Submitted:
22 March 2025
Posted:
24 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
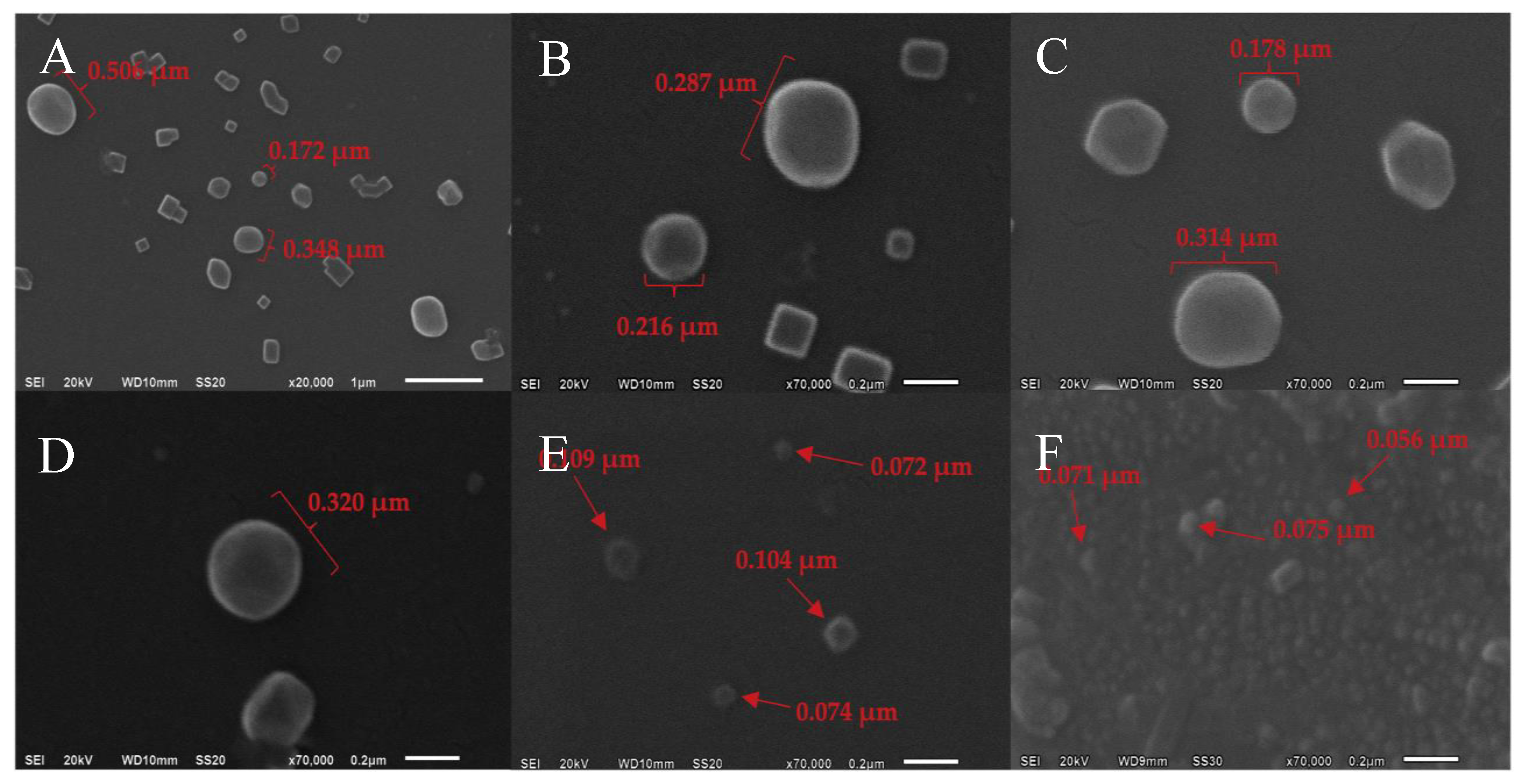
2.1. Morphological Study and Particle Size Measurement
2.2. Encapsulation Efficiency
2.3. Stability of Nanoliposomes by Centrifugation
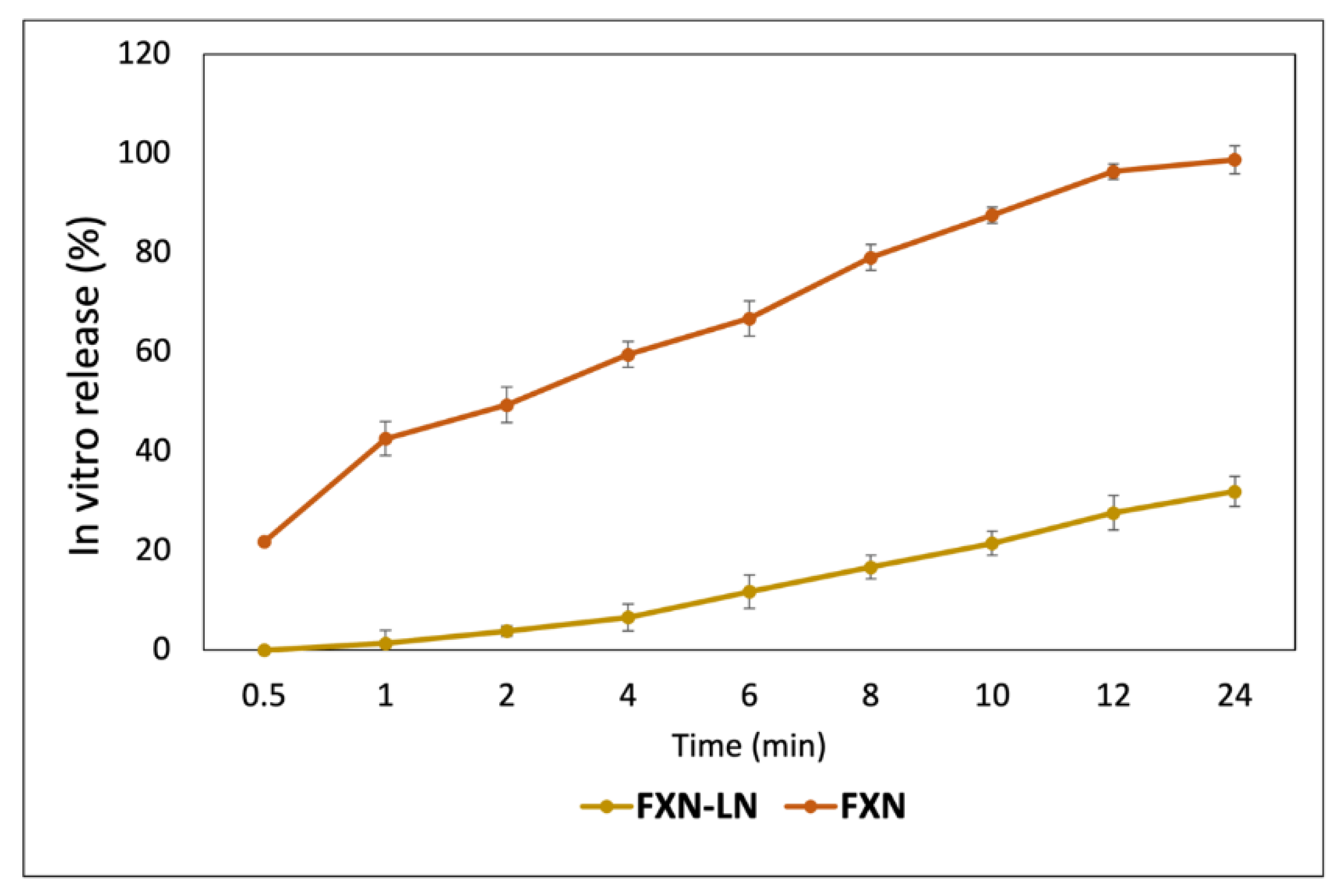
2.4. In Vitro Release
2.5. Impact of Cold Storage Conditions on Antioxidant, Physicochemical, and Rheological Properties of Different Yogurt Formulations
2.5.1. Antioxidant Properties
2.5.2. Physicochemical Properties
2.5.2.1. Electric Conductivity
2.5.2.2. pH
2.5.2.3. Titratable Acidity
2.5.2.4. Syneresis Susceptibility (STS)
2.5.2.5. Water-Holding Capacity (WHC)
2.5.2.6. Viscosity
2.5.2.7. Textural Properties (Firmness and Consistency)
2.5.2.8. Rheological Properties
2.5.2.9. Sensorial Analysis
3. Materials and Methods
3.1. Chemical Reagents
3.2. Biological Material and Ethical Considerations
3.3. Synthesis of Fucoxanthin-Loaded Nanoliposomes
3.4. Morphological Study
3.5. Encapsulation Efficiency
3.6. Centrifugal Stability Measurement
3.7. In Vitro Release
3.8. Preparation of Functional Yogurt
3.9. Effect of Cold Storage Conditions on the Antioxidant, Physicochemical, and Rheological Properties of Yogurt Enriched with FXN-LN
3.9.1. Antioxidant Properties
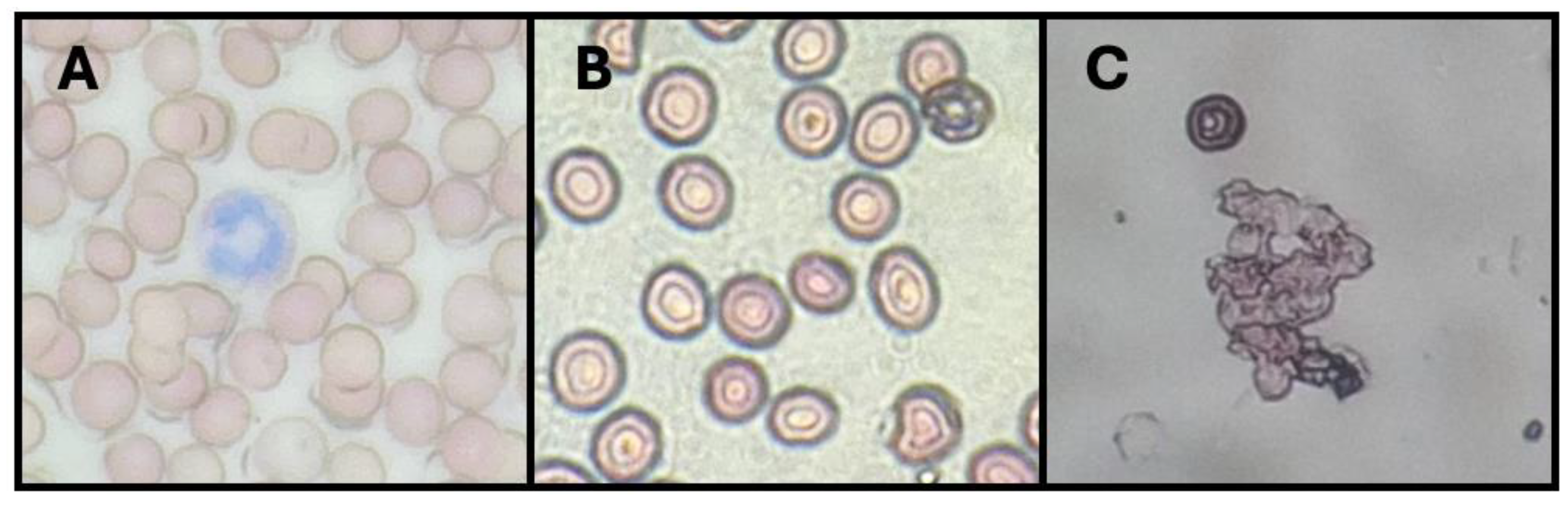
3.9.2. Erythroprotective Potential
3.9.3. Physicochemical Properties
3.9.3.1. Electrical Conductivity
3.9.3.2. pH
3.9.3.3. Titratable Acidity
3.9.3.4. Syneresis Susceptibility (STS)
3.9.3.5. Water Holding Capacity (WHC)
3.9.3.6. Texture
3.9.4. Rheological Analysis
3.9.5. Sensory Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashwan, A. K., Osman, A. I., & Chen, W. (2023). Natural nutraceuticals for enhancing yogurt properties: A review. Environmental Chemistry Letters, 21, 1907–1931. [CrossRef]
- Alkobeisi, F.; Varidi, M. J., Varidi, M., & Nooshkam, M. (2022). Quinoa flour as a skim milk powder replacer in concentrated yogurts: Effect on their physicochemical, technological, and sensory properties. Food Science and Nutrition, 10, 1113–1125. [CrossRef]
- Almutairi, B.; Turner, M. S., Fletcher, M. T., & Sultanbawa, Y. (2021). The impact of commercial prebiotics on the growth, survival and nisin production by Lactococcuslactis 537 in milk. LWT - Food Science and Technology, 137, Article 110356. [CrossRef]
- González-Vega, R.I.; Robles-García, M.Á.; Mendoza- Urizabel, L.Y.; Cárdenas-Enríquez, K.N.; Ruiz-Cruz, S.; Gutiérrez- Lomelí,, M.; Iturralde-García, R.D.; Avila-Novoa, M.G.; Villalpando- Vargas, F.V.; Del-Toro-Sánchez, C.L. Impact of the ABO and RhD Blood Groups on the Evaluation of the Erythroprotective Potential of Fucoxanthin, β-Carotene, Gallic Acid, Quercetin and Ascorbic Acid as Therapeutic Agents against Oxidative Stress. Antioxidants 2023, 12,2092. [CrossRef]
- Peng, J.; Jian-Ping, Y.; Chou-Fei, W.; Jiang-Hai, W. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs. 2011, 9:1806-1828. doi:10.3390/md9101806.
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181.
- Rostamabadi, H.; Reza Falsafi, S.; Mahdi Jafari, S. 2019. Nanoencapsulation of carotenoids within lipid-based nanocarriers. Journal of controlled release. 298: 38-67. [CrossRef]
- Sun, Y.; Chi, J.; Ye, X.; Wang, S.; Liang, J.; Yue, P.; Xiao, H.; Gao, X. (2021). Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. LWT, 139, 110554. [CrossRef]
- Bhosale, S.; Fulpagare, Y. G., & Desale, R. J. (2019). Nanoliposomes: Applications in food and dairy industry. 6(11), 79-84. DOI:. [CrossRef]
- Cheng, X.; Zang, M.; Wang, S.; Zhao, X.; Zhai, G.; Wang, L.; Li, X.; Zhao, Y.; Yue, Y. Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering 2022, 9, 818. [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; et al. (2017). Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT-Food Science & Technology, 84, 323–330. [CrossRef]
- da, S.i.l.v.a. D. F., Junior, N. N. T., Gomes, R. G., dos Santos Pozza, M. S., Britten, M., & Matumoto-Pintro, P. T. (2017). Physical, microbiological and rheological properties of probiotic yogurt supplemented with grape extract. Journal of Food Science & Technology, 54(6), 1608–1615. [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. (2020). Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT. 129:109571. [CrossRef]
- Megrous, S.; Al-Dalali, S.; Yang, Z. 2024. Physicochemical and functional properties of yoghurt supplemented with bioactive low-molecular-weight casein hydrolysates. International Dairy Journal. 155, 105956. [CrossRef]
- Lee, W. J., & Lucey, J. A. (2010). Formation and physical properties of yogurt. Asian-Australasian Journal of Animal Sciences, 23, 1127–1136. [CrossRef]
- Oh, N. S., Lee, J. Y., Joung, J. Y., Kim, K. S., Shin, Y. K., Lee, K. W., et al. (2016). Microbiological characterization and functionality of set-type yogurt fermented with potential prebiotic substrates Cudrania tricuspidata and Morus alba L. leaf extracts. Journal of Dairy Science, 99(8), 6014–6025. [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. (2014). Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. Journal of Dairy Science, 97(5), 2578–2590. [CrossRef]
- Liu, D.; Lv, X.X. (2019). Effect of blueberry flower pulp on sensory, physicochemical properties, lactic acid bacteria, and antioxidant activity of set-type yogurt during refrigeration. Journal of Food Processing and Preservation, 43(1), e13856. [CrossRef]
- Liu, X. T., Zhang, H., Wang, F., Luo, J., Guo, H. Y., & Ren, F. Z. (2014). Rheological and structural properties of differently acidified and renneted milk gels. Journal of Dairy Science, 97, 3292–3299. [CrossRef]
- Brodziak, A.; Krol, J.; Barłowska, J.; Teter, A.; Florek, M. (2020). Changes in the physicochemical parameters of yoghurts with added whey protein in relation to the starter bacteria strains and storage time. Animals, 10(8), 1350. [CrossRef]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019;6:191184. [CrossRef]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017;227:9–15. [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core-Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019;67:5113–5121. [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov. Food Sci. Emerg. 2014, 26, 366–374. [CrossRef]
- Pan, L. H., Liu, F., Luo, S. Z., & Luo, J. P. (2019). Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: Structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT-Food Science and Technology, 115, 108479. [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Venegas, J.B.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules. 2018;23:2601. [CrossRef]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer andapplication in yogurt as evaluated by consumer sensory profile. LWT-Food Sci. Technol. 2015, 62, 431–437. [CrossRef]
- Mozafari, M.R. (2010). Nanoliposomes: Preparation and Analysis. In: Weissig, V. (eds) Liposomes. Methods in Molecular Biology, vol 605. Humana Press. [CrossRef]
- Mozafari MR (ed) (2006) Nanocarrier technologies: frontiers of nanotherapy. Springer, The Netherlands. [CrossRef]
- Thien Trung Le, Thi Thanh Que Phan, John Van Camp, Koen Dewettinck. Milk and Dairy Polar Lipids: Occurrence, Purification, and Nutritional and Technological Properties. 2015, 91-143. [CrossRef]
- Aguilar-Pérez, K.M.; Medina, D.I.; Narayanan, J.; Parra-Saldívar, R.; Iqbal, H.M.N. Synthesis and Nano-Sized Characterization of Bioactive Oregano Essential Oil Molecule-Loaded Small Unilamellar Nanoliposomes with Antifungal Potentialities. Molecules 2021, 26, 2880. [CrossRef]
- Matos,J; Afonso,C; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [CrossRef]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthin by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [CrossRef]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Bilia, A.R. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol. Int. J. Pharm. 2019, 561, 161–170. [CrossRef]
- Hamad, I.; Harb, A.A.; Bustanji, Y. Liposome-Based Drug Delivery Systems in Cancer Research: An Analysis of Global Landscape Efforts and Achievements. Pharmaceutics. 2024; 16(3):400. [CrossRef]
- Corrêa, R.C. G., Barros, L., Fernandes, Â., Sokovic, M., Bracht, A., Peralta, R. M., & Ferreira, I. C. F. R. (2018). A natural food ingredient based on ergosterol: Optimization of the extraction from Agaricus blazei, evaluation of bioactive properties and incorporation in yogurts. Food & Function, 9(3), 1465-1474. [CrossRef]
- Bourne, M.C. 2002. Food Texture and Viscosity: Concept and Measurement. 2nd ed. Academic Press, San Diego, CA.
- Lee, J.-W.; Lucey, J.A. 2006. Impact of Gelation Conditions and Structural Breakdown on the Physical and Sensory Properties of Stirred Yogurts. J. Dairy Sci. 89:2374–2385. [CrossRef]
- Jeong, C. H., Ryu, H., Zhang, T., Lee, C. H., Seo, H. G., & Han, S. G. (2018). Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Science and Biotechnology, 27(5), 1419-1427. [CrossRef]
- Zahoor, I.; Allai, F.M. (2020). Food Antioxidants: Functional Aspects and Preservation During Food Processing. In: Ahmad, S., Al-Shabib, N. (eds) Functional Food Products and Sustainable Health. Springer, Singapore. [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.P.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving Antioxidant and Antimicrobial Properties of Curcumin by Means of Encapsulation in Gelatin through Electrohydrodynamic Atomization. Food Hydrocoll. 2017, 70, 313–320.
- Kristl, J.; K Teskac, C. Caddeo, Z. Abramovic, and M. Sentjurc. 2009. Improvements of cellular stress response on resveratrol in liposomes. European Journal of Pharmaceutics and Biopharmaceutics 73 (2):253–9. [CrossRef]
- Abd El-Emam, M.M.; Mostafa, M.; Farag, A.A.; Youssef, H.S.; El-Demerdash, A.S.; Bayoumi, H.; Gebba, M.A.; El-Halawani, S.M.; Saleh, A.M.; Badr, A.M.; et al. The Potential Effects of Quercetin-Loaded Nanoliposomes on Amoxicillin/Clavulanate-Induced Hepatic Damage: Targeting the SIRT1/Nrf2/NF-κB Signaling Pathway and Microbiota Modulation. Antioxidants 2023, 12, 1487. [CrossRef]
- Alimentarius, Codex (2010). Codex standard for fermented milks (CODEX STAN 243-2003), Adopted in 2003, Revision 2008. Rome: FAO.
- Wu, Y.; Wang, K.; Liu, Q.; Liu, X.; Mou, B.; Lai, O.-M.; Tan, C.-P.; Cheong, L.-Z. (2022). Selective antibacterial activities and storage stability of curcumin-loaded nanoliposomes prepared from bovine milk phospholipid and cholesterol. Food Chemistry, 367, 130700. [CrossRef]
- Hasan, M.; Elkhoury, K.; Kahn, C.J. F., Arab-Tehrany, E., & Linder, M. (2019). Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules, 24(10), 2023. [CrossRef]
- Linder, M.; Arab-Tehrany, E. (2018). The Positive Role of Curcumin-Loaded Salmon Nanoliposomes on the Culture of Primary Cortical Neurons. Marine Drugs, 16(7), 218. [CrossRef]
- Yakoubi, S.; Kobayashi, I.; Uemura, K.; Nakajima, M.; Isoda, H.; Ksouri, R.; Saidani-Tounsi, M.; Neves, M.A. Essential-Oil-Loaded Nanoemulsion Lipidic-Phase Optimization and Modeling by Response Surface Methodology (RSM): Enhancement of Their Antimicrobial Potential and Bioavailability in Nanoscale Food Delivery System. Foods2021, 10, 3149. [CrossRef]
- Chen, Y.; He, N.; Yang, T.; Cai, S.; Zhang, Y.; Lin, J.; Huang, M.; Chen, W.; Zhang, Y.; Hong, Z. Fucoxanthin Loaded in Palm Stearin- and Cholesterol-Based Solid Lipid Nanoparticle-Microcapsules, with Improved Stability and Bioavailability In Vivo. Mar. Drugs 2022, 20, 237. [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.-H. Recent Advances on Nanoparticle Based Strategies for Improving Carotenoid Stability and Biological Activity. Antioxidants 2021, 10, 713. [CrossRef]
- Verardi, A.; Sangiorgio, P.; Lopresto, C.G.; Casella, P.; Errico, S. Enhancing Carotenoids’ Efficacy by Using Chitosan-Based Delivery Systems. Nutraceuticals 2023, 3, 451-480. [CrossRef]
- Núñez de González, M.T.; Attaie, R.; Woldesenbet, S.; Mora-Gutierrez, A.; Jung, Y. Fucoxanthin as a Biofunctional Compound in Goat Milk Yogurt: Stability and Physicochemical Effects. Fermentation 2023, 9, 273. [CrossRef]
- Mok, I.K.; Lee, J.K.; Kim, J.H.; Pan, C.H.; Kim, S.M. 2018. Fucoxanthin bioavailability from fucoxanthin-fortified milk: In vivo and in vitro study. Food Chem. 258, 79–86. [CrossRef]
- Mok, I.K.; Yoon, J.R.; Pan, C.H.; Kim, S.M. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. J. Agric. Food Chem. 2016, 64, 6196–6202. [CrossRef]
- Gürbüz, Z.; Erkaya-Kotan, T.; Şengül, M. 2021. Evaluation of physicochemical, microbiological, texture and microstructure characteristics of set-style yoghurt supplemented with quince seed mucilage powder as a novel natural stabiliser. International Dairy Journal 114:104938. [CrossRef]
- Gyawali, R.; Ibrahim, S.A. 2016. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends in Food Science & Technology 56(S1):61–76. [CrossRef]
- Mehra, R.; Kumar, H.; Rafiq, S.; Kumar, N.; Buttar, H.S.; Leicht, K.; Okpala, C.O.R.; Korzeniowska, M. 2022. Enhancing yogurt products’ ingredients: preservation strategies, processing conditions, analytical detection methods, and therapeutic delivery—an overview. PeerJ 10:e14177. [CrossRef]
- Aportela-Palacios, A.; Sosa-Morales, M.; Vélez-Ruiz, J. (2005). Rheological and physicochemical behavior of fortified yogurt, with fiber and calcium. Journal of Texture Studies, 36(3), 333–349. [CrossRef]
- Mahomud et al., (2016)Role of whey protein-casein complexes on yoghurt texture. Reviews in Agricultural Science, 5:1- 12, 2017. [CrossRef]
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage Stability of Texture and Sensory Properties of Yogurt with the Addition of Polymerized Whey Proteins. Foods 2019, 8, 548. [CrossRef]
- Gharibzahedi, S.M.T.; Chronakis, I.S. 2018. Crosslinking of milk proteins by microbial transglutaminase: Utilization in functional yogurt products. Food Chem. 245, 620–632. [CrossRef]
- Vital, A.C.P.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, functional and rheological properties of low-fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT-Food Sci. Technol. 2015, 64, 1028–1035. [CrossRef]
- Guggisberg, D.; Cuthbert-Steven, J.; Piccinali, P.; Bütikofer, U.; Eberhard, P. 2009. Rheological, microstructural and sensory characterization of low-fat and whole milk set yoghurt as influenced by inulin addition. International Dairy. Volume 19 (2):107-115. [CrossRef]
- Shaker, R.; Jumah, R.; Abu-Jdayil, B. (2000). Rheological properties of plain yogurt during coagulation process: Impact of fat content and preheat treatment of milk. Journal of Food Engineering, 44(3), 175–180. [CrossRef]
- Shokery ES, El-Ziney MG, Yossef AH, Mashaly RI (2017). Effect of Green Tea and Moringa Leave Extracts Fortification on the Physicochemical, Rheological, Sensory and Antioxidant Properties of Set-Type Yoghurt. J Adv Dairy Res 5: 179. [CrossRef]
- Najgebauer-Lejko, D.; Żmudziński, D.; Ptaszek, A.; Socha, R. (2013). Textural properties of yogurts with green tea and Pu-erh tea additive. International Journal of Food Science & Technology. 49 (4). [CrossRef]
- de Campo, C.; Assis R. Q., da Silva, M. M., Costa, T. M. H., Paese, K., Guterres, S. S., et al. (2019). Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physico- chemical properties, carotenoid stability and sensory analysis. Food Chemistry, 301, 125230. [CrossRef]
- Athar, I.H.; Shah, M.A.; Khan, U.N. 2000. Effect of various stabilizers on whey separation (syneresis)and quality of yogurt. Pakisatn Journal of Biological Science, 3:1336-1338. [CrossRef]
- Raftani Amiri, Z.; Rezaei Erami, S.; Jafari, S. M., & Ahmadian, S. (2024). Physicochemical properties of yogurt enriched with nanoliposomes containing bitter melon extract. LWT - Food Science and Technology, 198, 116091. [CrossRef]
- Asaduzzaman, M.; Mahomud, M. S., & Haque, M. E. (2021). Heat-induced interaction of milk proteins: Impact on yoghurt structure. International Journal of Food Science, 2021, Article 5569917. [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S. M., & Katouzian, I. (2018). Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. Journal of Agricultural and Food Chemistry, 66(35). [CrossRef]
- Borjizadeh, Z.; Ahari, H.; Özdal, T.; Khosravi-Darani, K.; Mohammadi Nafchi, A. (2024). Saffron nanoencapsulation (Crocus sativus) and its role in food science: Types and techniques. ACS Food Science & Technology, 4(6), 1310–1333. [CrossRef]
- Radi, U.M. A., & Doosh, K. S. (2023). Study of the physiochemical and sensory properties of therapeutic low-cholesterol yoghurt fortified with zinc nanoparticles. IOP Conference Series: Earth and Environmental Science, 1262(6), 062029. [CrossRef]
- Schram, L.B.; Nielsen, C.J.; Porsgaard, T.; Nielsen, N.S.; Holm, R.; Mu, H. Food matrices affect the bioavailability of (n-3) polyunsaturated fatty acids in a single meal study in humans. Food Res. Int. 2007, 40, 1062–1068. [CrossRef]
- Ghorbanzade, T.; Mahdi Jafari, M.S.; Akhavan, S.; Hadavi, R. 2017. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chemistry 216 (2017) 146–152. [CrossRef]
- Domoto, N.; Koenen, M.E.; Havenaar, R.; Mikajiri, A.; Chu, B.S. 2013. The bioaccessibility of eicosapentaenoic acid was higher from phospholipid food products than from mono and triacylglycerol food products in a dynamic gastrointestinal model. Food Sci. Nutr. 1, 409–415. [CrossRef]
- Sahin, O. I., Dundar, A. N., Ozdemir, S., Uzuner, K., Parlak, M. E., Dagdelen, A. F., & Saricaoglu, F. T. (2022). Nanophytosomes as a protection system to improve the gastrointestinal stability and bioavailability of phycocyanin. Food Bioscience, 50(Part A), 102052. [CrossRef]
- Mahfoudhi, N.; Ksouri, R.; Hamdi, S. (2016). Nanoemulsions as potential delivery systems for bioactive compounds in food systems: Preparation, characterization, and applications in food industry. En Nanotechnology in the Agri-Food Industry. 365-403. [CrossRef]
- McClements, D.J. (2018). Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Advances in Colloid and Interface Science, 253, 1-22. [CrossRef]
- Li, S.; Lo, C.-Y.; Pan, M.-H.; Lai, C.-S.; Ho, C.-T. (2013). Black tea: Chemical analysis and stability. Food & Function, 4(1), 10-18. [CrossRef]
- ISO-IEC 17,025; Mexican Standard PROY-NMX-EC17,025 prepared by the Technical Committee for National Standardization of the Quality System INMC/CTNN 9. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/obp/ui/#iso:std: iso-iec:17025:ed-3:v2:es (accessed on 28 October 2022).
- ISO 15,189; Medical laboratories. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/obp/ui/#iso:std:iso: 15189:ed-4:v1:en (accessed on 28 October 2022).
- ISO/TC 212; Clinical laboratory testing and in vitro diagnostic test systems. ISO: Geneva, Switzerland, 2022. Available online: https://www.iso.org/committee/54916.html (accessed on 28 October 2022).
- ISO 9001; Quality Management. ISO: Geneva, Switzerland, 2015. Available online: https://www.iso.org/obp/ui/#iso:std:iso: 9001:ed-5:v1:en (accessed on 28 October 2022).
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro- Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective potential of phycobiliproteins extracted from Porphyridium cruentum.Metabolites. 2023, 13,366. [CrossRef]
- Ruiz-Cruz, S.; González-Vega, R.I.; Robles-Zepeda, R.E.; Reyes-Díaz, A.; López-Elías, J.A.; Alvarez-Ainza, M.L.; Cinco-Moroyoqui, F.J.; Moreno-Corral, R.A.; Wong-Corral, F.J.; Borboa-Flores, J.; et al. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula Incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites 2022, 12, 1203. [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.C.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [CrossRef]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract T and its validation using a computational simulation approach. Inform. Med. Unlocked 2019, 14, 6–14. [CrossRef]
- AOCS (2007). Official methods and recommended practices of the American oil chemist’s society (6th ed.). Champaign, IL: AOCS Press.
- AOAC (2000). The official methods of analysis (17th ed.). Maryland, USA: Association of Official Analytical Chemists.
- Achanta, K.; Aryana, K. J., & Boeneke, C. A. (2007). Fat free plain set yogurts fortified with various minerals. LWT – Food Science and Technology, 40(3), 424–429. [CrossRef]
- Lawless, H. T., & Heymann, H. (2010). Sensory evaluation of food: Principles and practices. Springer Science & Business Media.
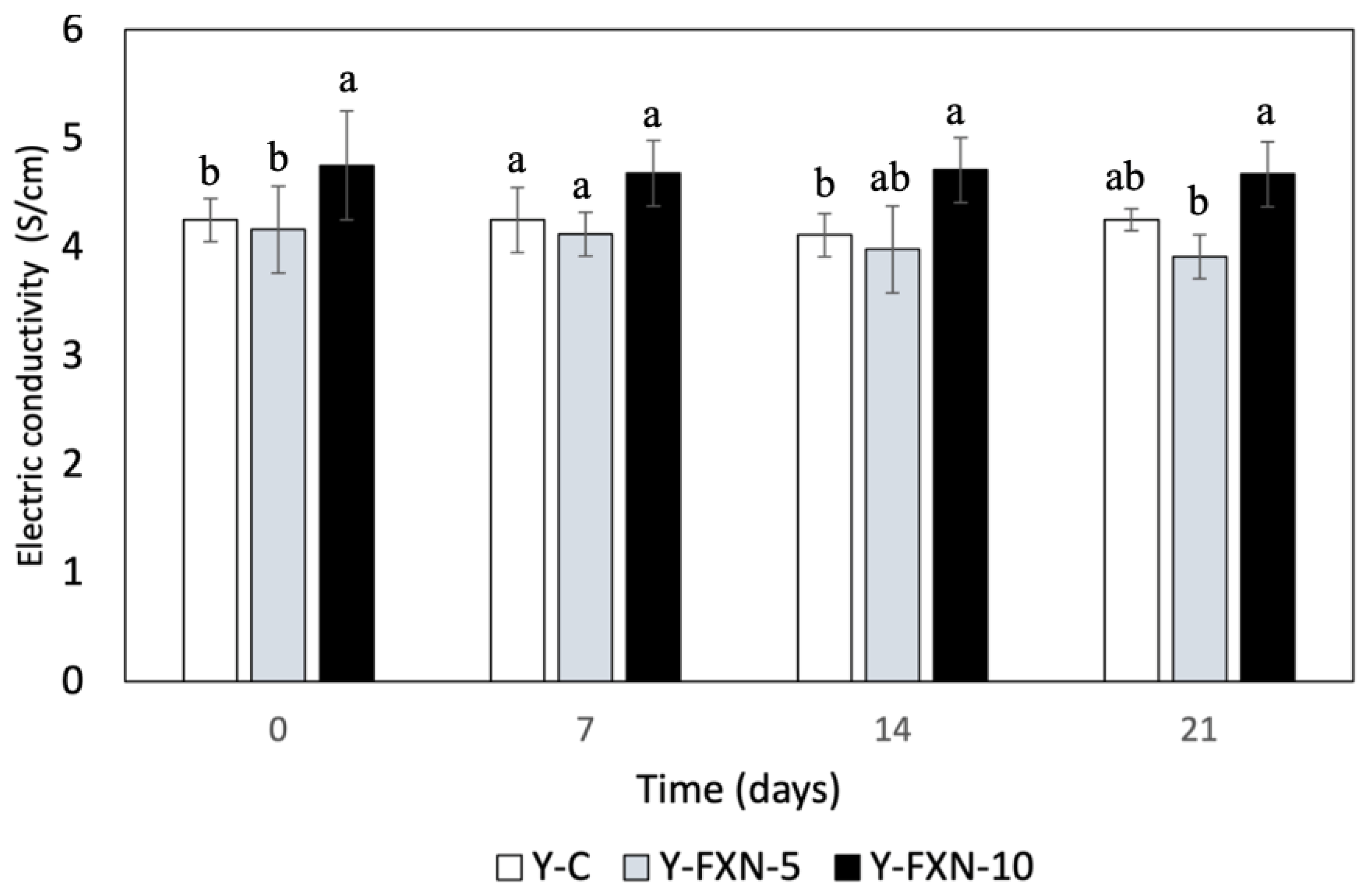
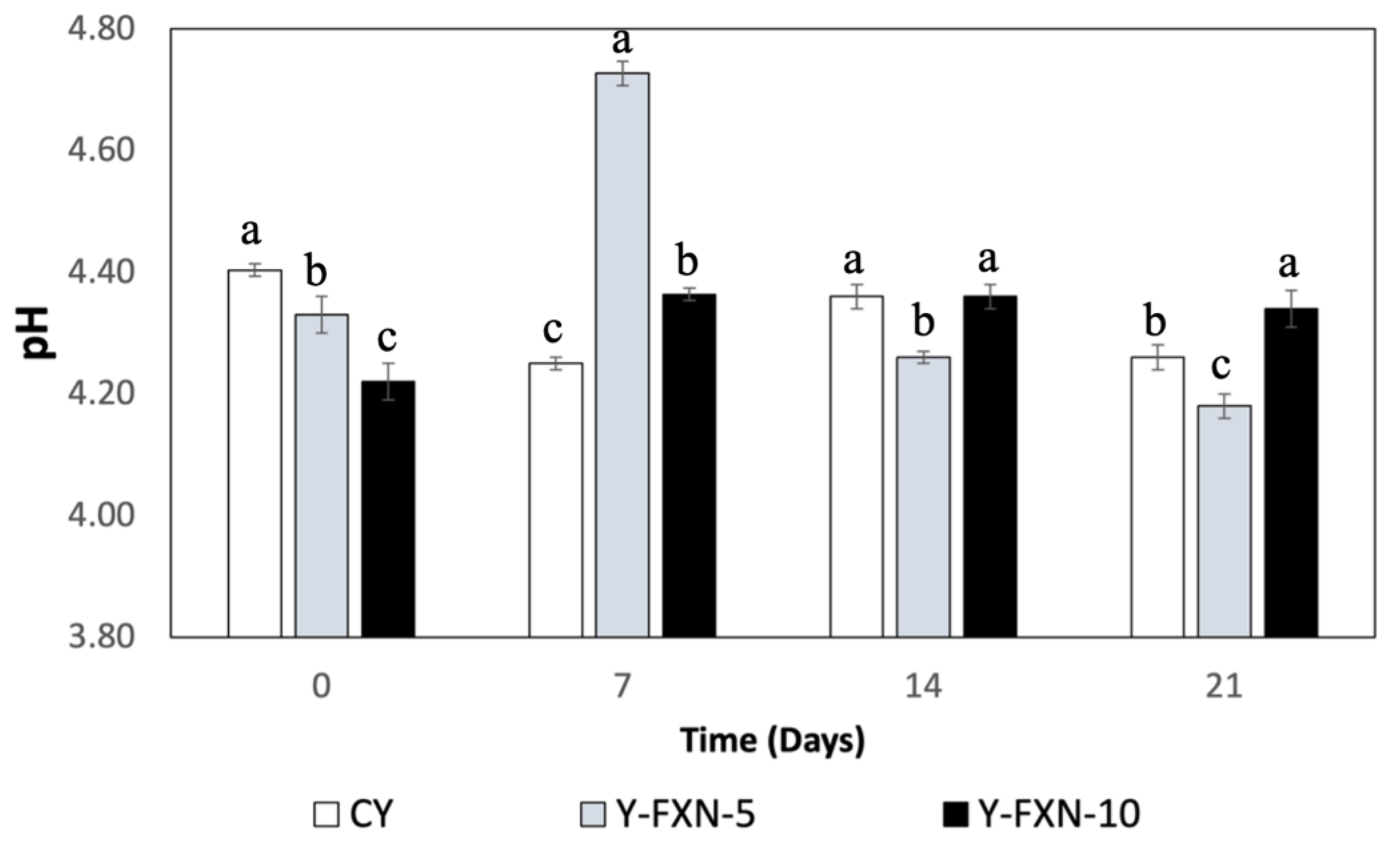
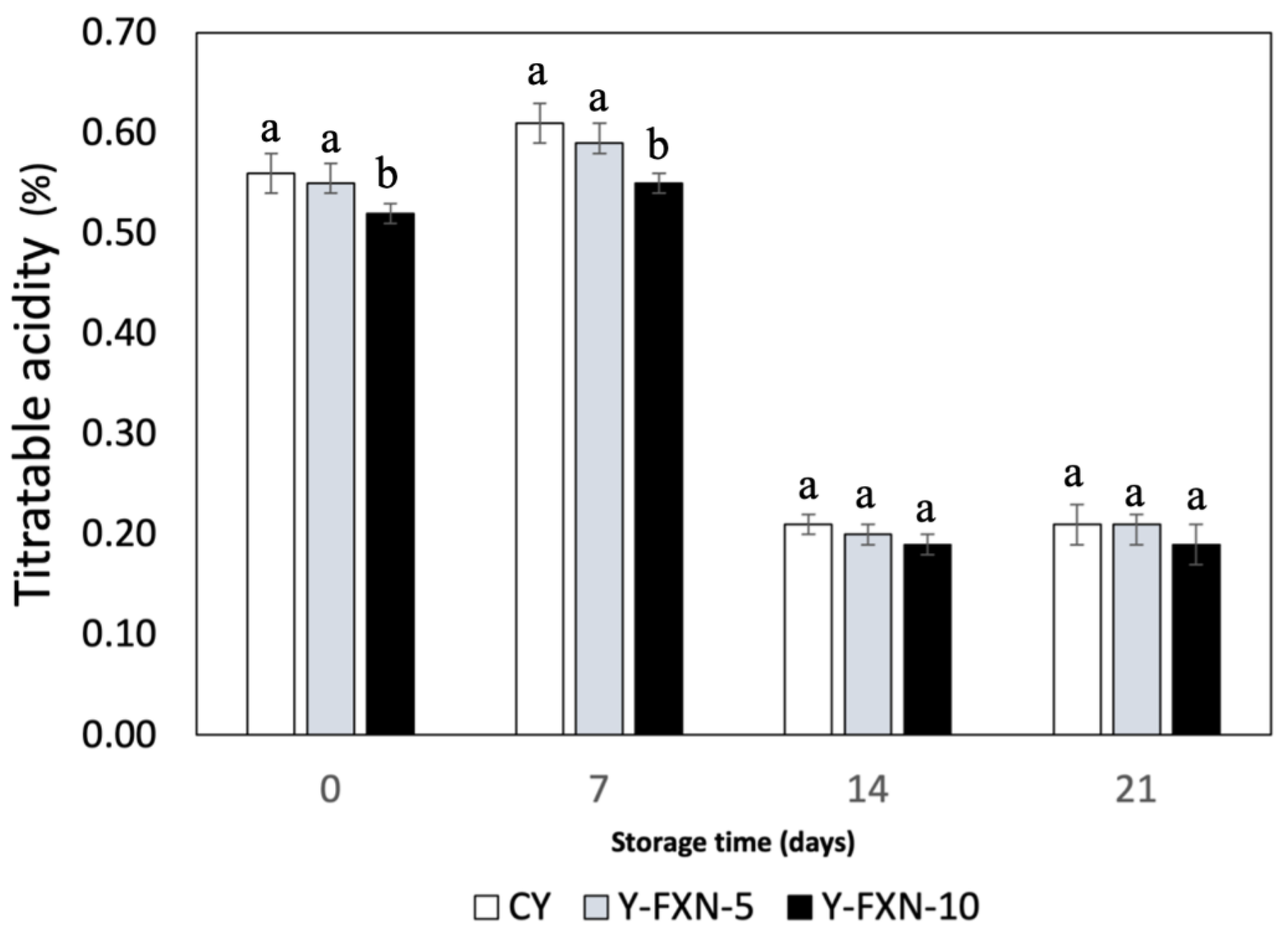
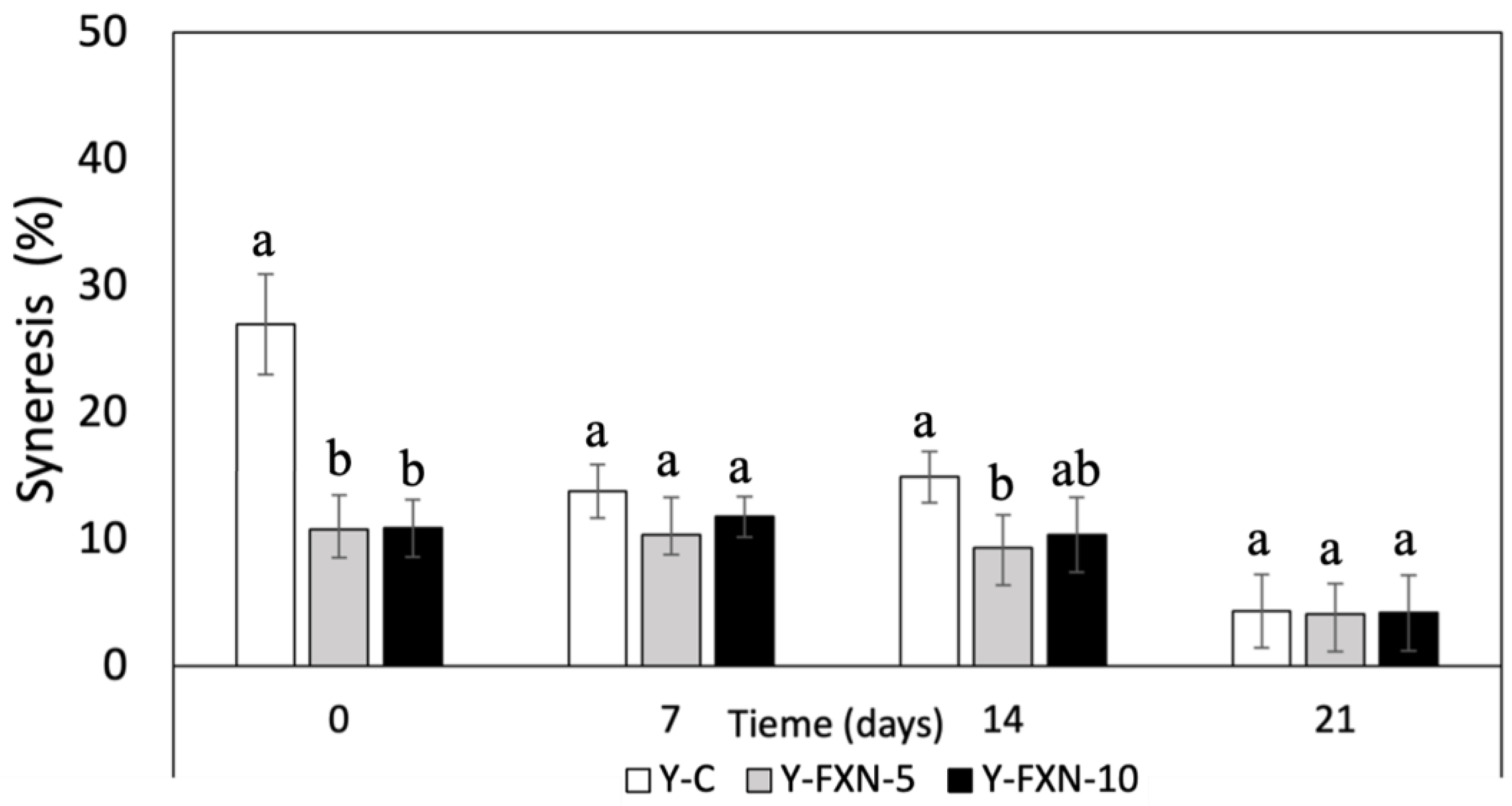
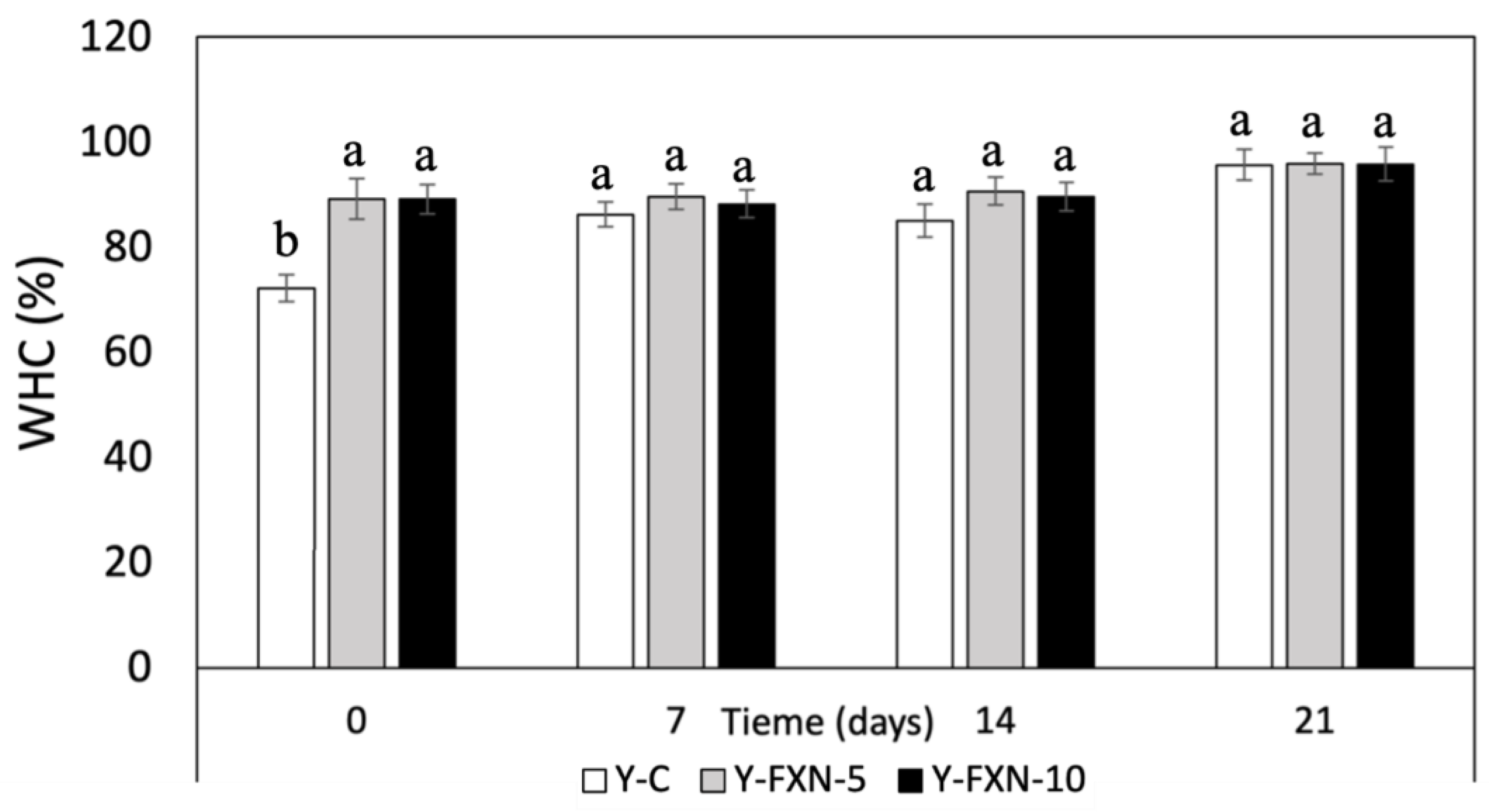
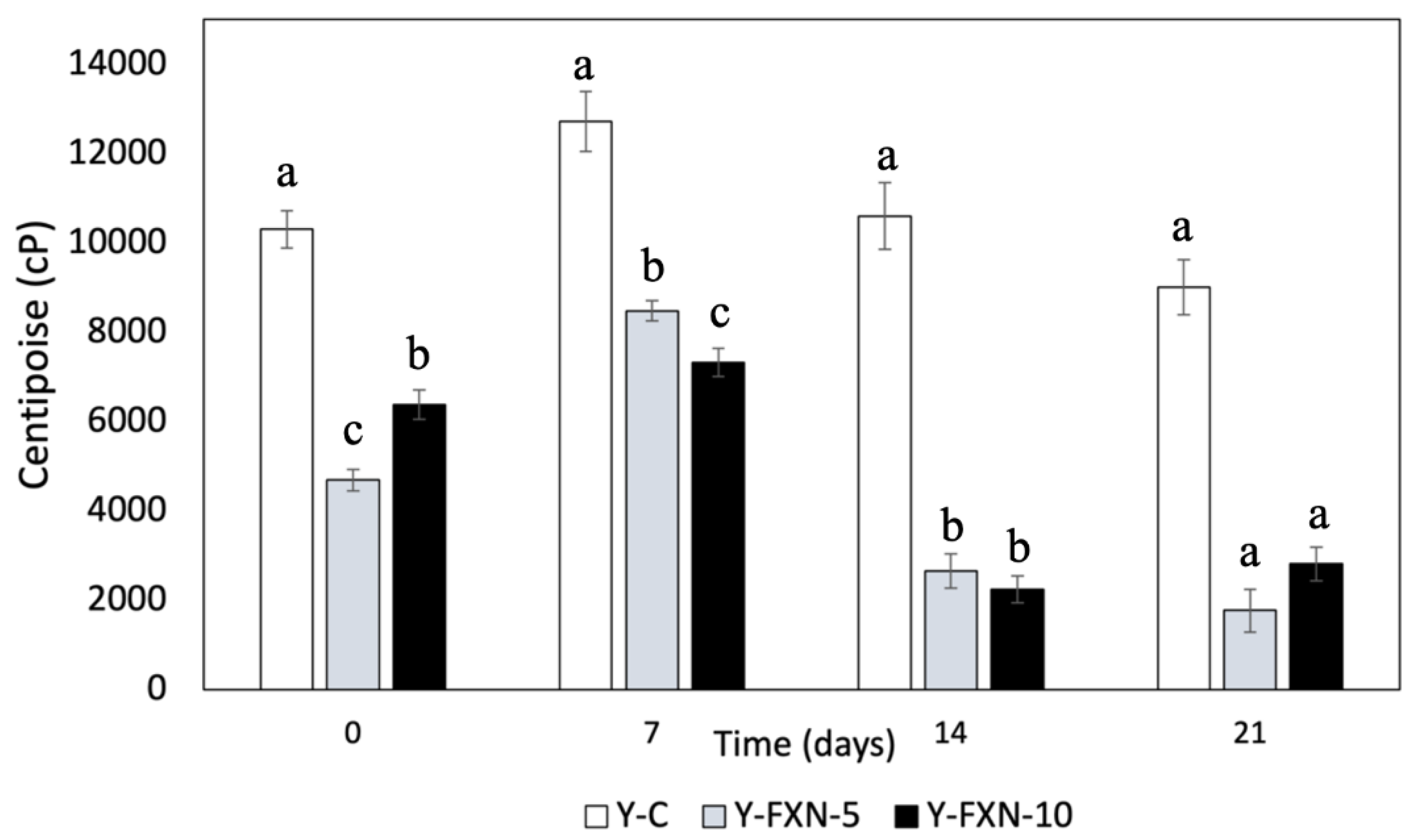
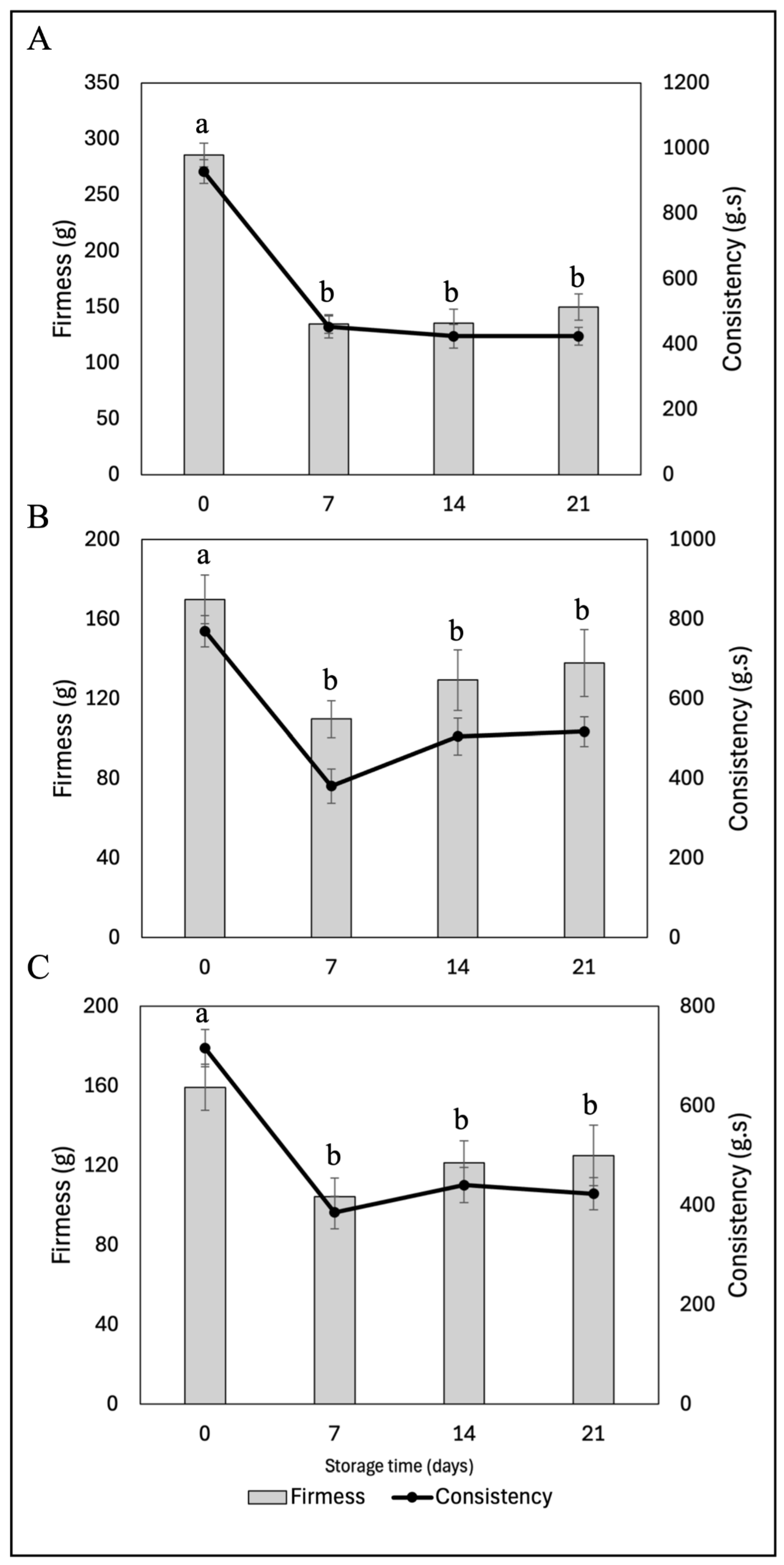
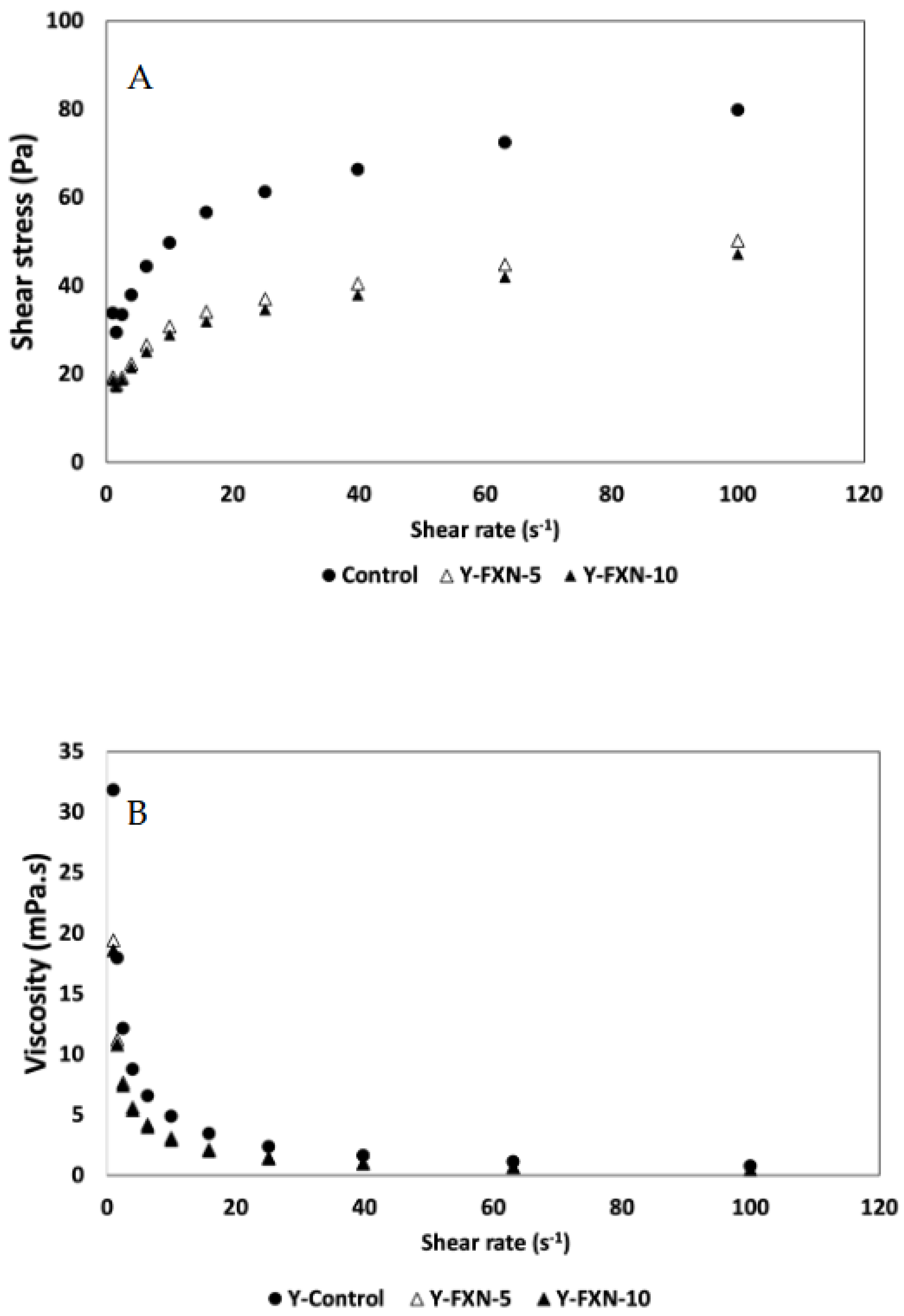
| Samples | Storage time (days) | Free radical-scavenging (%) | mmol TE/g | |
| DPPH• | ABTS+• | FRAP | ||
| Y-C | 0 | 24.77 e±1.25 | 33.72 f±1.75 | 2.85 c±0.13 |
| 7 | 24.32 e±2.45 | 30.36 fg±2.35 | 2.42 d±0.11 | |
| 14 | 21.68 f±1.74 | 28.41 g±2.95 | 2.23 d±0.10 | |
| 21 | 19.23 f±2.84 | 25.98 g±1.24 | 2.25 d±0.09 | |
| Y-FXN-5 | 0 | 34.99 c±0.95 | 43.38 d±1.64 | 3.11 ab±0.07 |
| 7 | 34.12 c±1.81 | 41.64 de±1.74 | 3.21 a±0.11 | |
| 14 | 32.58 cd±2.76 | 39.32 e±1.63 | 3.03 b±0.03 | |
| 21 | 30.23 d±1.63 | 39.64 e±2.87 | 2.98 b±0.08 | |
| Y-FXN-10 | 0 | 52.96 a±1.24 | 97.97 a±2.93 | 3.16 a±0.05 |
| 7 | 50.23 b±0.35 | 92.54 b±2.26 | 3.14 a±0.03 | |
| 14 | 51.25 ab±1.55 | 90.23 bc±1.15 | 3.15 a±0.06 | |
| 21 | 50.34 b±1.23 | 88.42 c±0.74 | 3.04 b±0.03 | |
| Samples | HI (%) | PHI (%) | Heat-IH | Hypo-IH |
| Y-C | 17.29 c ±2.67 | 61.07 b ±2.53 | 25.50 b ±2.35 | 81.46 b ±1.23 |
| Y-FXN-5 | 63.83 b ±2.34 | 54.93 c ±1.98 | 25.17 b ±2.87 | 80.87 b ±2.85 |
| Y-FXN-10 | 82.41 a ±1.54 | 82.40 a ±2.63 | 46.80 a ±3.63 | 93.62 a ±2.16 |
| Sensory quality attributes | Treatments | ||
| Y-C | Y-FXN-5 | Y-FXN-10 | |
| Color | 8.56a ±0.16 | 7.491a ±0.26 | 8.73a ±0.47 |
| Flavor | 7.83a ±0.23 | 7.64.73b ±0.42 | 8.09c ±0.83 |
| Aftertaste | 7.24b ±0.26 | 8.00a ±0.25 | 8.18a ±1.08 |
| Scent | 8.61a ±0.45 | 7.73ab ±0.45 | 8.64b ±0.67 |
| Consistency | 8.29a ±0.35 | 7.55ab ±0.34 | 8.19b ±0.94 |
| Texture | 8.22a ±0.23 | 7.91a ±0.65 | 8.36a ±0.92 |
| Appearance | 8.29a ±0.32 | 7.64a ±0.32 | 8.82b ±0.40 |
| General acceptance | 7.88a ±0.15 | 8.00a ±0.21 | 8.36a ±0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





