Submitted:
19 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
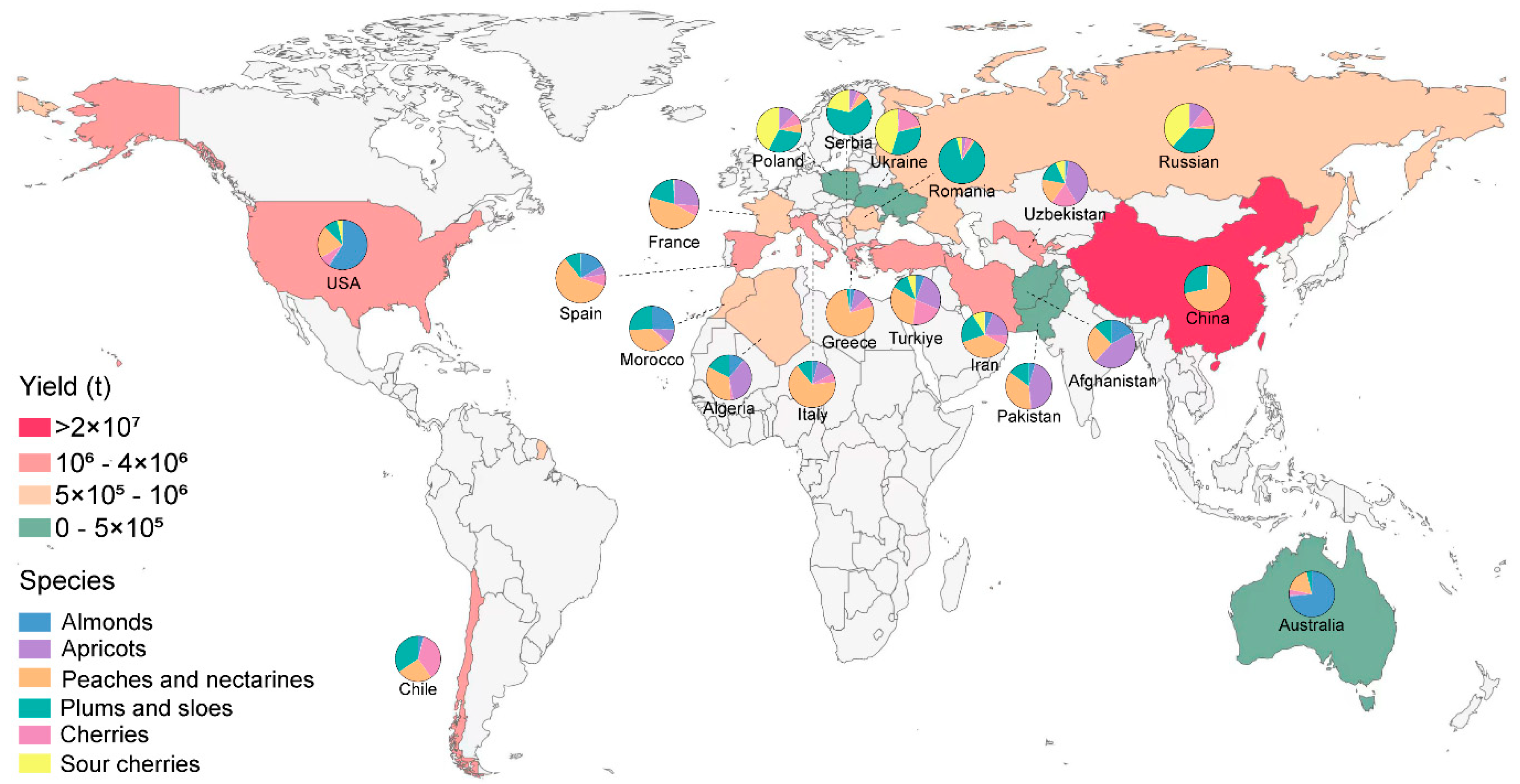
2. Modern Trends in Fruit Tree Cultivation Regime and the Demand for Advanced Rootstock Breeding
3. Breeding Objectives for Rootstocks of Stone Fruits
3.1. Efficient Clonal Propagation
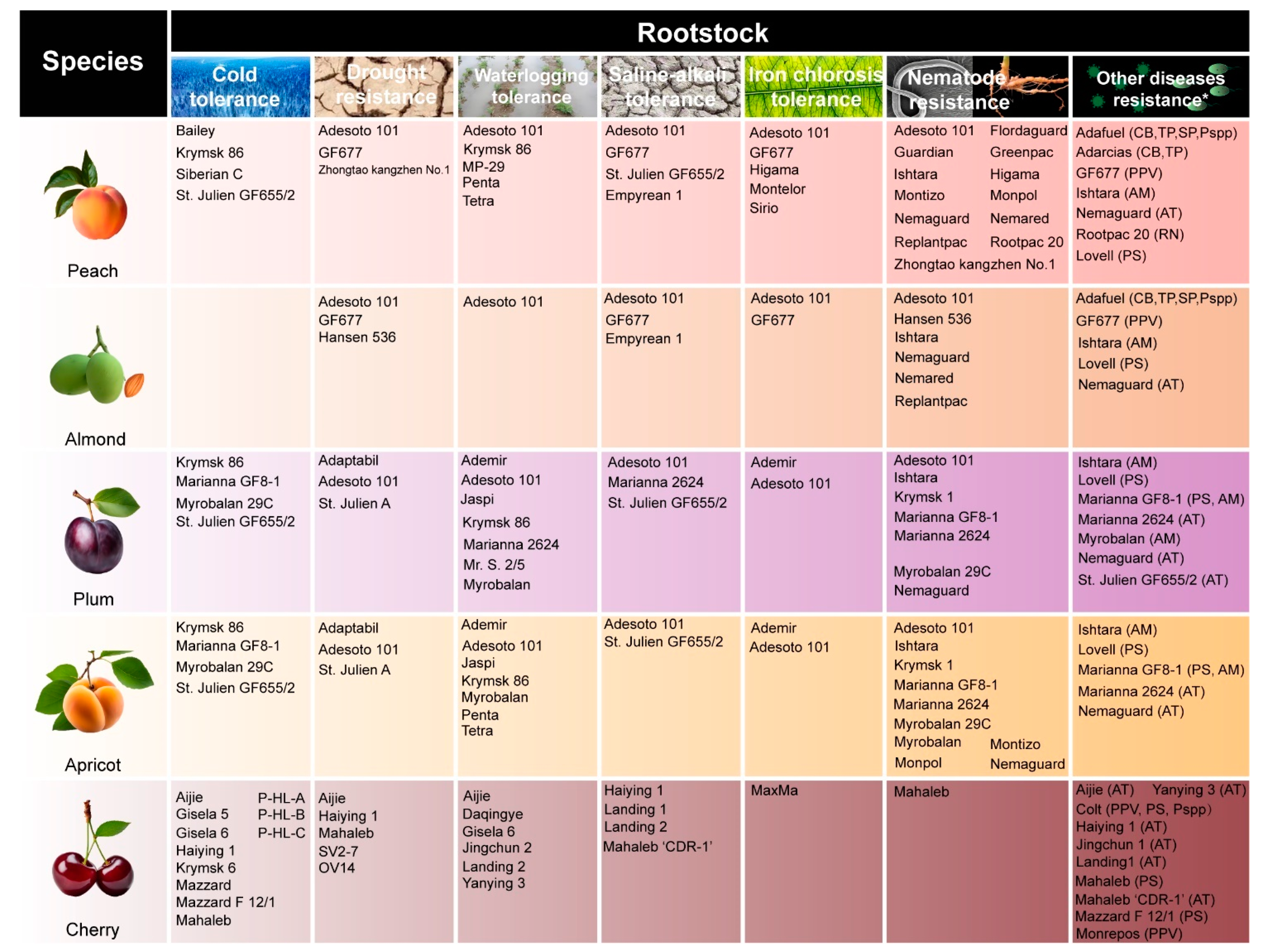
3.2. Abiotic Stress Tolerance
3.3. Biotic Stress Tolerance
3.4. Graft Compatibility
3.5. Dwarfing
3.6. Others
4. Breeding Achievements in Rootstocks of Stone Fruit Trees
5. Molecular Breeding Techniques for Rootstocks of Stone Fruit Trees
5.1. Marker-Assisted Breeding
5.2. Genetic Engineering Breeding
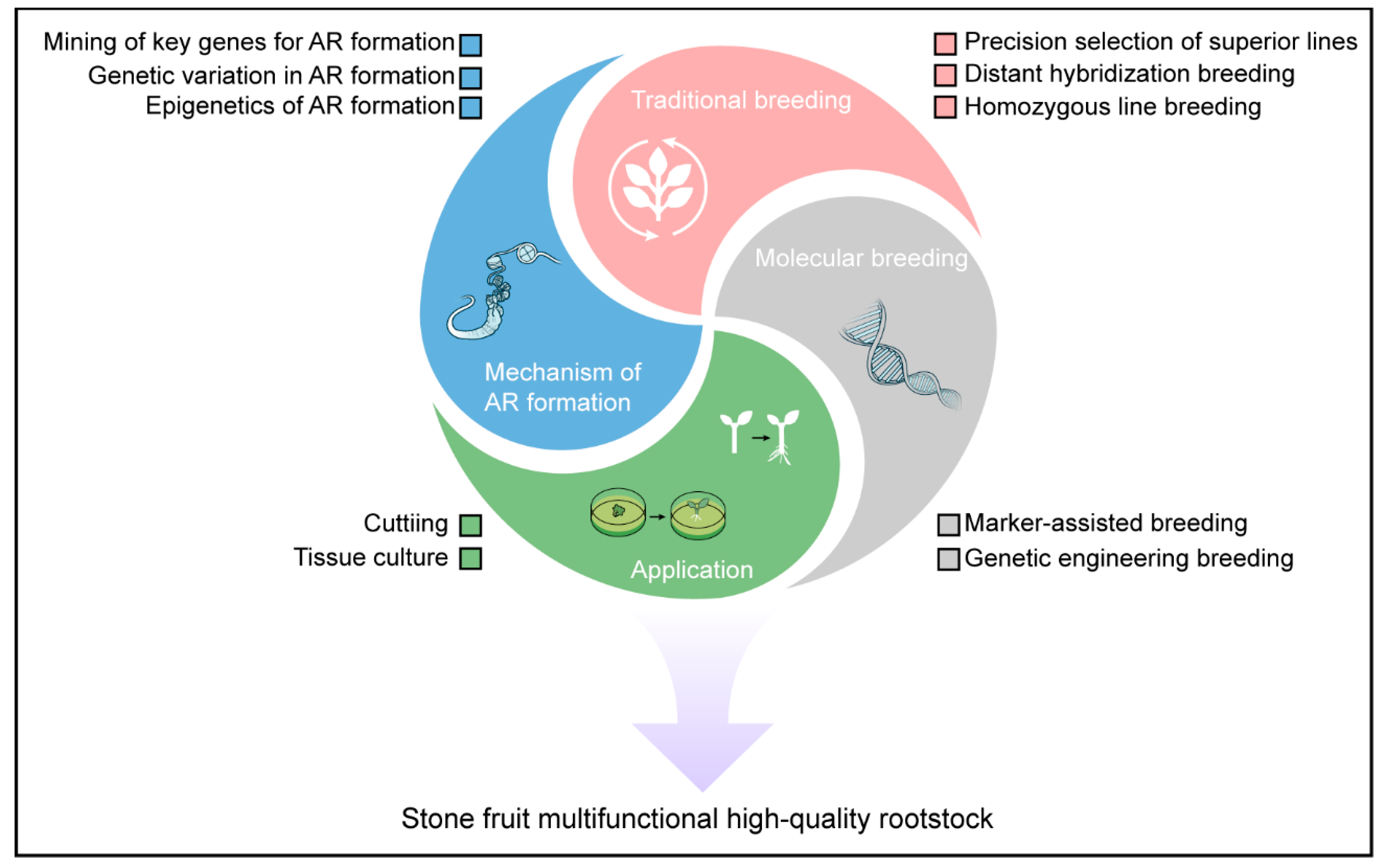
6. Future Perspectives in Stone Fruit Rootstock Research
6.1. Revealing the Molecular Mechanism of Adventitious Root Formation in Rootstocks
6.2. Developing of Efficient Breeding Strategies and New Techniques for Rootstocks
6.3. Optimizing Clonal Propagation Techniques for Rootstocks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riva, S. C.; Opara, U. O.; Fawole, O. A. , Recent developments on postharvest application of edible coatings on stone fruit: A review. Scientia Horticulturae 2020, 262. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Database (FAOSTAT). In 2022.
- Chen, G.; Boddu, R.; Aadil, R. M. , Study on Double-Layer Stereo Ecological Cultivation Technology of Greenhouse Gardening Fruit Trees. Journal of Food Quality 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Scalisi, A.; O'Connell, M. G.; Stefanelli, D.; Zhou, S.; Pitt, T.; Graetz, D.; Dodds, K.; Han, L.; De Bei, R.; Stanley, J.; Breen, K.; Goodwin, I. , Narrow orchard systems for pome and stone fruit—a review. Scientia Horticulturae 2024, 338. [Google Scholar] [CrossRef]
- Korkmaz, K.; Bolat, I.; Uzun, A.; Sahin, M.; Kaya, O. , Selection and Molecular Characterization of Promising Plum Rootstocks (Prunus cerasifera L.) among Seedling-Origin Trees. Life, 2023; 13, 1476. [Google Scholar]
- Mayer, N. A.; Ueno, B.; Rickes, T. B.; de Resende, M. V. L. A. , Cloning of rootstock selections and Prunus spp. cultivars by softwood cuttings. Scientia Horticulturae 2020, 273. [Google Scholar] [CrossRef]
- Rosa, G. G. d.; Zanandrea, I.; Mayer, N. A.; Bianchi, V. J., Propagação de porta-enxerto de Prunus spp. por estaquia: efeito do genótipo, do estádio de desenvolvimento do ramo e tipo de estaca. Revista Ceres 2017, 64, 90-97.
- Justamante, M. S.; Mhimdi, M.; Molina-Pérez, M.; Albacete, A.; Moreno, M. Á.; Mataix, I.; Pérez-Pérez, J. M. , Effects of Auxin (Indole-3-butyric Acid) on Adventitious Root Formation in Peach-Based Prunus Rootstocks. Plants 2022, 11, 913. [Google Scholar] [CrossRef]
- Tsafouros, A.; Frantzeskaki, A.; Assimakopoulou, A.; Roussos, P. A. , Spatial and temporal changes of mineral nutrients and carbohydrates in cuttings of four stone fruit rootstocks and their contribution to rooting potential. Scientia Horticulturae 2019, 253, 227–240. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P. A. , The possible bottleneck effect of polyamines' catabolic enzymes in efficient adventitious rooting of two stone fruit rootstocks. Journal of Plant Physiology 2020, 244. [Google Scholar] [CrossRef]
- Blažková, J.; Hlušičková, I. , Testing of wood hardiness to winter freezes in selections from progenies of Cerapadus × Prunus avium L. crosses. Horticultural Science 2002, 29, 133–142. [Google Scholar] [CrossRef]
- Turhan, E.; Ergin, S. , Soluble Sugars and Sucrose-Metabolizing Enzymes Related to Cold Acclimation of Sweet Cherry Cultivars Grafted on Different Rootstocks. The Scientific World Journal 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Dogan, M.; Bolat, I.; Turan, M.; Kaya, O. , Elucidating stress responses in Prunus rootstocks through comprehensive evaluation under drought, heat shock and combined stress conditions. Scientia Horticulturae 2025, 339. [Google Scholar] [CrossRef]
- Ljubojević, M.; Zorić, L.; Maksimović, I.; Dulić, J.; Miodragović, M.; Barać, G.; Ognjanov, V. , Anatomically assisted cherry rootstock selection. Scientia Horticulturae 2017, 217, 197–208. [Google Scholar] [CrossRef]
- Jia, L. t.; Qin, X.; Lyu, D. g.; Qin, S. j.; Zhang, P. , ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Scientia Horticulturae 2019, 257, 108647. [Google Scholar] [CrossRef]
- Gerbi, H.; Paudel, I.; Zisovich, A.; Sapir, G.; Ben-Dor, S.; Klein, T. , Physiological drought resistance mechanisms in wild species vs. rootstocks of almond and plum. Trees 2021, 36, 669–683. [Google Scholar] [CrossRef]
- McGee, T.; Schaffer, B.; Shahid, M. A.; Chaparro, J. X.; Sarkhosh, A. , Carbon and nitrogen metabolism in peach trees on different Prunus rootstocks in response to flooding. Plant and Soil 2022, 475, 427–441. [Google Scholar] [CrossRef]
- Ziegler, V. H.; Ploschuk, E.; Weibel, A.; Insausti, P. , Short-term responses to flooding stress of three Prunus rootstocks. Scientia Horticulturae 2017, 224, 135–141. [Google Scholar] [CrossRef]
- Klumb, E. K.; Braga, E. J. B.; Bianchi, V. J. , Differential expression of genes involved in the response of Prunus spp. rootstocks under soil flooding. Scientia Horticulturae 2020, 261. [Google Scholar] [CrossRef]
- Toro, G.; Pimentel, P.; Salvatierra, A. , Effective Categorization of Tolerance to Salt Stress through Clustering Prunus Rootstocks According to Their Physiological Performances. Horticulturae 2021, 7, 542. [Google Scholar] [CrossRef]
- Sandhu, D.; Kaundal, A.; Acharya, B. R.; Forest, T.; Pudussery, M. V.; Liu, X.; Ferreira, J. F. S.; Suarez, D. L. , Linking diverse salinity responses of 14 almond rootstocks with physiological, biochemical, and genetic determinants. Scientific Reports 2020, 10, 21087. [Google Scholar] [CrossRef]
- Shao, Y. h.; Cheng, Y. k.; Pang, H. g.; Chang, M. q.; He, F.; Wang, M. m.; Davis, D. J.; Zhang, S. x.; Betz, O.; Fleck, C.; Dai, T.; Madahhosseini, S.; Wilkop, T.; Jernstedt, J.; Drakakaki, G. , Investigation of Salt Tolerance Mechanisms Across a Root Developmental Gradient in Almond Rootstocks. Frontiers in Plant Science 2021, 11, 595055. [Google Scholar] [CrossRef]
- Paula, B. V. d.; Marques, A. C. R.; Rodrigues, L. A. T.; Souza, R. O. S. d.; Kulmann, M. S. d. S.; Kaminski, J.; Ceretta, C. A.; Melo, G. W. B. d.; Mayer, N. A.; Antunes, L. E.; Ricachenevsky, F. K.; Nicoloso, F. T.; Brunetto, G. , Morphological and kinetic parameters of the uptake of nitrogen forms in clonal peach rootstocks. Scientia Horticulturae 2018, 239, 205–209. [Google Scholar] [CrossRef]
- Chen, Q. j.; Lian, M.; Guo, J.; Zhang, B. b.; Yang, S. k.; Huang, K. x.; Peng, F. t.; Xiao, Y. s. , Comparative Transcriptome Analysis of Two Peach Rootstocks Uncovers the Effect of Gene Differential Expression on Nitrogen Use Efficiency. International Journal of Molecular Sciences 2022, 23, 11144. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Keles, H.; Bozkurt, E. , Physiological and histological responses of peach plants grafted onto different rootstocks under calcium deficiency conditions. Scientia Horticulturae 2021, 281, 109967. [Google Scholar] [CrossRef]
- Sun, S. x.; Li, J.; Song, H. y.; Chen, D.; Tu, M. y.; Chen, Q. y.; Jiang, G. l.; Zhou, Z. q. , Comparative transcriptome and physiological analyses reveal key factors in the tolerance of peach rootstocks to iron deficiency chlorosis. 3 Biotech 2022, 12, 38. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Abadía, A.; Moreno, M. Á.; Gogorcena, Y. , Tolerance Response to Iron Chlorosis of Prunus Selections as Rootstocks. HortScience 2008, 43, 304–309. [Google Scholar] [CrossRef]
- Somavilla, L. M.; Simão, D. G.; Tiecher, T. L.; Hammerschimitt, R. K.; de Oliveira, J. M. S.; Mayer, N. A.; Pavanello, E. P.; Trentin, E.; Belles, S. W.; Brunetto, G. , Structural changes in roots of peach rootstock cultivars grown in soil with high zinc content. Scientia Horticulturae 2018, 237, 1–10. [Google Scholar] [CrossRef]
- Usenik, V.; Marn, M. V. , Sugars and organic acids in plum fruit affected by Plum pox virus. Journal of the Science of Food and Agriculture 2016, 97, 2154–2158. [Google Scholar] [CrossRef]
- Zhou, J.; Xing, F.; Wang, H.; Li, S. , Occurrence, Distribution, and Genomic Characteristics of Plum Pox Virus Isolates from Common Apricot (Prunus armeniaca) and Japanese Apricot (Prunus mume) in China. Plant Disease 2021, 105, 3474–3480. [Google Scholar] [CrossRef]
- Polák, J.; oukroPec, I. , Identification of Interspecific Peach and Prunus sp. Hybrids Resistant to Plum Pox Virus Infection. Plant Protection Science 2010, 46, 139–144. [Google Scholar] [CrossRef]
- Polák, J.; Komínek, P. , Evaluation of rootstocks of stone fruits for resistance to natural Plum pox virus infection. Canadian Journal of Plant Pathology 2014, 36, 116–120. [Google Scholar] [CrossRef]
- Cinar, C. T.; Gazel, M.; Kaya, K.; Olmos, A.; Caglayan, K. , Susceptibility of different prunus rootstocks to natural infection of plum pox virus-Turkey (PPV-T) in Central Anatolia. Physiological and Molecular Plant Pathology 2022, 119. [Google Scholar] [CrossRef]
- T, T.; E, E.; C, T. , Susceptibility of five Prunus rootstocks to Agrobacterium tumefaciens. New Zealand Journal of Crop and Horticultural Science 2005, 33, 343–345. [Google Scholar]
- Beckman, T. G. , ‘Sharpe’, a Clonal Plum Rootstock for Peach. HortScience 2008, 43, 2236–2237. [Google Scholar] [CrossRef]
- Beckman, T. G. , ‘MP-29’, a Clonal Interspecific Hybrid Rootstock for Peach. HortScience 2012, 47, 128–131. [Google Scholar] [CrossRef]
- Baumgartner, K.; Fujiyoshi, P.; Ledbetter, C.; Duncan, R.; Kluepfel, D. A. , Screening Almond Rootstocks for Sources of Resistance to Armillaria Root Disease. HortScience 2018, 53, 4–8. [Google Scholar] [CrossRef]
- Lesmes-Vesga, R. A.; Cano, L. M.; Ritenour, M. A.; Sarkhosh, A.; Chaparro, J. X.; Rossi, L. , Rootstocks for Commercial Peach Production in the Southeastern United States: Current Research, Challenges, and Opportunities. Horticulturae 2022, 8, 602. [Google Scholar] [CrossRef]
- Eliwa, G. I.; Hagag, E. S. , Approach to New peach rootstocks resistant to root-knot nematodes (Meloidogyne species) selected from local Mit-Ghamer peach cultivar. Scientia Horticulturae 2021, 284. [Google Scholar] [CrossRef]
- Rubio-Cabetas, M. J.; Lecouls, A. C.; Salesses, G.; Bonnet, A.; Esmenjaud, D. , Evidence of a new gene for high resistance to Meloidogyne spp.in Myrobalan plum, Prunus cerasifera. Plant Breeding, 1998; 117, 567–571. [Google Scholar]
- Lecouls, A. C.; Rubio-Cabetas, M. J.; Minot, J. C.; Voisin, R.; Bonnet, A.; Salesses, G.; Dirlewanger, E.; Esmenjaud, D. , RAPD and SCAR markers linked to the Ma1root-knot nematode resistance gene in Myrobalan plum (Prunus cerasifera Ehr.). Theoretical & Applied Genetics 1999, 99, 328–335. [Google Scholar]
- Liu, J.; Zhu, J.; Li, H.; Luo, D.; Xie, J.; Li, H.; Liu, S.; Zhang, Y.; Chen, L.; Xie, X.; Wang, D.; Li, K.; Yao, M.; Zhang, G. , A preliminary study on the root-knot nematode resistance of a cherry plum cultivar Mirabolano 29C. Czech Journal of Genetics and Plant Breeding 2023, 59, 133–140. [Google Scholar] [CrossRef]
- Irisarri, P.; Errea, P.; Pina, A. , Physiological and Molecular Characterization of New Apricot Cultivars Grafted on Different Prunus Rootstocks. Agronomy 2021, 11. [Google Scholar] [CrossRef]
- Reig, G.; Zarrouk, O.; Font i Forcada, C.; Moreno, M. Á. , Anatomical graft compatibility study between apricot cultivars and different plum based rootstocks. Scientia Horticulturae 2018, 237, 67–73. [Google Scholar] [CrossRef]
- Mendelné Pászti, E.; Bujdoso, G.; Ercisli, S.; Hrotkó, K.; Mendel, Á. , Apricot Rootstocks with Potential in Hungary. Horticulturae 2023, 9, 720. [Google Scholar] [CrossRef]
- Reig, G.; Salazar, A.; Zarrouk, O.; Forcada, C. F. i.; Val, J.; Moreno, M. Á. , Long-term graft compatibility study of peach-almond hybrid and plum based rootstocks budded with European and Japanese plums. Scientia Horticulturae 2019, 243, 392–400. [Google Scholar] [CrossRef]
- Liu Qing-zhong; Dong-zi, Z.; Jia-wei, W.; Li-si, Z.; Po, H.; Qing-dang, G., The evaluation of sweet cherry rootstocks and their application prospects in the world. Deciduous Fruits 2023, 55, 1–7.
- Skočajić, D.; Gašić, U.; Dabić Zagorac, D.; Nešić, M.; Tešić, Ž.; Meland, M.; Fotirić Akšić, M. , Analysis of Phenolic Compounds for the Determination of Grafts (in) Compatibility Using In Vitro Callus Cultures of Sato-Zakura Cherries. Plants 2021, 10. [Google Scholar] [CrossRef]
- Jalali, A.; Moghaddam, E. G.; Marjani, A. , Early detection of graft incompatibility in sweet cherry by internode association and callus fusion techniques. Plant Cell, Tissue and Organ Culture (PCTOC), 2024; 156. [Google Scholar]
- Iglesias, I.; Botet, R. , The selection of appropriate rootstock and training system towards sustainable production of stone fruits. Italus Hortus 2024, 31. [Google Scholar] [CrossRef]
- Clark, J. R.; Finn, C. E. , Register of New Fruit and Nut Cultivars List 43. HortScience 2006, 41, 1101–1133. [Google Scholar] [CrossRef]
- Lordan, J.; Zazurca, L.; Maldonado, M.; Torguet, L.; Alegre, S.; Miarnau, X. , Horticultural performance of ‘Marinada’ and ‘Vairo’ almond cultivars grown on a genetically diverse set of rootstocks. Scientia Horticulturae 2019, 256. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Tan, B.; Jiang, Y.; Zheng, X.; Ye, X.; Guo, Z.; Xiong, T.; Wang, W.; Li, J.; Feng, J. , A single nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotechnology Journal 2019, 17, 1723–1735. [Google Scholar] [CrossRef]
- Reig, G.; Font i Forcada, C.; Mestre, L.; Betrán, J. A.; Moreno, M. Á. , Potential of new Prunus cerasifera based rootstocks for adapting under heavy and calcareous soil conditions. Scientia Horticulturae 2018, 234, 193–200. [Google Scholar] [CrossRef]
- Sottile, F.; Monte, M.; De Michele, A. , Effect of Different Rootstocks on Vegetative Growth of Japanese and European Plum Cultivars in Southern Italy: Preliminary Results. Acta Horticulturae 2007, 375–380. [Google Scholar] [CrossRef]
- Yaman, M.; UĞUr, R.; SÜMbÜL, A.; KeÇE, Y.; GÖNÜLtaŞ, M.; ÜNsal, H. T.; GÜNeŞ, A.; Yildiz, E.; Yilmaz, K. U. Determination of fruit characteristics, nutrients and biochemical contents of Transvalia (Prunus persica L.) peach cultivar grafted on different clonal rootstocks obtained by selection and hybridization. Scientia Horticulturae 2024, 330.
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M. A. , Fruit sugar profile and antioxidants of peach and nectarine cultivars on almond × peach hybrid rootstocks. Scientia Horticulturae 2013, 164, 563–572. [Google Scholar] [CrossRef]
- Reig, G.; Garanto, X.; Mas, N.; Iglesias, I. , Long-term agronomical performance and iron chlorosis susceptibility of several Prunus rootstocks grown under loamy and calcareous soil conditions. Scientia Horticulturae 2020, 262. [Google Scholar] [CrossRef]
- Giorgi, M.; Capocasa, F.; Scalzo, J.; Murri, G.; Battino, M.; Mezzetti, B. The rootstock effects on plant adaptability, production, fruit quality, and nutrition in the peach (cv. ‘Suncrest’). Scientia Horticulturae 2005, 107, 36-42.
- Iglesias, I.; Giné-Bordonaba, J.; Garanto, X.; Reig, G. , Rootstock affects quality and phytochemical composition of ‘Big Top’ nectarine fruits grown under hot climatic conditions. Scientia Horticulturae 2019, 256. [Google Scholar] [CrossRef]
- López-Ortega, G.; García-Montiel, F.; Bayo-Canha, A.; Frutos-Ruiz, C.; Frutos-Tomás, D. Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the Region of Murcia. Scientia Horticulturae 2016, 198, 326-335.
- Milošević, T.; Milošević, N.; Mladenović, J. , Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Scientia Horticulturae 2020, 265. [Google Scholar] [CrossRef]
- Bujdosó, G.; Magyar, L.; Hrotkó, K. Long term evaluation of growth and cropping of sweet cherry (Prunus avium L.) varieties on different rootstocks under Hungarian soil and climatic conditions. Scientia Horticulturae 2019, 256.
- Hernández, F.; Pinochet, J.; Moreno, M. A.; Martínez, J. J.; Legua, P. , Performance of Prunus rootstocks for apricot in Mediterranean conditions. Scientia Horticulturae 2010, 124, 354–359. [Google Scholar] [CrossRef]
- Gürcan, K.; Çetinsağ, N.; Pınar, H.; Macit, T. , Molecular and biological assessment reveals sources of resistance to Plum pox virus - Turkey strain in Turkish apricot (Prunus armeniaca) germplasm. Scientia Horticulturae 2019, 252, 348–353. [Google Scholar] [CrossRef]
- Polo-Oltra, Á.; Romero, C.; López, I.; Badenes, M.; Zuriaga, E. , Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Boris, K.; Yang, L.; Jiang, F.; Zhang, M.; Wang, Y. , RT-PCR Detection of Plum pox virus and the Screening of DNA Markers Linked to PPV-resistance in Apricot. Acta Agriculturae Boreali-Sinica, 2018; 33, 31–38. [Google Scholar]
- Blenda, A. V.; Verde, I.; Georgi, L. L.; Reighard, G. L.; Forrest, S. D.; Muñoz-Torres, M.; Baird, W. V.; Abbott, A. G. , Construction of a genetic linkage map and identification of molecular markers in peach rootstocks for response to peach tree short life syndrome. Tree Genetics & Genomes, 2007; 3, 341–350. [Google Scholar]
- Maquilan, M. A. D.; Olmstead, M. A.; Olmstead, J. W.; Dickson, D. W.; Chaparro, J. X. , Genetic analyses of resistance to the peach root-knot nematode (Meloidogyne floridensis) using microsatellite markers. Tree Genetics & Genomes, 2018; 14. [Google Scholar]
- Duval, H.; Van Ghelder, C.; Portier, U.; Confolent, C.; Meza, P.; Esmenjaud, D. , New Data Completing the Spectrum of the Ma, RMia, and RMja Genes for Resistance to Root-Knot Nematodes (Meloidogyne spp.) in Prunus. Phytopathology, 2019; 109, 615–622. [Google Scholar]
- Duval, H.; Heurtevin, L.; Dlalah, N.; Caravel, C.; Callot, C.; Van Ghelder, C. , Identification and Expression of the peach TNL RMia genes for the Resistance to the Root-knot Nematode Meloidogyne incognita. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lu, Z.; Niu, L.; Chagné, D.; Cui, G.; Pan, L.; Foster, T.; Zhang, R.; Zeng, W.; Wang, Z. , Fine mapping of the temperature-sensitive semi-dwarf (Tssd) locus regulating the internode length in peach (Prunus persica). Molecular Breeding 2016, 36. [Google Scholar] [CrossRef]
- Cantín, C. M.; Arús, P.; Eduardo, I. , Identification of a new allele of the Dw gene causing brachytic dwarfing in peach. BMC Research Notes 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- García-Almodóvar, R. C.; Clemente-Moreno, M. J.; Díaz-Vivancos, P.; Petri, C.; Rubio, M.; Padilla, I. M. G.; Ilardi, V.; Burgos, L. , Greenhouse evaluation confirms in vitro sharka resistance of genetically engineered h-UTR/P1 plum plants. Plant Cell, Tissue and Organ Culture (PCTOC), 2014; 120, 791–796. [Google Scholar]
- Sidorova, T.; Pushin, A.; Miroshnichenko, D.; Dolgov, S. , Generation of Transgenic Rootstock Plum ((Prunus pumila L. × P. salicina Lindl.) × (P. cerasifera Ehrh.)) Using Hairpin-RNA Construct for Resistance to the Plum pox virus. Agronomy, 2017; 8. [Google Scholar]
- Sidorova, T.; Mikhailov, R.; Pushin, A.; Miroshnichenko, D.; Dolgov, S. , Agrobacterium-Mediated Transformation of Russian Commercial Plum cv. “Startovaya” (Prunus domestica L.) With Virus-Derived Hairpin RNA Construct Confers Durable Resistance to PPV Infection in Mature Plants. Frontiers in Plant Science, 2019; 10. [Google Scholar]
- Alburquerque, N.; Pérez-Caselles, C.; Faize, L.; Ilardi, V.; Burgos, L. , Trans-grafting plum pox virus resistance from transgenic plum rootstocks to apricot scions. Frontiers in Plant Science 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Mourenets, L.; Pushin, A.; Timerbaev, V.; Khmelnitskaya, T.; Gribkov, E.; Andreev, N.; Dolgov, S. , Effect of Gene Silencing of Translation Initiation Factors eIF(iso)4G and eIF(iso)4E on Sour Cherry Rootstock Resistance to Sharka Disease. International Journal of Molecular Sciences 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Zong, X. j.; Xu, L.; Tan, Y.; Wei, H. r. , Development of genetically modified sweet cherry rootstock ‘Gisela 6’ with overexpression of PcMPK3-HA gene by Agrobacterium-mediated genetic transformation. Plant Cell, Tissue and Organ Culture, 2022; 151, 375–384. [Google Scholar]
- Jedličková, V.; Štefková, M.; Sánchez López, J. F.; Grimplet, J.; Rubio Cabetas, M. J.; Robert, H. S. , Genome editing in almond using hairy root transformation system. Plant Cell, Tissue and Organ Culture (PCTOC), 2024; 159. [Google Scholar]
| Rootstock | Origin | Developed | Compatible scion | Vigour control |
|---|---|---|---|---|
| Hansen 536 | USA | P. dulcis × P. persica | almond | Standard |
| Rootpac 20 | Spain | P. besseyi × P. cerasifera | peach | Dwarf |
| Sirio | Italy | Seedling and open pollinated seedling of GF 557 | peach | Semi-dwarf |
| Siberian C | Canada | P. persica | peach | Semi-vigorous |
| Greenpac | Spain | Cross of Felinem (almond × peach) × Cadaman (peach × P. davidiana) | peach | Semi-vigorous |
| Bailey | USA | Naturalized peach selection | peach | semi-Vigorous |
| Adarcias | Spain | An almond-peach hybrid selected from open pollinated seedling population | peach | Semi-vigorous |
| Higama | France | P. persica | peach | Standard |
| Guardian | USA | P. persica × P. davidiana | peach | very Vigorous |
| Montelor | France | P. spersica | peach | Vigorous |
| Zhong Tao Kang Zhen No. 1 | China | P. spersica × P. kansuensis | peach | vigorous |
| Nemared | USA | P. persica × P. davidiana | peach, almond | Standard |
| GF677 | France | P. dulcis × P. persica | peach, almond | Standard |
| Adafuel | Spain | Seedling population obtained by open pollination of ‘Marcona’ almond cultivars | peach, almond | vigorous |
| Replantpac | Spain | Plum × almond rootstock | peach, almond | Vigorous |
| Penta | Italy | P. domestica × P. cerasifera | peach, apricot | Semi-dwarf |
| Tetra | Italy | P. domestica | peach, apricot | Semi-dwarf |
| Montizo | Spain | Clone selected from Pollizo plum rootstock | peach, apricot | Semi-vigorous |
| Monpol | Spain | Clone selected from Pollizo plum rootstock | peach, apricot | Semi-vigorous |
| Krymsk 1 | Russia | P. tomentosa × P. cerasifera | plum, apricot | Semi-dwarf |
| Jaspi | France | P. salicina × P. spinosa | plum, apricot | Semi-vigorous |
| Adaptabil | Romania | P. besseyi | plum, apricot | Semi-vigorous |
| Ademir | Spain | P. cerasifera | plum, apricot | Semi-vigorous |
| St. Julien A | UK | P. insititia | plum, apricot | Semi-vigorous |
| Myrobalan 29C | USA | P. cerasifera | plum, apricot | standard |
| Marianna GF8-1 | France | P. cerasifera × P.munsoniana | plum, apricot | very Vigorous |
| Marianna 2624 | USA | P. cerasifera × P. musonianna | plum, apricot | very Vigorous |
| Myrobalan | USA | P. cerasifera | plum, apricot | Vigorous |
| St. Julien GF 655/2 | France | P. insititia | plum, apricot, peach, | Semi-dwarf |
| Krymsk 86 | Russia | P. persica × P. cerasifera | plum, apricot, peach, | Standard |
| Ishtara | France | (P. cerasifera × P. salicina) × (P. cerasifera × P. persica) | plum, apricot, peach, almond | Semi-vigorous |
| Adesoto 101 | Spain | P. insititia | plum, apricot, peach, almond | Semi-vigorous |
| Lovell | USA | P. persica | plum, apricot, peach, almond | Standard |
| Nemaguard | USA | P. persica × P. davidiana | plum, apricot, peach, almond | very Vigorous |
| MaxMa | USA | P. Mahaleb × P. avium | sweet cherry | semi-dwarf |
| P-HL-C | Czech Republic | P. avium × P. cerasus | sweet cherry | Semi-dwarf |
| Jingchun 1 | China | P. cerusus × P. pseudocerasus | sweet cherry | Semi-vigorous |
| P-HL-A | Czech Republic | P. avium × P. cerasus | sweet cherry | Semi-vigorous |
| Aijie | China | P. cerasus × P. canescens | sweet cherry | Semi-vigorous |
| Monrepos | Spain | P. cerasifera | sweet cherry | Semi-vigorous |
| Mazzard | USA | P. avium | sweet cherry | very Vigorous |
| P-HL-B | Czech Republic | P. avium × P. cerasus | sweet cherry | Vigorous |
| Mazzard F 12/1 | USA | P. avium | sweet cherry | Vigorous |
| Landing 1 | China | P. avium × P. pseudocerasus | sweet cherry | Vigorous |
| Landing 2 | China | P. avium × P. pseudocerasus | sweet cherry | Vigorous |
| Yanying 3 | China | P. pseudocerasus | sweet cherry | vigorous |
| Haiying 1 | China | P. pseudocerasus | sweet cherry | #N/A |
| Gisela 5 | Germany | P.cerasus × P.canescens | sweet cherry, sour cherry | Semi-dwarf |
| Mahaleb ‘CDR-1’ | USA | P. mahaleb | sweet cherry, sour cherry | Semi-vigorous |
| Gisela 6 | Germany | P.cerasus × P.canescens | sweet cherry, sour cherry | Semi-vigorous |
| Krymsk 6 | Russia | P. cerasus × (P. cerasus × P. maackii) | sweet cherry, sour cherry | Semi-vigorous |
| Mahaleb | USA | P. Mahaleb | sweet cherry, sour cherry | very Vigorous |
| Colt | UK | P. avium × P. pseudocerasus | sweet cherry, sour cherry | vigorous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





