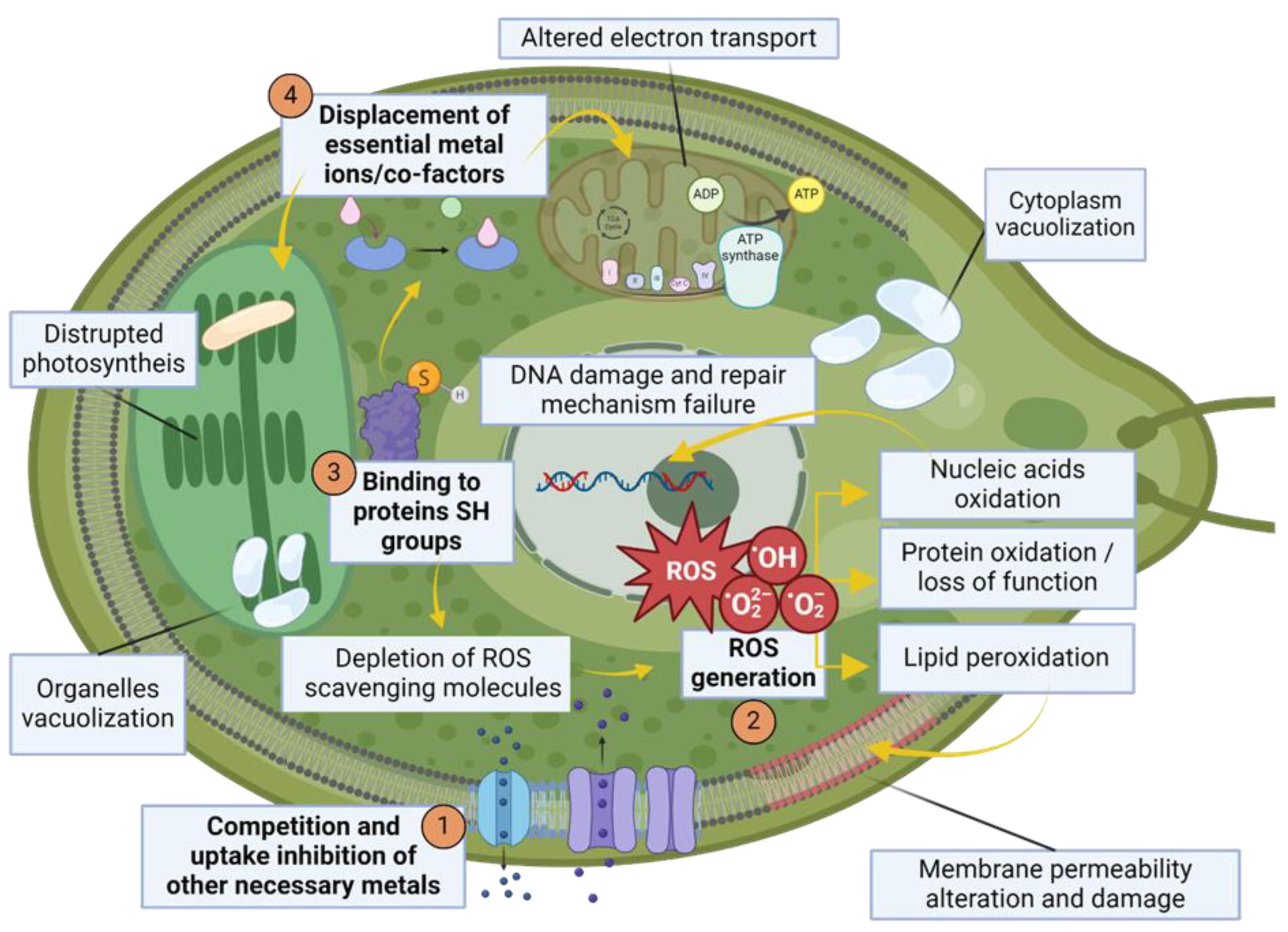
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Pang, F.M.; Teng, S.P.; Teng, T.T.; Omar, A.M. Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Quality Research Journal 2009, 44, 174–182. [Google Scholar] [CrossRef]
- Lewis, A. Precipitation of Heavy Metals. In Sustainable Heavy Metal Remediation: Volume 1: Principles and Processes, Rene, E.R., Sahinkaya, E., Lewis, A., Lens, P.N.L., Eds.; Springer International Publishing: Cham, 2017; pp. 101–120. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environmental Chemistry Letters 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: a review. Journal of environmental management 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Maarof, H.I.; Daud, W.M.A.W.; Aroua, M.K. Recent trends in removal and recovery of heavy metals from wastewater by electrochemical technologies. Reviews in Chemical Engineering 2017, 33, 359–386. [Google Scholar] [CrossRef]
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Frontiers in Energy Research 2019, 7. [Google Scholar] [CrossRef]
- Wang, S. A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes and Pigments 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Applied microbiology and biotechnology 2016, 100, 6509–6518. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Xia, B.; Du, Q.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. Journal of Hazardous Materials 2010, 177, 876–880. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: a critical review. Critical reviews in environmental science and technology 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and lignin based materials for the removal of heavy metals from waste water-an overview. Zeitschrift für Physikalische Chemie 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World Journal of Microbiology and Biotechnology 2016, 32, 180. [Google Scholar] [CrossRef]
- Benoiston, A.S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Philos Trans R Soc Lond B Biol Sci 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Vargas, C.A.; Martínez, R.A.; Escribano, R.; Lagos, N.A. Seasonal relative influence of food quantity, quality, and feeding behaviour on zooplankton growth regulation in coastal food webs. Journal of the Marine Biological Association of the United Kingdom 2010, 90, 1189–1201. [Google Scholar] [CrossRef]
- Lauringson, V.; Kotta, J.; Orav-Kotta, H.; Kaljurand, K. Diet of mussels Mytilus trossulus and Dreissena polymorpha in a brackish nontidal environment. Marine Ecology 2013, 35. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal Physiology and Large-Scale Outdoor Cultures of Microalgae. In The Physiology of Microalgae. In The Physiology of Microalgae, Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, 2016; pp. 601–652. [Google Scholar]
- Metting, F.B. Biodiversity and application of microalgae. Journal of Industrial Microbiology 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae cultivation and metabolites production: a comprehensive review. Biofuels, Bioproducts and Biorefining 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renewable and Sustainable Energy Reviews 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. Journal of Bioscience and Bioengineering 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- SureshKumar, P.; Thomas, J.; Poornima, V. Structural insights on bioremediation of polycyclic aromatic hydrocarbons using microalgae: a modelling-based computational study. Environmental Monitoring and Assessment 2018, 190, 92. [Google Scholar] [CrossRef]
- Nayak, J.K.; Ghosh, U.K. Post treatment of microalgae treated pharmaceutical wastewater in photosynthetic microbial fuel cell (PMFC) and biodiesel production. Biomass and Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Frontiers in Marine Science 2019, 6. [Google Scholar] [CrossRef]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic engineering of algae for fourth generation biofuels production. Energy & Environmental Science 2011, 4, 2451–2466. [Google Scholar] [CrossRef]
- Pessôa, L.C.; Deamici, K.M.; Pontes, L.A.M.; Druzian, J.I.; Assis, D.d.J. Technological prospection of microalgae-based biorefinery approach for effluent treatment. Algal Research 2021, 60, 102504. [Google Scholar] [CrossRef]
- Abomohra, A.; Hanelt, D. Recent Advances in Micro-/Nanoplastic (MNPs) Removal by Microalgae and Possible Integrated Routes of Energy Recovery. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Sardo, A.; Moros, M.; Smerilli, A.; Chiaiese, P.; Percopo, I.; Cavalletti, E.; Castro-Hinojosa, C.; Balzano, S. Identification of a Green Algal Strain Collected from the Sarno River Mouth (Gulf of Naples, Italy) and Its Exploitation for Heavy Metal Remediation. Microorganisms 2022, 10, 2445. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Wang, H.Y. Highly effective removal of microplastics by microalgae <i>Scenedesmus abundans</i>. Chemical Engineering Journal 2022, 435. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Ismail, A.F.; Samawati, Z.; Liang, Y.Y.; Kanakaraju, D. Microalgae-Enabled Wastewater Treatment: A Sustainable Strategy for Bioremediation of Pesticides. Water 2023, 15. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y. Batch vs continuous-feeding operational mode for the removal of pesticides from agricultural run-off by microalgae systems: A laboratory scale study. Journal of Hazardous Materials 2016, 309, 126–132. [Google Scholar] [CrossRef]
- Webster, L.J.; Villa-Gomez, D.; Brown, R.; Clarke, W.; Schenk, P.M. A synthetic biology approach for the treatment of pollutants with microalgae. Frontiers in Bioengineering and Biotechnology 2024, 12. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. Journal of Environmental Chemical Engineering 2020, 8, 104460. [Google Scholar] [CrossRef]
- Levy, J.L.; Angel, B.M.; Stauber, J.L.; Poon, W.L.; Simpson, S.L.; Cheng, S.H.; Jolley, D.F. Uptake and internalisation of copper by three marine microalgae: Comparison of copper-sensitive and copper-tolerant species. Aquatic Toxicology 2008, 89, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr Environ Assess Manag 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B. Toxic effects of fluoranthene and copper on marine diatom Phaeodactylum tricornutum. Journal of environmental sciences (China) 2008, 20, 1363–1372. [Google Scholar] [CrossRef]
- Shivaji, S.; VL Dronamaraju, S. Scenedesnus rotundus isolated from the petroleum effluent employs alternate mechanisms of tolerance to elevated levels of cadmium and zinc. Scientific Reports 2019, 9, 8485. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; Ashok Prabu, V.; Sampathkumar, P. Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull Environ Contam Toxicol 2012, 88, 30–37. [Google Scholar] [CrossRef]
- Sendra, M.; Yeste, M.P.; Gatica, J.M.; Moreno-Garrido, I.; Blasco, J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 2017, 179, 279–289. [Google Scholar] [CrossRef]
- Pillai, S.; Behra, R.; Nestler, H.; Suter, M.J.-F.; Sigg, L.; Schirmer, K. Linking toxicity and adaptive responses across the transcriptome, proteome, and phenotype of <i>Chlamydomonas reinhardtii</i> exposed to silver. Proceedings of the National Academy of Sciences 2014, 111, 3490–3495. [Google Scholar] [CrossRef]
- Dewez, D.; Oukarroum, A. Silver nanoparticles toxicity effect on photosystem II photochemistry of the green alga Chlamydomonas reinhardtii treated in light and dark conditions. Toxicological & Environmental Chemistry 2012, 94, 1536–1546. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicology and Environmental Safety 2012, 78, 80–85. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juarez, A.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; de Molina, M.d.C.R. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicology and environmental safety 2009, 72, 1200–1206. [Google Scholar] [CrossRef]
- Okamoto, O.K.; Pinto, E.; Latorre, L.; Bechara, E.J.H.; Colepicolo, P. Antioxidant modulation in response to metal-induced oxidative stress in algal chloroplasts. Archives of Environmental Contamination and Toxicology 2001, 40, 18–24. [Google Scholar] [PubMed]
- Strejckova, A.; Dvorak, M.; Klejdus, B.; Krystofova, O.; Hedbavny, J.; Adam, V.; Huska, D. The strong reaction of simple phenolic acids during oxidative stress caused by nickel, cadmium and copper in the microalga Scenedesmus quadricauda. New biotechnology 2019, 48, 66–75. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis. Environmental Pollution 2021, 285, 117443. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Xing, G.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresource technology 2015, 175, 537–544. [Google Scholar] [CrossRef]
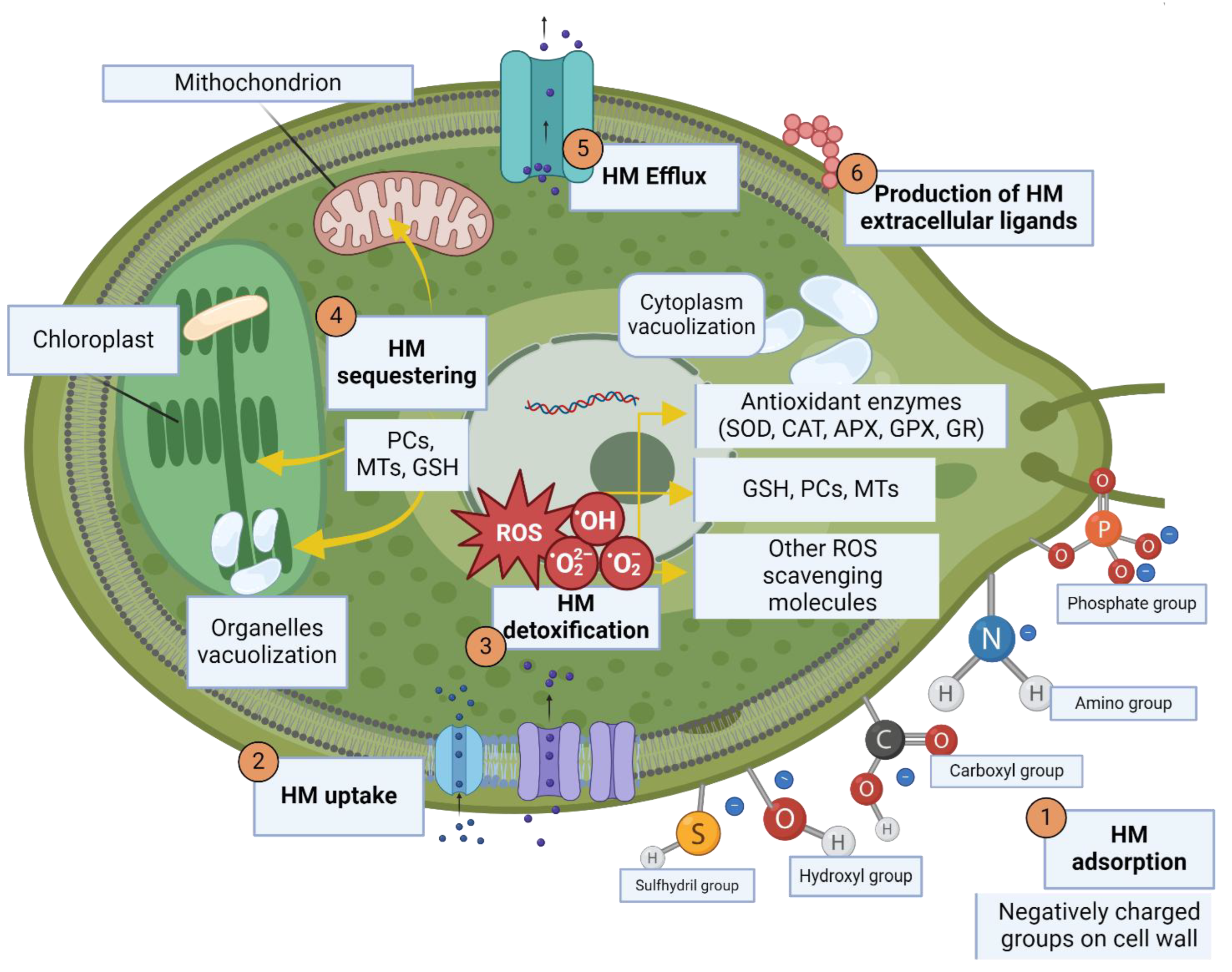
- Spain, O.; Plöhn, M.; Funk, C. The cell wall of green microalgae and its role in heavy metal removal. Physiologia Plantarum 2021, 173, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Science and Technology 2014, 69, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for biofuel production and removal of heavy metals: a review. Environmental Chemistry Letters 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7. [Google Scholar] [CrossRef]
- García-García, J.D.; Sánchez-Thomas, R.; Moreno-Sánchez, R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnology Advances 2016, 34, 859–873. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnology Progress 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Sigaud-Kutner, T.; Zajac, M.A.; Okamoto, O.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. Journal of Phycology 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghatchouli, N.; Arroussi, H. Overview of the management of heavy metals toxicity by microalgae. Journal of Applied Phycology 2022, 34, 475–488. [Google Scholar] [CrossRef]
- Ugya, A.Y.; Imam, T.S.; Li, A.; Ma, J.; Hua, X. Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: a mini review. Chemistry and Ecology 2020, 36, 174–193. [Google Scholar] [CrossRef]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, W.; Jin, M.; Zhang, L.; Qin, L.; Geng, W. Responses and tolerance mechanisms of microalgae to heavy metal stress: A review. Mar Environ Res 2023, 183, 105805. [Google Scholar] [CrossRef]
- Arora, N.; Gulati, K.; Patel, A.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Research 2017, 24, 29–39. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Jolley, D.F. Sensitivity of marine microalgae to copper: the effect of biotic factors on copper adsorption and toxicity. Sci Total Environ 2007, 387, 141–154. [Google Scholar] [CrossRef]
- Díaz, S.; De Francisco, P.; Olsson, S.; Aguilera, Á.; González-Toril, E.; Martín-González, A. Toxicity, Physiological, and Ultrastructural Effects of Arsenic and Cadmium on the Extremophilic Microalga Chlamydomonas acidophila. International Journal of Environmental Research and Public Health 2020, 17, 1650. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquatic Toxicology 2021, 239, 105941. [Google Scholar] [CrossRef]
- Gillet, S.; Decottignies, P.; Chardonnet, S.; Le Maréchal, P. Cadmium response and redoxin targets in Chlamydomonas reinhardtii: a proteomic approach. Photosynthesis Research 2006, 89, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Arora, N.; Pruthi, V.; Poluri, K.M. Elucidating the bioremediation mechanism of Scenedesmus sp. IITRIND2 under cadmium stress. Chemosphere 2021, 283, 131196. [Google Scholar] [CrossRef]
- Desai, S.R.; Verlecar, X.N.; Nagarajappa; Goswami, U. Genotoxicity of cadmium in marine diatom Chaetoceros tenuissimus using the alkaline Comet assay. Ecotoxicology 2006, 15, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, P.; Qian, W.; Tang, X.; Zhu, X.; Wang, Z.; Cai, Z.; Wang, J. Mitigation effects of CO2-driven ocean acidification on Cd toxicity to the marine diatom Skeletonema costatum. Environmental Pollution 2020, 259, 113850. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chai, X.; Shao, Y.; Hu, L.; Xie, Q.; Wu, H. Toxicity of copper, lead, and cadmium on the motility of two marine microalgae Isochrysis galbana and Tetraselmis chui. Journal of Environmental Sciences 2011, 23, 330–335. [Google Scholar] [CrossRef]
- Novosel, N.; Kasum, D.; Žutinić, P.; Legović, T.; Ivošević DeNardis, N. Short-term effect of cadmium on the motility of three flagellated algal species. Journal of Applied Phycology 2020, 32, 4057–4067. [Google Scholar] [CrossRef]
- Wang, M.-J.; Wang, W.-X. Cadmium sensitivity, uptake, subcellular distribution and thiol induction in a marine diatom: exposure to cadmium. Aquatic toxicology 2011, 101, 377–386. [Google Scholar] [CrossRef]
- Lu, M.-M.; Gao, F.; Li, C.; Yang, H.-L. Response of microalgae Chlorella vulgaris to Cr stress and continuous Cr removal in a membrane photobioreactor. Chemosphere 2021, 262, 128422. [Google Scholar] [CrossRef]
- Arun, N.; Vidyalaxmi; Singh, D. P. Chromium (VI) induced oxidative stress in halotolerant alga Dunaliella salina and D. tertiolecta isolated from sambhar salt lake of Rajasthan (India). Cell Mol Biol (Noisy-le-grand) 2014, 60, 90–96. [Google Scholar]
- Takami, R.; Almeida, J.V.; Vardaris, C.V.; Colepicolo, P.; Barros, M.P. The interplay between thiol-compounds against chromium (VI) in the freshwater green alga Monoraphidium convolutum: Toxicology, photosynthesis, and oxidative stress at a glance. Aquatic Toxicology 2012, 118-119, 80–87. [Google Scholar] [CrossRef]
- Li, N.; Qin, L.; Jin, M.; Zhang, L.; Geng, W.; Xiao, X. Extracellular adsorption, intracellular accumulation and tolerance mechanisms of Cyclotella sp. to Cr(VI) stress. Chemosphere 2021, 270, 128662. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Scarano, G. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Science 2004, 167, 289–296. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Hu, Z.; Lei, A.; Wang, J. Towards elucidation of the toxic mechanism of copper on the model green alga Chlamydomonas reinhardtii. Ecotoxicology 2016, 25, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, Q.; Hu, C.-w.; Chen, L.; Liu, Z.-l.; Kong, Z.-m. Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): Effects on lipid peroxidation and antioxidant enzymes. Journal of Environmental Sciences 2007, 19, 1330–1335. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Tan, L.; Wang, J. Toxicity of Co nanoparticles on three species of marine microalgae. Environ Pollut 2018, 236, 454–461. [Google Scholar] [CrossRef]
- Zada, S.; Lu, H.; Khan, S.; Iqbal, A.; Ahmad, A.; Ahmad, A.; Ali, H.; Fu, P.; Dong, H.; Zhang, X. Biosorption of iron ions through microalgae from wastewater and soil: Optimization and comparative study. Chemosphere 2021, 265, 129172. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Arroussi, H. Phycoremediation mechanisms of heavy metals using living green microalgae: physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 2021, 7, e07609. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Talarek, M.; Bralska, M.; Zambrzycka, E. The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environmental Science and Pollution Research 2015, 22, 19112–19123. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.B.; Puthumana, J.; Lee, J.-S. Effects of zinc and mercury on ROS-mediated oxidative stress-induced physiological impairments and antioxidant responses in the microalga Chlorella vulgaris. Environmental Science and Pollution Research 2021, 28, 32475–32492. [Google Scholar] [CrossRef]
- Kováčik, J.; Rotková, G.; Bujdoš, M.; Babula, P.; Peterková, V.; Matúš, P. Ascorbic acid protects Coccomyxa subellipsoidea against metal toxicity through modulation of ROS/NO balance and metal uptake. Journal of Hazardous Materials 2017, 339, 200–207. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, D.; Zheng, X.; Sheng, K.; Luo, Y.; Chen, B.; Huang, B.; Yang, X.; Pan, X. The ecotoxicity of single-layer molybdenum disulfide nanosheets on freshwater microalgae Selenastrum capricornutum. Process Safety and Environmental Protection 2023, 175, 426–436. [Google Scholar] [CrossRef]
- Luo, S.-W.; Alimujiang, A.; Balamurugan, S.; Zheng, J.-W.; Wang, X.; Yang, W.-D.; Cui, J.; Li, H.-Y. Physiological and molecular responses in halotolerant Dunaliella salina exposed to molybdenum disulfide nanoparticles. Journal of Hazardous Materials 2021, 404, 124014. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, Z.Z.; Yang, Z.M. HISN3 Mediates Adaptive Response of Chlamydomonas reinhardtii to Excess Nickel. Plant and Cell Physiology 2013, 54, 1951–1962. [Google Scholar] [CrossRef]
- Li, H.; Yao, J.; Duran, R.; Liu, J.; Min, N.; Chen, Z.; Zhu, X.; Zhao, C.; Ma, B.; Pang, W.; et al. Toxic response of the freshwater green algae Chlorella pyrenoidosa to combined effect of flotation reagent butyl xanthate and nickel. Environmental Pollution 2021, 286, 117285. [Google Scholar] [CrossRef]
- Dahmen-Ben Moussa, I.; Athmouni, K.; Chtourou, H.; Ayadi, H.; Sayadi, S.; Dhouib, A. Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. Journal of Applied Phycology 2018, 30, 931–941. [Google Scholar] [CrossRef]
- Kalehjahi, H.A.; Kosari-Nasab, M.; Amini, M.; Movafeghi, A. Defense responses of the green microalgae to the vanadium pentoxide nanoparticles. Oceanological and Hydrobiological Studies 2023, 52, 446–460. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicology and Environmental Safety 2017, 140, 256–263. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environmental Science and Pollution Research 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chemical communications 2021, 57, 7215–7231. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chemical Society Reviews 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Perelonia, K.B.S.; Benitez, K.C.D.; Banicod, R.J.S.; Tadifa, G.C.; Cambia, F.D.; Montojo, U.M. Validation of an analytical method for the determination of cadmium, lead and mercury in fish and fishery resources by graphite furnace and Cold Vapor Atomic Absorption Spectrometry. Food Control 2021, 130, 108363. [Google Scholar] [CrossRef]
- Knecht, M.R.; Sethi, M. Bio-inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles. Anal Bioanal Chem 2009, 394, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ichinoki, S.; Kitahata, N.; Fujii, Y. Selective Determination of Mercury(II) Ion in Water by Solvent Extraction Followed by Reversed-Phase HPLC. Journal of Liquid Chromatography & Related Technologies—J LIQ CHROMATOGR RELAT TECHNO 2004, 27, 1785–1798. [Google Scholar] [CrossRef]
- Stortini, A.M.; Baldo, M.A.; Moro, G.; Polo, F.; Moretto, L.M. Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors (Basel) 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Z.; Wang, L.; Dong, J.; Xue, J.; Yu, J.; Wang, Y.; Hua, X.; Wang, M.; Zhang, C.; et al. SERS Assay for Copper(II) Ions Based on Dual Hot-Spot Model Coupling with MarR Protein: New Cu(2+)-Specific Biorecognition Element. Anal Chem 2017, 89, 6392–6398. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environmental Science: Water Research & Technology 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Lace, A.; Cleary, J. A Review of Microfluidic Detection Strategies for Heavy Metals in Water. Chemosensors 2021, 9, 60. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef]
- Plácido, J.; Bustamante-López, S.; Meissner, K.E.; Kelly, D.E.; Kelly, S.L. Microalgae biochar-derived carbon dots and their application in heavy metal sensing in aqueous systems. Sci Total Environ 2019, 656, 531–539. [Google Scholar] [CrossRef]
- Quigg, A. Micronutrients. The physiology of microalgae 2016, 211–231. [Google Scholar]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicology and Environmental Safety 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings — 1st Conference on Culture of Marine Invertebrate Animals Greenport, Smith, W.L., Chanley, M.H., Eds.; Springer US: Boston, MA, 1975; pp. 29–60. [Google Scholar]
- Gregorin, C.; Albarano, L.; Somma, E.; Costantini, M.; Zupo, V. Assessing the Ecotoxicity of Copper and Polycyclic Aromatic Hydrocarbons: Comparison of Effects on Paracentrotus lividus and Botryllus schlosseri, as Alternative Bioassay Methods. Water 2021, 13, 711. [Google Scholar] [CrossRef]
- Stauber, J.L.; Davies, C.M. Use and limitations of microbial bioassays for assessing copper bioavailability in the aquatic environment. Environmental Reviews 2000, 8, 255–301. [Google Scholar] [CrossRef]
- Serra, A.; Corcoll, N.; Guasch, H. Copper accumulation and toxicity in fluvial periphyton: the influence of exposure history. Chemosphere 2009, 74, 633–641. [Google Scholar] [CrossRef]
- Belghith, T.; Athmouni, K.; Bellassoued, K.; El Feki, A.; Ayadi, H. Physiological and biochemical response of Dunaliella salina to cadmium pollution. Journal of Applied Phycology 2016, 28, 991–999. [Google Scholar] [CrossRef]
- Kadim, M.K.; Risjani, Y. Biomarker for monitoring heavy metal pollution in aquatic environment: An overview toward molecular perspectives. Emerging Contaminants 2022, 8, 195–205. [Google Scholar] [CrossRef]
- Sharan, A.; Nara, S. Exposure-based ecotoxicity assessment of Co(3)O(4) nanoparticles in marine microalgae. Environ Sci Pollut Res Int 2021, 28, 54802–54810. [Google Scholar] [CrossRef]
- Rajalakshmi, A.M.; Silambarasan, T.; Dhandapani, R. Small scale photo bioreactor treatment of tannery wastewater, heavy metal biosorption and CO2 sequestration using microalga Chlorella sp.: a biodegradation approach. Applied Water Science 2021, 11, 108. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah, X.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K. Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Manikandan, A.; Suresh Babu, P.; Shyamalagowri, S.; Kamaraj, M.; Muthukumaran, P.; Aravind, J. Emerging role of microalgae in heavy metal bioremediation. J Basic Microbiol 2022, 62, 330–347. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renewable and Sustainable Energy Reviews 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Velazquez-Lucio, J.; Rodríguez-Jasso, R.M.; Colla, L.M.; Sáenz-Galindo, A.; Cervantes-Cisneros, D.E.; Aguilar, C.N.; Fernandes, B.D.; Ruiz, H.A. Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Research Journal 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography And Marine Biology: An Annual Review. 1988, 26, 259–315. [Google Scholar]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis gaditana Cell Wall. Eukaryotic Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochemical Engineering Journal 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Applied Microbiology and Biotechnology 2019, 103, 3297–3316. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour Technol 2020, 303, 122886. [Google Scholar] [CrossRef]
- Das, N.; Vimala, R.; Karthika, P. Biosorption of heavy metals–an overview. 2008.
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresource technology 2020, 303, 122886. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–a promising tool for heavy metal remediation. Ecotoxicology and environmental safety 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Nateras-Ramírez, O.; Martínez-Macias, M.R.; Sánchez-Machado, D.I.; López-Cervantes, J.; Aguilar-Ruiz, R.J. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresource Technology Reports 2022, 17, 100932. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnology progress 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.R.F.; Veloso, T.; Macário, I.P.E.; Pereira, J.L.; Ventura, S.P.M.; Passos, H.; Coutinho, J.A.P. The role of biomass elemental composition and ion-exchange in metal sorption by algae. Chemosphere 2023, 314, 137675. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.-S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal Phycoremediation: A Glimpse into a Sustainable Environment. Toxics 2022, 10, 525. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Ranjbar, S.; Malcata, F.X. Is Genetic Engineering a Route to Enhance Microalgae-Mediated Bioremediation of Heavy Metal-Containing Effluents? Molecules 2022, 27, 1473. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol Prog 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: a current perspective on microalgae-based future. Letters in Applied Microbiology 2022, 75, 701–717. [Google Scholar] [CrossRef]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: a potential tool for remediating aquatic environments from toxic metals. International Journal of Environmental Science and Technology 2018, 15, 1815–1830. [Google Scholar] [CrossRef]
- Blaby-Haas, C.E.; Merchant, S.S. The ins and outs of algal metal transport. Biochim Biophys Acta 2012, 1823, 1531–1552. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Frontiers in Microbiology 2020, 11. [Google Scholar] [CrossRef]
- Croot, P.L.; Moffett, J.W.; Brand, L.E. Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnology and Oceanography 2000, 45, 619–627. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renewable and Sustainable Energy Reviews 2021, 137, 110603. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Palmieri, M.C.; Garcia, O. Biosorption of Metals: State of the Art, General Features, and Potential Applications for Environmental and Technological Processes; 2011; pp. 151-176.
- Kumar, K.S.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicology and Environmental Safety 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnology Advances 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnology Advances 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Arica, Y.; Tüzün, İ.; Yalcin, E.; İnce, Ö.; Bayramoglu, G. Utilisation of Native, Heat and Acid-Treated Microalgae Chlamydomonas reinhardtii Preparations for Biosorption of Cr(VI) Ions. Process Biochemistry 2005, 40, 2351–2358. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnology Advances 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Microalga-Mediated Bioremediation of Heavy Metal–Contaminated Surface Waters. In Biomanagement of Metal-Contaminated Soils, Khan, M.S., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer Netherlands: Dordrecht, 2011; pp. 365–385. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rai, L.; Gaur, J.; Kumar, H. Phycology and heavy-metal pollution. Biological reviews 1981, 56, 99–151. [Google Scholar] [CrossRef]
- Schiariti, A.; Juárez, A.B.; Rodriguez, M.C. Effects of sublethal concentrations of copper on three strains of green microalgae under autotrophic and mixotrophic culture conditions. Algological Studies 2004, 114, 143–157. [Google Scholar] [CrossRef]
- Kaushik, G.; Raza, K. Potential of novel Dunaliella salina from sambhar salt lake, India, for bioremediation of hexavalent chromium from aqueous effluents: An optimized green approach. Ecotoxicology and environmental safety 2019, 180, 430–438. [Google Scholar]
- Bishnoi, N.R.; Anju Pant, A.P.; Garima, G. Biosorption of copper from aqueous solution using algal biomass. 2004.
- Hein, M.; Pedersen, M.F.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro-and macroalgae. Marine ecology progress series. Oldendorf 1995, 118, 247–253. [Google Scholar] [CrossRef]
- Quigg, A.; Reinfelder, J.R.; Fisher, N.S. Copper uptake kinetics in diverse marine phytoplankton. Limnology and oceanography 2006, 51, 893–899. [Google Scholar] [CrossRef]
- Khoshmanesh, A.; Lawson, F.; Prince, I.G. Cell surface area as a major parameter in the uptake of cadmium by unicellular green microalgae. The Chemical Engineering Journal and the Biochemical Engineering Journal 1997, 65, 13–19. [Google Scholar] [CrossRef]
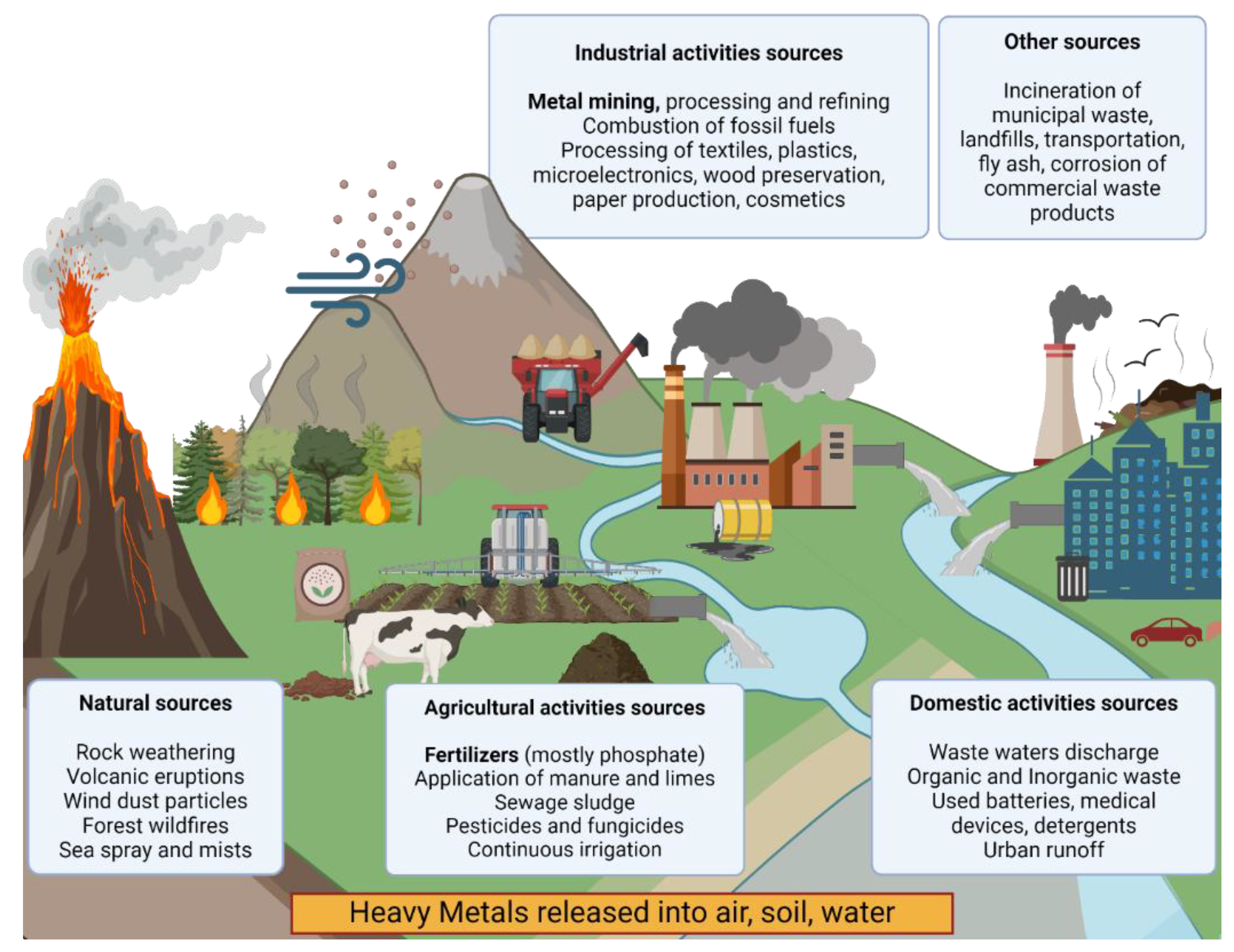
| Source type | HM release type | HMs released |
| Natural | Weathering of geologic parent material and rock outcroppings | Depends on rock composition; generally high Cr, Mn, Co Ni, Cu, Zn, Cd, Sn, Hg, Pb |
| Volcanic eruptions | High Zn, Mn, Pb, Ni, Cu, Cd, Hg. Particulate deposited contains high Cd and Hg | |
| Wind dust particles from desertic regions like Sahara | High Fe; Mn, Zn, Cr, Ni, Pb to a lesser extent | |
| Forest wild fires and prairie fires | Volatile HMs (Hg) | |
| Sea spray and mists | Mostly Cu and Zn from bubble bursting, in addition Cd, Ni, Pb from salt particles | |
| Anthropic | Industrial | |
| Mining ores | Coal mines: Cd, Fe. Gold mines: Hg | |
| Metal processing and refinement | Cu, Cd, Pb, Sn aerosols (smelting and dry/wet deposition), Cd (leachates in soil/water: Zn and Pb refining) | |
| Combustion of fossil fuels in refineries | Cd, Pb and Hg released from coal burning and petroleum combustion | |
| Agricultural | ||
| Organic and inorganic fertilizers to improve crop yield | Cd (high level from phosphate-based type), Cr, Ni, Pb, Zn | |
| Application of manure and limes | Cd, Mn, Zn, Cu and Co | |
| Sewage sludge | Cd, Zn, Cr, Pb, Ni and Cu | |
| Prolonged water irrigation | Cd and Pb from deep wells water sources | |
| Pesticides and fungicides | Cu, Pb, Zn, Fe, Mn and Hg | |
| Domestic | ||
| Urban runoff | Pb, Cu leaching | |
| Detergents | Fe, Mn, Cr, Co, Zn | |
| Used batteries | Pb, Zn, Cd, Ni, Hg | |
| Electronics | Pb, Cd, Hg, Pt, Au, Cr, Ni | |
| Others | ||
| Refuse incineration of municipal waste | Zn, Pb, Al, Sn, Fe, Cu | |
| Transportation (automobiles, diesel-powered vehicles, aircraft) | Ni, Zn, Cd (in engines and Cd from lubricants), Ni and Zn (aerosols emissions), Pb (leaded gasoline) | |
| Corrosion of commercial waste products | Cr, Cu, Pb from galvanized metals, Zn | |
| Fly ash from coal burning | Cd, Hg, Mn, Ni, Fe | |
| Technique | Methodology/Principle | Metals | Advantages | Bottlenecks |
| Chemical precipitation | Reaction of metals with different compounds, mostly involving hydroxides or sulfides, to produce insoluble precipitates | Cu, Cd, Pb, Zn, Mg, Mn | Effective, relatively cheap | Toxic by-products (H2S fumes) |
| Coagulation/Flocculation | Removal of soluble compounds, destabilization of colloids, flocs formation | Zn, Cd, Mn, Ni, Mg | Excellent HM removal under specific conditions | Toxicity of some reagents, strong influence of pH that limits, in some cases, coagulation |
| Ion-exchange | Ion-exchange resin, either synthetic or natural solid resin, has the specific ability to exchange its cations with the metals in the wastewater |
Ce, Fe, Pb, Co, Ni, Cr | High removal efficiency and fast kinetics | Influence of initial metal concentration, ionic charge, contact time, temperature, pH; Resins must be regenerated by expensive procedures |
| Membrane filtration | Use of various kinds of membranes as selective barriers, allowing for selection of retained material | Cd, Cu, Ni, Pb, Zn, Cr | High efficiency, easy operation and space saving | Use of surfactants or water-soluble polymers to aggregate metal ions into micelles or complexes with high molecular weight is mandatory to exceed membrane pore size |
| Electrochemical technologies | Plating-out of metal ions on a cathode surface |
Zn, Cu, Ni, Ag, Cr | Minimum solid waste | Large capital investment and expensive electricity supply |
| HM | Microalgal species | Side-effect | References |
| Arsenic (As) | Chlamydomonas acidophila | As(V) and As(III) (higher extent) 10 and 20 μM generate superoxide anions. TEM underlined ultrastructural changes in As(V) (stigma severely affected but mitochondria preserved, chloroplast intact but thylakoid structure sometimes disrupted, increment in starch granules, vacuolization, excretion of EPS with entrapped As nanoparticles, chromatin masses but no nucleus detection) and As(III) (intense disorganization in stigma and thylakoids, damaged mitochondria, increased lipid droplets and starch granules, nucleolus invisible, formation of nanoparticles, excretion of EPS) | [115] |
| Chlorella sorokiniana | 1 mM As(III) and 5 mM As(V) generated significant inhibition in ammonium consumption (proxy for photosynthetic assimilation of nitrogen), increase in APX and CAT gene expression and activity (higher in As(III), more toxic). | [116] | |
| Cadmium (Cd) | Chlamydomonas reinhardtii | Exposure for 4-5 days 150 μM Cd increases APX MnSOD and GST activity, decreases FeSOD activity | [117] |
| Chlamydomonas acidophila | No significant ROS generation, but severe ultrastructural alteration observed by TEM at 0.05 μM (increase in starch reserves around pyrenoid, cytoplasmic vacuoles with Cd particles later expelled, excretion of EPS, alteration in chromatin structure in nucleus) | [115] | |
| Chlorella sorokiniana | No inhibition of photosynthetic assimilation of nitrogen at 500 μM, APX increase in gene expression but decrease in enzymatic activity, sign of hyperaccumulator | [116] | |
| Scenedesmus sp. | Formation of ROS and lipid peroxidation, increase in APX, GR and CAT activity, increase in lipid content and in saturated and polyunsaturated fatty acids, SEM shown no variation in cell size but surface aberration (rough and ridged) due to Cd adsorption, changes in chlorophyll a, b and carotenoid | [118] | |
| Chaetoceros tenuissimus | DNA damages (detected by Comet assay) increased at increasing Cd concentrations: more than 50% apoptotic cells at day 8 at 10 mg/L Cd | [119] | |
| Skeletonema costatum | 1.2 mg/L Cd increases lipid peroxidation and SOD activity, deregulates genes involved in photosynthesis, increases PC synthesis and upregulates gens involved in peroxisome formation | [120] | |
| Tetraselmis chui, Isochrysis galbana | Reduction in motility: EC50 based on % of motile cells was 121.6 μM for I. galbana and 37,8 μM for T. chui | [121] | |
| Dunaliella tertiolecta, Rhodomonas maculata, Tetraselmis suecica | Cd 1 mg/L after 1 hour results in significant decrease in swimming speed and search radius for all observed species: movement was partly restored after 3 hours | [122] | |
| Thalassiosira nordenskioeldii | Cd exposure for long period influences synthesis of different PC oligomers: PC2 triggered at day 1 but constant afterwards. PC3 and PC4 are produced increasingly in time. Intra-Cd/PC-SH ratio increased with Cd dose and duration suggesting decreased detoxification ability | [123] | |
| Chromium (Cr) | Chlorella vulgaris | Cr(VI) lead to decrease in chlorophyll a content and increase of MDA (lipid peroxidation), which peaks at 2 mg/L. SOD and CAT increase at 0,5 mg/L peaking at 1 mg/L then gradually decrease at increasing Cr(VI) | [124] |
| Dunaliella salina and Dunaliella tertiolecta | Cr(VI) in D. tertiolecta decreased photosynthetic pigments, protein and carbohydrate, lead to higher increase in lipid peroxidation and H2O2 production, increase in rate of RNO bleaching, loss of pigments and thiol (-SH) group than D. salina. SEM with 40 ppm Cr(VI) show damages in both, higher in D. tertiolecta. | [125] | |
| Monoraphidium convolutum | Cr(VI) decreases photosynthetic efficiency, triggers thiols antioxidant response and lipid peroxidation, increases GSH and activity of GR and APX | [126] | |
| Cyclotella sp. | Cr(VI) 5.0 mg/L significantly decreases chlorophyll a, soluble protein content decreases and lipid peroxidation increased with time, SOD and CAT increased with concentration, peaking at 0.5 and 1.0 mg/L, and then decreased. Dissolution of cell membrane sand loss of cellular structure at Cr(VI) 2.0 mg/L observed by TEM | [127] | |
| Copper (Cu) | Phaeodactylum tricornutum, Dunaniella tertiolecta, Tetraselmis sp. | P. tricornutum increases in size and volume upon Cu 10 μg/L and after 72 h at Cu 15 μg/L swells and form cell clumps. D. tertiolecta exposed to Cu 500 μg/L produces exudates. | [87] |
| Phaeodactylum tricornutum | Cu 10 μM: rapid synthesis of PCs of various degree of polymerization, initial decrease in GHS pool, formation of Cu-PC complexes after 1 h, SOD and CAT rapid increase. GR first inhibited and then enhanced. Prolonged Cu exposure increased lipid peroxidation. | [128] | |
| Odontella mobiliensis | After 72 h: chlorophyll a decreased with dose, at Cu 574 μg/L increase of cell length and absent spines, at Cu 926 μg/L structure completely damaged. After 7 days: increase of CAT and peroxidase activities, increase in lipid peroxidation, decrease in nitrate reductase activity at Cu 21.5 μg/L | [91] | |
| Isochrysis galbana, Tetraselmis chui | Significant reduction in motility: EC50 based on % of motile cells was 1.3 μM for I. galbana and 31.4 μM L for T. chui | [121] | |
| Scenedesmus vacuolatus, Chlorella kessleri | S. vacuolatus increases protein and MDA content, and decreases chlorophyll a/b ratio. Increased CAT activity at 210 μM Cu and SOD activity and GSH content at 414 μM Cu | [96] | |
| Chlamydomonas reinhardtii | Photochemical efficiency decreased at 250 μM Cu, lipid peroxidation at its highest after 3 days, GSH content increased at 100 μM and GPX, SOD, glutathione S-transferase induced by Cu 100 and 250 μM | [129] | |
| Chlorella sorokiniana | 1 mM Cu generated significant inhibition in ammonium consumption (proxy for photosynthetic assimilation of nitrogen), APX increase in gene expression but decrease in enzymatic activity, sign of hyperaccumulator | [116] | |
| Cobalt (Co) | Pavlova viridis | Lipid peroxidation, deregulated SOD activity, increase in CAT activity and in and GSH, GPX activity markedly increased | [130] |
| Platymonas subcordiforus, Chaetoceros curvisetus and Skeletonema costatum | Co nanoparticles released Co2+ in solution, aggregated to microalgal cells as observed by SEM. Decrease in chlorophyll a and photosynthetic efficiency observed in S. costatum, TEM images showed increased agglomerates compared to other species due to smaller size and rougher surface. | [131] | |
| Iron (Fe) | Scenedesmus obliquus, Chlorella fusca, Chlorella saccharophila, A. braunii | 50 ppm Fe2+ induced nucleic acid leakage due to cell lysis and cellular ultrastructure changes observed by SEM: rougher surface and cell shrinkage | [132] |
| Lead (Pb) | Scenedesmus obliquus | Pb (141 ppm) caused increase in H2O2 and lipid peroxidation, increase in enzymatic activity of SOD, CAT, APX, GR, guaiacol peroxidase and increase in non-enzymatic ROS response (proline and polyphenols) | [133] |
| Acutodesmus obliquus | Decrease in protein content and chlorosis due to loss of chlorophyll a and b at 500 μM at day 6 and 7, increasing lipid peroxidation with dose. Pb above 10 μM decreased ascorbate and GSH. Low Pb dose (0,1 μM ) increased the activity of SOD, CAT, APX, and GR and higher concentration inhibited activities of all enzymes | [134] | |
| Isochrysis galbana, Tetraselmis chui | Reduction in motility: EC50 based on % of motile cells was 37,8 μM for I. galbana and 10,9 μM for T. chui | [121] | |
| Manganese (Mn) | Pavlova viridis | increase in CAT activity and in and GSH, GPX decreased at increasing concentrations, but at concentrations higher 50 μmol/L the trend was inverted, and it increased rapidly | [130] |
| Mercury (Hg) | Chlamydomonas reinhardtii | Chlorophyll content decrease at 4 μM Hg, lipid peroxidation at increasing Hg concentrations, activity of CAT, APX and SOD increased up to 6 μM but decreased at higher concentrations | [100] |
| Chlorella vulgaris | SEM imaging shows cell shrinkage and structural damages from 2.5% Zn treatment, decreased photosynthetic pigments, concentration-dependent reduction in protein content, ROS increasing with increasing doses, increase of CAT, initial increase of SOD and decrease over time for highest concentrations tested | [135] | |
| Coccomyxa subellipsoidea | 100 μM Hg decrease pigments, potassium ions, soluble proteins, ascorbic acid, non-protein thiols and nitric oxide signal and increased ROS production and CAT and SOD activity | [136] | |
| Molybdenum (Mn) | Selenastrum capricornutum | MoS2 nanosheets at low concentrations (≤ 1.0 mg/L) increase photosynthesis, at concentrations > 1 mg/L induce oxidative stress and membrane damages, EPS production. MoS2 nanosheets aggregates on cell and damaging/penetrating the membrane observed by TEM. | [137] |
| Dunaliella salina | MoS2 NPs enhance photosynthetic efficiency, increases protein and carbohydrate content, induce activity of CATand SOD | [138] | |
| Nickel (Ni) | Chlamydomonas reinhardtii | Ni 90 μM or higher induced generation of ROS and lipid peroxidation, proline and other non-protein thiols were increased while ascorbate was decreased | [139] |
| Chlorella pyrenoidosa | Decrease in chlorophyll a content, formation of ROS and lipid peroxidation, increased SOD and CAT activity | [140] | |
| Dunaliella sp., Amphora subtropica | Reduction in carbohydrate and protein increased total lipids, increase in phenolic compounds and carotenoid content. Increased lipid peroxidation and of SOD, CAT, GPX activity. Reductions in total chlorophyll and chlorophyll a and b at higher metal concentrations, increase in carotenoids | [141] | |
| Silver (Ag) | Dunaliella tertiolecta, Chlorella vulgaris | ROS formation and lipid peroxidation in the presence of AgNPs (1-10 mg/L) | [95] |
| Phaeodactylum tricornutum, Chlamydomonas reinhardtii | Growth rate reduction, ROS generation, membrane damages in the presence of AgCl (aq,) AgCl- and Ag+ | [92] | |
| Vanadium (V) | Chlorella sorokiniana | V2O5 NPs 200 mg/L caused plasmolysis and cell shrinkage observed by SEM. Decrease in photosynthetic pigments and phenolic content and increase of SOD, CAT and APX activity were also observed. | [142] |
| Zinc (Zn) | Chlorella sorokiniana, Scenedesmus acuminatus | Sublethal doses trigger oxidative stress in both species (H2O2 and lipid peroxidation). C. sorokiniana has more efficient antioxidant defense (accumulates more flavonoids, polyphenols, tocopherols, GSH, and ascorbate and GST, GR, SOD, POX, and APX enzyme activity higher) | [143] |
| Chlorella vulgaris | SEM imaging shows cell shrinkage and structural damages from 2,5% Zn treatment, decreased photosynthetic pigments, concentration-dependent reduction in protein content, ROS increasing with increasing doses, increase of CAT, initial increase of SOD and decrease over time for highest concentrations tested | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





