1. Introduction
Airway stents, hollow cylindrical implants made from biocompatible materials such as silicone or metal alloys, have evolved significantly since the introduction of the Dumon silicone stent in the 1990s [
1,
2,
3,
4,
5].
Recent advancements in stent technology, including custom-designed and biodegradeable 3D-printed stents, have gained prominence [
6,
7,
8,
9,
10,
11,
12,
13].
Additionally, “active” stents, which incorporate drug elution, antimicrobial coatings, or radiation delivery, are under investigation [
14]
.
Traditional airway stents are available in fixed diameters and lengths, but selecting the appropriate stent size is far more complex than matching lumen dimensions [
3,
15,
16,
17,
18,
19,
20].
The decision is influenced by technical, anatomical, and clinical factors, with the underlying indication—whether malignant airway obstruction, post-transplant complications, or benign diseases like tracheobronchomalacia—guiding design parameters and desired outcomes. Stents also vary by region, with differences in availability and regulatory approvals shaping clinical practice [
15,
16,
17,
18,
19,
21].
In the United States, most airway stents are cylindrical, except for a few branched configurations like Y-stents and T-tubes [
13,
16,
22]
. Despite their widespread use, airway stents remain prone to complications, including migration, infection, mucus plugging, and granulation, largely due to poor fit—an area that remains inadequately understood but is recognized as critical [
5,
11,
23,
24,
25,
26,
27,
28,
29]. Recent surveys highlight global variability in stent sizing techniques, underscoring the challenge of achieving an optimal fit [
15,
16,
17,
18,
19,
21]. Modifying stent fit features may help mitigate these risks [
30,
31,
32]. However, the complexity of airway disease further complicates stent selection. Pathologies such as cancer, prior airway resections, or lung transplantation can lead to asymmetrical or multifocal involvement, with variations in airway diameter the airway, angulation, and structural integrity [
33,
34,
35,
36]. Lung transplantation presents an extreme case, where stenosis, dehiscence, and malacia frequently coexist near the carina and extend into non-anastomotic regions, demanding highly individualized stent solutions.
To address these challenges, patient-specific (PS) implants have been developed in the US and Europe. ³² These custom-designed stents aim to improve fit by accommodating each patient’s unique anatomy and pathology. This study examines the limitations of pre-manufactured stents, the challenges of airway stent sizing, and expert-driven strategies for customization. Although the primary focus is on the design and implementation of 3D-printed PS stents, we also consider the critical post-deployment factors, including radiographic fit, bronchoscopic evaluations, and the “settling phase,” where stents and airways gradually conform to one another. By exploring these factors, we aim to establish a systematic approach for optimizing airway stent fit and improving patient outcomes.
5. Results
Nine cases were selected for review, each involving a 3DPS stent tailored to complex airway anatomy. These cases were chosen due to diverse pathology and unique anatomical to provide a comprehensive overview of different stent design callenges for different diagnoses, indications, and locations (
Figure 1). To understand the decision-making regarding the size and design, the treating physician provided a background and the technical issues associated with the airway and stentingThe primary challenges identified included:
Shape mismatches requiring stent contouring (9/9 cases)
Branching angles requiring custom modification (6/9 cases)
Sizing discrepancies necessitating variable tapering (8/9 cases)
Jailed airway segments requiring fenestrations (1/9 cases)
Migration risk requiring anchoring adjustments (1/9 cases)
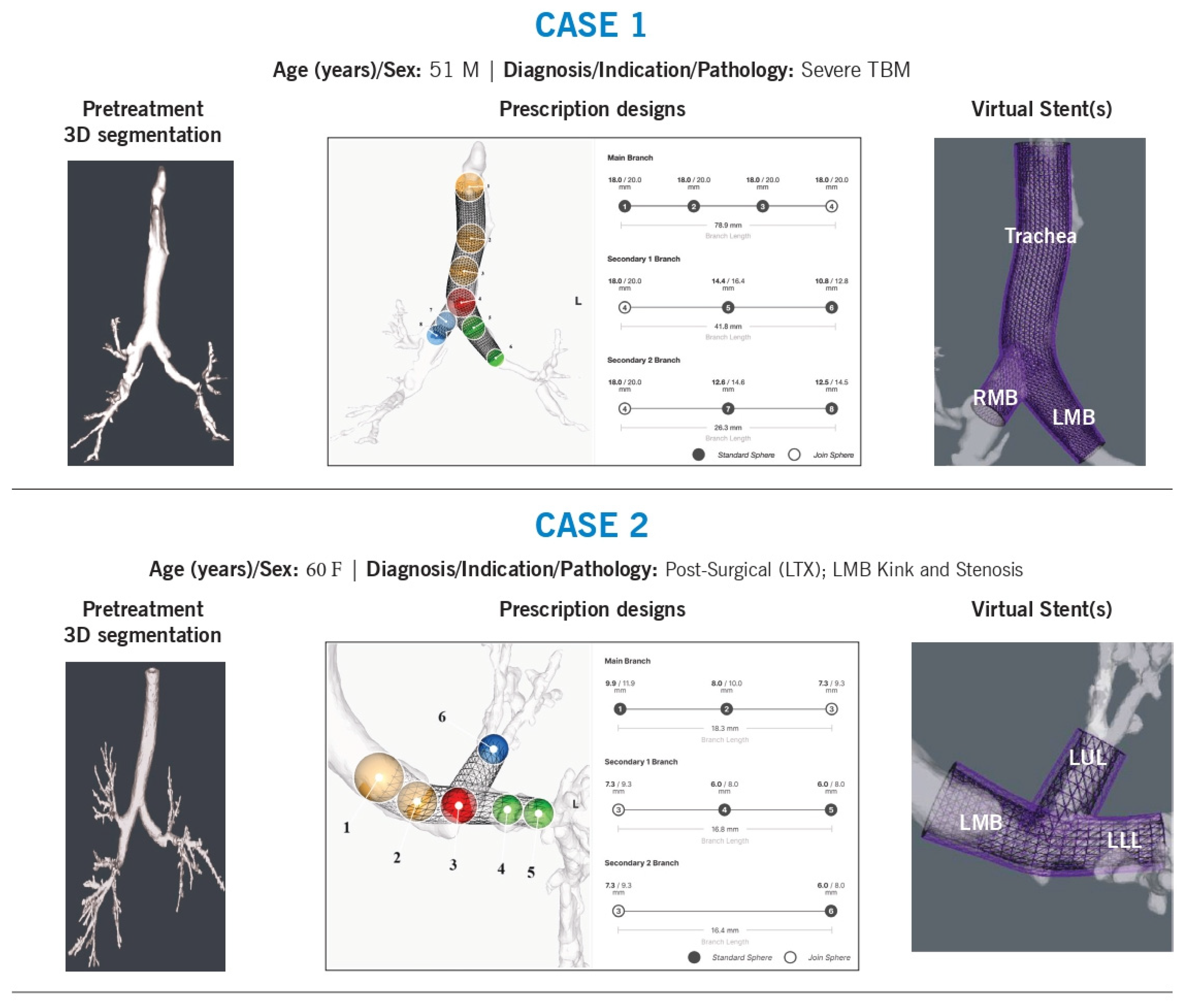
Case 1
Background: A 51-year-old man with tracheobronchomalacia underwent multiple 3D-printed patient-specific (3DPS) stent placements to support the trachea, right mainstem, and left mainstem. Prior Y-stents were manually customized by sewing together different sizes to achieve the required shape, angle, and diameter.
Technical Challenges:
Shape and angulation
Tapering of diameters
Variability in branch sizing, particularly in secondary airways
Intervention & Design Rationale:
The stent length was determined based on the extent of malacia, which originated at the thoracic inlet and extended distally into the left mainstem. The tracheal stent diameter was restricted by a maximum inner diameter of 18 mm. Because the airway had a flattened elliptical shape, diameter estimation required averaging the long and short axes, a challenge that persisted throughout the airway. Beyond the main carina, additional customization was necessary due to significant asymmetry between the right and left mainstem bronchi. The right mainstem was notably larger than the left, requiring precise contouring to ensure the stent closely approximated the medial airway walls while maintaining curvature aligned with native anatomy. Where not limited by maximum diameter specifications, the left mainstem stent wall was intentionally oversized to allow direct luminal interaction, with a 1-mm wall thickness providing additional support and dilation.
To prevent misalignment or excessive airway wall pressure, the stent was designed to follow the natural airway path, avoiding sharp edges or rigid structures that could cause localized stress. This patient-specific approach aimed to optimize airway stabilization while minimizing complications.
Case 2
Background: A 60-year-old lung transplant recipient developed left mainstem stenosis due to a post-anastomotic kink extending beyond the left upper lobe bronchus (LC1).
Technical Challenges:
Shape and angulation
Branch diameter and length
Intervention & Design Rationale:
The left mainstem stenosis and post-transplant kink extended distally into each lobar bronchus. The stent was designed to be long enough to cover the stenotic region while slightly oversized at the narrowest point to prevent restenosis. Proximally, it was tapered to match the native airway before the anastomosis.
To ensure a proper fit beyond the anastomosis, the stent design incorporated rotational adjustments to align lumen size with expected distal airway diameters. At the carina, the positioning of the join-sphere was refined to optimize the angles of each limb. The stent limb lengths were minimized to prevent obstruction of distal branches.
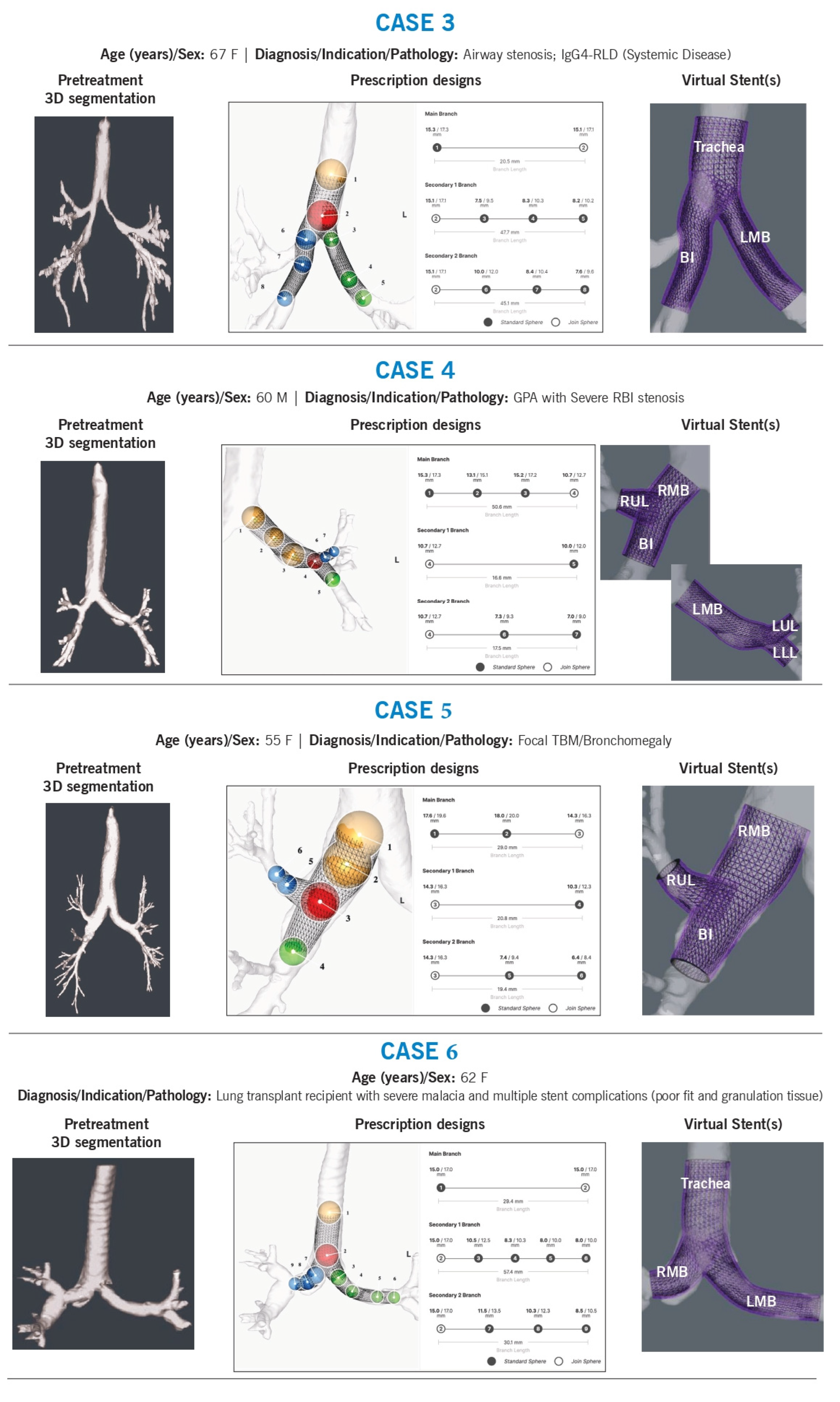
Case 3
Background: A 67-year-old woman with diffuse, multilevel airway stenosis received multiple 3DPS stents for the trachea, right bronchus intermedius (RBI), right lower lobe (RLL), and left mainstem.
Technical Challenges:
Size, shape, and angulation
Tapering
Airway preservation (avoiding luminal obstruction or “jailed airway segments”)
Intervention & Design Rationale:
The patient had a significantly distorted main carina with an acute angle due to prior upper tracheal resection. Disease extended distally into the left mainstem and right bronchus intermedius, beyond the right upper lobe level.
To accommodate stenotic regions, the stents were oversized by approximately 2 mm, allowing for planned balloon dilation before implantation. The right mainstem stent was contoured along the medial wall and extended past the right upper lobe, with a fenestration designed to maintain ventilation. The tapered design and strategic sizing ensured adequate luminal patency while minimizing airway obstruction. The stent design also accommodated the abnormal main carina by ensuring precise contouring to prevent migration.
Case 4
Background: A 60-year-old man with granulomatosis with polyangiitis (GPA) had recurrent granulation tissue formation despite prior commercial stents. This was a complex case with mixed airway disease and several years of stent complications.
Technical Challenges:
Size, shape, and angulation
Intervention & Design Rationale:
Left Mainstem:
The left airway presented with significant malacia in a curved mainstem, potentially exacerbated by prior stents. The proximal stent was designed to match airway volume, while the distal portion was oversized (by 2–4 mm) to accommodate known strictures at the secondary carina. The sphere at the carina was positioned to extend into both the left upper and lower lobes while avoiding excessive distal airway oversizing. The stent met a minimum branch length of 15 mm but was excess length was intended to be trimmed before implantation.
Right mainstem:
A stent was designed to replace a previously customized hourglass stent, addressing a GPA-related stricture at the right bronchus intermedius (RBI) orifice. Minimal oversizing (<1 mm) was used in the right mainstem. The right upper lobe limb was slightly tapered and oversized by 1 mm, with precise angulation to ensure central placement. The RBI stent was contoured to restore near-normal luminal dimensions, with a slight distal taper to reduce granulation tissue formation. The stent was positioned 1–2 mm above the right middle lobe (RML) and RB6 orifice.
Case 5
Background: A 55-year-old woman with bronchomegaly at RC1 was managed for right mainstem malacia.
Technical Challenges:
Size, and shape
Intervention & Design Rationale:
The patient had focal right-sided malacia with recurrent infections. Previous stents were intentionally oversized to prevent migration but may have contributed to airway dilation over time. The airway stent was designed to match, rather than significantly oversize, the right mainstem. It tapered distally into the right bronchus intermedius (RBI) and right upper lobe to ensure proper fit. Given the short segmental anatomy of the right upper lobe, the stent was centered but extended through the segmental trifurcation, with the intention of trimming the stent prior to implantation, to maintain ventilation into segmental bronchi. The RBI stent was extended to the mid-RBI, ensuring adequate coverage of the malacic region.
Case 6
Background: A 62-year-old lung transplant recipient with non-anastomotic airway complications, received an airway stent treating the trachea, right mainstem, and left mainstem. She had a history of severe malacia and multiple prior stent failures due to poor fit and granulation tissue formation.
Technical Challenges:
Shape adaptation of abnormal airway anatomy
Intervention & Design Rationale:
The patient exhibited severe distal tracheal malacia, along with combined stenosis and malacia of both mainstem bronchi. The left mainstem bronchus had an almost horizontal orientation, while the right mainstem anastomosis was highly curved. The stent was designed to conform to these unique airway angles, with distal limbs extending just beyond both anastomoses to ensure proper support while preventing migration and airway obstruction.
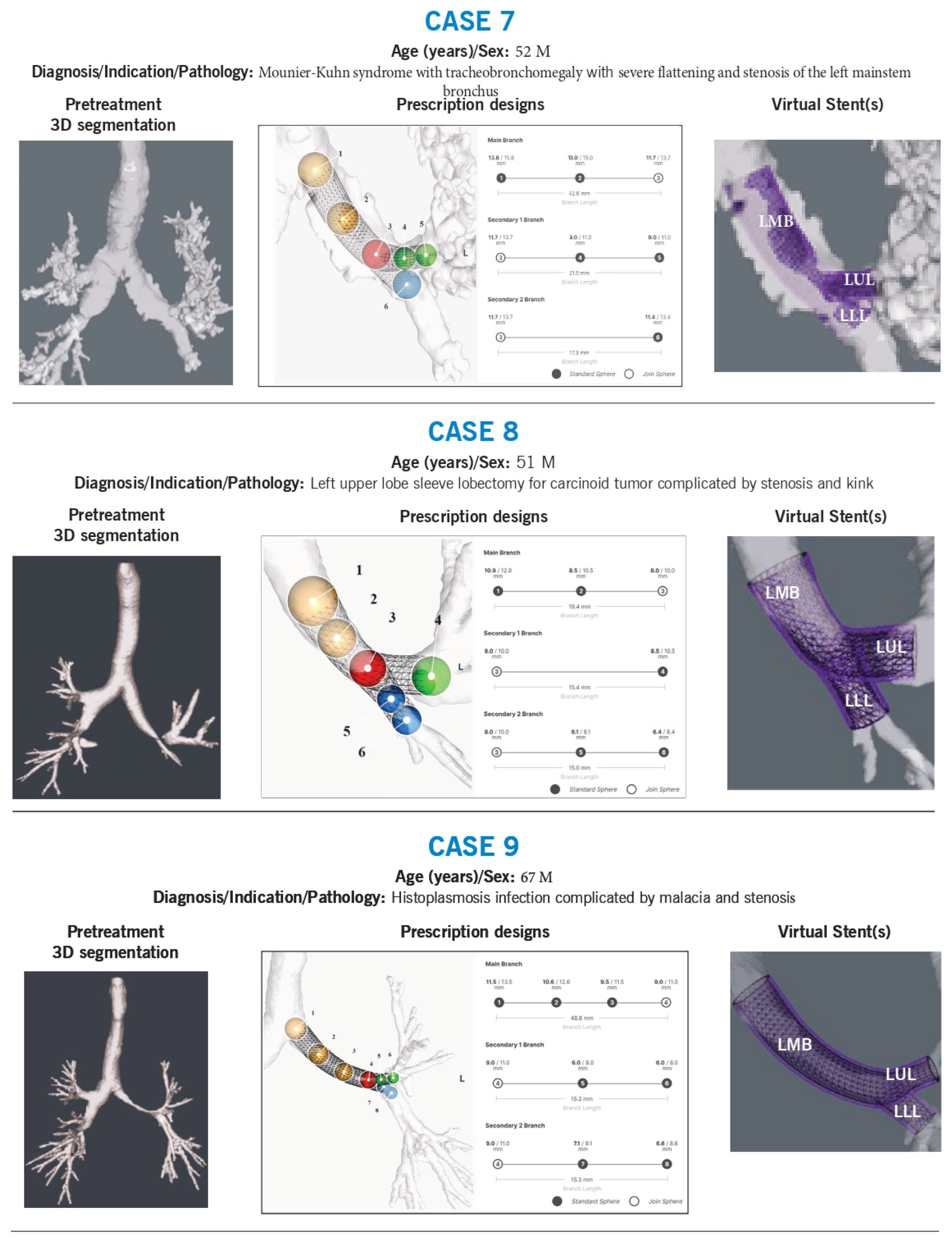
Case 7
Background: A 52-year-old man with Mounier-Kuhn syndrome and tracheobronchomegaly had severe flattening and stenosis of the left mainstem bronchus. Multiple prior stent placements were complicated by proximal migration.
Technical Challenges:
Size, and shape adaption
Stent migration risk
Intervention & Design Rationale:
The patient’s airways were markedly dilated, characteristic of Mounier-Kuhn syndrome. The left mainstem bronchus was particularly flattened (1 mm × 30 mm). Stent sizing was based on previous CT measurements and the fit of prior silicone stents (up to 16 mm). The stent was shaped to match the left mainstem morphology, with left upper and lower lobe limbs contoured for both stenting and anchoring, reducing the risk of migration.
Case 8
Background: A 51-year-old man with a history of left upper lobe sleeve lobectomy for carcinoid tumor developed a stenotic kink in the left mainstem.
Technical Challenges:
Size, shape, and angulation
Intervention & Design Rationale:
The left lower lobe exhibited stenosis at the sleeve anastomosis, with a non-anatomical airway conformation. Previous stenting attempts had failed. The new stent was designed with an outer diameter approximating the unaffected left mainstem (~12 mm) to maintain luminal integrity. The left lower lobe received two branches, one into the superior segment and another into the basilar bronchus, with dimensions based on preoperative CT measurements. The stent design evolved with subsequent placements to optimize fit and function.
Case 9
Background: A 67-year-old man with severe left mainstem and left upper lobe malacia due to histoplasmosis underwent multiple airway stent placements.
Technical Challenges:
Size, shape, length, and branch point alignment
Intervention & Design Rationale:
The first stent was designed using a 3D airway reconstruction in the setting of malacia but failed to fit properly in the left upper lobe and was removed. The second stent was modeled using a pre-malacia CT scan, ensuring anatomic precision and a superior fit, effectively restoring airway patency.
6. Discussion
Currently available stents are designed primarily in a single linear plane, which is suitable for short-segment stenosis but inadequate for complex airway pathologies. Metallic stents offer flexibility but may exert uneven pressure on the airway, while stock Y-stents can accommodate extrinsic compression without significant carinal distortion. However, many airway diseases result in multifocal or extensive involvement, leading to occlusion, distortion, and a combination of stenosis and malacia of varying lengths and diameters. Disease affecting the carina further complicates stent angulation and transitions between different lumen diameters and shapes. Thus, contouring a stent to the airway’s abnormal morphology while strategically adding volume to optimize airflow and reduce mechanical stress is crucial for improving its function and durability.
Since airway diseases are often non-linear, particularly at the carina, more adaptable solutions are required. Silicone stents allow further modifications, including shortening or creating side holes for branch access, enhancing their adaptability. These stents also possess specific mechanical properties such as durometer, modulus, hoop strength, and tear resistance that influence clinical outcomes; however, these parameters remain poorly understood and underutilized in clinical practice. Each stent on the market has some variability in these characteristics, and while experienced bronchoscopists may consider these properties during selection, the lack of standardized data limits evidence-based decision-making regarding optimal stent choice.
Proper stent sizing is crucial, as undersizing can lead to poor airflow, mucus buildup, and migration, whereas oversizing may cause airway trauma, rupture, or undue pressure on surrounding structures. Optimal selection involves placing a slightly oversized stent that maintains luminal patency while generating sufficient friction to resist migration. Diameter selection is typically guided by CT imaging, direct visualization, or sizing tools such as balloons or rigid bronchoscopes with known diameters to get a “feel” for the lesion [
5,
6,
20]. Endoscopic sizing tools are also available to assist in ensuring appropriate fit [
9,
18,
19].
The required stent length depends on disease extent and anatomical constraints; however, longer stents may impair mucociliary function and increase the risk of mucus occlusion. While metallic stents have fixed lengths due to prepacking and mesh integrity concerns, silicone stents can be trimmed and shaped intraoperatively to accommodate patient-specific needs [
37].
Achieving optimal stent fit requires more than simply matching diameter and length; clinical judgement is essential to contour a prosthesis that provides adequate support while minimizing adverse tissue interactions. Advances in computational modeling offer promising avenues for stent customization by enabling precise volumetric adjustments tailored to complex airway morphologies.
Stent Deployment and Procedural Considerations
Stent deployment remains a critical aspect of the overall success of airway stenting. Standard rigid bronchoscopy tools, designed for linear, pre-manufactured stents, may not be well suited for unique contours of patient-specific designs. Regarding stent delivery, metal stents are preloaded for ease of placement, often utilizing guidewires or flexible bronchoscopic instruments that do not require rigid bronchoscopy. This makes them advantageous in certain scenarios. In contrast, silicone stents require manual loading and deployment, often necessating post-deployment adjustments or even removal and repositioning. Unlike metal stents, silicone stents can be reloaded, modified, or replaced, offering greater flexibility in complex cases.
From our experience, meticulous pre-procedural planning—incorporating 3D airway reconstructions before and after stent insertion—helps anticipate challenges and optimize fit. Intra operative stent trimming was required in several cases, highlighting the need for more flexible deployment systems that can accommodate customized stents. Future research should focus on developing dedicated deployment kits for 3D-printed stents and systematically evaluating post-deployment “settling” phase through serial imaging and bronchoscopic follow up.
Implications for 3DPS Stents and Future Directions
Our case series highlights that optimal stent fitting involves more than simply matching diameter and length—it requires a detailed understanding of the patient’s airway pathology and the dynamic interplay between the stent and airway structures. By leveraging 3D printing, we can tailor stent contours to provide targeted airway support while minimizing adverse tissue interactions. However, future investigations must systematically assess post-deployment outcomes, including the ‘settling’ phase, to determine whether these design principles translate into improved clinical performance. Respiration and coughing further influence stent positioning and function, necessitating ongoing assessment to validate these design strategies. This study emphasizes the importance of the design process, and the clinical decision making involved, while acknowledging that the final fit requires continuous evaluation.
Limitations
This study has notable limitations. As all cases involved Y-stents, we were unable to assess design-related factors influencing migration, which is typically less concerning with this configuration. Additionally, material properties were not extensively analyzed. The current stent model features a single wall thickness and base material, meaning its mechanical properties—such as softness (flexibility) and hoop strengh—are dictated primarily by diameter rather than intentional design variations. Furthermore, clinical outcomes were beyond the scope of this study, preventing meaningful evaluation of potential complications. The dynamic interaction between the stent and airway, particularly the post deployment settling phase, was not captured in our current analysis. Future work will integrate serial radiographic and bronchoscopic follow-up to better quantify stent fit and function.
Ongoing studies, including those registered under NCT03111888, aim to correlate stent parameters with objective oiutcomes such as migration, mucus clearance, and respiratory function. Although our current study focuses on design methodology, we recognize that the ultimate test of a stent’s efficacy lies in its clinical performance. Whether these advancements truly address the challenge of stent fit will require further post-deployment analysis in prospective studies.