1. Introduction
The mining and metallurgical complex plays a vital role in the global economy and, in particular, in Kazakhstan, serving as the cornerstone of the national economy. At present, zinc and lead are key metals that play an important role both in Kazakhstan and in the global industry, the metallurgical sector, and the development of the circular economy. Kazakhstan is among the top ten world leaders in the extraction and production of mineral raw materials, including polymetallic ores. Approximately 16% of the worl
d’s lead reserves and 10% of the world’s zinc reserves are concentrated in Kazakhstan’s subsoil, which gives the country a strategic advantage in the international market [
1].
The major polymetallic ore deposits, such as Ridder-Sokolnoye, Zyryanovskoye, Maleevskoye, and Achisai, contain rich ores that are actively developed and processed. However, the reserves of rich and easily beneficiable ores are being depleted, and the supply of reserves from the developed deposits does not exceed 25 years. The prospects for the discovery of new lead and zinc deposits with high concentrations of valuable components remain relevant for nearly all regions of the republic. In recent years, the Shymerden deposit in Northern Kazakhstan, which is characterized by an exceptionally high zinc content, has been involved in development. According to literature sources, 41% of lead-zinc deposits in Kazakhstan are concentrated in the Central region, 33% in the Eastern region, and 21% in Southern Kazakhstan. Promising areas for discovering new deposits include Central and Southern Kazakhstan, the Rudny Altai, and border areas with Russia and China [
2].
Currently, increasingly complex and difficult-to-enrich oxidized and mixed ore are being introduced into production, and extracting non-ferrous metals from them presents a technological challenge.
Oxidized polymetallic ores are complex ores containing metals such as lead, zinc, copper, and precious metals. They are formed as a result of the weathering of sulfide ores and are characterized by a high content of oxide minerals. Difficult-to-enrich ores represent a significant challenge in ensuring a high level of valuable component extraction and comprehensive utilization. The involvement of oxidized polymetallic ores in processing is an urgent task for the mining and metallurgical industry. These ores are characterized by a complex mineral composition and low reactivity of their valuable components, requiring the development of specialized technologies. To process oxidized polymetallic ores, it is necessary to integrate modern technologies (hydrometallurgy, autoclave leaching, bioleaching) considering the mineral composition and availability of the ores. This will enable the efficient use of resources, reduce environmental damage, and ensure the sustainable development of the mining and metallurgical industry [
2,
3,
4,
5,
6,
7,
8,
9,
10].
The most reasonable method for enriching oxidized polymetallic ores is flotation after preliminary sulfidization. Sulfidization of oxidized minerals is a process of chemically modifying the surface of minerals, wherein their oxidized compounds are converted into sulfides. This method is used to enhance the hydrophobicity of minerals, improve their flotation properties, and increase the extraction of non-ferrous metals from ores. The main goal of sulfidization is to transform the oxidized forms of metals (e.g., carbonates, sulfates, oxides) into sulfide forms, which are more easily floated. This is especially important for difficult-to-enrich oxidized and mixed ores containing metals such as copper, lead, or zinc.
Sulfidization occurs in several stages [
11,
12]:
It has also been proven that the stabilization of the electrochemical state of the dispersed system before flotation is necessary, controlled by measuring the oxidative-reductive potential (ORP) of the pulp. The oxidative-reductive potential (ORP) is an important indicator that reflects the ratio of oxidized and reduced forms in the liquid phase of the pulp. This parameter characterizes the prevailing direction of chemical processes in the system, whether oxidation or reduction. ORP significantly influences the ion-molecular composition of the liquid phase, altering it due to the occurrence of oxidation-reduction reactions. The ORP value is measured in volts or millivolts and serves as an indicator of the oxidative or reductive activity of the medium. A positive value indicates a tendency toward oxidation, while a negative value signals the predominance of reduction processes. Thus, ORP serves as an important additional factor determining the chemical behavior of the liquid phase of the pulp and the direction of the reactions occurring [
8,
9,
10].
Regulation of Oxidative-Reduction Potential (ORP) is a key factor in the enrichment of oxidized polymetallic ores. A comprehensive study of ORP changes during flotation, leaching, and precipitation processes allows for the development of effective enrichment technologies and enhances the extraction of valuable metals. Proper understanding and control of ORP help optimize the selectivity of separation and increase the efficiency of extracting target components.
The aim of this study is to investigate the effect of sulfidization on the enrichment processes of oxidized polymetallic ores from the Koskudyk deposit, determine the optimal conditions for converting oxidized minerals into sulfide forms, improve the flotation properties of ores, and enhance the extraction of valuable components, such as lead and zinc. The study also aims to determine the optimal ORP values to ensure the hydrophobization of the surface of oxidized minerals [
8,
9,
10,
11,
12].
2. Materials and Methods
The object of study is the oxidized lead-zinc ores from the Koskudyk deposit in Kazakhstan.
The material composition of the ore and the enrichment products was determined using the group trial gravimetric analysis method in representative samples specially prepared for atomic absorption analysis, atomic emission analysis, classical chemical analysis, and photometric analysis. The phase composition was determined using a “D2 Phaser” diffractometer from “Bruker.” The study of ore minerals was conducted in reflected light on polished thin sections using the OLYMPUS BX 53 microscope, the SIMAGIS XS-3CU video camera, and image analysis software, Mineral C7 by SIAMS.
According to
Table 1, the ore sample contains 0.85% lead, 0.65% zinc, 5.4 g/t silver, and 0.6 g/t gold.
Table 2—Results of Phase Analysis of Lead, Zinc, and Iron
According to the data of the phase chemical analysis (
Table 2), the sample is classified as of the oxidized type. This is confirmed by the fact that oxidized compounds make up 79.69% of the total lead mass, while for zinc, this value reaches 84.72%.
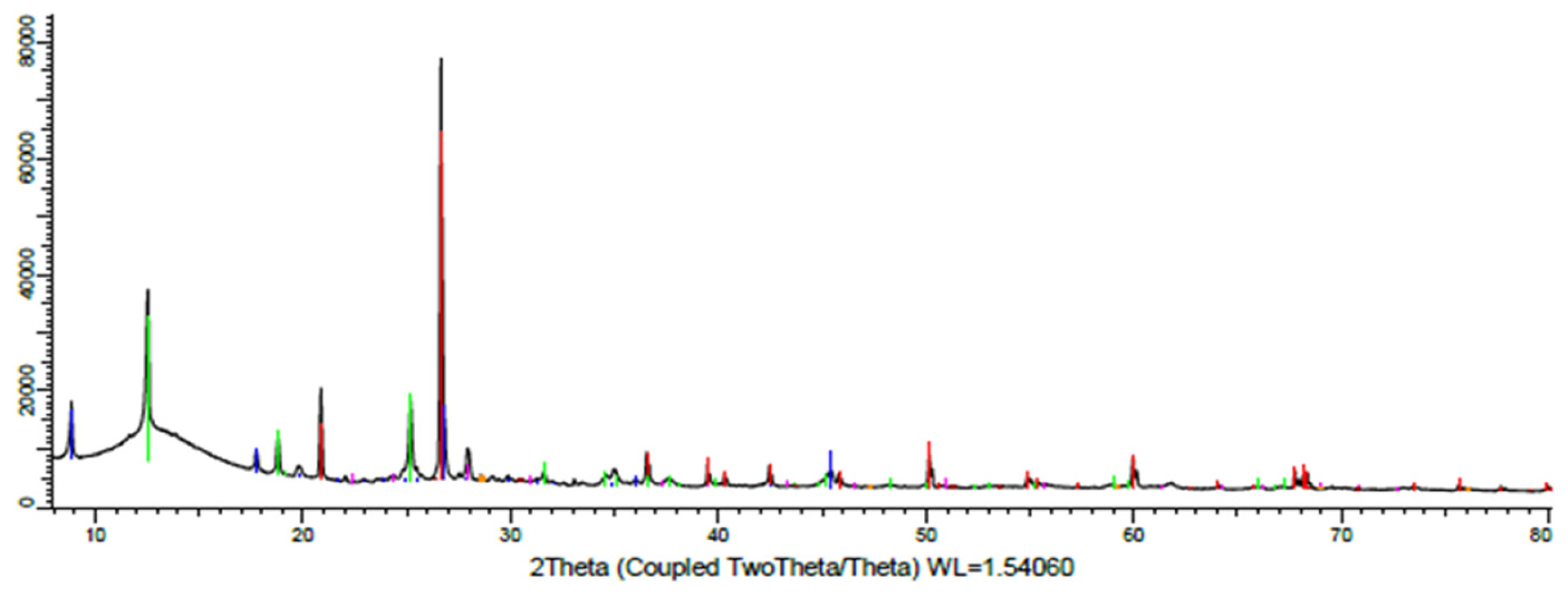
The X-ray phase composition of the ore sample is shown in
Figure 1, and the data in numerical form are presented in
Table 3.
The ore minerals in the sample are represented by dull ores, chalcosine, covellite, goethite, and hematite. Sulfides present include chalcopyrite, galena, pyrite, and sphalerite.
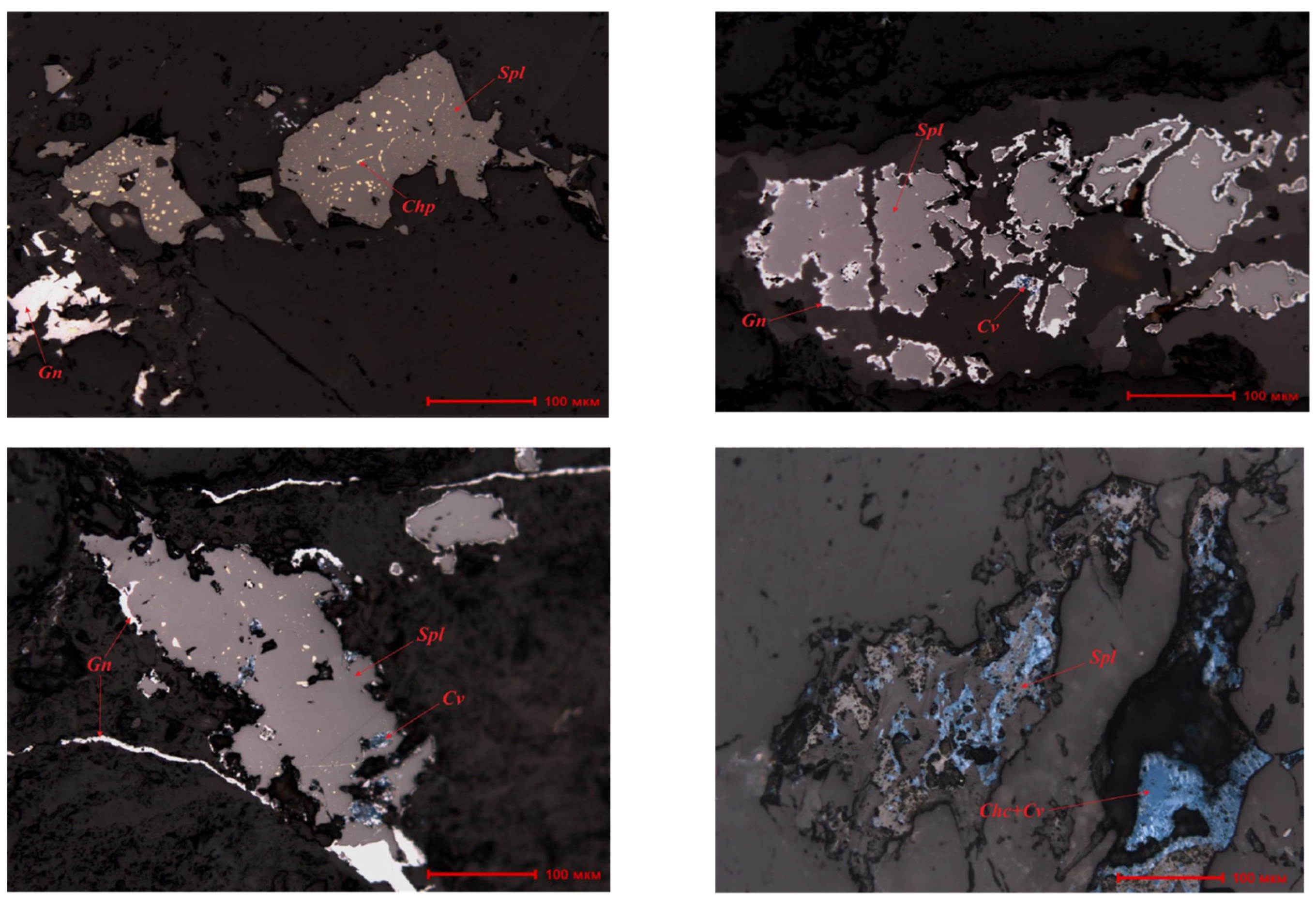
Sphalerite contains fine inclusions of chalcopyrite (0.003-0.01 mm) and galena (0.005-0.05 mm) (
Figure 2). Galena films with a thickness of 0.003-0.005 mm are observed along the contours of sphalerite grains, with inclusions of covellite (0.011-0.03 mm). Galena veins range in length from 0.01 to 0.30 mm. Along the contours of the sphalerite grains, there are galena films (0.003-0.010 mm) and covellite inclusions (0.03 mm). Rare sphalerite veins have a thickness of 0.18 mm. Occasional galena crystals measuring 0.15 mm and discontinuous galena veins with a thickness of 0.02 mm are also observed.
The oxidized ores of the Koskudyk deposit are characterized by a high degree of oxidation, fine inclusions, as well as specific structural-textural features, which classify them as finely disseminated and difficult-to-beneficiate ores.
Flotation enrichment was carried out on standard laboratory mechanical flotation machines of the Mechanobr type with chamber volumes of 3, 1.0, and 0.5 liters. The following reagents were used for the study: sodium sulfide (Na2S), xanthate (Kx), methyl isobutyl ketone (MIBK), and AEROFLOAT® MX-515. The flotation time was 10 minutes, with a solid content in the flotation feed of 30% and the content of the final class of -0.071 mm being 70%. During the laboratory experiment, pH level and redox potential (ORP) were monitored (pH level was controlled with the HANNA HI 1230 electrode and ORP with the HI 3131 electrode).
3. Results and Discussion
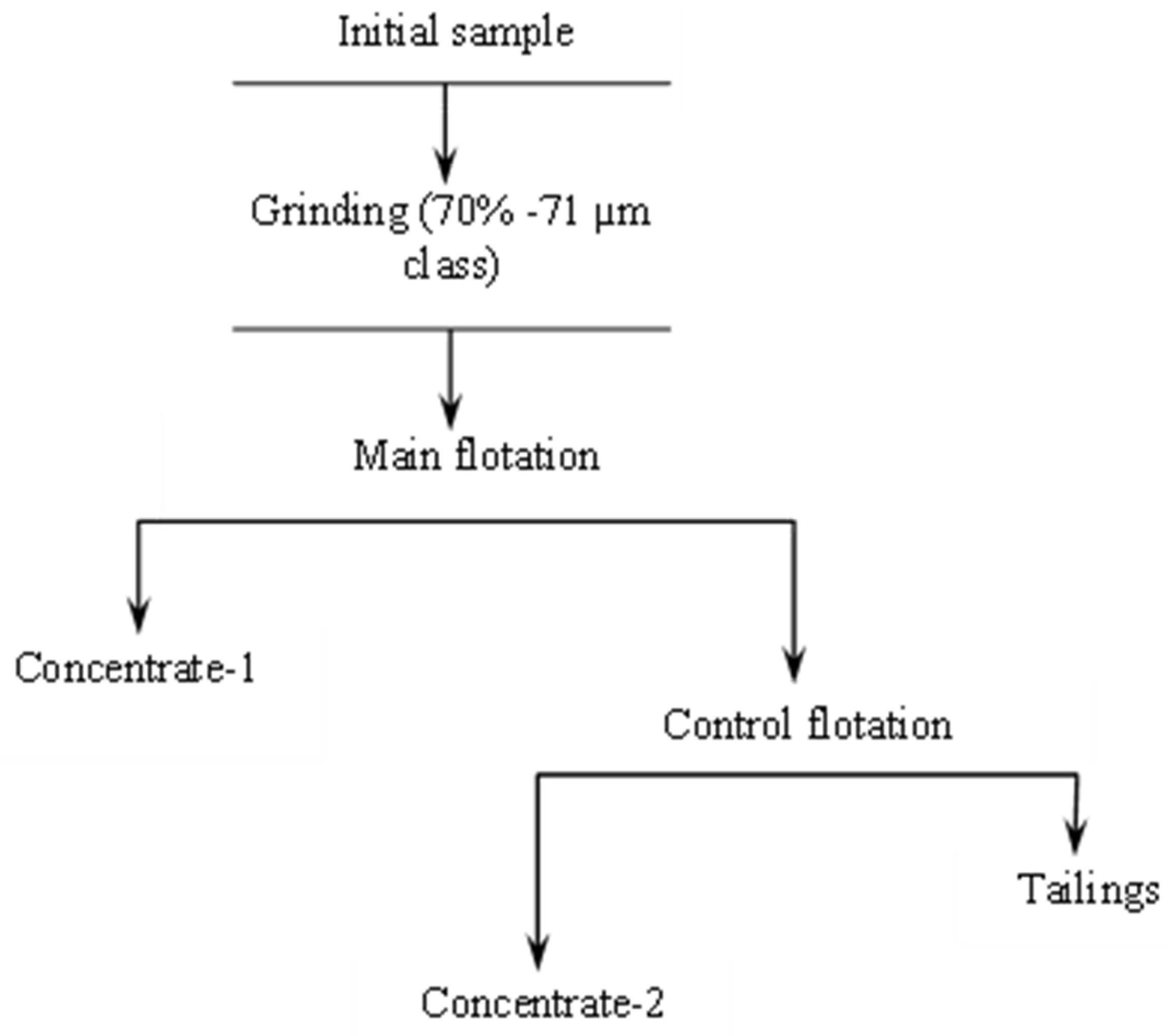
The principal technological flowchart of the laboratory experiment is shown in
Figure 3.
Sodium sulfide was used to activate the oxidized minerals [
13,
14,
15]. The results of the open laboratory experiments are presented in
Table 4.
The results of the beneficiation efficiency calculation using the Houcock-Luyken (or Hankok-Luyken) are presented in
Figure 4.
The maximum beneficiation efficiency is observed for lead and gold at a sodium sulfide consumption of about 700 g/t. These consumption levels are considered optimal for Na2S (active). It should be noted that sulfidization does not significantly improve the beneficiation results for zinc.
The redox potential (ORP) level is 200 mV. This value of the ORP is quite consistent with the data from literature sources. In literature, it is recommended to maintain the ORP in the range of 200-300 mV for mineral activation [
11,
12,
13,
14,
15,
16,
17]. The optimal values of redox potential during the flotation of oxidized ores can vary depending on the mineral composition of the ore and the reagents used.
5. Conclusions
The oxidized ores of the Koskudyk deposit are characterized by a high degree of oxidation, fine dissemination, as well as specific structural-textural features, which classify them as finely disseminated and difficult-to-beneficiate ores.
The ore sample for the study contains 0.85% lead, 0.65% zinc, 5.4 g/t silver, and 0.6 g/t gold.
According to the data from phase chemical analysis, the sample is classified as of the oxidized type. This is confirmed by the fact that oxidized compounds constitute 79.69% of the total lead mass, while for zinc, this value reaches 84.72%.
The ore minerals in the sample are represented by dull ores, chalcosine, covellite, goethite, and hematite. Sulfides present include chalcopyrite, galena, pyrite, and sphalerite.
Studies were carried out on the sulfidization of oxidized minerals with sodium sulfide, and the redox potential (ORP) levels were determined for each consumption rate. Determining the optimal redox potential level will significantly improve both the qualitative and quantitative beneficiation indicators, which will greatly simplify the regulation of the process.
The flotation data for the oxidized ore showed that with an increase in sodium sulfide consumption to 700 g/t and the ORP level to 200 mV, lead recovery increased to 50.07%, while the qualitative and quantitative indicators for zinc remained at the same level. Further increases in sodium sulfide consumption and ORP levels led to a decrease in lead recovery to 48.04%.
The study of changes in the redox potential during the beneficiation of oxidized polymetallic ores is an important direction in mining and metallurgy. It not only allows for a deeper understanding of the electrochemical aspects of beneficiation processes but also helps to create efficient technologies for processing ores with a highly complex composition. The obtained data could lead to improvements in the selectivity of metal recovery and an increase in the economic efficiency of production processes.
Author Contributions
Conceptualization, T.T. and A.M.; methodology, G. M.; formal analysis L.S.; investigation, A.M., data curation, A.M. and L.S.; writing—original draft preparation, T.T.; writing—review and editing, T.T.; project administration, A.M., M.B., and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out within the framework of the grant project AR 23489765, funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors state that the study was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
References
- https://dknews.kz/ru/ekonomika/105991-v-kazahstane-ezhegodno-proizvoditsya-300-tysyach-tonn.
- Merkibayev, Y.S. Processing of poor hard-to-process complex lead-zinc ores and enrichment intermediate products // Dissertation for the degree of Doctor of Philosophy (PhD), Almaty, 2024, p.187. Available online: https://official.satbayev.university/ru/protection.
- Nayak, A., Jena, M.S., Mandre, N.R. Beneficiation of lead–zinc ores—a review // Miner. Process. Extr. Metall. Rev., 43 (5) (2022), pp. 564-583. [CrossRef]
- Yuangan Chen, Yongsheng Suna, Yuexin Hana. Efficient flotation separation of lead–zinc oxide ores using mineral sulfidation reconstruction technology: A review // Green and Smart Mining Engineering 1 (2024), pp.175–189. Available online: https://www.sciencedirect.com/.
- Turysbekov D., Semushkina L., Narbekovа S., Mukhamedilova A. On the use of environmentally safe sulfidizator in the beneficiation of ores // Proceedings of the Universities of Kyrgyzstan, № 8, 2019, p. 24-29. Available online: https://elibrary.ru.
- Hongliang Zhang, Heng Yu, Wei Sun, Shangyong Lin, Chenyang Zhang. Beneficiation of silver and silver-bearing lead–zinc ores: A review // Minerals Engineering Volume 208, March 2024.
- Chepushtanova T.A., Motovilov I.Yu, Merkibayev Y.S., Polyakov K.V., Gostu S. Flotation studies of the middling product of lead-zinc ores with preliminary sulfidizing roasting of oxidized lead and zinc compounds // KIMS. Kazakhstan №4 (323), 2022, p. 78-83. Available online: https://kims-imio.kz/wp-content/uploads/2024/04/2022-4-10.pdf.
- Motovilov I.Yu., Barmenshinova M.B., Telkov Sh.A., Omar R.S. Study of the chemical composition and assessment of the gravity beneficiation of oxidized polymetallic ores of the RK deposit // Mining Journal of Kazakhstan, No. 9, 2024. pp. 51-58. Available online: https://minmag.kz/wp-content/uploads/2024/10/2409_51-58.pdf.
- Majid Ejtemaei, Mahdi Gharabaghi, Mehdi Irannajad. A review of zinc oxide mineral beneficiation using flotation method // Advances in Colloid and Interface Science Volume 206, April 2014, pp. 68-78.
- Wonder Chimonyo, Kirsten Corin, Jenny Wiese and Cyril O’Connor. Redox potential control during flotation of sulphide minerals // Centre for Minerals Research, Department of Chemical Engineering, University of Cape Town.—November, 2015. Available online: https://www.researchgate.net/publication/283510716_Redox_potential_control_during_flotation_of_sulphide_minerals.
- Oskembekov I.M., Bekturganov N.S., Katkeeva G.L., Burkitseterkyzy G., Gizatullina D.R. Use of the sulphidation process in the processing of oxidised copper ores // KIMS, 2017, рр.16-22. Available online: https://kims-imio.com.
- Dospaev M.M., Figurinene I.V., Gabdullin S.T. Combined electrochemical sulphidisation of difficult to enrich oxidised copper ores // Obogashchenie Rud, 2024, pp. 19-24.
- Xojimuratova X. B., Abdusamieva L. N. Method for Processing Sulphide-Oxidized Copper Ores with Copper and Silver Extraction //EUROPEAN JOURNAL OF INNOVATION IN NONFORMAL EDUCATION.—2024.—Т. 4.—№. 3.—р. 510-513. https://api.scienceweb.uz/storage/publication_files/9820/26493/666bdfded87a5___510_513+Method+for+Processing+Sulphide_Oxidized+Copper+Ores+wit.pdf.
- Li, J.L., Liu, S.Y., Liu, D.W., Liu, R.Z., Liu, Z.C., Jia, X.D., Chang, T.C., Sulfidization mechanism in the flotation of cerussite: a heterogeneous solid-liquid reaction that yields PbCO3/PbS core–shell particles, Miner. Eng. 153 (2020).
- Yuan, W.Y., Li, J.H., Zhang, Q.W., Saito, F., Mechanochemical sulfidization of lead oxides by grinding with sulfur, Powder Technol. 230 (2012), p.63–66. Available online: https://www.researchgate.net/publication/262725367_Mechanochemical_Sulfidization_of_Lead_Oxides_by_Grinding_with_Sulfur.
- Anthropova, I.G., Merinov, А.A., Gulyashinov, Р.A., Damdinov, B.B.. Sulfidation of oxidized lead and zinc with pyrite-bearing lead-and-zinc ore // Physical and technical problems of mineral resources development. July 2023.
- Oskembekov, I.M., Burkitseterkyzy G., Akubaeva, M.A., Gizatullina D.R., Zhunussov E.M. Use of the sulphidation process in the processing of oxidised copper ores // KIMS, 2017, рр.16-22. Available online: https://kims-imio.com.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).