Submitted:
21 March 2025
Posted:
24 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Cohort
- age ≥ 18 years
- kidney stone disease: NL and/or NC
- onset of kidney stone disease before 40 years of age
- the presence of at least one of the following: family history for NL and/or NC, indicative phenotype (such as distal renal tubular acidosis, particular stone composition), recurrent NL, and chronic kidney disease (CKD).
- patients diagnosed with a known non-genetic cause of kidney stone-related disease.
2.2. Clinical Assessments
2.3. Genetic Testing
- genes related to calcium metabolism: ADCY10, CASR, CLCN5, CLDN16, CLDN19, CYP24A1, GNA11, HNF4A, OCRL, VDR;
- genes related to renal tubular acidosis: ATP6V0A4, ATP6V1B1, SLC4A1, CA2, FOXI1;
- genes related to phosphate metabolism: SLC9A3R1, ALPL, SLC34A1, SLC34A3;
- genes related to uric acid metabolism: UMOD, HPRT1, PRPS1, SLC22A12, SLC2A9;
- Bartter syndrome genes: BSND, CLCNKA, CLCNKB, KCNJ1, MAGED2, SLC12A1;
- Cystinuria genes: SLC3A1, SLC7A9;
- genes related to oxalate metabolism: AGXT, GRHPR, HOGA1, SLC26A1;
- other genes: XDH, MOCOS, MOCS1, MOCS2, APRT, FAM20A, GPHN, CLPB, ATP7B.
2.4. Study Endpoints
- -
- to review the genotype and phenotype of hereditary NL and/or NC in adult patients from a single center;
- -
- to compare the clinical and biological characteristics of patients with positive and negative genetic diagnosis;
- -
- to evaluate the clinical utility of the molecular genetic diagnosis.
2.5. Statistical Analysis
3. Results
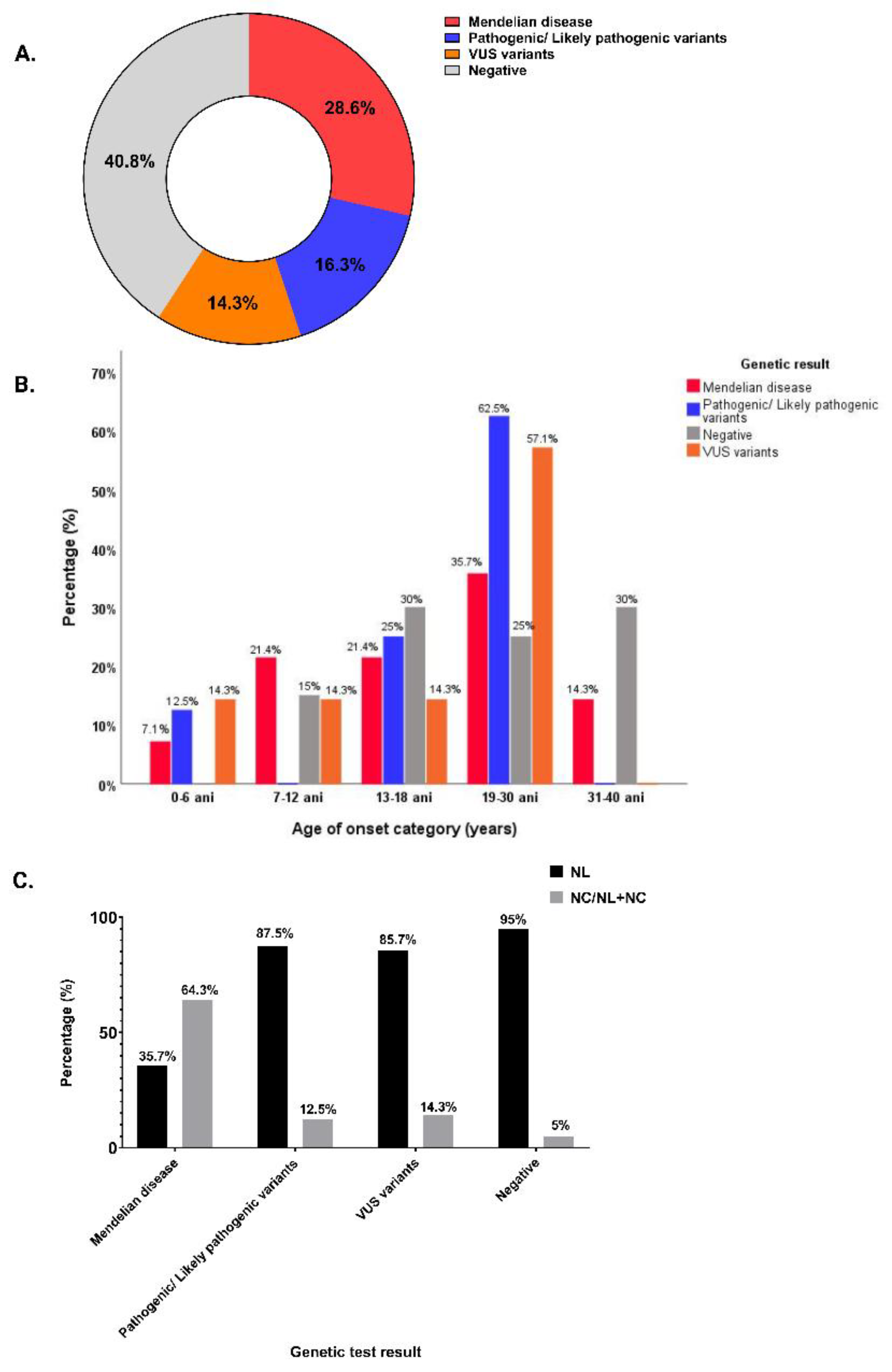
3.1. Study Cohort and Genetic Analysis
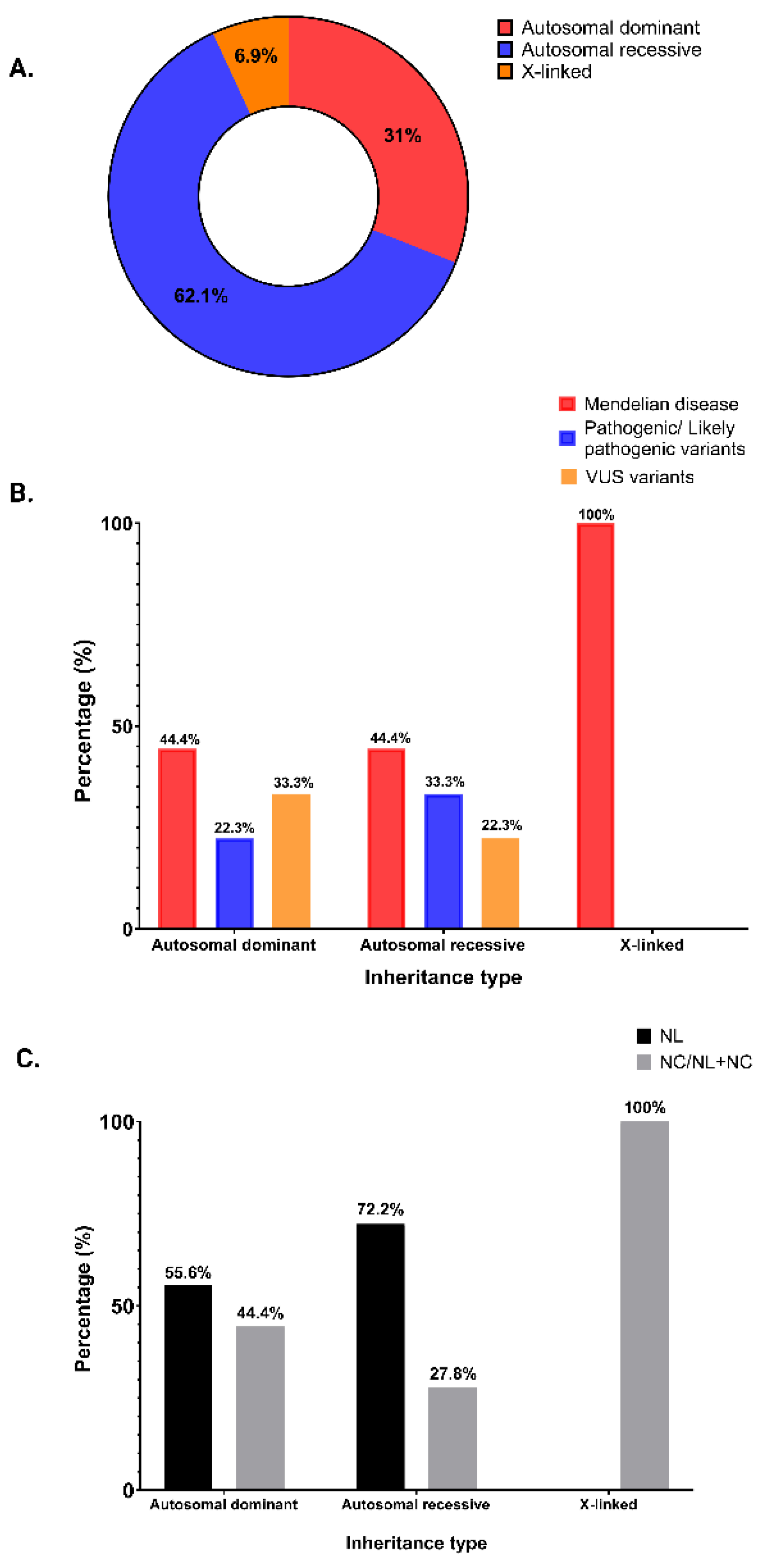
3.2. Genotype and Phenotype of the Positive Patients
3.2.1. Mendelian Forms of NL/NC
- Cystinuria
- Hereditary Distal Renal Tubular Acidosis
- Dent Disease
- Familial hypomagnesemia with hypercalciuria and nephrocalcinosis
- Infantile hypercalcemia type 1
- Primary hyperoxaluria type 1
- Bartter syndrome type 2
- Autosomal dominant tubulointerstitial kidney disease
3.2.2. Patients with a Possible Genetic Diagnosis for NL/NC
3.2.3. P/LP Monoallelic Variants Predisposing to NL/NC
3.2.4. Monoallelic VUS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chewcharat, A.; Curhan, G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis. 2021, 49, 27-39. [CrossRef]
- Liu, Y.; Chen, Y.; Liao, B.; Luo, D.; Wang, K.; Li, H.; Zengb, G. Epidemiology of urolithiasis in Asia. Asian J Urol. 2018, 5, 205–214. [CrossRef]
- Wang, K.; Ge, J.; Han, W.; Wang, D.; Zhao, Y.; Shen, Y.; Chen, J.; Chen, D.; Wu, J.; Shen, N.; Zhu, S.; Xue, B.; Xu, X. Risk factors for kidney stone disease recurrence: a comprehensive meta-analysis. BMC Urol 2022, 22, 62. [CrossRef]
- Daudon, M.; Jungers, P.; Bazin, D.; Williams, J.C. Jr. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis. 2018, 46, 459-470. [CrossRef]
- Hao, X.; Shao, Z.; Zhang, N.; Jiang, M.; Cao, X.; Li, S.; Guan, Y.; Wang, C. Integrative genome-wide analyses identify novel loci associated with kidney stones and provide insights into its genetic architecture. Nat Commun. 2023, 14, 7498. [CrossRef]
- Halbritter J. Genetics of kidney stone disease-Polygenic meets monogenic. Nephrol Ther. 2021, 17S, S88-S94. [CrossRef]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. Family history and risk of kidney stones. J Am Soc Nephrol. 1997, 8, 1568–1573. [CrossRef]
- Koyuncu, H.H.; Yencilek, F.; Eryildirim, B.; Sarica, K. Family history in stone disease: how important is it for the onset of the disease and the incidence of recurrence? Urol Res. 2010, 38, 105–109. [CrossRef]
- Goldfarb, D.S.; Avery, A.R.; Beara-Lasic, L.; Duncan, G.E.; Goldberg, J. A Twin Study of Genetic Influences on Nephrolithiasis in Women and Men. Kidney Int Rep. 2018, 4, 535-540. [CrossRef]
- Geraghty, R.; Lovegrove, C.; Howles, S.; Sayer, J.A. Role of Genetic Testing in Kidney Stone Disease: A Narrative Review. Curr Urol Rep. 2024, 25, 311-323. [CrossRef]
- Hill, F.; Sayer, J.A. Precision medicine in renal stone-formers. Urolithiasis. 2019, 47, 99-105. [CrossRef]
- Skolarikos, A.; Jung, H.; Neisius, A.; Petřík, A.; Somani, B.; Tailly, T.; Gambaro, G. Uroweb-European Association of Urology [Internet]. EAU Guidelines on Urolithiasis. [(accessed on 27 August 2024)]. Available online: https://uroweb.org/guidelines/urolithiasis.
- American Urological Association Kidney Stones: Medical Mangement Guideline. [(accessed on 27 August 2024)]. Available online: https://www.auanet.org/guidelines-and-quality/guidelines/kidney-stones-medical-mangement-guideline.
- Ferraro, P.M.; D’Addessi, A.; Gambaro, G. When to suspect a genetic disorder in a patient with renal stones, and why. Nephrol Dial Transplant. 2013, 28, 811–820. [CrossRef]
- Edvardsson, V.O.; Goldfarb, D.S.; Lieske, J.C.; Beara-Lasic, L.; Anglani, F.; Milliner, D.S.; Palsson, R. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol. 2013, 28, 1923–1942. [CrossRef]
- Policastro, L.J.; Saggi, S.J.; Goldfarb, D.S.; Weiss, J.P. Personalized Intervention in Monogenic Stone Formers. J Urol. 2018, 199, 623-632. [CrossRef]
- Halbritter, J.; Baum, M.; Hynes, A.M.; Rice, S.J.; Thwaites, D.T.; Gucev, Z.S.; Fisher, B.; Spaneas, L.; Porath, J.D.; Braun, D.A.; Wassner, A.J.; Nelson, C.P.; Tasic, V.; Sayer, J.A.; Hildebrandt, F. Fourteen monogenic genes account for 15% of nephrolithiasis/nephrocalcinosis. J Am Soc Nephrol. 2015, 26, 543–551. [CrossRef]
- Cogal, A.G.; Arroyo, J.; Shah, R.J.; Reese K.J.; Walton, B.N.; Reynolds, L.M; Kennedy, G.N.; Seide B.M.; Senum, S.R.; Baum, M.; Erickson, S.B.; Jagadeesh, S.; Soliman, N.A.; Goldfarb, D.S.; Beara-Lasic, L.; Edvardsson, V.O.; Palsson, R.; Milliner, D.S.; Sas, D.J.; Lieske, J.C.; Harris, P.C.; Investigators of the Rare Kidney Stone Consortium. Comprehensive Genetic Analysis Reveals Complexity of Monogenic Urinary Stone Disease. Kidney Int Rep. 2021, 6, 2862-2884. [CrossRef]
- Anderegg, M.A.; Olinger, E.G.; Bargagli, M.; Geraghty, R.; Taylor, L.; Nater, A.; Bruggmann, R.; Sayer, J.A.; Vogt, B.; Schaller, A.; Fuster, D.G. Prevalence and characteristics of genetic disease in adult kidney stone formers. Nephrol Dial Transplant. 2024, 39, 1426-1441. [CrossRef]
- Santoro, G.; Lombardi, G.; Andreola, S.; Salvagno, G.L.; Treccani, M.; Locatelli, E.; Ferraro, P.M.; Lippi, G.; Malerba, G.; Gambaro, G. Association analysis of 10 candidate genes causing Mendelian calcium nephrolithiasis in the INCIPE study: a South European general population cohort. Clin Kidney J. 2023, 16, 521-527. [CrossRef]
- Schönauer, R.; Scherer, L.; Nemitz-Kliemchen, M.; Hagemann, T.; Hantmann, E.; Seidel, A.; Müller, L.; Kehr, S.; Voigt, C.; Stolzenburg, J.-U.; Halbritter, J. Systematic assessment of monogenic etiology in adult-onset kidney stone formers undergoing urological intervention–evidence for genetic pretest probability. Am J Med Genet C Semin Med Genet. 2022, 190, 279-288. [CrossRef]
- Payne, N.G.; Boddu, S.P.; Wymer, K.M.; Heidenberg, D.J.; Van Der Walt, C.; Mi, L.; Keddis, M.; Stern, K.L. The Use of Genetic Testing in Nephrolithiasis Evaluation: A Retrospective Review from a Multidisciplinary Kidney Stone Clinic. Urology. 2024, 193, 20-26. [CrossRef]
- KDIGO CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 136–150.
- www.blueprintgenetics.com. Available online: https://blueprintgenetics.com/tests/panels/metabolic-disorders/nephrolithiasis-panel/ (accessed on 16 August 2024).
- www.invitae.com. Available online: https://www.invitae.com/us/providers/test-catalog/test-72037 (accessed on 16 August 2024).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; Voelkerding, k.; Rehm, H.L. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015, 17, 405–424. [CrossRef]
- Pearce R.J.; Sui W.; Yang, H.; Chi, T.; Stoller, M. The Yield of Genetic Testing in Management of Nephrolithiasis. Urology. 2024, 193, 27-34. [CrossRef]
- Daga, A.; Majmundar, A.J.; Braun, D.A.; Gee, H.Y.; Lawson, J.A.; Shril, S.; Jobst-Schwan, T.; Vivante, A.; Schapiro, D.; Tan, W.; Warejko, J.K.; Widmeier, E.; Nelson, C.P.; Fathy, H.M.; Gucev, Z.; Soliman, N.A.; Hashmi, S., Halbritter, J.; Halty, M.; Kari, J.A.; El-Desoky, S.; Ferguson, M.A.; Somers, M.J.G.; Traum, A.Z.; Stein, D.R.; Daouk, G.H.; Rodig, N.M.; Katz, A.; Hanna, C.; Schwaderer, A.L.; Sayer, J.A.; Wassner, A.J.; Mane, S.; Lifton, R.P.; Milosevic, D.; Tasic, V.; Baum, M.A.; Hildebrandt, F. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018, 93, 204-213. [CrossRef]
- Spasiano, A.; Treccani, M.; De Tomi, E.; Malerba, G.; Gambaro, G.; Ferraro, P.M. Characteristics and Yield of Modern Approaches for the Diagnosis of Genetic Causes of Kidney Stone Disease. Genes 2024, 15, 1470. [CrossRef]
- Martins, M.C.; Meyers, A.A.; Whalley, N.A.; Rodgers, A.L. Cystine: A promoter of the growth and aggregation of calcium oxalate crystals in normal undiluted human urine. J Urol. 2002, 167, 317–321.
- Elkoushy, M.A.; Andonian S. Characterization of patients with heterozygous cystinuria. Urology 2012, 80, 795-799. [CrossRef]
- Rhodes, H.L.; Yarram-Smith, L.; Rice, S.J.; Tabaksert, A.; Edwards, N.; Hartley, A.; Woodward, M.N.; Smithson, S.L.; Tomson, C.; Welsh, G.I.; Williams, M.; Thwaites, D.T.; Sayer, J.A.; Coward, R.J.M. Clinical and Genetic Analysis of Patients with Cystinuria in the United Kingdom. Clin J Am Soc Nephrol. 2015, 10, 1235–1245. [CrossRef]
- Alghamdi, M.; Alhasan, K.A.; Taha Elawad, A.; Salim, S.; Abdelhakim, M., Nashabat, M.; Raina, R.; Kari, J.; Alfadhel, M. Diversity of Phenotype and Genetic Etiology of 23 Cystinuria Saudi Patients: A Retrospective Study. Front. Pediatr. 2020, 8:569389. [CrossRef]
- Dasgupta, D.; Wee, M.J.; Reyes, M.; Li, Y.; Simm, P.J.; Sharma, A.; Schlingmann, K.P.; Janner, M.; Biggin, A.; Lazier, J.; Gessner, M.; Chrysis, D.; Tuchman, S.; Baluarte, H.J.; Levine, M.A.; Tiosano, D.; Insogna, K.; Hanley, D.A.; Carpenter, T.O.; Ichikawa, S.; Hoppe, B.; Konrad, M.; Sävendahl, L.; Munns, C.F.; Lee, H.; Jüppner, H.; Bergwitz, C. Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol. 2014, 25, 2366-2375. [CrossRef]
- Nwachukwu, C.; Singh, G.; Moore, B.; Strande, N.T.; Bucaloiu, I.D.; Chang, A.R. Risk of Nephrolithiasis in Adults Heterozygous for SLC34A3 Ser192Leu in an Unselected Health System Cohort. J Am Soc Nephrol. 2023, 34, 1819-1821. [CrossRef]
- Sadeghi-Alavijeh O, Chan MMY, Moochhala SH. Genomics England Research Consortium; Howles S, Gale DP, Böckenhauer D. Rare variants in the sodium-dependent phosphate transporter gene SLC34A3 explain missing heritability of urinary stone disease. Kidney Int. 2023, 104, 975-984. [CrossRef]
- Wang, Q.; Chen, J.; Wei, L. et al. Biallelic and monoallelic pathogenic variants in CYP24A1 and SLC34A1 genes cause idiopathic infantile hypercalcemia. Orphanet J Rare Dis. 2024, 19, 126. [CrossRef]
- Oddsson, A.; Sulem, P.; Helgason, H.; Edvardsson, V.O.; Thorleifsson, G.; Sveinbjörnsson, G.; Haraldsdottir, E.; Eyjolfsson, G.I.; Sigurdardottir, O.; Olafsson, I.; Masson, G.; Holm, H.; Gudbjartsson, D.F.; Thorsteinsdottir, U.; Indridason, O.S.; Palsson, R.; Stefansson, K. Common and rare variants associated with kidney stones and biochemical traits. Nat Commun. 2015, 6, 7975. [CrossRef]
- Stephan, R.; Hoppe, B. Genetic kidney stones disease in adults. Nephrol Dial Transplant. 2024, 39, 1381-1383. [CrossRef]
| Characteristics | Positive genetic test (N=29) |
Negative genetic test (N=20) |
p value | |
|---|---|---|---|---|
| General Features |
Age at genetic test, years, mean ± SD | 34.86 ± 9.23 | 35.50 ± 11.65 | 0.83 |
| Age at onset, years, mean ± SD | 20.52 ± 9.68 | 24.20 ± 10.71 | 0.21 | |
| Gender, n (%) | 0.25 | |||
| Male | 12 (41.4%) | 11 (55%) | ||
| Female | 17 (58.6%) | 9 (45%) | ||
| Hypertension, n (%) | 1 (3.4%) | 5 (25%) | 0.03 | |
| Diabetes, n (%) | 3 (10.3%) | 0 (0%) | 0.26 | |
| Obesity (BMI ≥ 30 kg/m2), n (%) | 2 (6.9%) | 1 (5%) | 1 | |
| Dyslipidemia, n (%) | 3 (10.3%) | 1 (5%) | 0.63 | |
| Hyperuricemia, n (%) | ||||
| Family history of NL/NC, n (%) | 20 (69%) | 14 (70%) | 0.93 | |
| Nephrolithiasis, n (%) | 18 (62.1%) | 19 (95%) | 0.10 | |
| Recurrent NL, n (%) | 23 (79.3%) | 15 (75%) | 0.75 | |
| Bilateral NL, n (%) | 22 (75.9%) | 13 (65%) | 0.45 | |
| Nephrocalcinosis, n (%) | 2 (6.9%) | 1 (5%) | 1 | |
| NL + NC, n (%) | 9 (31%) | 0 (0%) | 0.007 | |
| CKD, n (%) | 0.03 | |||
| G1 | 9 (31%) | 8 (40%) | ||
| G2 | 5 (17.2%) | 10 (50%) | ||
| G3 | 7 (24.1%) | 1 (5%) | ||
| G4 | 4 (13.8%) | 1 (5%) | ||
| G5 Dialysis/KT |
2 (6.9%) 2 (6.9%) |
0 (0%) 0 (0%) |
||
| Blood Parameters |
Total calcium, mg/dl, mean ± SD | 9.36 ± 0.71 | 9.73 ± 0.70 | 0.10 |
| Ionized calcium, mg/dl, mean ± SD | 4.04 ± 0.18 | 4.05 ± 0.15 | 0.83 | |
| Phosphorus, mg/dl, mean ± SD | 3.40 ± 0.76 | 3.20 ± 0.72 | 0.45 | |
| Magnesium (xx) mean ± SD | 2.00 ± 0.26 | 1.96 ± 0.18 | 0.59 | |
| Uric acid, mg/dl, mean ± SD | 5.88 ± 1.79 | 5.74 ± 1.25 | 0.76 | |
| Intact parathormone, ng/l, median (IQR) | 86.5 (47.5 - 132.9) | 64.3 (55.6 - 93.8) | 0.72 | |
| eGFR, CKD-EPI no race 2021 equation, ml/min/1.73m2, median (IQR) | 69.7 (41.0- 98.0) | 85.5 (77.0- 121.3) | 0.03 | |
| Composition | 0.10 | |||
| Stone Composition* |
Calcium oxalate monohydrate, n (%) | 7 (25.9%) | 3 (15%) | |
| Calcium oxalate dihydrate, n (%) | 2 (7.4%) | 2 (10%) | ||
| Uric acid, n (%) | 2 (6.9%) | 1 (5%) | ||
| Cystine, n (%) | 3 (11.1%) | 0 (0%) | ||
| Calcium phosphate, n (%) | 7 (24.1%) | 1 (5%) | ||
| Unknown, n (%) | 5 (17.2%) | 11 (55%) | ||
| History of surgical interventions | Ureteroscopy with lithotripsy, n (%) | 14 (48.3%) | 9 (45%) | 0.82 |
| ESWL, n (%) | 7 (24.1%) | 1 (5%) | 0.11 | |
| Percutaneous nephrolithotomy, n (%) | 4 (13.8%) | 3 (15%) | 0.90 | |
| Pielolithotomy, n (%) | 5 (17.2%) | 1 (5%) | 0.37 | |
| Nephrectomy, n (%) | 2 (6.9%) | 0 (%) | 1 |
| Genetic Diagnosis |
Patient No. |
Sex/ Age (yrs) |
Genetic Testing (Gene, variant) |
Inheritance | Allelic state | ACMG class | On- set Age (yrs) |
Clinical diagnosis before genetic test |
Phenotype |
|---|---|---|---|---|---|---|---|---|---|
| Cystinuria type A |
1 | B/45 |
SLC3A1, c.1354C>T, p.Arg452Trp; c.1094G>T, p.Arg365Leu |
AR | com het |
P | 35 |
Cystinuria | Cystine NL, CKD G5, unilateral renal cysts |
| 2 | F/43 |
SLC3A1, c.1400T>C, p.Met437Thr |
AR | hom | P | 25 |
Cystinuria, polycystic kidney disease (PKD1 mutation c.4175_4176del, p.Val1392Alafs*38) |
Cystine NL, CKD G3a, Bilateral kidney cysts |
|
| Cystinuria type B |
3 | M/53 |
SLC7A9, c.313G>A, p.Gly105Arg; c.690G>A , p.Trp230* |
AR | com het |
P, LP |
14 |
Cystinuria |
Cystine NL, CKD G4 |
| 4 | F/52 |
SLC7A9, c.313G>A, p.Gly105Arg; c.217G>A, p.Gly73Arg |
AR/ AD |
com het |
P, LP |
29 |
ESKD, NL |
NL, ESKD, hemodialysis | |
| Autosomal dominant distal renal tubular acidosis |
5 | F/27 |
SLC4A1, c.1765C>T, p.Arg589Cys | AD/AR | het | P | 18 |
dRTA | Carbapatite NL, medullary NC, dRTA, CKD G3a |
| 6 | M/39 |
SLC4A1, c.1825G>A, p.Gly609Arg |
AD | het | P | 12 |
Medullary sponge kidney, dRTA | Carbapatite NL, medullary NC, dRTA, developmental delay, CKD stage G3a | |
| 7 |
M/35 |
SLC4A1, c.1825G>A, p.Gly609Arg |
AD | het | P | 13 |
dRTA | Carbapatite NL, medullary NC, developmental delay dRTA, CKD G3b | |
| Dent disease | 8 | M/53 |
CLCN5, c.1561C>T, p.Leu521Phe |
XL | hem | LP | 28 |
NL, NC Polycystic kidney disease |
NL and NC, ESKD starting at age 28, receiving RRT (HD, afterwards KT) |
| 9 |
F/37 |
CLCN5, c.794G>A, p.Ser265Asn |
XL | het | LP | 37 |
NC | Severe medullary NC, mild proteinuria, CKD G1 |
|
| Familial hypomagnesemia with hypercalciuria and nephrocalcinosis | 10 | F/20 |
CLDN16 c.646C>T, p.Arg216Cys |
AR |
hom |
P |
7 |
NL, NC, Tubulo-interstitial disease |
NL and NC, hypomagnesemia, mild hypocalcemia, hypophosphatemia, hypercalcemia, proteinuria, CKD G4 |
| Infantile Hypercalcemia type 1 |
11 | M/25 |
CYP24A1, c.428_430del, p.Glu143del; c.443T>C, p.Leu148Pro |
AR | com het |
P | 24 |
NC | Hypercalcemia, low iPTH, bilateral NC, CKD G2, right kidney cyst |
| Primary hyperoxaluria type 1 |
12 |
F/42 |
AGXT, c.33del, p.Lys12Argfs*34; c.508G>A, p.Gly170Arg |
AR |
com het |
P | 6 |
PH1, NL, NC |
Calcium oxalate monohydrate NL, increased urinary and plasma oxalate, medullary NC, CKD G4 |
| Bartter syndrome type 2 |
13 | F/32 |
KCNJ1, c.658C>T, p. Leu220Phe |
AR | hom | P | 12 |
NC, NL, Type 3 RTA, diabetes insipi- dus, hyperreninemic hyperaldosteronism |
NL, NC, hypokalemia, hypercalciuria, weakness, polyuria, polydipsia, hyperreninemic hyperaldosteronism, CKD G3a, parathyroid adenoma |
| Autosomal dominant tubulointerstitial kidney disease | 14 | F/36 |
UMOD, c.686>A, p.(Met229Lys) |
AD | het | P | 28 |
ADTKD, NL |
Progressive CKD, bland urinalysis, hyperuricemia, sporadic kidney stones, CKD G2 |
| M, male; F, female; AD, autosomal dominant; AR, autosomal recessive; hem, hemizygous; het, heterozygous; hom, homozygous; com het, compound heterozygous; CKD, chronic kidney disease; NL, nephrolithiasis; NC, nephrocalcinosis; dRTA, distal renal tubular acidosis; ESKD, end-stage kidney disease; RRT, renal replacement therapy; HD, hemodialysis; KT, kidney transplant; iPTH, intact parathyroid hormone. | |||||||||
| Possible genetic diagnosis |
Patient No. |
Sex/ Age (yrs) |
Genetic Testing |
Inhe-ritance | Allelic state | ACMG class | Onset Age (yrs) |
Clinical diagnosis before genetic test |
Phenotype |
|---|---|---|---|---|---|---|---|---|---|
| Autosomal dominant familial idiopathic hypercalciuria | 1 | F/31 |
ADCY10, c.4558G>A, p.Val1520Ile |
AD | Het | VUS | 15 |
PH, NL |
NL with calcium oxalate monohydrate stone composition, and normal kidney function |
| Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) |
2 | B/40 |
SLC34A3, c.274G>A, p.Val92Ile ; c.286G>A, p.Ala96Thr |
AR | com het |
VUS | 37 |
Idiopathic NL | Recurrent NL with multiple surgical interventions, hypophosphatemia, and normal kidney function |
| M, male; F, female; AD, autosomal dominant; AR, autosomal recessive; het, heterozygous; com het, compound heterozygous; VUS, variant of uncertain significance; PH, primary hyperoxaluria; NL, nephrolithiasis. | |||||||||
| Patient No. |
Sex/ Age (yrs) |
Genetic Testing (gene, variant) |
Inheritance | Allelic state | ACMG class | Onset age (yrs) |
Clinical diagnosis before genetic test |
Phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 |
F/32 |
SLC3A1, c.1400T>C, p.Met467Thr |
AR | het | P | 30 |
NL | Calcium carbonate and struvite NL, normal kidney function, many episodes of renal colic (~20/year) with multiple urological interventions |
| 2 | M/30 |
SLC3A1, c.(891+1_892-1)_(1617+1_1618-1)dup |
AR | het | P | 28 |
NL | Calcium oxalate dihydrate NL, normal kidney function |
| 3 | M/26 |
SLC7A9, c.217G>A, p.Gly73Arg |
AD | het | LP | 25 |
NL | Calcium oxalate NL, normal kidney function |
| 4 | F/30 |
SLC7A9, c.313G>A, p.Gly105Arg |
AD | het | P | 20 |
NL | Calcium oxalate NL, normal kidney function |
| 5 |
F/28 |
AGXT, c.107G>A, p.Arg36His and SLC34A3, c.575C>T, p.Ser192Leu |
AR | het | P | 18 |
NL | Calcium oxalate monohydrate NL, slight increase in urinary oxalate, CKD G2 |
| 6 | F/25 |
AGXT, c.107G>A, p.Arg36His and SLC34A3, c.575C>T, p.Ser192Leu |
AR | het | P | 20 |
NL | Calcium oxalate monohydrate NL, slight increase in urinary oxalate, CKD G2 |
| 7 | M/18 |
SLC34A3, c.1304del, p.Ser435Thrfs*46 |
AR | het | P | 17 |
NC | Bilateral medullary NC, borderline phosphorus, metabolic alkalosis, hypercalciuria, normal kidney function |
| 8 | M/23 |
CLCNKB, c.(?_-1)_(*1_?)del, p.0 |
AR | het | P | 5 |
dRTA | NL with calcium oxalate and carbapatite stone composition, dRTA, normal kidney function |
| Patient No. |
Sex/ Age (yrs) |
Genetic Testing |
Inheritance | Allelic state | ACMG class | On- set Age (yrs) |
Clinical diagnosis before genetic test |
Phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 | F/39 |
SLC3A1 c.1684G>C, p.Glu562Gln |
AR | het | VUS | 33 |
NL, NC | Oxalate and phosphate NL, bilateral severe medullary NC, CKD G2 |
| 2 | F/37 |
SLC22A12, c.412G>A, p.Val138Met |
AR | het | VUS | 29 |
NL | Calcium phosphate and struvite NL, multiple urological interventions, hypouricemia, normal kidney function |
| 3 | F/35 |
SLC22A12, c.431T>C, p.Leu144Pro |
AR | het | VUS | 31 |
NL, ESKD | Staghorn NL with obstructive nephropathy, ESKD |
| 4 | F/28 |
SLC22A12, c.1427C>A, p.Ala476Asp, and HNF1B c.544+5G>A, het, VUS |
AR | het | VUS | 11 |
Genetic tubulo- interstitial disease. CKD G4 |
NL, dysplastic kidney, renal cysts, tubule-interstitial kidney disease, diabetes mellitus, hyperparathyroidism, CKD G4. |
| 5 |
B/42 |
SLC26A1 c.2007C>G, p.Asp669Glu and HNF1B c.867C>G, p.Asn289Lys, het, VUS |
AR | het | VUS | 4 |
NL, ADTKD |
Bilateral NL, uric and oxalic diathesis, multicystic and dysplastic kidney, tubulointerstitial kidney disease, CKD G3a, diabetes mellitus, pancreatic hypoplasia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





