Submitted:
21 March 2025
Posted:
25 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
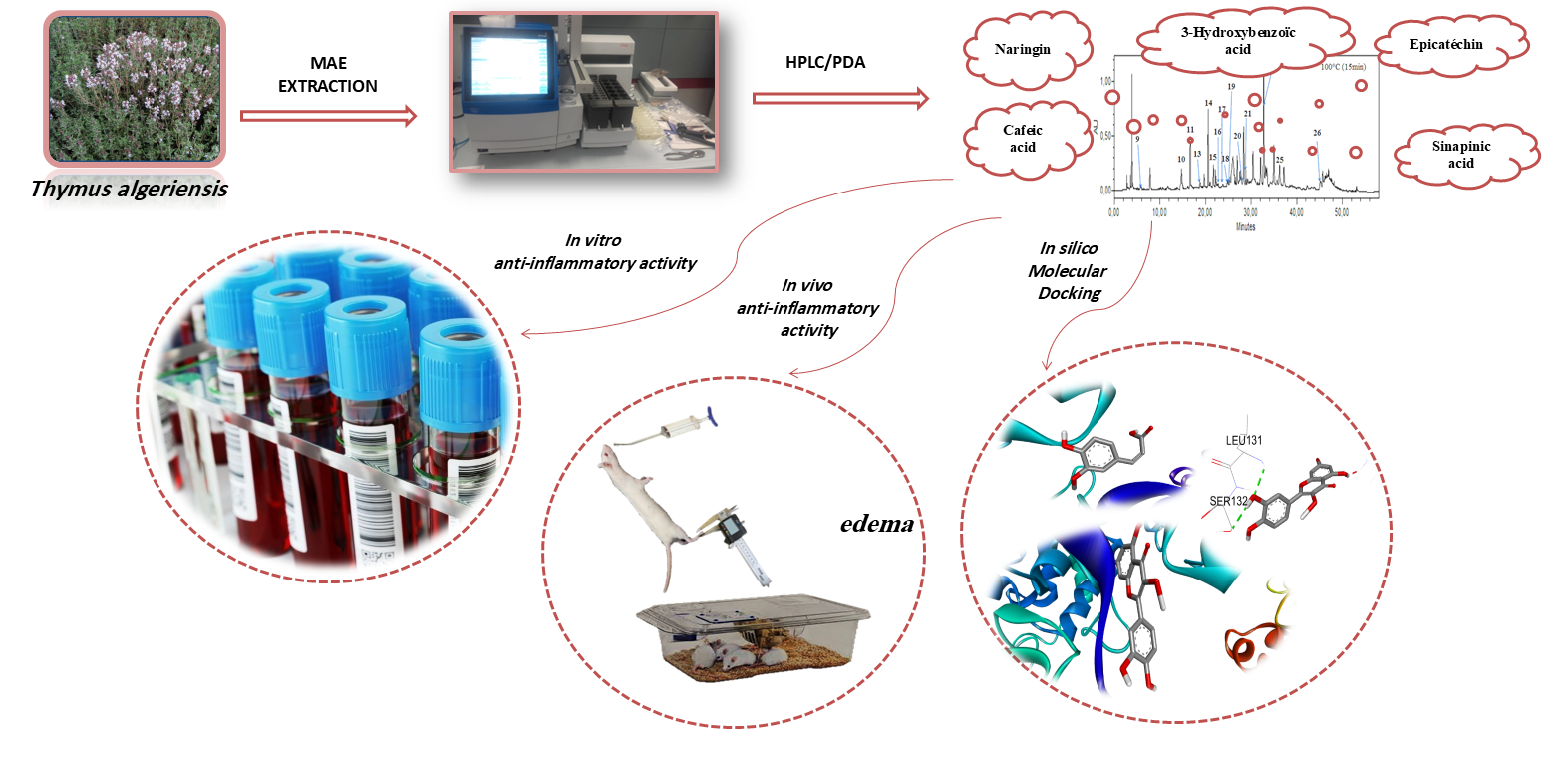
2.1. Plant Material and Preparation of Extracts
2.2. Profile of Bioactive Compounds
2.3. In Vitro Anti-Inflammatory Activity
2.4. Inflammatory Paw Edema Test in Rats
2.5. Molecular Docking Study
2.6. Statistical Analysis
3. Results and Discussion
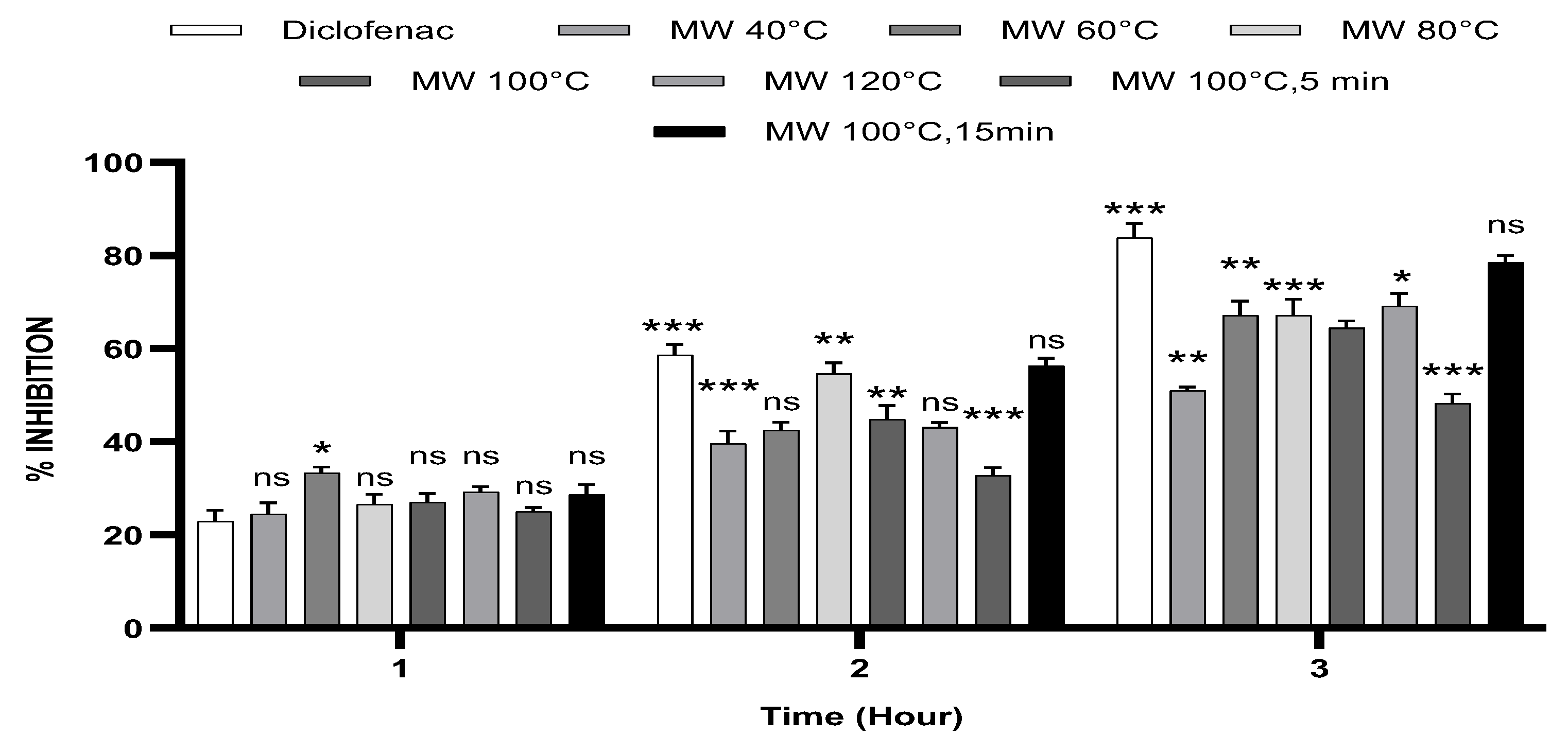
3.1. In Vitro Anti-Inflammatory Activity in Human RBCs (HBRC)
3.2. Carrageenan-Induced Paw Oedema
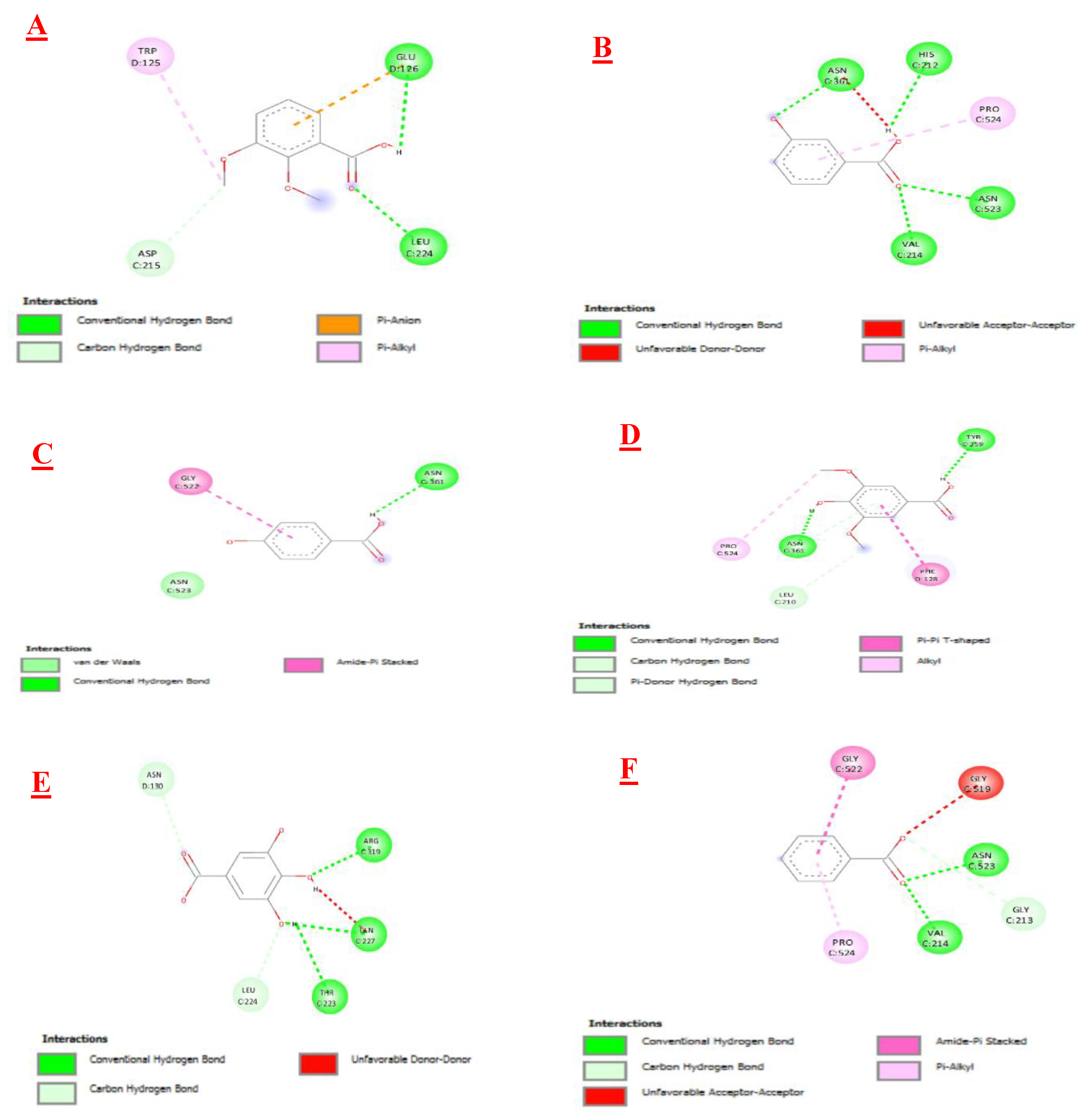
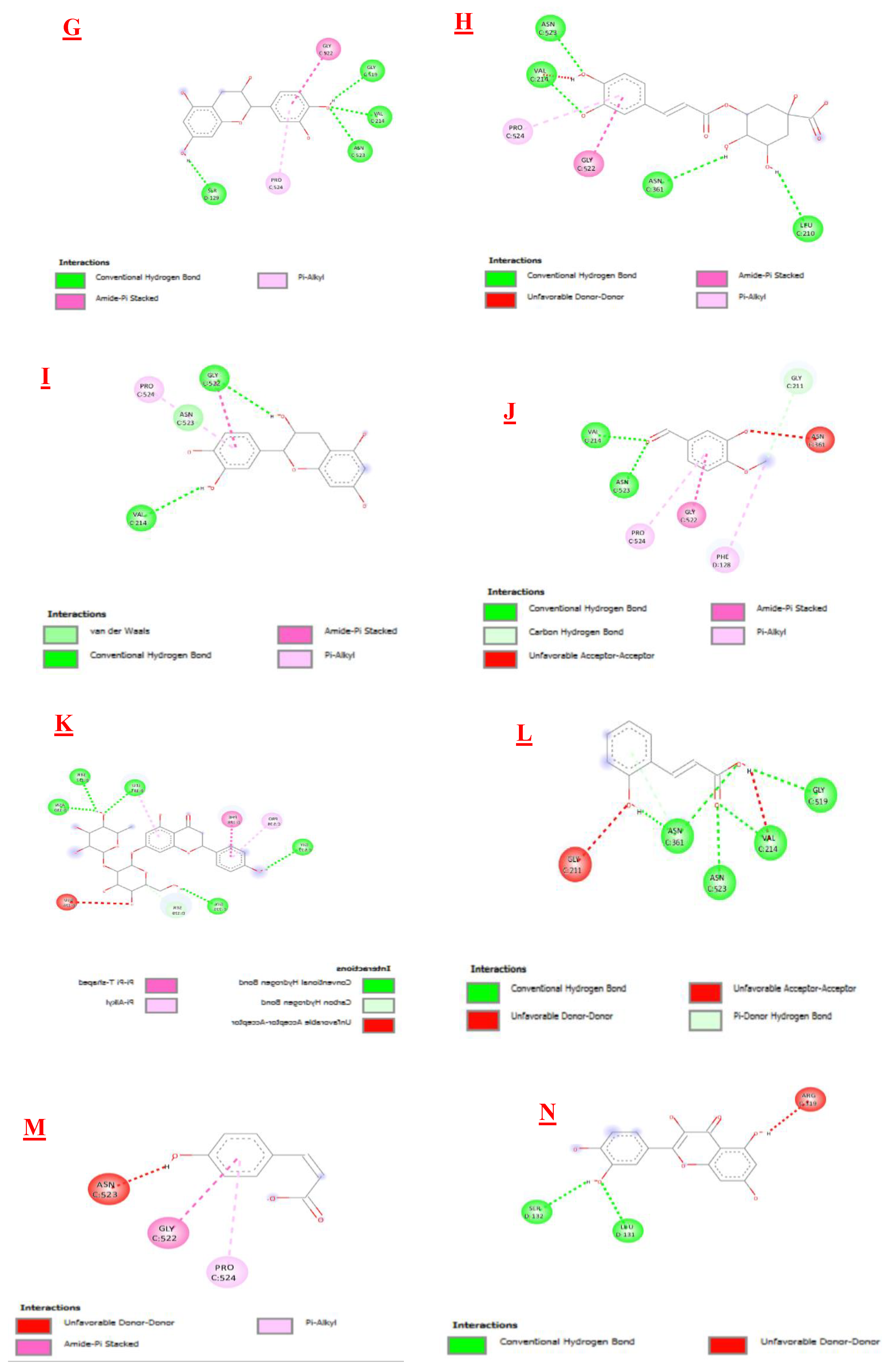
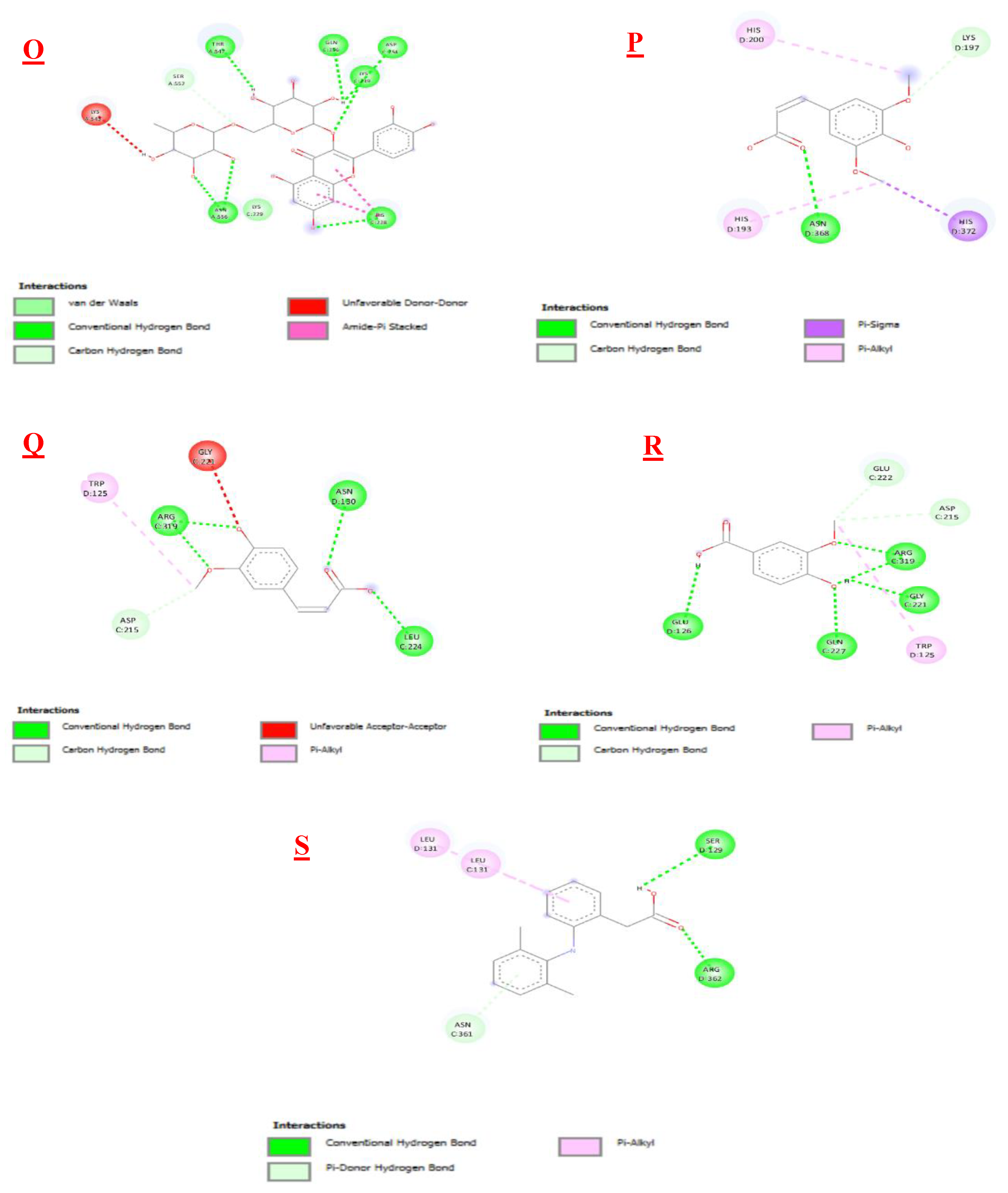
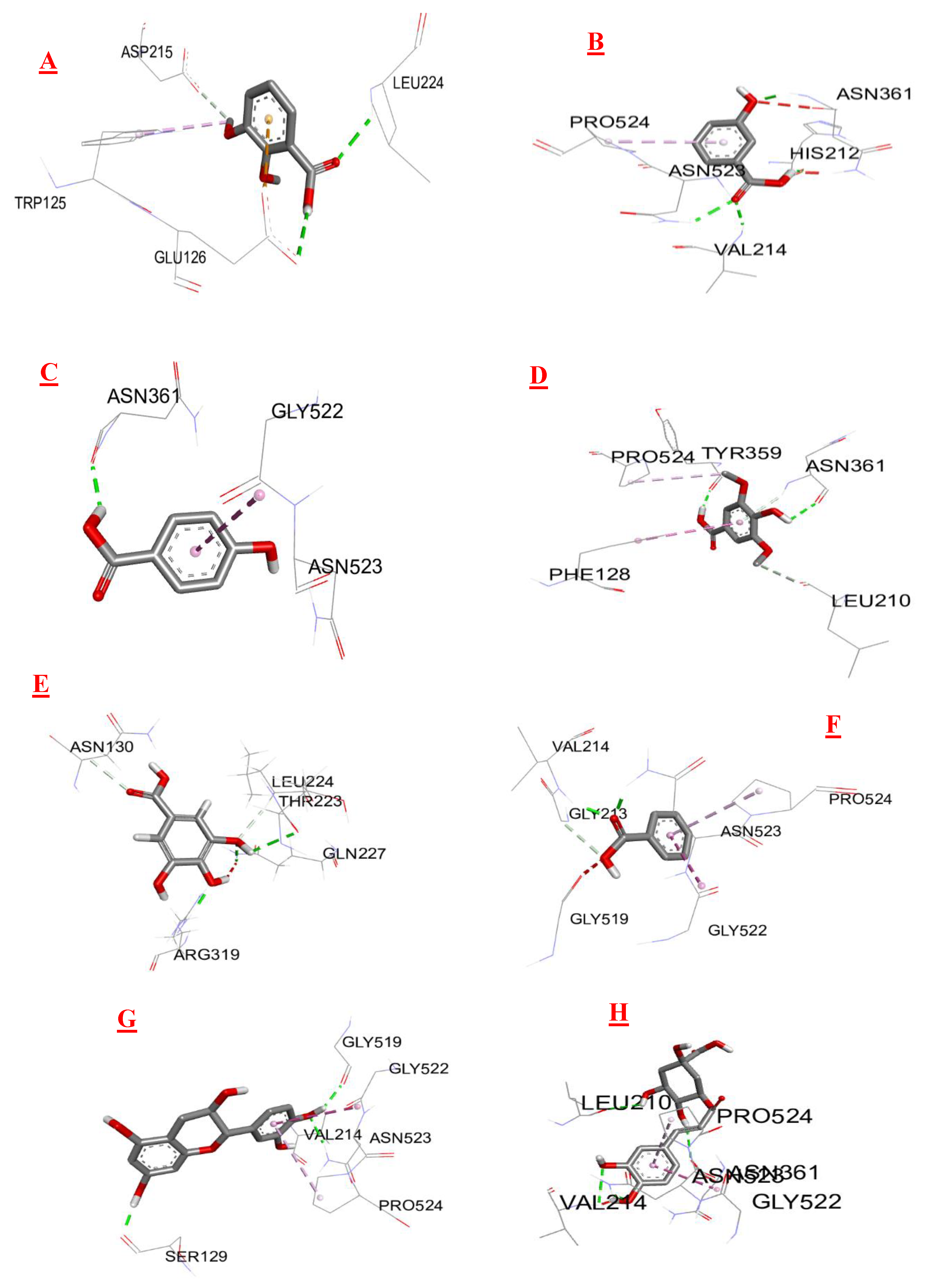
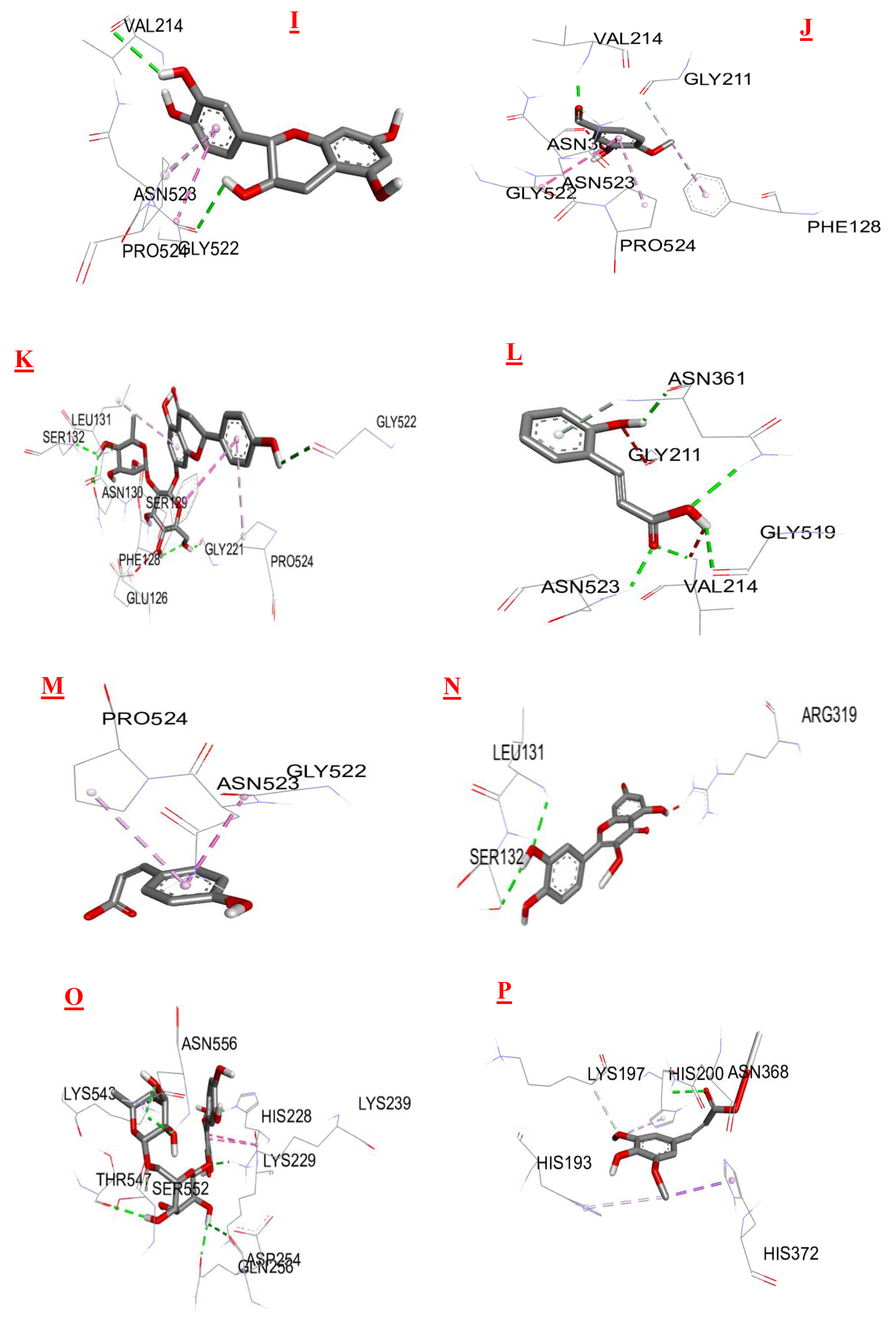
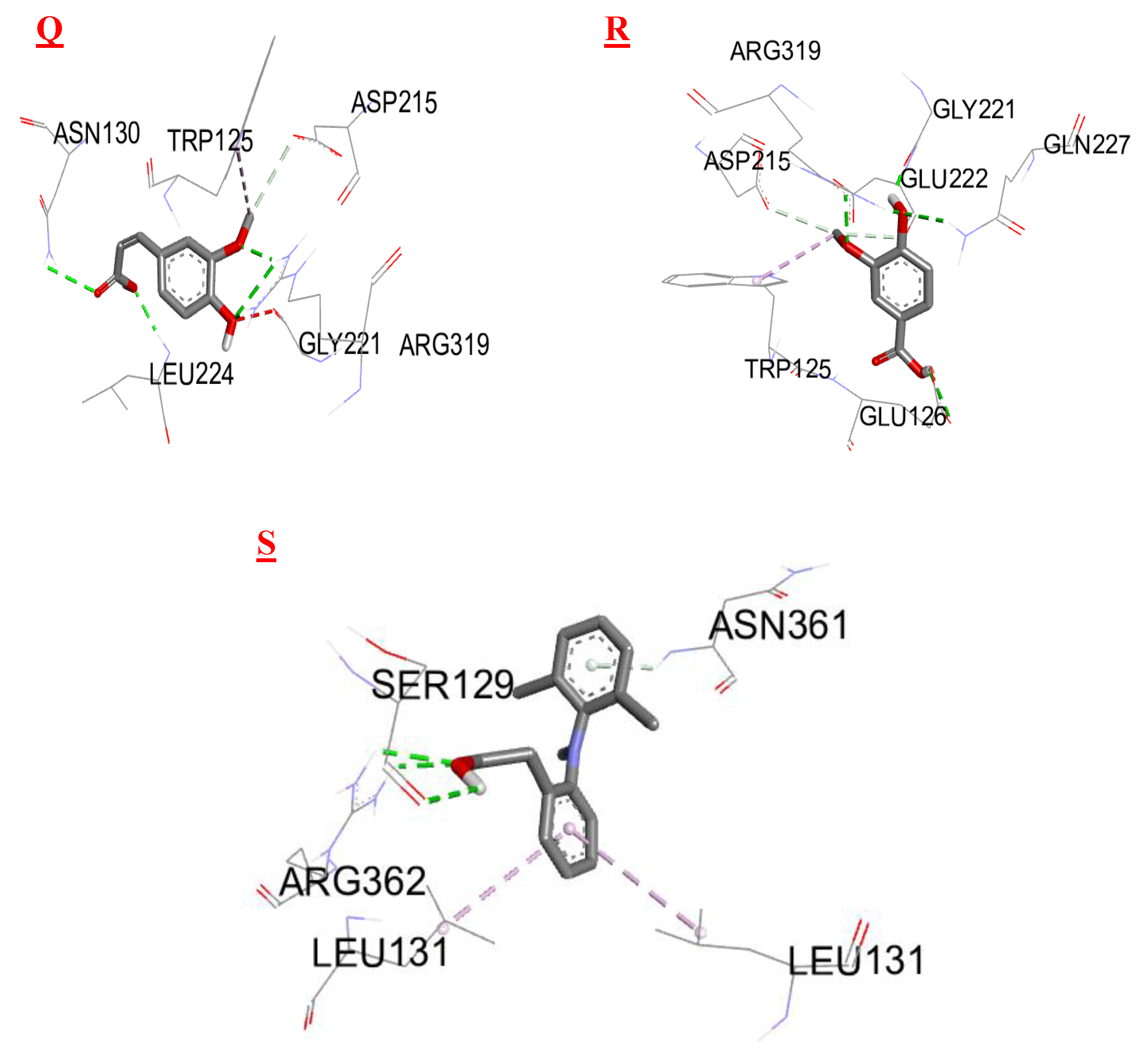
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| The following abbreviations are used in this manuscript: | . |
| MW | Microwave extracts |
| RBC | Red Blood Cell |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
References
- Halvorsen, B.L., et al., Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. The American journal of clinical nutrition, 2006. 84(1): p. 95-135. [CrossRef]
- Halvorsen, B.L., et al., A systematic screening of total antioxidants in dietary plants. The Journal of nutrition, 2002. 132(3): p. 461-471. [CrossRef]
- Lindsay, D.G. and S.B. Astley, European research on the functional effects of dietary antioxidants-EUROFEDA. Molecular Aspects of Medicine, 2002. 1(23): p. 1-38. [CrossRef]
- Chen, L., et al., Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2018. 9(6): p. 7204. [CrossRef]
- Fioranelli, M., et al., Regulation of inflammatory reaction in health and disease. International Journal of Molecular Sciences, 2021. 22(10): p. 5277. [CrossRef]
- Pezone, A., et al., Inflammation and DNA damage: Cause, effect or both. Nature Reviews Rheumatology, 2023. 19(4): p. 200-211. [CrossRef]
- Cronquist, A., The evolution and classification of flowering plants. Bronx: New York Botanical Garden, 1988. 556. [CrossRef]
- Bourlière, F., Mabberley, DJ—The Plant Book. A Portable Dictionary of the Higher Plants. Cambridge University Press, Cambridge, 1987. Revue d'Écologie (La Terre et La Vie), 1988. 43(2): p. 198-199.
- Benjilali, B., et al., Essential oil composition of different Moroccan thyme varieties. 2. Principal component analysis. Sciences des Aliments (France), 1987.
- Guesmi, F., et al., Thymus hirtus Sp. algeriensis Boiss. and Reut. volatile oil enhances Trail/Apo2l induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging (Albany NY), 2021. 13(18): p. 21975. [CrossRef]
- Guesmi, F., et al., Histopathological and biochemical effects of thyme essential oil on H2O2 stress in heart tissues. Heart, Lung and Circulation, 2020. 29(2): p. 308-314.
- Bukvicki, D., et al., Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. Journal of dairy science, 2018. 101(5): p. 3859-3865. [CrossRef]
- Zaïri, A., et al., Phytochemical analysis and assessment of biological properties of essential oils obtained from Thyme and Rosmarinus species. Current pharmaceutical biotechnology, 2020. 21(5): p. 414-424. [CrossRef]
- Fatma, G., B.H.A. Sami, and L. Ahmed, Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomedical and Environmental Sciences, 2017. 30(11): p. 811-824.
- Ouakouak, H., et al., Biological properties of essential oils from Thymus algeriensis Boiss. Plants, 2021. 10(4): p. 786. [CrossRef]
- Zouari, N., et al., Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. et Reut., a North African endemic Species. Lipids in health and disease, 2012. 11(1): p. 1-12. [CrossRef]
- Maissa, B.J. and H. Walid, Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Natural product research, 2015. 29(9): p. 869-873. [CrossRef]
- Guesmi, F., et al., Effects of Thymus hirtus sp. algeriensis Boiss. et Reut.(Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids in Health and Disease, 2014. 13(1): p. 1-14. [CrossRef]
- Ait-Ouazzou, A., et al., Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. Journal of the Science of Food and Agriculture, 2011. 91(14): p. 2643-2651.
- Ahmed, S.B.H., et al., Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia). Natural Product Research, 2011. 25(12): p. 1195-1201. [CrossRef]
- Rezq, S., et al., Thymus algeriensis and Thymus fontanesii exert neuroprotective effect against chronic constriction injury-induced neuropathic pain in rats. Scientific Reports, 2020. 10(1): p. 1-15. [CrossRef]
- Sobeh, M., et al., Thymus algeriensis and Thymus fontanesii: chemical composition, in vivo antiinflammatory, pain killing and antipyretic activities: a comprehensive comparison. Biomolecules, 2020. 10(4): p. 599. [CrossRef]
- Fatma, G., B. Houda, and L. Ahmed, H2O2-Induced Oxidative Stress, AChE inhibition and mediated brain injury attenuated by Thymus algeriensis. 2018.
- Kouache, B., et al., Chemical composition and acaricidal activity of Thymus algeriensis essential oil against Varroa destructor. Natural product communications, 2017. 12(1): p. 1934578X1701200138. [CrossRef]
- Fatma, G., et al., Antioxidant machinery related to decreased MDA generation by Thymus algeriensis essential oil-induced liver and kidney regeneration. Biomedical and Environmental Sciences, 2016. 29(9): p. 639-649.
- Guesmi, F., et al., Prevention of H2O2 induced oxidative damages of rat testis by Thymus algeriensis. Biomedical and Environmental Sciences, 2016. 29(4): p. 275-285. [CrossRef]
- Fatma, G., et al., In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis. Lipids in Health and Disease, 2014. 13(1): p. 1-12. [CrossRef]
- Jayari, A., et al., Nanoencapsulation of thyme essential oils: Formulation, characterization, storage stability, and biological activity. Foods, 2022. 11(13): p. 1858. [CrossRef]
- Nedjimi, B., Trace element quantification in two Algerian thymes (Thymus algeriensis Boiss & Reut. and Thymus capitatus (L.) Hoffm. & Link) using EDXRF spectrometry. Biological Trace Element Research, 2023. 201(1): p. 455-463. [CrossRef]
- El Ouahdani, K., et al., Thymus algeriensis and Artemisia herba-alba essential oils: chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules, 2021. 26(22): p. 6780.
- Rezzoug, M., et al., Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC complementary and alternative medicine, 2019. 19(1): p. 1-10. [CrossRef]
- Mahdi, I., et al., Unraveling the phytochemistry, traditional uses, and biological and pharmacological activities of Thymus algeriensis Boiss. & Reut. Oxidative Medicine and Cellular Longevity, 2022. 2022. [CrossRef]
- Bouafia, M., et al., Ethnobotanical and ethnomedicinal analysis of wild medicinal plants traditionally used in Naâma, southwest Algeria. Vegetos, 2021. 34: p. 654-662.
- Righi, N., et al., Thymus algeriensis Bioss & Reut: Relationship of phenolic compounds composition with in vitro/in vivo antioxidant and antibacterial activity. Food Research International, 2020. 136: p. 109500. [CrossRef]
- Jaouadi, R., et al., Differentiation of phenolic composition among tunisian thymus algeriensis boiss. Et reut.(lamiaceae) populations: correlation to bioactive activities. Antioxidants, 2019. 8(11): p. 515.
- Ziani, B.E., et al., Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Research International, 2019. 116: p. 312-319.
- Zaïri, A., et al., Antioxidant, antimicrobial and the phenolic content of infusion, decoction and methanolic extracts of Thyme and Rosmarinus species. Current Pharmaceutical Biotechnology, 2018. 19(7): p. 590-599. [CrossRef]
- Boutaoui, N., et al., Qualitative and quantitative phytochemical analysis of different extracts from Thymus algeriensis aerial parts. Molecules, 2018. 23(2): p. 463. [CrossRef]
- Herowati, R. and G.P. Widodo, Molecular docking analysis: Interaction studies of natural compounds to anti-inflammatory targets. Quantitative structure-activity relationship, 2017. 63(10.5772). [CrossRef]
- Thamaraiselvi, L., et al., In-silico molecular docking analysis of some plant derived molecules for anti-inflammatory inhibitory activity. Curr. Bot, 2021. 12: p. 22-27. [CrossRef]
- Zhang, Q., et al., Mechanism of anti-inflammatory and antibacterial effects of QingXiaoWuWei decoction based on network pharmacology, molecular docking and in vitro experiments. Frontiers in Pharmacology, 2021. 12: p. 678685. [CrossRef]
- Laloo, D., J.M. Kalita, and S.K. Prasad, Molecular docking studies of plant-derived bioactive compounds: a comprehensive in silico standardization approach. Evidence Based Validation of Traditional Medicines: A comprehensive Approach, 2021: p. 371-404.
- Brown, J., H. Mackey, and D. Riggilo, A novel in vitro assay for anti-inflammatory agents based on stabilization of erythrocytes. Proceedings of the Society for Experimental Biology and Medicine, 1967. 125(3): p. 837-843. [CrossRef]
- Trovato, A., et al., Anti-inflammatory and analgesic activity of Hypericum empetrifolium Willd.(Guttiferae). Il Farmaco, 2001. 56(5-7): p. 455-457. [CrossRef]
- Wang, J.L., et al., The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorganic & medicinal chemistry letters, 2010. 20(23): p. 7159-7163. [CrossRef]
- Trott, O. and A.J. Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of computational chemistry, 2010. 31(2): p. 455-461. [CrossRef]
- Chou, C.T., The Antiinflammatory Effect of an Extract of Tripterygium wilfordii Hook F on Adjuvant-induced Paw Oedema in Rats and Inflammatory Mediators Release. Phytotherapy Research: An International Journal Devoted to Medical and Scientific Research on Plants and Plant Products, 1997. 11(2): p. 152-154. [CrossRef]
- Murugesh, N., S. Vembar, and C. Damodaran, Studies on erythrocyte membrane IV: in vitro haemolytic activity of oleander extract. Toxicology letters, 1981. 8(1-2): p. 33-38. [CrossRef]
- David, S., Studies force new view on biology of flavonoids. Biol Med, 2007. 541: p. 737-87.
- González-Gallego, J., et al., Fruit polyphenols, immunity and inflammation. British journal of nutrition, 2010. 104(S3): p. S15-S27.
- Rosa, M.D. and D. Willoughby, Screens for anti-inflammatory drugs. Journal of Pharmacy and Pharmacology, 1971. 23(4): p. 297-298.
- Salvemini, D., et al., Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. European journal of pharmacology, 1996. 303(3): p. 217-220. [CrossRef]
- Rayego-Mateos, S., et al., Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. International journal of molecular sciences, 2020. 21(11): p. 3798. [CrossRef]
- Morris, C., Carrageenan-induced paw edema in the rat and mouse. Inflammation Protocols. Edited by: Winyard PG, Wiloughby DA, 2003, Totowa, New Jersey: Humana Press Inc.
- Tai, F.W.D. and M.E. McAlindon, Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clinical Medicine, 2021. 21(2): p. 131-134. [CrossRef]
- Srivastava, S., et al., Antiinflammatory, analgesic and antipyretic activities of aerial parts of Costus speciosus Koen. Indian journal of pharmaceutical sciences, 2013. 75(1): p. 83.
- Silva, G.N., et al., Investigation of anti-inflammatory and antinociceptive activities of Lantana trifolia. Journal of ethnopharmacology, 2005. 100(3): p. 254-259. [CrossRef]
- Mahdi, I., et al., Unraveling the phytochemistry, traditional uses, and biological and pharmacological activities of Thymus algeriensis Boiss. & Reut. Oxidative Medicine and Cellular Longevity, 2022. 2022(1): p. 6487430. [CrossRef]
- Kumar, S. and S. Kumar, Molecular docking: a structure-based approach for drug repurposing, in In silico drug design2019, Elsevier. p. 161-189.
- Yung-Chi, C. and W.H. Prusoff, Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical pharmacology, 1973. 22(23): p. 3099-3108.
- Salentin, S., et al., PLIP: fully automated protein–ligand interaction profiler. Nucleic acids research, 2015. 43(W1): p. W443-W447.
- Stojanović, S. and S. Zarić, Hydrogen bonds and hydrophobic interactions of porphyrins in porphyrin-containing proteins. The Open Structural Biology Journal, 2009. 3: p. 34-41. [CrossRef]
- Omoboyowa, D.A., et al., Identification of terpenoids from Abrus precatorius against Parkinson’s disease proteins using in silico approach. Bioinformatics and Biology Insights, 2021. 15: p. 11779322211050757. [CrossRef]
- Mohapatra, S., et al., In silico investigation of black tea components on α-amylase, α-glucosidase and lipase. Journal of Applied Pharmaceutical Science, 2015. 5(12): p. 042-047.
| 0,25 mg/ml | 0,5 mg/ml | 1 mg/ml | |
|---|---|---|---|
| MW 40°C | 77,18±0,17 *** | 84,92±0,30 *** | 89,28±0,20 *** |
| MW 60°C | 78,59±0,18 *** | 87,43±0,22*** | 90,67±0,10 *** |
| MW 80°C | 80,53±0,20 *** | 88,12±0,13 *** | 91,97±0,15 *** |
| MW 100°C | 77,06±3,04 *** | 88,99±0,31 *** | 94,94±0,25 *** |
| MW 120°C | 86,25±0,12 ns | 91,13±0,25 ns | 96,88±0,15 ns |
| MW 100°C, 5min | 76,92±0,20 *** | 85,09±0,22 *** | 88,64±0,18 *** |
| MW 100°C,15min | 88,96± 0,17 ns | 90,95±0,15 ns | 96,85±0,17 ns |
| Diclofenac | 89,91±0,17 | 91,91±0,2 | 96,67±0,15 |
| Mean increase in paw thickness (mm) ± SD | |||
|---|---|---|---|
| Group | 1h | 2h | 3h |
| Control | 1,92 ±0,04 | 1,74 ±0,09 | 1,49 ± 0,07 |
| Diclofenac | 1,48 ±0,04*** | 0,72 ± 0,04*** | 0,24 ± 0,04*** |
| MW 40°C | 1,45 ±0,04*** | 1,05 ±0,04*** | 0,73 ±0,01*** |
| MW 60°C | 1,28 ±0,02*** | 1 ±0,02*** | 0,49 ±0,04*** |
| MW 80°C | 1,41 ±0,04*** | 0,79 ±0,04*** | 0,49 ±0,05*** |
| MW 100°C | 1,4 ±0,03*** | 0,96 ±0,05*** | 0,53 ±0,02*** |
| MW 120°C | 1,36 ±0,02*** | 0,99 ±0,01*** | 0,46 ±0,04*** |
| MW 100°C 5min | 1,44 ±0,01*** | 1,17 ±0,02*** | 0,77 ±0,02*** |
| MW 100°C 15min | 1,37 ±0,04*** | 0,76 ±0,02*** | 0,32 ±0,02*** |
| Biomolecules | Anti-inflammatory activity | |
|---|---|---|
| Protein : COX-2 (3LN0) | ||
| ΔG (Kcal/mol1-) | Ki (µmol) | |
| 2,3- dimethoxybenzoic acid | -6,0 | 39,95 |
| 3-hydroxybenzoic acid | -6,1 | 33,74 |
| 4-hydroxybenzoic acid | -5,7 | 66,28 |
| Syringic acid | -5,9 | 47,29 |
| Gallic acid | -6,4 | 20,33 |
| Benzoic acid | -5,5 | 92,90 |
| Catechin | -9,2 | 0,18 |
| Chlorogenic acid | -8,5 | 0,58 |
| Epicatechin | -8,9 | 0,29 |
| Isovanillin | -5,9 | 47,29 |
| Naringin | -10,3 | 0,028 |
| o-coumaric acid | -6,3 | 24,07 |
| p-coumaric acid | -6,1 | 33,74 |
| Quercetin | -7,9 | 1,61 |
| Rutin | -8,8 | 0,35 |
| sinapinic acid | -5,8 | 55,99 |
| t-ferulic acid | -6,1 | 33,74 |
| Vannilic acid | -6,2 | 28,50 |
| Dichlofenac | -7,1 | 6,23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





