Submitted:
20 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- syphilis AND differentiation AND anthropology
- syphilis AND molecular AND anthropology
- syphilis AND markers AND anthropology
- syphilis AND imaging AND anthropology
- syphilis AND diagnostics AND anthropology
- The record was an original, peer-reviewed, and published study.
- The full text was available.
- The full text was in English.
- The full text was relevant to the topic of our review: it discusses how described diagnostic methods are being or can be used to detect osteological lesions or molecular marks caused by Treponema pallidum infection in anthropological samples and/or possibilities of differential diagnosis of those lesions.
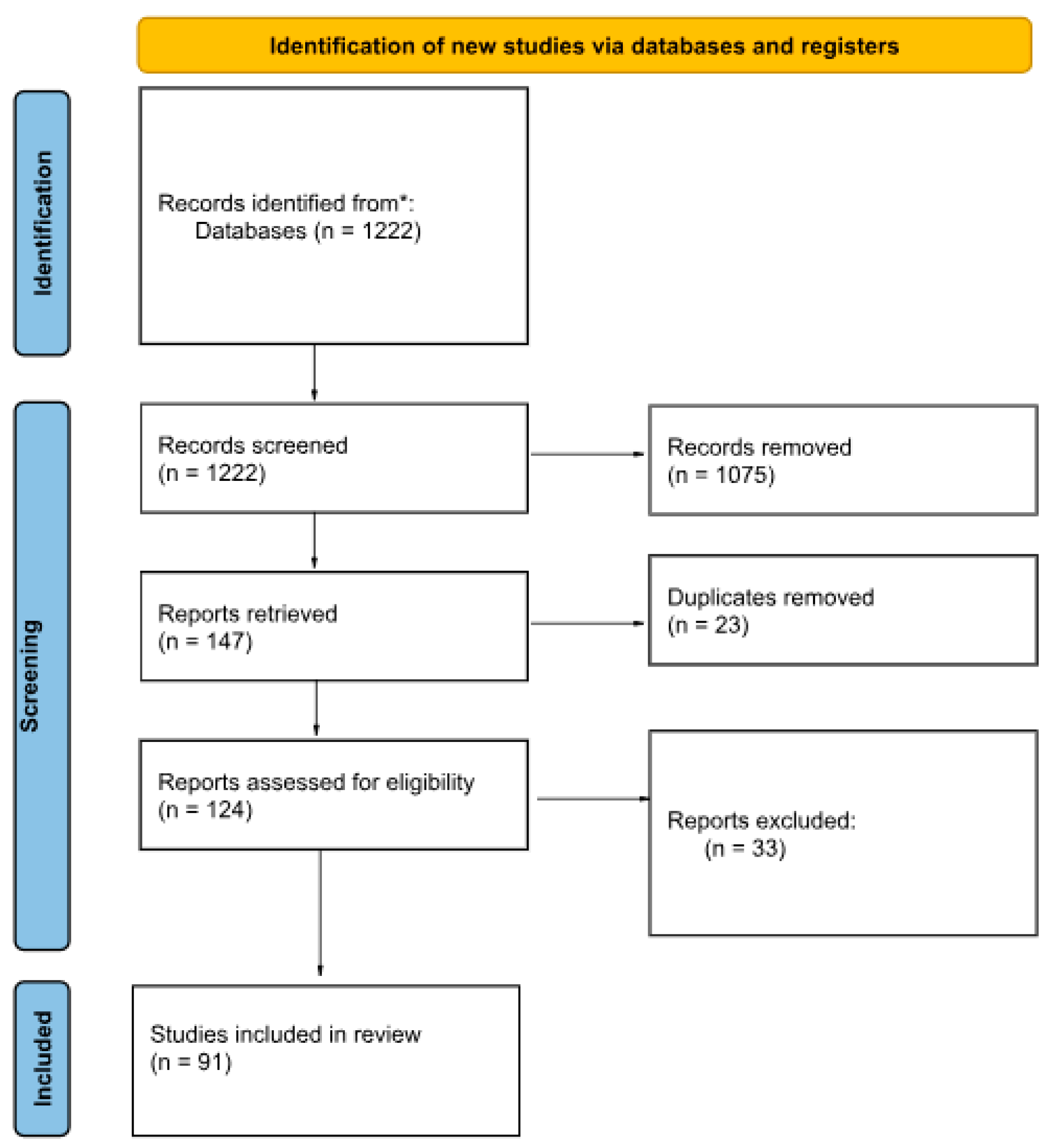
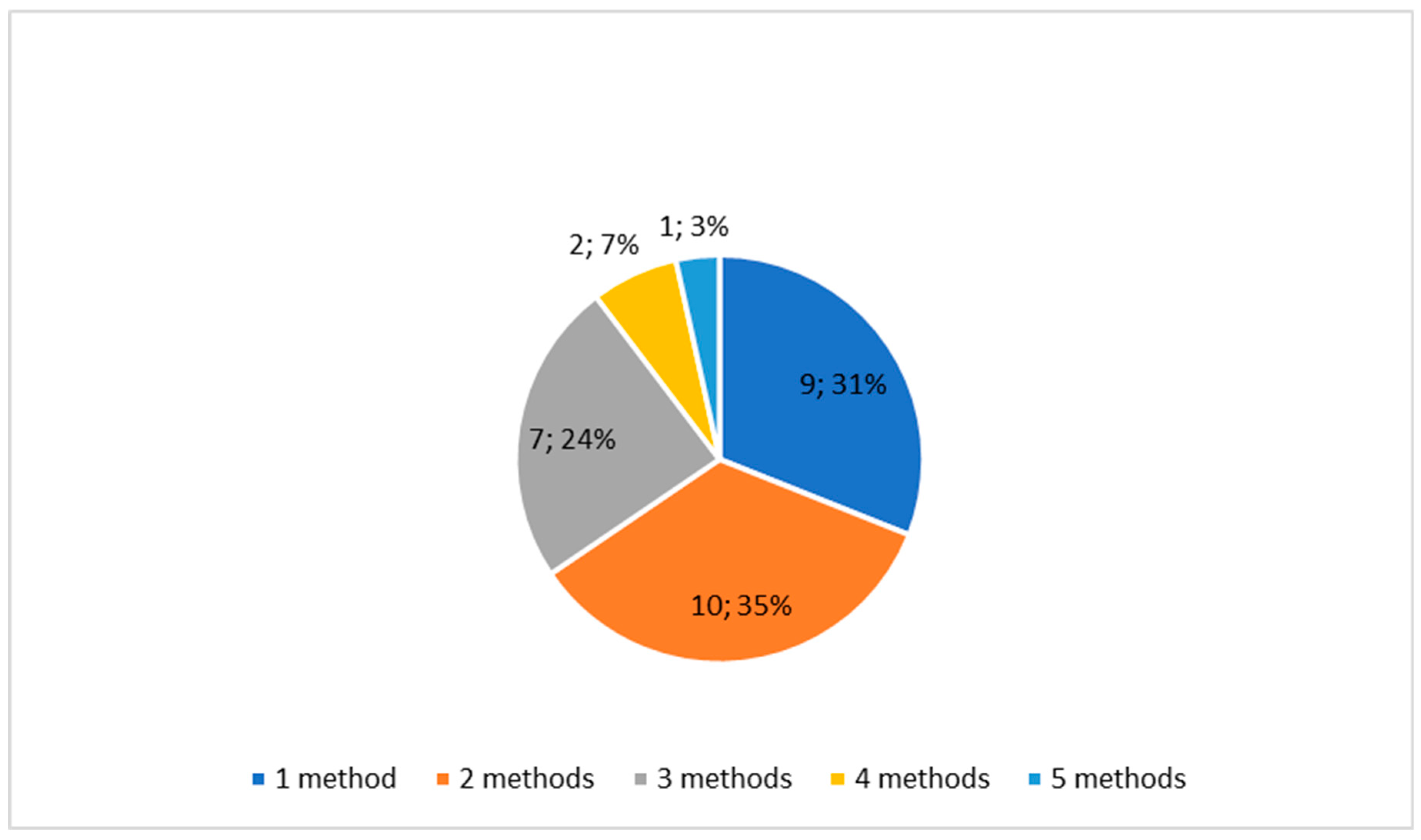
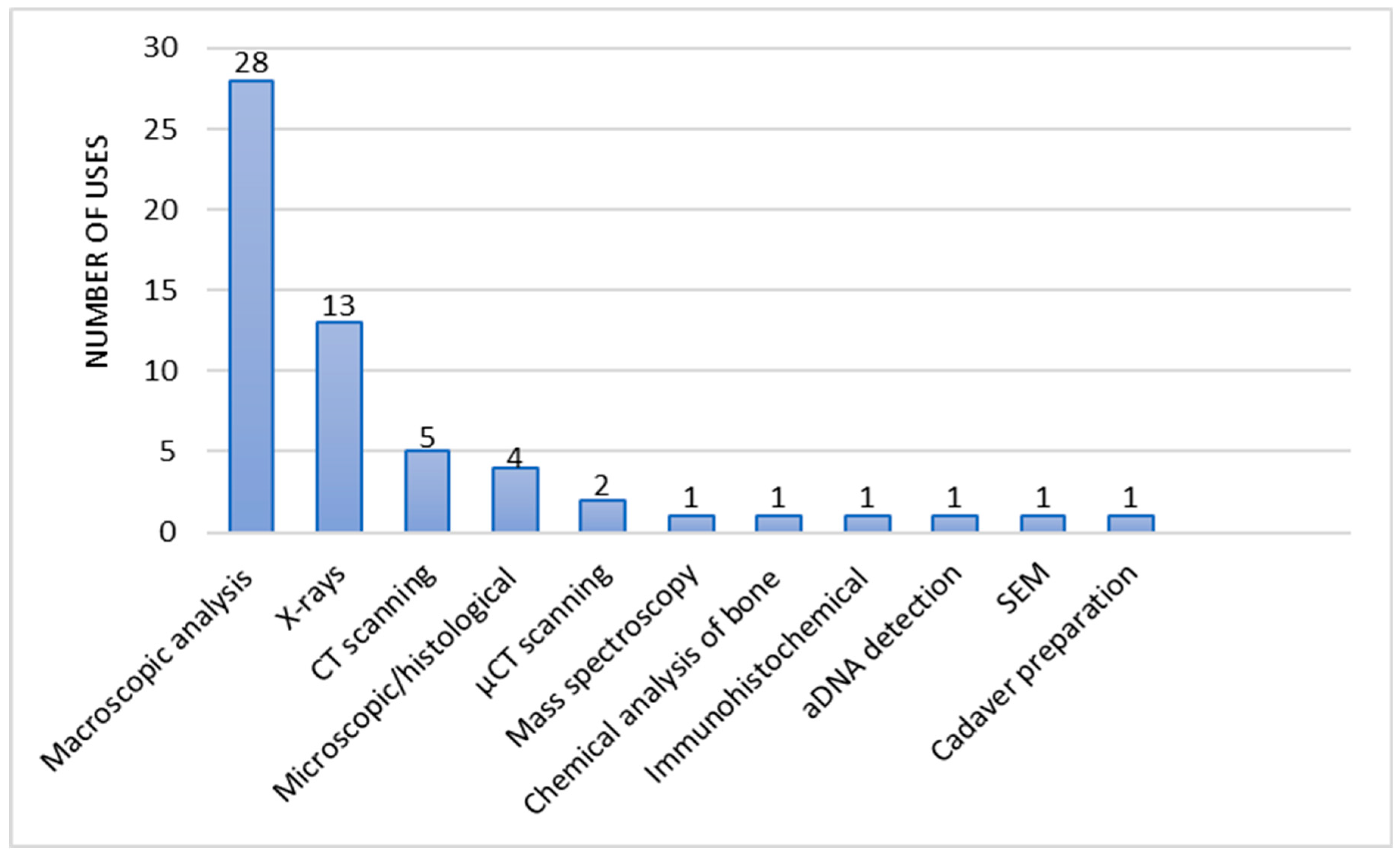
3. Results
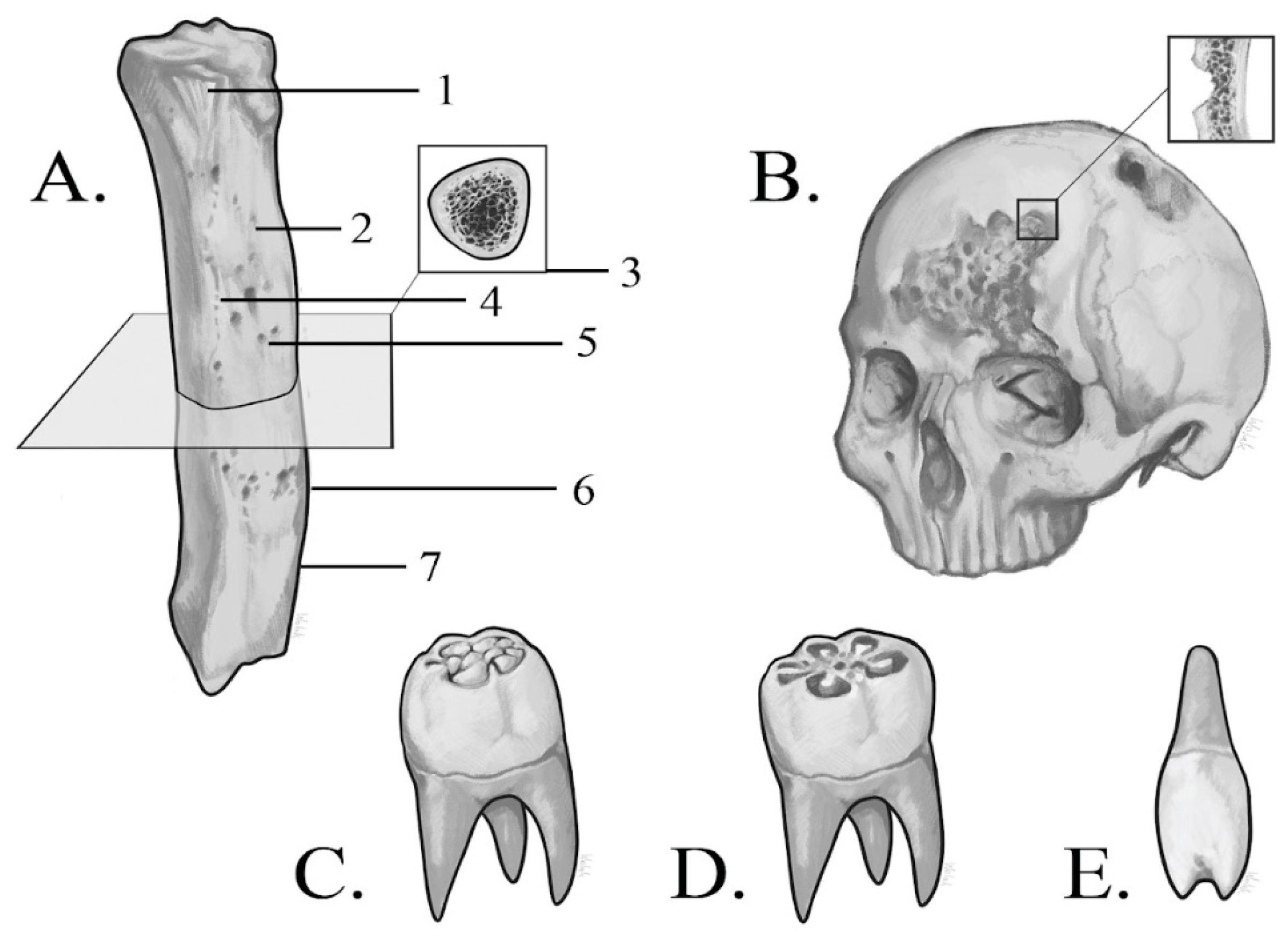
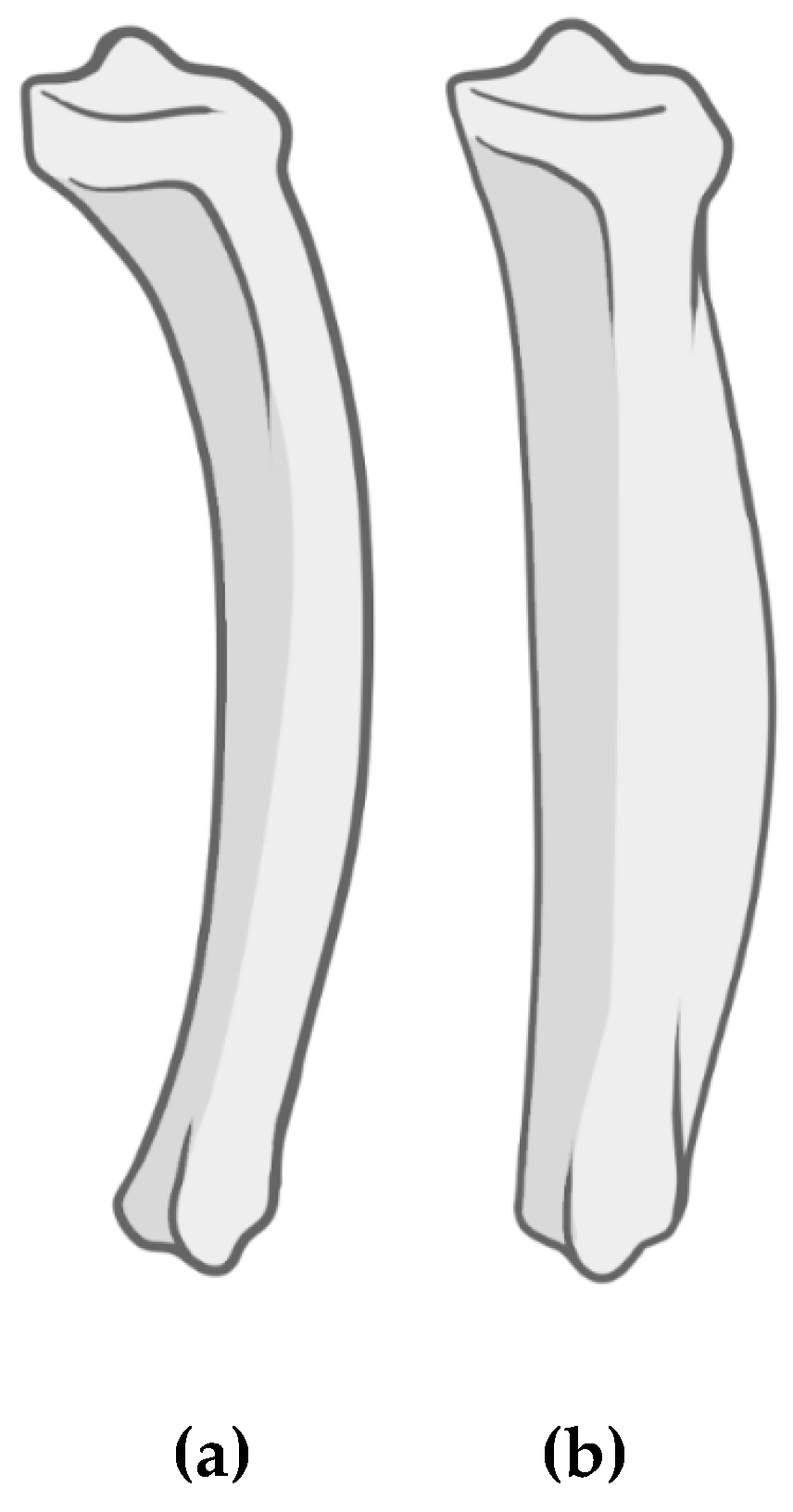
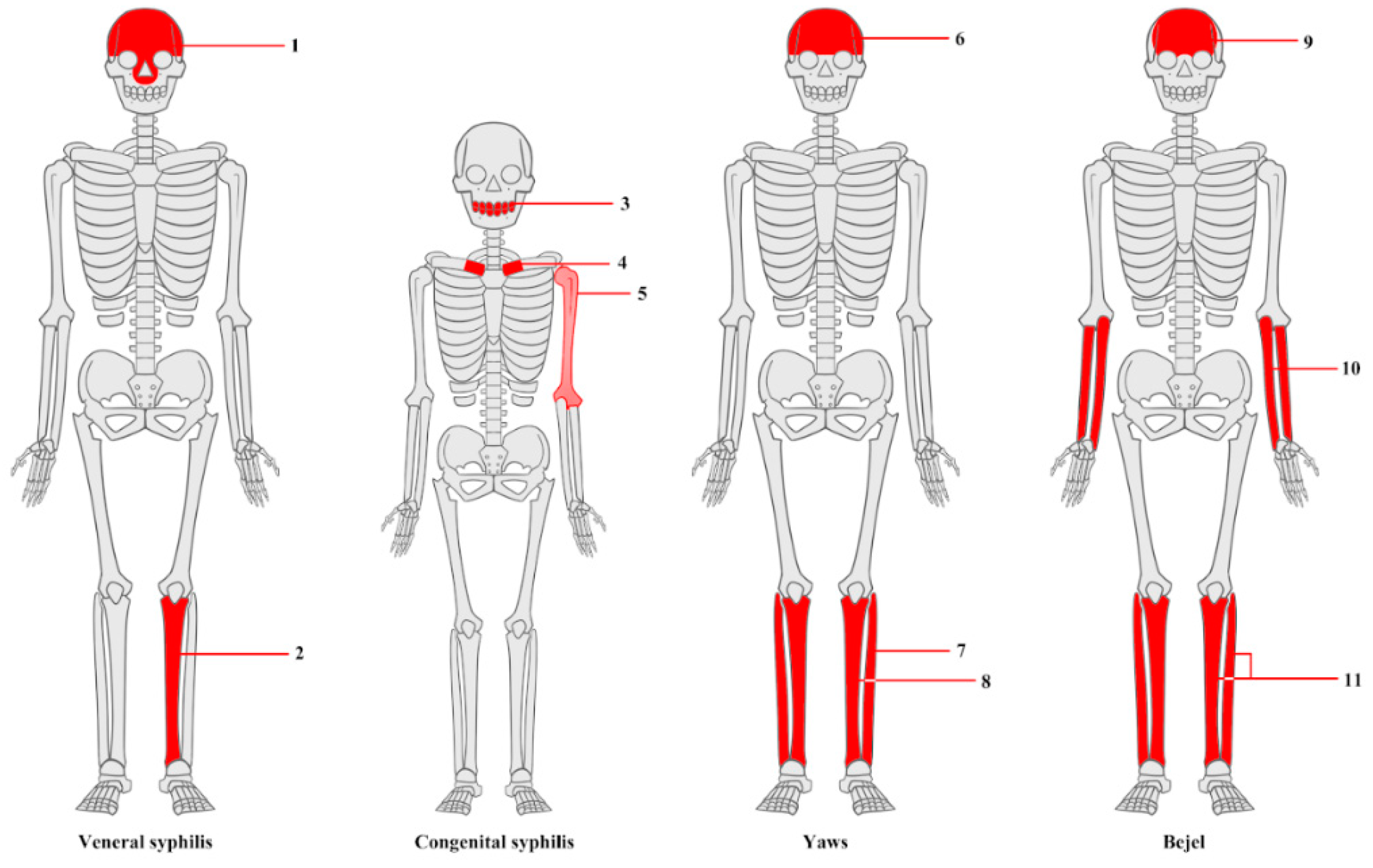
3.1. Macroscopic Analysis of Bone Lesions
3.2. Microscopic Analysis of Bone Lesions
3.3. Radiological Examinations
3.3.1. X-Ray
3.3.2. CT Imaging
3.3.3. Micro-CT Imaging
3.4. Genetic Techniques
3.5. Detection of Heavy Metals
3.6. Case Reports
4. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| ICT | Micro-computed tomography |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| aDNA | Ancient deoxyribonucleic acid |
| SEM | Scanning electron microscope |
| LPA | Linear polyacrylamide |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| LA-ICP-MS | Laser ablation inductively coupled mass spectrometry |
| pXRF | Portable X-ray fluorescence |
References
- Cole, H.N.; Harkin, J.C.; Kraus, B.S.; Moritz, A.R. Pre-Columbian Osseous Syphilis Skeletal Remains Found at Kinishba and Vandal Cave, Arizona, with Some Comments on Pertinent Literature [Internet]. 1955. Available from: http://archderm.jamanetwork.com/.
- Dabernat, H.; Reis, T.M.; Tarasov, A.Y.; Artyukhov, I.P.; Nikolaev, V.G.; Medvedeva, N.N.; et al. Paleopathology of the population of Krasnoyarsk, central Siberia (Pokrovskiy and Voskresensko-Preobrazhenskiy cemeteries of the 17th- early 20th centuries). Archaeology, Ethnology and Anthropology of Eurasia. 2013, 41, 140–150. [Google Scholar] [CrossRef]
- Hernandez, M.; Hudson, M.J. Diagnosis and evaluation of causative factors for the presence of endemic treponemal disease in a Japanese sub-tropical island population from the Tokugawa period. Int J Paleopathol. 2015, 10, 16–25. [Google Scholar] [CrossRef]
- Fraberger, S.; Dockner, M.; Winter, E.; Pretterklieber, M.; Weber, G.W.; Teschler-Nicola, M.; et al. Micro-CT evaluation of historical human skulls presenting signs of syphilitic infection. Wien Klin Wochenschr. 2021, 133, 602–609. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Vol. 10, Frontiers in Cellular and Infection Microbiology. Frontiers Media S.A.; 2021.
- Austin, R.M.; Honap, T.P.; Mann, A.E.; Hübner, A.; DeGaglia, C.M.S.; Warinner, C.; et al. Metagenomic and paleopathological analyses of a historic documented collection explore ancient dental calculus as a diagnostic tool. Sci Rep. 2024, 14, 14720. [Google Scholar] [CrossRef] [PubMed]
- Malgosa, A.; Aluja, M.P.; Isidro, A. Pathological Evidence in Newborn Children from the Sixteenth Century in Huelva (Spain). International Journal of Osteoarcbaeology. 1996, 6. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Rothschild, C. Treponemal Disease Revisited: Skeletal Discriminators for Yaws, Bejel, and Venereal Syphilis [Internet]. 1995. Available from: http://cid.oxfordjournals.org/.
- Schwarz, S.; Skytte, L.; Rasmussen, K.L. Pre-Columbian treponemal infection in Denmark?- a paleopathological and archaeometric approach. Herit Sci. 2013, 1. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Calderon, F.L.; Coppa, A.; Rothschild, C. First European Exposure to Syphilis: The Dominican Republic at the Time of Columbian Contact [Internet]. 2000. Available from: http://cid.oxfordjournals.org/.
- Shuler, K.A. Life and death on a Barbadian sugar plantation: Historic and bioarchaeological views of infection and mortality at Newton Plantation. Int J Osteoarchaeol. 2011, 21, 66–81. [Google Scholar] [CrossRef]
- Castro, M.; Pacheco, A.; Kuzmanic, I.; Clarot, A.; Díaz, P. Treponematosis in a pre-Columbian hunter-gatherer male from Antofagasta (1830 ± 20 BP, Northern Coast of Chile). Int J Paleopathol. 2020, 30, 10–16. [Google Scholar] [CrossRef]
- Dabrowski, P.; Kulus, M.J.; Cieslik, A.; Domagala, Z.; Wiglusz, R.J.; Kuropka, P.; et al. A case of syphilis with high bone arsenic concentration from early modern cemetery (Wroclaw, Poland). Open Life Sci. 2019, 14, 427–439. [Google Scholar] [CrossRef]
- Vargová, L.; Vymazalová, K.; Horáčková, L. Evidences of children’s inflammatory diseases, trauma and tumours from the 13th to the 19th centuries in the czech lands. Anthropologie. 2021, 155–170. [Google Scholar] [CrossRef]
- Tomczyk, J.; Mańkowska-Pliszka, H.; Palczewski, P.; Olczak-Kowalczyk, D. Congenital syphilis in the skeleton of a child from Poland (Radom, 18th-19th century AD). Vol. 78, Anthropological Review. Versita; 2015. p. 79–90.
- Marden, K.; Ortner, D.J. A case of treponematosis from pre-Columbian Chaco Canyon, New Mexico. Int J Osteoarchaeol. 2011, 21, 19–31. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Mariotti, V.; Fusari, S.; Gasparini, A.; Bettuzzi, M.; Morigi, M.P.; et al. Syphilis in an Italian medieval jewish community: A bioarchaeological and cultural perspective. Int J Paleopathol. 2020, 30, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Anteric, I.; Basic, Z.; Vilovic, K.; Kolic, K.; Andjelinovic, S. Which theory for the origin of syphilis is true? Journal of Sexual Medicine. 2014, 11, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- P C Gerszten, E Gerszten, M J Allison. Diseases of the skull in pre-columbian South American mummies. Neurosurgery. 1998, 42. [Google Scholar]
- Walker, D.; Powers, N.; Connell, B.; Redfern, R. Evidence of skeletal treponematosis from the medieval burial ground of St. Mary Spital, London, and implications for the origins of the disease in Europe. Am J Phys Anthropol. 2015, 156, 90–101. [Google Scholar] [CrossRef]
- Somers, J.; Cooper, C.; Alterauge, A.; Lösch, S. A Medieval/Early Modern Alpine Population from Zweisimmen, Switzerland: A Comparative Study of Anthropology and Palaeopathology. Int J Osteoarchaeol. 2017, 27, 958–972. [Google Scholar] [CrossRef]
- El Najjar, M.Y. Human Treponematosis and Tuberculosis: Evidence from the New World. 1979.
- Hackett, C.J. Diagnostic Criteria of Syphilis, Yaws and Treponarid (Treponematoses) and of Some Other Diseases in Dry Bones. Diagnostic Criteria of Syphilis, Yaws and Treponarid (Treponematoses) and of Some Other Diseases in Dry Bones. 1976.
- Williams, H.U. The origin and antiquity of syphilis. The evidence from diseased bones. Arch. Pathol. 1932, 13, 931–83. [Google Scholar]
- Weston, D.A. Investigating the specificity of periosteal reactions in pathology museum specimens. Am J Phys Anthropol. 2008, 137, 48–59. [Google Scholar] [CrossRef]
- Buckley, H.R. Subadult Health and Disease in Prehistoric Tonga, Polynesia. J Phys Anthropol. 2000, 113. [Google Scholar] [CrossRef]
- Mays, S.; Crane-Kramer, G.; Bayliss, A. Two probable cases of treponemal disease of medieval date from England. Am J Phys Anthropol. 2003, 120, 133–143. [Google Scholar] [CrossRef]
- Stirland, A. Pre-Columbian Treponematosis in Medieval Britain. International lournal of Osteoarchaeology. 1991, 1. [Google Scholar] [CrossRef]
- Suzuki, T. Typical Osseous Syphilis in a Medieval Skeletal Remains from Hokkaido. 1984.
- Palfi, G.; Dutour, O.; Borreani, M.; Brun, J.P.; Berato, J. Pre-Columbian Congenital Syphilis from the Late Antiquity in France. Int J Osteoarchaeol. 1992, 2, 245–261. [Google Scholar] [CrossRef]
- Lewis, B. Treponematosis and Lyme Borreliosis Connections: Explanation for Tchefuncte Disease Syndromes? American Journal Of Physical Anthropology. 1994, 93. [Google Scholar] [CrossRef]
- Maryna Steyn, M.H. Pre-Columbian Presence of Treponemal Disease: A Possible Case from Iron Age Southern Africa [Internet]. 1995. Available from: http://www.journals.uchicago.edu/t-and-c.
- Buzhilova, A. Medieval Examples of Syphilis from European Russia. Int. J. Osteoarchaeol. 1999, 9. [Google Scholar] [CrossRef]
- Hackett, C.J. An Introduction to Diagnostic Criteria of Syphilis, Treponarid and Yaws (Treponematoses) in Dry Bones, and Some Implications*. Virchows Arch. A Path. Anat. and ttistol. 1975, 368. [Google Scholar] [CrossRef]
- Mitchell, P.D. Pre-Columbian treponemal disease from 14th century AD Safed, Israel, and implications for the medieval eastern Mediterranean. Am J Phys Anthropol. 2003, 121, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pineda, C.; Mansilla-Lory, J.; Martínez-Lavín, M.; Leboreiro, I.; Izaguirre, A.; Pijoan, C. Rheumatic diseases in the ancient americas: The skeletal manifestations of treponematoses. Journal of Clinical Rheumatology. 2009, 15, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.G. Evidence for Prehistoric Cardiovascular Disease of Syphilitic Origin on the Northern Plains. American Journal Of Physical Anthropology. 1983. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Heathcote, G.M. Characterization of the Skeletal Manifestations of the Treponemal Disease Yaws as a Population Phenomenon Downloaded from [Internet]. Vol. 17, University of Iowa Libraries/Serials Acquisitions. 1993. Available from: http://cid.oxfordjournals.org/.
- Fournier, A. Syphilitic teeth. Dental Cosmos. 1884, 26, 12–25. [Google Scholar]
- Hillson, S.; Grigson, C.; Bond, S. Dental Defects of Congenital Syphilis. 1998.
- Jacobi, K.P.; Cook, D.C.; Corruccini, R.S.; Handler, J.S. Congenital Syphilis in the Past: Slaves at Newton Plantation, Barbados, West lndies. American Journal O F Physical Anthropology. 1992, 89. [Google Scholar] [CrossRef]
- Hutchinson, J. Syphilis. Prepubescent syphilis. 1885.
- Nystrom, K.C. Dental evidence of congenital syphilis in a 19th century cemetery from the mid-hudson valley. Int J Osteoarchaeol. 2011, 21, 371–378. [Google Scholar] [CrossRef]
- Moon, H. On irregular and defective tooth development. Trans Odontol Great Britain. 1877, 9, 223–243. [Google Scholar]
- Ioannou, S.; Hunt, D.; Henneberg, M. Five Cases of Dental Anomalies Attributable to Congenital Syphilis from Early 20th Century American Anatomical Collections. 2017. [Google Scholar]
- Rothschild, B.M.; Rothschild, C. Congenital Syphilis in the Archaeological Record: Diagnostic Insensitivity of Osseous Lesions. 1997.
- Frangos, C.C.; Lavranos, G.M.; Frangos, C.C. Higoumenakis’ sign in the diagnosis of congenital syphilis in anthropological specimens. Med Hypotheses. 2011, 77, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Vargová, L.; Vymazalová, K.; Vargová, L.; Horáčková, L.; Vymazalová, K.; Svoboda, J. Inflammatory changes on skeletons from the 16th to 17th century in Veselí nad Moravou, Czech Republic [Internet]. 2014. Available from: https://www.researchgate.net/publication/309041529.
- Rothschild, B.M.; Rothschild, C.; Doran, G. Virgin Texas: Treponematosis-Associated Periosteal Reaction 6 Millenia in the Past. Advances in Anthropology. 2011, 01, 15–18. [Google Scholar] [CrossRef]
- Radu, C.; Soficaru, A.D. Dental developmental defects in a subadult from 16th–19th centuries Bucharest, Romania. Int J Paleopathol. 2016, 15, 33–38. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, M.; Henneberg, R.J.; Anson, T. Diagnosis of Mercurial Teeth in a Possible Case of Congenital Syphilis and Tuberculosis in a 19th Century Child Skeleton. Journal of Anthropology. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Zuckerman, M.K. More harm than healing? Investigating the iatrogenic effects of mercury treatment on acquired syphilis in post-medieval London. Open Archaeology. 2016, 2, 42–55. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, M. Dental signs attributed to congenital syphilis and its treatments in the Hamann-Todd Skeletal Collection. Anthropological Review. 2017, 80, 449–465. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Zhang, X.; Gao, B.; Duan, C.; Zhu, H.; et al. Identifying treponemal disease in early East Asia. American Journal of Biological Anthropology. 2022, 178, 530–543. [Google Scholar] [CrossRef]
- Jäger, H.Y.; Maixner, F.; Pap, I.; Szikossy, I.; Pálfi, G.; Zink, A.R. Metagenomic analysis reveals mixed Mycobacterium tuberculosis infection in a 18th century Hungarian midwife. Tuberculosis. 2022, 137. [Google Scholar] [CrossRef] [PubMed]
- Schuenemann, V.J.; Kumar Lankapalli, A.; Barquera, R.; Nelson, E.A.; Iraíz Hernández, D.; Acuña Alonzo, V.; et al. Historic Treponema pallidum genomes from Colonial Mexico retrieved from archaeological remains. PLoS Negl Trop Dis. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Rühli, F.J.; Kuhn, G.; Evison, R.; Müller, R.; Schultz, M. Diagnostic value of micro-CT in comparison with histology in the qualitative assessment of historical human skull bone pathologies. Am J Phys Anthropol. 2007, 133, 1099–1111. [Google Scholar] [CrossRef]
- Guedes, L.; Dias, O.; Neto, J.; Ribeiro Da Silva, L.D.P.; Mendonça De Souza, S.M.F.; Iñiguez, A.M. First Paleogenetic Evidence of Probable Syphilis and Treponematoses Cases in the Brazilian Colonial Period. Biomed Res Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Henneberg, R.J.; Henneberg, M. Presence of dental signs of congenital syphilis in pre-modern specimens. Vol. 85, Archives of Oral Biology. Elsevier Ltd; 2018. p. 192–200.
- Barnes, I.; Thomas, M.G. Evaluating bacterial pathogen DNA preservation in museum osteological collections. Proceedings of the Royal Society B: Biological Sciences. 2006, 273, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Von Hunnius, T.E.; Roberts, C.A.; Boylston, A.; Saunders, S.R. Histological identification of syphilis in pre-Columbian England. Am J Phys Anthropol. 2006, 129, 559–566. [Google Scholar] [CrossRef]
- Rothschild, B.; Jellema, L. Periosteal reaction recognition and specificity assessed by surface microscopy. Int J Osteoarchaeol. 2020, 30, 355–361. [Google Scholar] [CrossRef]
- Mansilia, J.; Pijoan, C.M. Brief Communication: A Case of Congenital Syphilis During the Colonial Period in Mexico City. American Journal Of Physical Anthropology. 1995, 97. [Google Scholar]
- Andreu-Arasa, V.C.; Chapman, M.N.; Kuno, H.; Fujita, A.; Sakai, O. Craniofacial manifestations of systemic disorders: CT and MR imaging findings and imaging approach. Radiographics. 2018, 38, 890–911. [Google Scholar] [CrossRef]
- Biehler-Gomez, L.; Mattia, M.; Sala, C.; Giordano, G.; Di Candia, D.; Messina, C.; et al. Mercury poisoning in two patients with tertiary syphilis from the Ca’ Granda hospital (17th-century Milan). Archaeometry. 2022, 64, 500–510. [Google Scholar] [CrossRef]
- Sarhan, M.S.; Wurst, C.; Tzankov, A.; Bircher, A.J.; Wittig, H.; Briellmann, T.; et al. A nontuberculous mycobacterium could solve the mystery of the lady from the Franciscan church in Basel, Switzerland. BMC Biol. 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; et al. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone. 2016, 93, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Waldron, T.; Shelmerdine, S.; Hutchinson, C.; McHugh, K.; Calder, A.; et al. The skeletal effects of congenital syphilis: The case of Parrot’s bones. Med Hist. 2020, 64, 467–477. [Google Scholar] [CrossRef]
- Montiel, R.; Solórzano, E.; Díaz, N.; Álvarez-Sandoval, B.A.; González-Ruiz, M.; Cañadas, M.P.; et al. Neonate human remains: A window of opportunity to the molecular study of ancient syphilis. PLoS One. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- von Hunnius, T.E.; Yang, D.; Eng, B.; Waye, J.S.; Saunders, S.R. Digging deeper into the limits of ancient DNA research on syphilis. J Archaeol Sci. 2007, 34, 2091–2100. [Google Scholar] [CrossRef]
- Meffray, A.; Perrin, M.; Richier, A.; Schmitt, A.; Ardagna, Y.; Biagini, P. Molecular detection of Treponema Pallidum subspecies Pallidum in 150-year-old foetal remains, southeastern France. J Med Microbiol. 2019, 68, 761–769. [Google Scholar] [CrossRef]
- Giffin, K.; Lankapalli, A.K.; Sabin, S.; Spyrou, M.A.; Posth, C.; Kozakaitė, J.; et al. A treponemal genome from an historic plague victim supports a recent emergence of yaws and its presence in 15th century Europe. Sci Rep. 2020, 10. [Google Scholar] [CrossRef]
- Kolman, C.J.; Centurion-Lara, A.; Lukehart, S.A.; Owsley, D.W.; Tuross, N. Identification of Treponema pallidum Subspecies pallidum in a 200-Year-Old Skeletal Specimen [Internet]. 1999. Available from: http://jid.oxfordjournals.org/.
- Pääbo, S.; Poinar, H.; Serre, D.; Jaenicke-Després, V.; Hebler, J.; Rohland, N.; et al. Genetic analyses from ancient DNA. Annual Review of Genetics. 2004, 38, 645–679. [Google Scholar] [CrossRef]
- Höss, M.; Jaruga, P.; Zastawny, T.H.; Dizdaroglu, M.; Pääbo, S. DNA damage and DNA sequence retrieval from ancient tissues. Vol. 24, Nucleic Acids Research. Oxford University Press; 1996.
- Kulik-Kupka, K.; Koszowska, A.; Brończyk-Puzoń, A.; Nowak, J.; Gwizdek, K.; Zubelewicz-Szkodzińska, B. Arsen – trucizna czy lek? Vol. 67, Medycyna Pracy. Nofer Institute of Occupational Medicine; 2016. p. 89–96.
- Kłys, M. Z rtęcią (i...) przez stulecia. 2010. [Google Scholar]
- Kepa, M.; Kozłowski, T.; Szostek, K.; Drozd, A.; Walas, S.; Mrowiec, H.; et al. Analysis of mercury levels in historical bone material from syphilitic subjects - Pilot studies (short report). Anthropologischer Anzeiger. 2012, 69, 367–377. [Google Scholar] [CrossRef]
- Erdal, Y.S. A pre-Columbian case of congenital syphilis from anatolia (Nicaea, 13th century AD). Int J Osteoarchaeol. 2006, 16, 16–33. [Google Scholar] [CrossRef]
- Ioannou, S.; Henneberg, M. A Rare Case of Congenital Syphilis and a Supernumerary Fourth Molar in an Early 20th Century African American Woman. Vol. 29. 2016. [CrossRef]
- Henkel, J.S.; Davis, J.; Farley, N. Anatomical and biochemical evidence for Treponema pallidum in a 19th to early twentieth century skeletal cadaver. Forensic Sci Med Pathol. 2020, 16, 557–561. [Google Scholar] [CrossRef]
- Mendonça De Souza/ + S, Codinha, S.; Cunha, E. The girl from the Church of the Sacrament: a case of congenital syphilis in XVIII century Lisbon. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2006, 101.
- Patel, R.; Mitchell, P.D. The Search for Rosa Pike: Congenital Syphilis in 1880s London. Archaeopress; 2007.
- Gaul, J.S.; Grossschmidt, K.; Gusenbauer, C.; Kanz, F. A probable case of congenital syphilis from pre-Columbian Austria. Anthropologischer Anzeiger. 2015, 72, 451–472. [Google Scholar] [CrossRef]
- Gaul, J.S.; Grossschmidt, K. A probable case of congenital syphilis from 18th century Vienna. Int J Paleopathol. 2014, 6, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Fornaciari, A.; Gaeta, R.; Minozzi, S.; Giuffra, V. Syphilis in Maria Salviati (1499–1543), Wife of Giovanni de’ Medici of the Black Bands. Emerg Infect Dis. 2020, 26, 1274–1282. [Google Scholar] [CrossRef]
- Lopez, B.; Lopez-Garcia, J.M.; Costilla, S.; Garcia-Vazquez, E.; Dopico, E.; Pardiñas, A.F. Treponemal disease in the old world? Integrated palaeopathological assessment of a 9th-11th century skeleton from north-central Spain. Anthropological Science. 2017, 125, 101–114. [Google Scholar] [CrossRef]
- Gino Fornaciari. Renaissance mummies in Italy. 1999.
- Mario Šlaus, Mn. A Case of Venereal Syphilis in the Modern Age Horizon of Graves near the Church of St. Lawrence in Crkvar. 2007.
- Rissech, C.; Roberts, C.; Tomás-Batlle, X.; Tomás-Gimeno, X.; Fuller, B.; Fernandez, P.L.; et al. A Roman Skeleton with Possible Treponematosis in the North-East of the Iberian Peninsula: A Morphological and Radiological Study. Int J Osteoarchaeol. 2013, 23, 651–663. [Google Scholar] [CrossRef]
- Cole, G.; Waldron, T. Apple Down 152: A putative case of syphilis from sixth century AD Anglo-Saxon England. Am J Phys Anthropol. 2011, 144, 72–79. [Google Scholar] [CrossRef]
- Carlos Pineda JCSFMML. Radiographs of an Ancient Mortuary Bundle Support Theory for the NewWorld Origin of Syphilis. 1998.
- Célia Lopes MLPALS. Syphilis and cirrhosis: a lethal combination in a XIX century individual identified from the Medical Schools Collection at the University of Coimbra (Portugal). 2010.
- Woo, E.J.; Kim, J.H.; Lee, W.J.; Cho, H.; Pak, S. Syphilitic infection in a pre-modern population from South Korea (19th century AD). Anthropological Science. 2019, 127, 55–63. [Google Scholar] [CrossRef]
- Castro, M.M.; Benavente, M.A.; Ortega, J.; Acuña, R.; Montero, C.; Thomas, C.; et al. Thoracic aortic aneurysm in a pre-Columbian (210 BC) inhabitant of Northern Chile: Implications for the origins of syphilis. Int J Paleopathol. 2016, 13, 20–26. [Google Scholar] [CrossRef]
- Klaus, H.D.; Ortner, D.J. Treponemal infection in Peru’s Early Colonial period: A case of complex lesion patterning and unusual funerary treatment. Int J Paleopathol. 2014, 4, 25–36. [Google Scholar] [CrossRef]
- Salesse, K.; Kaupová, S.; Brůžek, J.; Kuželka, V.; Velemínský, P. An isotopic case study of individuals with syphilis from the pathological-anatomical reference collection of the national museum in Prague (Czech Republic, 19th century A.D.). Int J Paleopathol. 2019, 25, 46–55. [Google Scholar] [CrossRef]
- Szczepanek, A.; Walocha, J.; Kochan, P. Cases of late syphilis documented at the cemetery of noblemen residents of the Knights of the Holy Sepulchre poorhouse (XVII-XVIII centuries) on Stradom in Cracow, Poland. World J Med Images. 2019, 5. [Google Scholar]
- Assis, S.; Casimiro, S.; Cardoso, F.A. A possible case of acquired syphilis at the former Royal Hospital of All-Saints (RHAS) in Lisbon, Portugal (18th century): A comparative methodological approach to differential diagnosis. Anthropologischer Anzeiger. 2015, 72, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.; Andreica, L.; Constantinescu, M.; Soficaru, A. Multiple Cases with Probable Treponemal Infection from 16th to 19th Centuries Romania. Int J Osteoarchaeol. 2015, 26, 563–573. [Google Scholar] [CrossRef]
- Zuckerman, M.K. The “Poxed” and the “Pure”: A Bioarchaeological Investigation of Community and Marginalization Relative to Infection with Acquired Syphilis in Post-Medieval London. Archeological Papers of the American Anthropological Association. 2017, 28, 91–103. [Google Scholar] [CrossRef]
- Gladykowska - Rzeczycka, J. J. , Kwiatkowska, B., Nowakowski, D., Trnka, J. Treponematosis in a 14 th century skeleton from Wroclaw, Poland. Journal of Paleopathology 2003, 15, 187–193. [Google Scholar]
- Suzuki, T.; Matsushita, T.; Han, K. On the possible case of treponematosis from the Bronze Age in China. Anthropological Science. 2005, 113, 253–258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




