Submitted:
20 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Background
2. Methodology
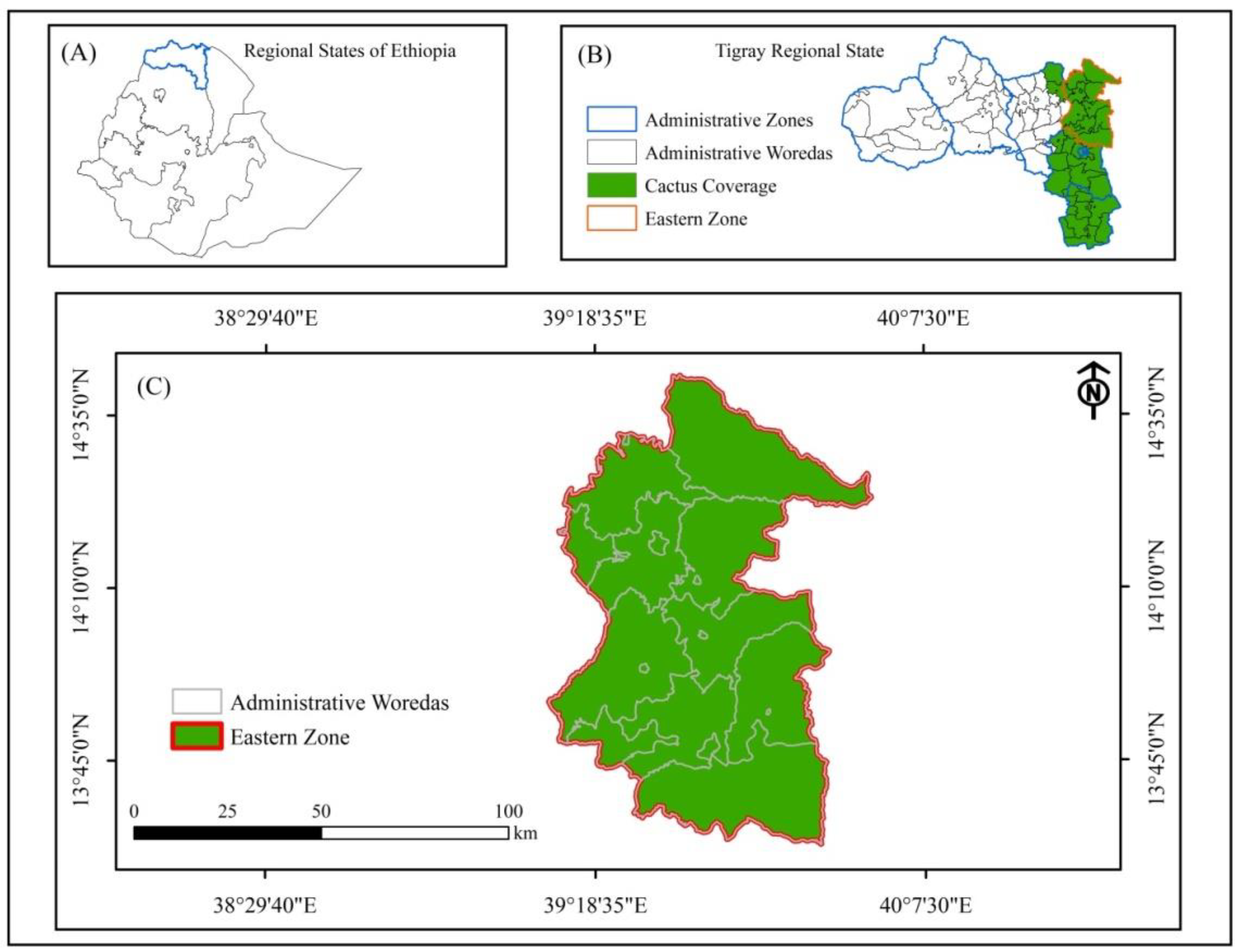
2.1. Description of the Study Area
2.2. Method of Data Collection
2.3. Data Analysis
3. Results and Discussion
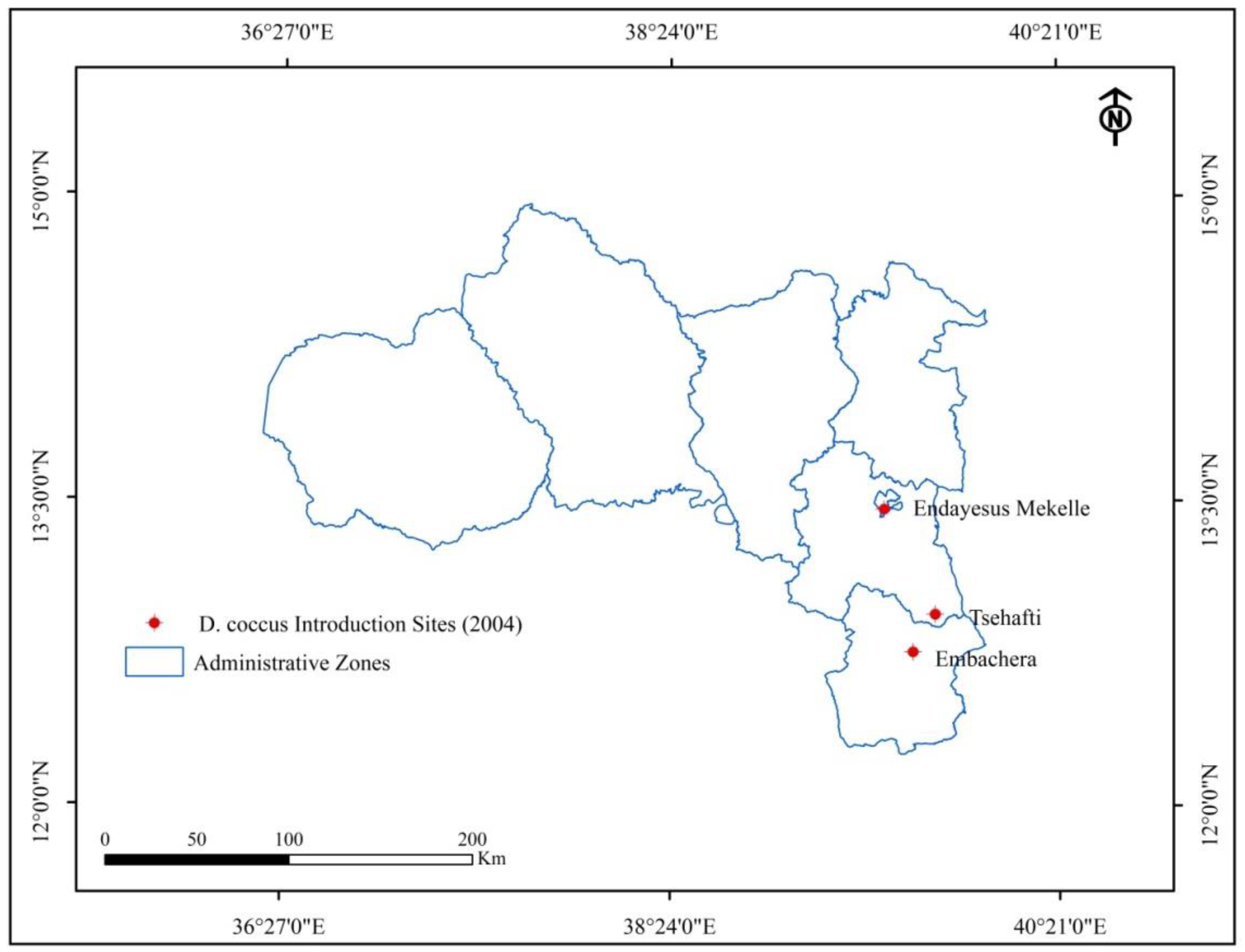
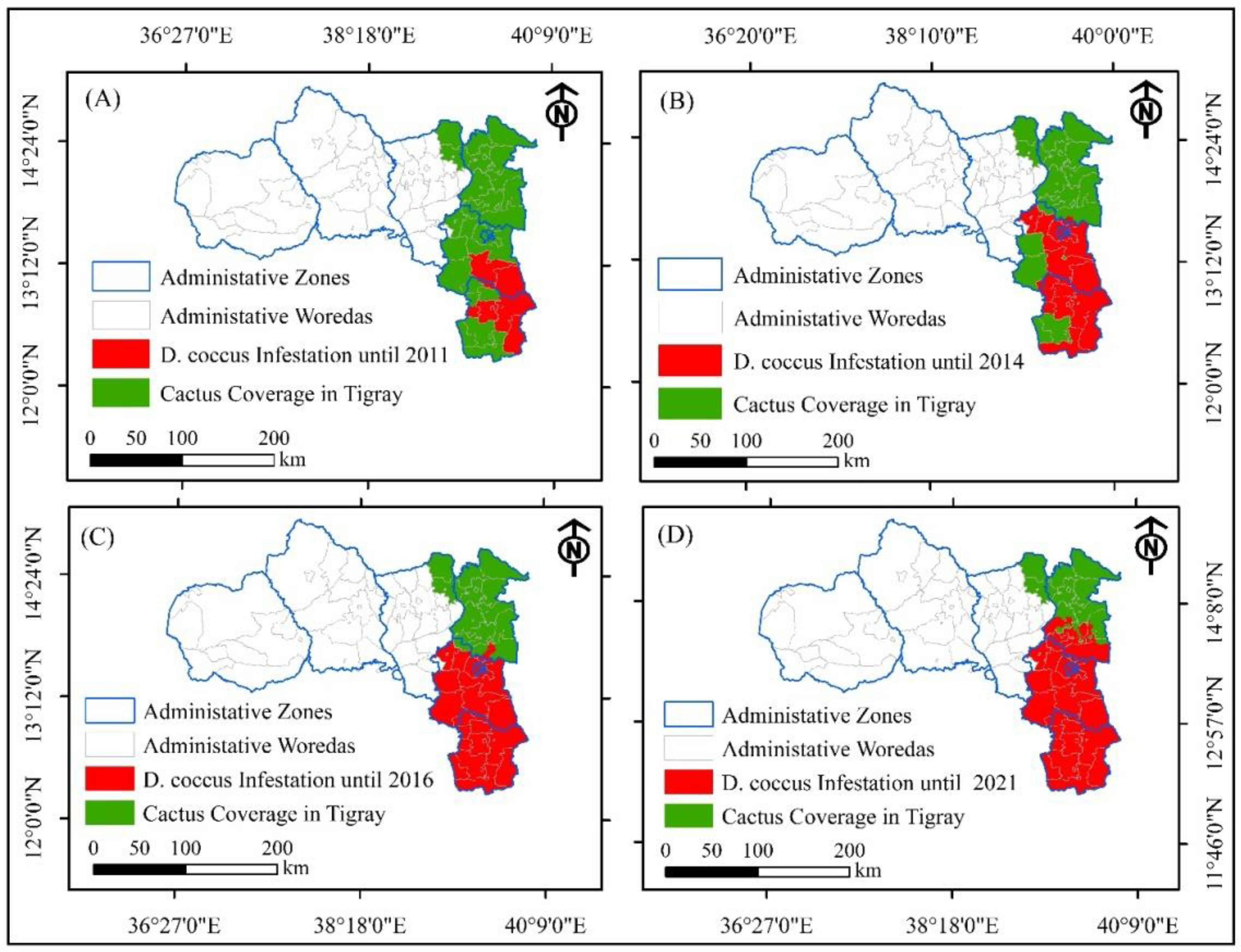
3.1. Trends of D. coccus Dissemination in Tigray Before the Conflict
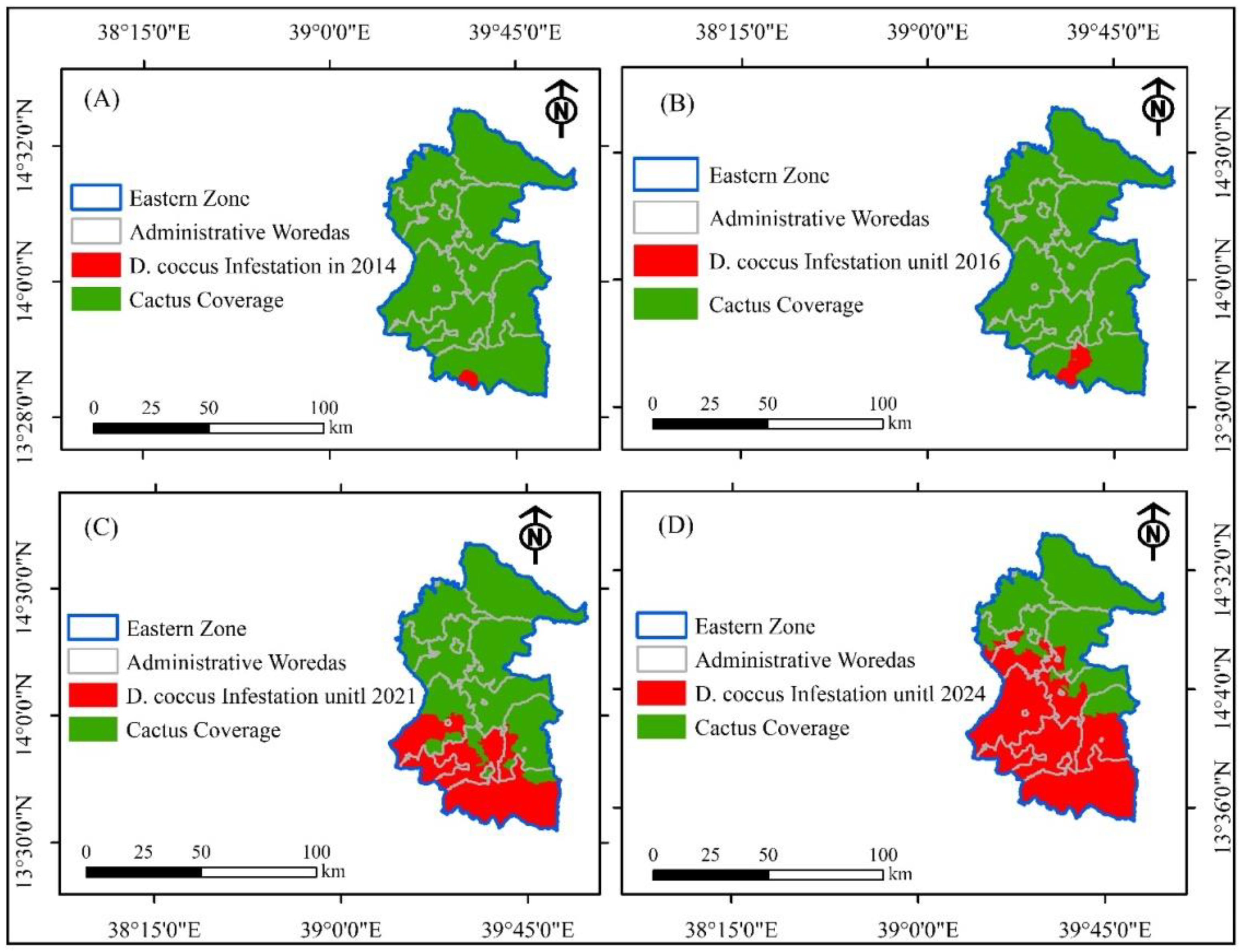
3.2. Trends of D. coccus Dissemination in the Eastern Zone of Tigray Before the Armed Conflict
3.3. The Effect of the Armed Conflict on the Management of D. coccus
3.3.1. The Neglected D. coccus Management Practices
3.3.2. Trends of D. coccus Infestation as Affected by the Armed Conflict
4. Existing Challenges on the Management of D. coccus
5. Way Forward for Integrated D. coccus Management to Restore and Sustainably Produce Cactus Pear in Tigray
5.1. Preventative Approach
5.2. Pest Suppression Approach
5.3. Eradication Approach
6. Conclusions
Author Contributions
Funding
Data availability
Conflicts of Interest
References
- Abrha, S.W. Cactus Pear (Opuntia ficus-indica L.) in Tigray, North Ethiopia: History, potential and challenges (Review paper). J. Biol. 2014, 4, 226–230. [Google Scholar]
- Belay, T.; Gebreselassie, M.; Abadi, T. Description of cactus pear ( Opuntia ficus-indica [ L.] Mill.) cultivars. Resear report 1; Tigray Agricultural Research Institute: Ethiopia, 2015. [Google Scholar]
- Berhe, Y.K.; Aymut, K.M.; Gebremariam, B.L.; Gebreziher, H.G. Introduction of carmine cochineal to Northern Ethiopia, Current status of infestation on Cactus Pear, and Control Measures. International Journal of Botany Studies 2020, 5, 32–38. [Google Scholar]
- Meaza, H.; Demissie, B.; Kahsay, Y.; Gebrehiwot, M.; Nyssen, J. Understanding cactus pear status for improved ecosystem services in northern Ethiopia. Discov. Environ. 2024. [Google Scholar] [CrossRef]
- Desta, T.Y. Farmers Practice in use of cactus as animal feed-in Tigray. In Improved utilization of cactus pear for food, feed, soil and water conservation and other products in Africa. Proceedings of the International Workshop held in Mekelle, Ethiopia, 19-21 October 2009.
- Belay, T. Carmine cochineal: Fortune wasted in northern Ethiopia. J. Prof. Assoc. Cactus Dev. 2015, 17, 61–80. [Google Scholar] [CrossRef]
- Ramdani, C.; Fakhouri, K.E.; Sbaghi, M.; Bouharroud, R.; Boulamtat, R.; Aasfar, A.; Mesfioui, A.; Bouhssini, M.E. Chemical composition and insecticidal potential of six essential oils from morocco against dactylopius opuntiae (Cockerell) under field and laboratory conditions. Insects 2021, 12, 1007. [Google Scholar] [CrossRef]
- Zeweld, S.W.; Ayimut, K.M.; Hiben, M.G. Efficacy of herbal extracts in the management of cactus pest, Dactylopius opuntiae (Hemiptera: Dactylopiidae) in Tigray Region, Ethiopia. Discov. Agric. 2024, 2, 19. [Google Scholar] [CrossRef]
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. , Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.P.; Porter, J.M. Phylogeny of Opuntioideae (Cactaceae). Int. J. Plant Sci. 2009, 170, 107–116. [Google Scholar] [CrossRef]
- Fitiwy, I.; Gebretsadkan, A.; Araya, A. Management of cochineal (Dactylopius coccus Costa) insect pest through botanical extraction in Tigray, north Ethiopia. J. Drylands 2016, 6, 499–505. [Google Scholar]
- Teklu, G.W.; Ayimut, K.M.; Abera, F.A. Evaluation of Opuntia species for their resistance to carmine cochineal (Dactylopius coccus) insect in Tigray, northern Ethiopia. Haseltonia 2024, 31, 88–88. [Google Scholar] [CrossRef]
- Gebretsadik, G.; Animut, G.; Tegegne, F. Assessment of Potential of cactus pear as livestock feed in northern Ethiopia. Livestock Research for Rural Development 2013, 25, 26. [Google Scholar]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Molecular Nutrition &Food Research 2005, 49, 175–194. [Google Scholar]
- Naorem, A.; Patel, A.; Hassan, S.; Louhaichi, M.; Jayaraman, S. Global research landscape of cactus pear (Opuntia ficus-indica) in agricultural science. Front. Sustain. Food Syst. 2024, 8, 1354395. [Google Scholar] [CrossRef]
- Ranjan, P.; Ranjan, J.K.; Misra, R.L.; Dutta, M.; Singh, B. Cacti: notes on their uses and potential for climate change mitigation. Genet. Resour. Crop Evol. 2016, 63, 901–917. [Google Scholar] [CrossRef]
- Manaye, A.; Afewerk, A.; Manjur, B.; Solomon, N. The Effect of the war on smallholder agriculture in Tigray, Northern Ethiopia. Cogent Food Agric. 2023, 9, 2247696. [Google Scholar] [CrossRef]
- Negash, E.; Birhane, E.; Gebrekirstos, A.; Gebremedhin, M.A.; Annys, S.; Rannestad, M.M.; Berhe, D.H.; Sisay, A.; Alemayehu, T.; Berhane, T.; et al. Remote sensing reveals how armed conflict regressed woody vegetation cover and ecosystem restoration efforts in Tigray, Ethiopia. Sci. Remote Sens. 2023, 8, 1–17. [Google Scholar] [CrossRef]
- Gebremedhin, A.; Solomon, B.; Misgna, H.; Gebre, G.; Abraha, S.; Abraha, A.Z. Variability and trend analysis of temperatures, rainfall, and characteristics of crop - growing season in the eastern zone of Tigray region, northern Ethiopia. Theor. Appl. Climatol. 2023, 152, 25–43. [Google Scholar] [CrossRef]
- Hailu, Z.; Gebreziher, H.G.; Abrha, E. Effect of tree tobacco leaf extracts on mortality rate of carmine cochineal. Acta Hortic. 2022, 1343, 569–576. [Google Scholar] [CrossRef]
- Fitiwy, I.; Gebretsadkan, A.; Ayimut, K. Evaluation of botanicals for onion thrips, Thrips tabaci Lindeman, (Thysanoptera: Thripidae) control at Gum Selassa, South Tigray, Ethiopia. Momona Ethiop. J. Sci. 2015, 7, 32. [Google Scholar] [CrossRef]
- Joseph, S.V.; De la Fuente, M. Evaluation of Insecticides Against Cochineal Insect on Prickly Pear Cactus. Arthropod Manag. Tests 2019, 44, tsz031. [Google Scholar] [CrossRef]
- Lopes, R.S.; Oliveira, L.G.; Costa, A.F.; Correia, M.T.S.; Lima, E.A.L.-A.; Lima, V.L.M. Efficacy of Libidibia ferrea var. ferrea and Agave sisalana Extracts against Dactylopius opuntiae (Hemiptera: Coccoidea). J. Agric. Sci. 2018, 10, 255. [Google Scholar] [CrossRef]
- Zeitoun, R.; Hayar, S.; Majed, L.; El-Omari, K.; Dousset, S. Comparison of the efficacy of two insecticides for the management of Dactylopius opuntiae on prickly pear cactus in Lebanon and monitoring of the insecticides residues dissipation rates in fruits and cladodes. SN Appl. Sci. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Berhe, Y.K.; Portillo, L.; Vigueras, A.L. Resistance of Opuntia ficus-indica cv “Rojo Pelon” to Dactylopius coccus (Hemiptera: Dactylopiidae) under greenhouse condition. J. Prof. Assoc. Cactus Dev. 2022, 24, 293–309. [Google Scholar] [CrossRef]
- Lores, A.F.; Lvera, H.O.; Odríguez, S.R.; Arranco, J.B. Predation Potential of Chilocorus cacti ( Coleoptera : Coccinellidae ) to the Prickly Pear Cacti Pest Dactylopius opuntiae ( Hemiptera : Dactylopiidae ). Neotrop Entomol 2013, 407–411. [Google Scholar] [CrossRef]
- Tawayah, M.; Mahasneh, A.; Haddad, N. First Record of the Predator Ladybeetle, Hyperaspis Trifurcata ( Schaeffer ) ( Coleoptera : Coccinellidae ) Feeding on the Cochineal Scale Insect, Dactylopius opuntiae ( Hemiptera : Dactylopiidae ), in Jordan. Int. J. Innov. Sci. Res. Tech. 2023. [Google Scholar]
- Kumar, K.; Singh, D.; Singh, R.S. Cactus Pear: Cultivation and uses; CIAH/Tech./Pub. No73’; 2018; pp. 1–38. [Google Scholar]
- IPPC. ISPM 5: Glossary Of Phytosanitary Terms; FAO, 2019; pp. 1–36. [Google Scholar]
- Myers, J.H.; Savoie, A.; Van Randen, E. Eradication and pest management. Annu. Rev. Entomol. 1998, 43, 471–491. [Google Scholar] [CrossRef]
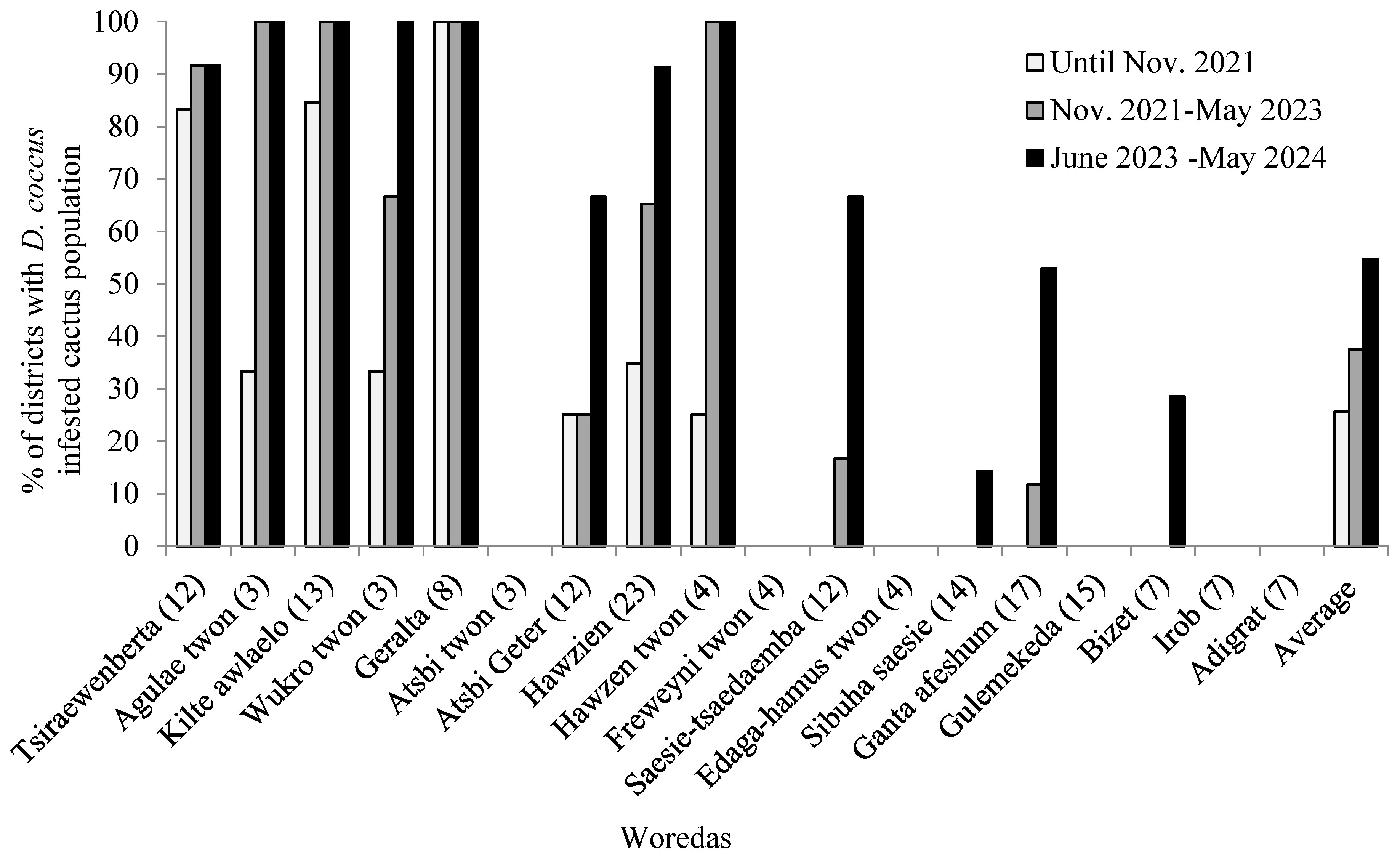
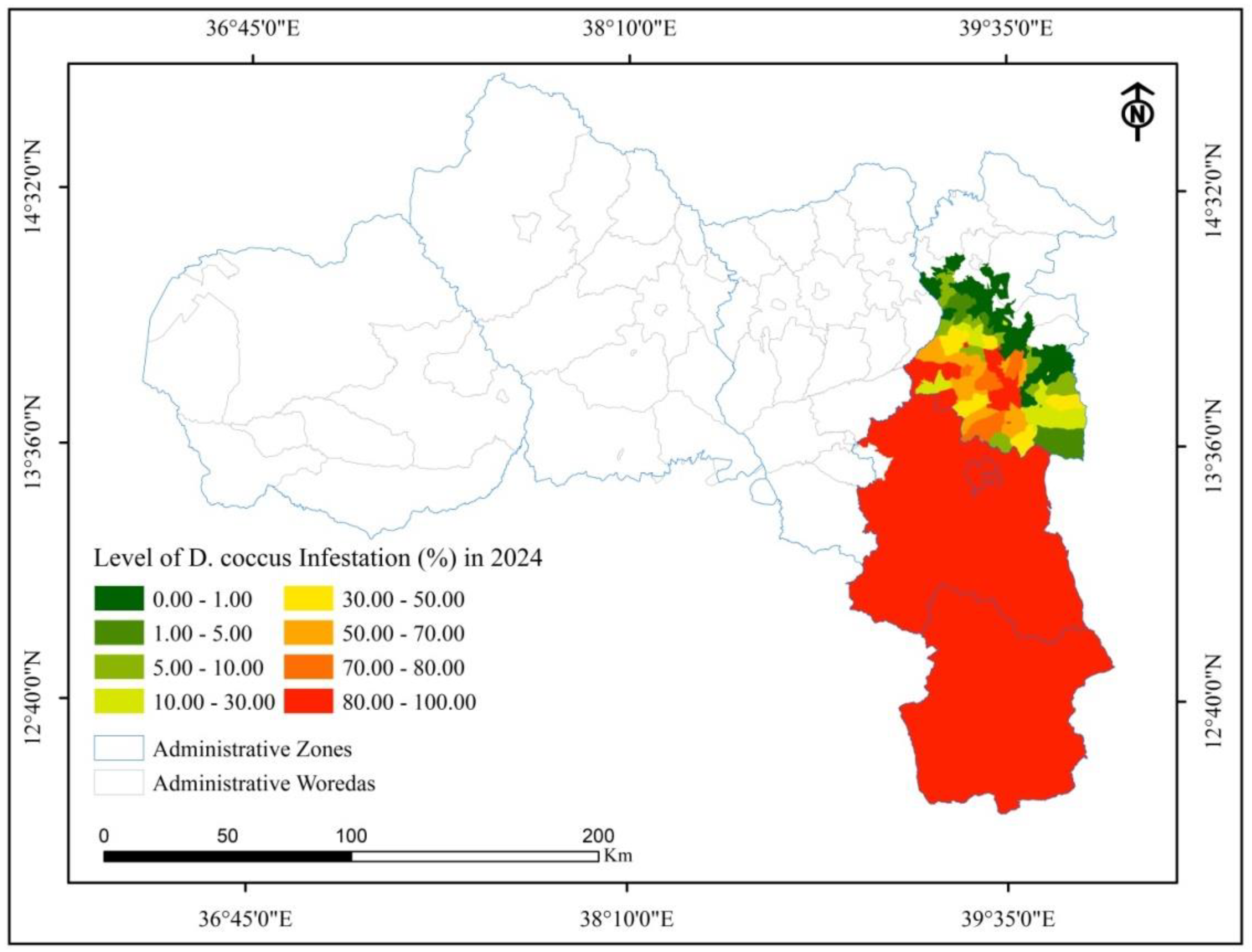
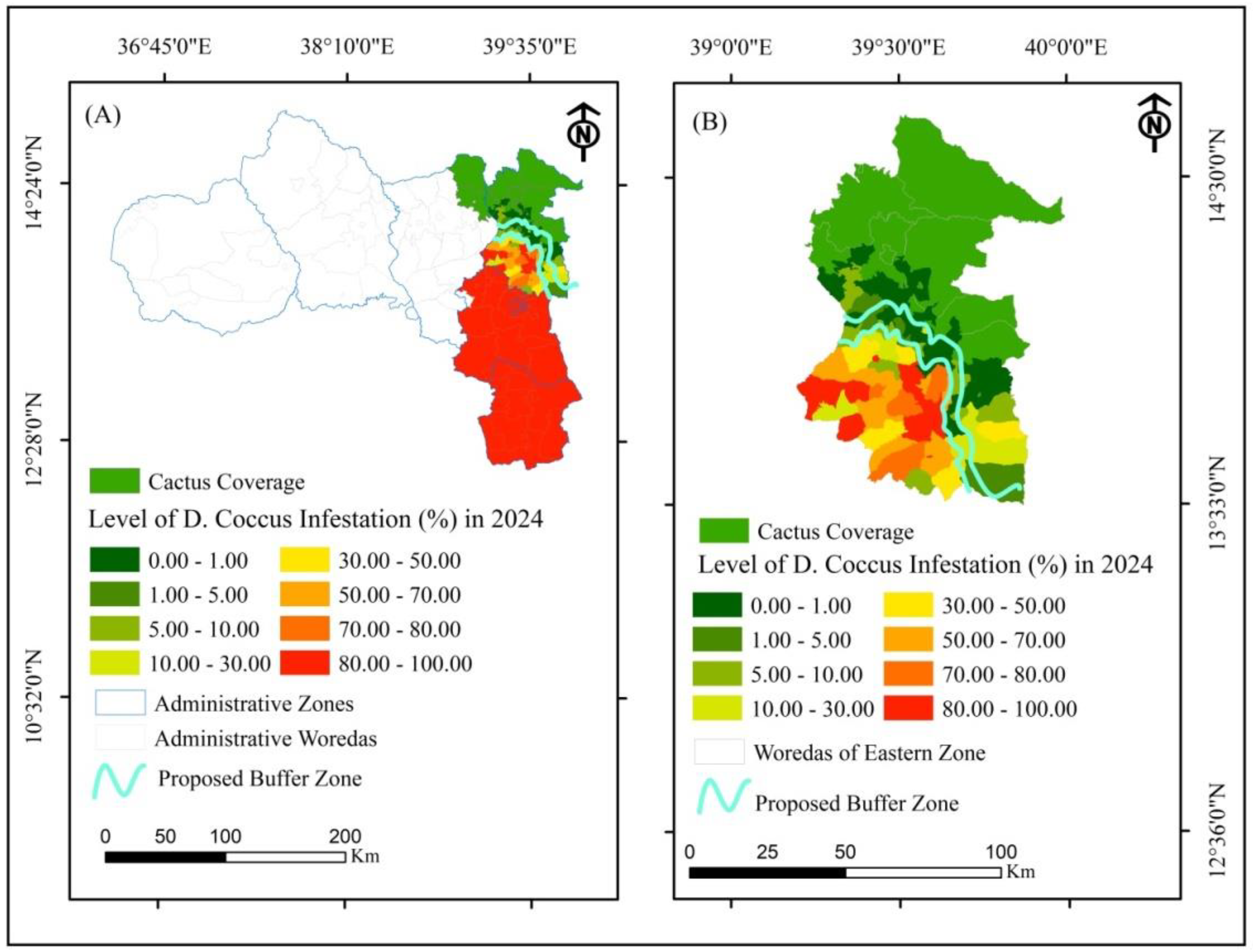
| Name of Woredas | Number of districts | Cactus pear area coverage (ha) | D. coccus affected districts | D. coccus-infested cactus pear coverage (ha) | ||||
|---|---|---|---|---|---|---|---|---|
| before the war (until Nov. 2021) | until May 2023 | until May 2024 | before the war (until Nov. 2021) | Nov 2021 - May 2023 | May 2021 - May 2024 | |||
| Tsiraewenberta | 12 | 1353.10 | 10 | 11 | 11 | 215.75 | 362.00 | 414.75 |
| Agulae town | 3 | 6.50 | 1 | 3 | 3 | 1.00 | 6.50 | 6.50 |
| Kilte awlaelo | 13 | 2060.99 | 11 | 13 | 13 | 87.76 | 794.61 | 1806.60 |
| Wukro town | 3 | 4.67 | 1 | 2 | 3 | 1.50 | 4.67 | 4.67 |
| Geralta | 8 | 2342.00 | 8 | 8 | 8 | 2342.00 | 2342.00 | 2342.00 |
| Atsbi town | 3 | 3.00 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Atsbi Geter | 12 | 2549.20 | 3 | 3 | 8 | 2.25 | 1.75 | 22.14 |
| Hawzien | 23 | 3580.25 | 8 | 15 | 21 | 27.59 | 131.26 | 607.63 |
| Hawzen town | 4 | 6.50 | 1 | 4 | 4 | 1.47 | 6.50 | 6.50 |
| Freweyni town | 4 | 7.00 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Saesie-tsaedaemba | 12 | 4109.25 | 0 | 2 | 8 | 0.00 | 2.00 | 27.59 |
| Edaga-hamus town | 4 | 5.00 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Sibuha saesie | 14 | 8321.00 | 0 | 0 | 2 | 0.00 | 0.00 | 0.80 |
| Gantaafeshum | 17 | 4151.50 | 0 | 2 | 9 | 0.00 | 0.00 | 5.59 |
| Gulemekeda | 15 | 4173.25 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Bizet | 7 | 664.63 | 0 | 0 | 2 | 0.00 | 0.00 | 1.20 |
| Irob | 7 | 14953.50 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Adigrat | 7 | 75.00 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Total | 168 | 48366.34 | 43 | 63 | 92 | 2676.35 | 3644.79 | 5239.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





