Submitted:
17 March 2025
Posted:
18 March 2025
You are already at the latest version
Abstract
Keywords:
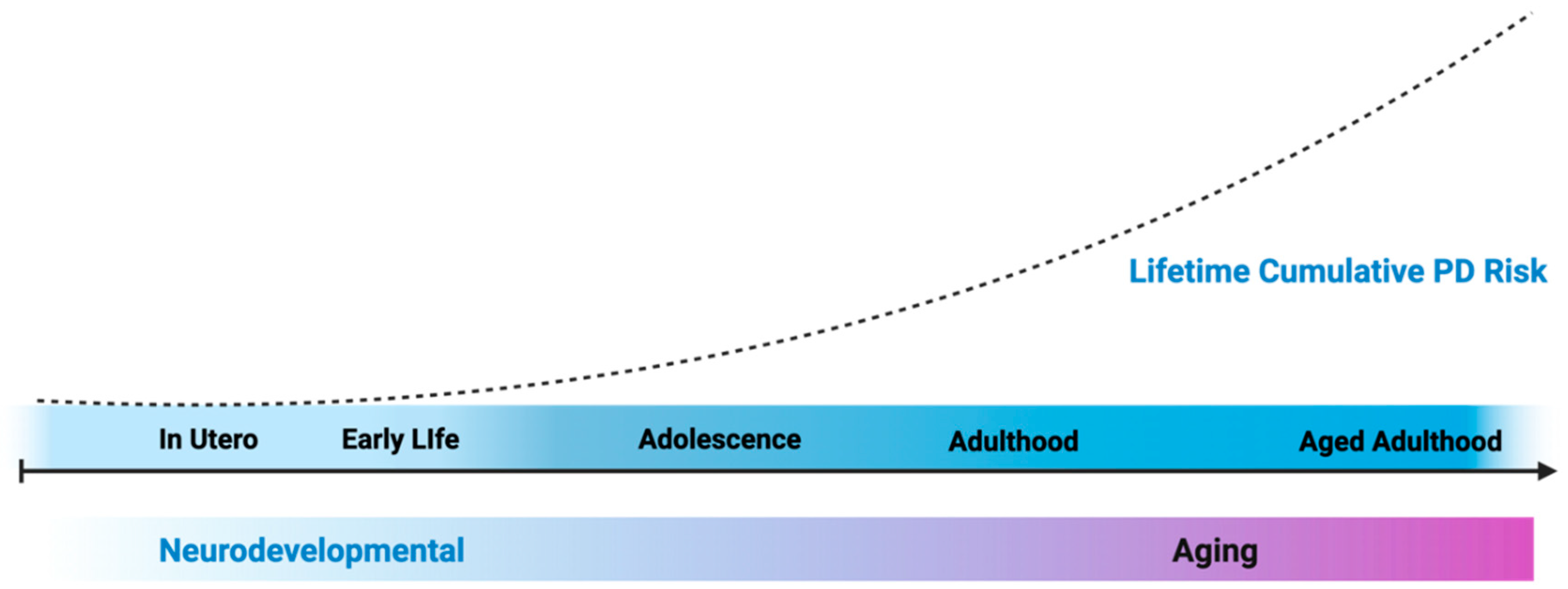
Introduction
Effects of Early Life Exposure to TCE
Persistent Consequences of Early Life and Developmental Exposure to Other PD-Related Toxicants
Dieldrin
Paraquat
Tetrachloroethylene
Rotenone
Next Steps for PD Prevention
Acknowledgements
Conflicts of Interest
References
- Doherty, R.E. A history of the production and use of carbon tetrachloride, tetrachloroethylene, trichloroethylene and 1, 1, 1-trichloroethane in the United States: Part 1—Historical background; carbon tetrachloride and tetrachloroethylene. Environmental forensics. 2000, 1, 69–81. [Google Scholar]
- Bakke, B.; Stewart, P.A.; Waters, M.A. Uses of and exposure to trichloroethylene in U.S. industry: A systematic literature review. J Occup Environ Hyg. 2007, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Schaum, J. Exposure assessment of trichloroethylene. Environmental health perspectives 2000, 108 (Suppl 2), 359–363. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Zafar, M.; Lettenberger, S.E.; Pawlik, M.E.; Kinel, D.; Frissen, M.; Schneider, R.B.; Kieburtz, K.; Tanner, C.M.; De Miranda, B.R.; Goldman, S.M.; Bloem, B.R. Trichloroethylene: An Invisible Cause of Parkinson's Disease? J Parkinsons Dis. 2023, 13, 203–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todd, G.D.; Ruiz, P.; Mumtaz, M.; Wohlers, D.; Klotzbach, J.M.; Diamond, G.L.; Coley, C.; Citra, M.J. Toxicological profile for trichloroethylene (TCE)2019.
- Trichloroethylene (TCE); Regulation Under the Toxic Substances Control Act (TSCA), (2024).
- Guha, N.; Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. The Lancet Oncology. 2012, 13, 1192–1193. [Google Scholar] [CrossRef]
- Karami, S.; Lan, Q.; Rothman, N.; Stewart, P.A.; Lee, K.-M.; Vermeulen, R.; Moore, L.E. Occupational trichloroethylene exposure and kidney cancer risk: A meta-analysis. Occupational and Environmental Medicine. 2012, 69, 858. [Google Scholar] [CrossRef]
- Siegel Scott, C.; Jinot, J. Trichloroethylene and Cancer: Systematic and Quantitative Review of Epidemiologic Evidence for Identifying Hazards. International Journal of Environmental Research and Public Health [Internet]. 2011, 8, 4238–4272. [Google Scholar] [CrossRef]
- Ruckart, P.Z.; Bove, F.J.; Shanley, E.; Maslia, M. Evaluation of contaminated drinking water and male breast cancer at Marine Corps Base Camp Lejeune, North Carolina: A case control study. Environmental Health. 2015, 14, 74. [Google Scholar] [CrossRef]
- Maltoni, C.; Lefemine, G.; Cotti, G.; Perino, G. Long-term carcinogenicity bioassays on trichloroethylene administered by inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice. Annals of the New York Academy of Sciences. 1988, 534, 316–42. [Google Scholar]
- Rusyn, I.; Chiu, W.A.; Lash, L.H.; Kromhout, H.; Hansen, J.; Guyton, K.Z. Trichloroethylene: Mechanistic, epidemiologic and other supporting evidence of carcinogenic hazard. Pharmacology & Therapeutics. 2014, 141, 55–68. [Google Scholar] [CrossRef]
- Bove, F.J.; Ruckart, P.Z.; Maslia, M.; Larson, T.C. Mortality study of civilian employees exposed to contaminated drinking water at USMC Base Camp Lejeune: A retrospective cohort study. Environ Health. 2014, 13, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guehl, D.; Bezard, E.; Dovero, S.; Boraud, T.; Bioulac, B.; Gross, C. Trichloroethylene and parkinsonism: A human and experimental observation. European journal of neurology. 1999, 6, 609–611. [Google Scholar] [CrossRef]
- Goldman, S.M.; Quinlan, P.J.; Ross, G.W.; Marras, C.; Meng, C.; Bhudhikanok, G.S.; Comyns, K.; Korell, M.; Chade, A.R.; Kasten, M.; Priestley, B.; Chou, K.L.; Fernandez, H.H.; Cambi, F.; Langston, J.W.; Tanner, C.M. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012, 71, 776–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gash, D.M.; Rutland, K.; Hudson, N.L.; Sullivan, P.G.; Bing, G.; Cass, W.A.; Pandya, J.D.; Liu, M.; Choi, D.-Y.; Hunter, R.L.; Gerhardt, G.A.; Smith, C.D.; Slevin, J.T.; Prince, T.S. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Annals of Neurology. 2008, 63, 184–192. [Google Scholar] [CrossRef]
- Goldman, S.M.; Weaver, F.M.; Stroupe, K.T.; Cao, L.; Gonzalez, B.; Colletta, K.; Brown, E.G.; Tanner, C.M. Risk of Parkinson Disease Among Service Members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023, 80, 673–681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldman, S.M.; Weaver, F.M.; Gonzalez, B.; Stroupe, K.T.; Cao, L.; Colletta, K.; Brown, E.G.; Tanner, C.M. Parkinson's Disease Progression and Exposure to Contaminated Water at Camp Lejeune. Movement Disorders. 2024, 39, 1732–1739. [Google Scholar] [CrossRef]
- Liu, M.; Choi, D.Y.; Hunter, R.L.; Pandya, J.D.; Cass, W.A.; Sullivan, P.G.; Kim, H.C.; Gash, D.M.; Bing, G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem. 2010, 112, 773–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Shin, E.-J.; Dang, D.-K.; Jin, C.-H.; Lee, P.H.; Jeong, J.H.; Park, S.-J.; Kim, Y.-S.; Xing, B.; Xin, T.; Bing, G.; Kim, H.-C. Trichloroethylene and Parkinson’s Disease: Risk Assessment. Molecular Neurobiology. 2018, 55, 6201–6214. [Google Scholar] [CrossRef]
- Adamson, A.; Ilieva, N.; Stone, W.J.; De Miranda, B.R. Low-dose inhalation exposure to trichloroethylene induces dopaminergic neurodegeneration in rodents. Toxicol Sci. 2023. [CrossRef] [PubMed]
- De Miranda, B.R.; Castro, S.L.; Rocha, E.M.; Bodle, C.R.; Johnson, K.E.; Greenamyre, J.T. The industrial solvent trichloroethylene induces LRRK2 kinase activity and dopaminergic neurodegeneration in a rat model of Parkinson's disease. Neurobiol Dis. 2021, 153, 105312. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, N.M.; Hoffman, E.K.; Ghalib, M.A.; Greenamyre, J.T.; De Miranda, B.R. LRRK2 kinase inhibition protects against Parkinson's disease-associated environmental toxicants. Neurobiol Dis. 2024, 196, 106522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Ritz, B. The Search for Environmental Causes of Parkinson's Disease: Moving Forward. J Parkinsons Dis. [CrossRef] [PubMed] [PubMed Central]
- Reuhl, K. Delayed expression of neurotoxicity: The problem of silent damage. Neurotoxicology. 1991, 12, 341–346. [Google Scholar]
- Kraft, A.D.; Aschner, M.; Cory-Slechta, D.A.; Bilbo, S.D.; Caudle, W.M.; Makris, S.L. Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicology and Teratology. 2016, 55, 38–44. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Thiruchelvam, M.; Barlow, B.K.; Richfield, E.K. Developmental pesticide models of the Parkinson disease phenotype. Environmental health perspectives. 2005, 113, 1263–1270. [Google Scholar] [PubMed]
- Sulzer, D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends in neurosciences. 2007, 30, 244–250. [Google Scholar]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Ageing as a primary risk factor for Parkinson's disease: Evidence from studies of non-human primates. Nature Reviews Neuroscience. 2011, 12, 359–366. [Google Scholar] [PubMed]
- Rodier, P.M. Developing brain as a target of toxicity. Environmental Health Perspectives. 1995; 103, (suppl 6), 73–76. [Google Scholar] [CrossRef]
- Lanphear, B.P. The Impact of Toxins on the Developing Brain. Annual Review of Public Health. 2015; 36, 211–230. [Google Scholar] [CrossRef]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; Scharfmann, R.; Sheppard, A.; Skinner, M.; Szyf, M.; Waterland, R.A.; Waxman, D.J.; Whitelaw, E.; Ong, K.; Albertsson-Wikland, K. Child Health, Developmental Plasticity, and Epigenetic Programming. Endocrine Reviews. 2011, 32, 159–224. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease: A paradigm for understanding disease cause and prevention. Current opinion in pediatrics. 2015, 27, 248–253. [Google Scholar]
- Ghantous, H.; Danielsson, B.R.; Dencker, L.; Gorczak, J.; Vesterberg, O. Trichloroacetic acid accumulates in murine amniotic fluid after tri- and tetrachloroethylene inhalation. Acta Pharmacol Toxicol (Copenh). 1986, 58, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Forand, S.P.; Lewis-Michl, E.L.; Gomez, M.I. Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in New York State. Environ Health Perspect. 2012, 120, 616–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldberg, S.J.; Lebowitz, M.D.; Graver, E.J.; Hicks, S. An association of human congenital cardiac malformations and drinking water contaminants. Journal of the American College of Cardiology. 1990, 16, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Beamer, P.I.; Luik, C.E.; Abrell, L.; Campos, S.; Martínez, M.E.; Sáez, A.E. Concentration of trichloroethylene in breast milk and household water from Nogales, Arizona. Environmental science & technology. 2012, 46, 9055–9061. [Google Scholar]
- Blossom, S.J.; Doss, J.C.; Hennings, L.J.; Jernigan, S.; Melnyk, S.; James, S.J. Developmental exposure to trichloroethylene promotes CD4+ T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice. Toxicology and Applied Pharmacology. 2008, 231, 344–353. [Google Scholar] [CrossRef]
- Blossom, S.J.; Doss, J.C. Trichloroethylene Alters Central and Peripheral Immune Function in Autoimmune-Prone MRL+/+ Mice Following Continuous Developmental and Early Life Exposure. Journal of Immunotoxicology. 2007, 4, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.M.; Woodruff, W.; Blossom, S.J. Differential immunotoxicity induced by two different windows of developmental trichloroethylene exposure. Autoimmune Dis. 2014, 2014, 982073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilbert, K.M.; Bai, S.; Barnette, D.; Blossom, S.J. Exposure Cessation During Adulthood Did Not Prevent Immunotoxicity Caused by Developmental Exposure to Low-Level Trichloroethylene in Drinking Water. Toxicological Sciences. 2017, 157, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Byrum, S.D.; Washam, C.L.; Patterson, J.D.; Vyas, K.K.; Gilbert, K.M.; Blossom, S.J. Continuous Developmental and Early Life Trichloroethylene Exposure Promoted DNA Methylation Alterations in Polycomb Protein Binding Sites in Effector/Memory CD4+ T Cells. Frontiers in Immunology. 2019, 10. [Google Scholar] [CrossRef]
- Blossom, S.J.; Cooney, C.A.; Melnyk, S.B.; Rau, J.L.; Swearingen, C.J.; Wessinger, W.D. Metabolic changes and DNA hypomethylation in cerebellum are associated with behavioral alterations in mice exposed to trichloroethylene postnatally. Toxicology and Applied Pharmacology. 2013, 269, 263–269. [Google Scholar] [CrossRef]
- Blossom, S.J.; Melnyk, S.B.; Li, M.; Wessinger, W.D.; Cooney, C.A. Inflammatory and oxidative stress-related effects associated with neurotoxicity are maintained after exclusively prenatal trichloroethylene exposure. Neurotoxicology. 2017, 59, 164–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blossom, S.J.; Melnyk, S.; Cooney, C.A.; Gilbert, K.M.; James, S.J. Postnatal exposure to trichloroethylene alters glutathione redox homeostasis, methylation potential, and neurotrophin expression in the mouse hippocampus. Neurotoxicology. 2012, 33, 1518–1527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, H.L.; Teismann, P. Glutathione--a review on its role and significance in Parkinson's disease. FASEB J. 2009, 23, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Zeevalk, G.D.; Razmpour, R.; Bernard, L.P. Glutathione and Parkinson's disease: Is this the elephant in the room? Biomed Pharmacother. 2008, 62, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.M.; Bai, S.; Barnette, D.; Blossom, S.J. Exposure Cessation During Adulthood Did Not Prevent Immunotoxicity Caused by Developmental Exposure to Low-Level Trichloroethylene in Drinking Water. Toxicol Sci. 2017, 157, 429–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanthasamy, A.G.; Kitazawa, M.; Kanthasamy, A.; Anantharam, V. Dieldrin-induced neurotoxicity: Relevance to Parkinson's disease pathogenesis. Neurotoxicology. 2005, 26, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Richardson, J.R.; Guillot, T.S.; McCormack, A.L.; Di Monte, D.A.; Jones, D.P.; Pennell, K.D.; Miller, G.W. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007, 204, 619–630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richardson, J.R.; Caudle, W.M.; Wang, M.; Dean, E.D.; Pennell, K.D.; Miller, G.W.; Richardson, J.R.; Caudle, W.M.; Wang, M.; Dean, E.D. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. The FASEB journal. 2006, 20, 1695–1697. [Google Scholar]
- Gezer, A.O.; Kochmanski, J.; VanOeveren, S.E.; Cole-Strauss, A.; Kemp, C.J.; Patterson, J.R.; Miller, K.M.; Kuhn, N.C.; Herman, D.E.; McIntire, A.; Lipton, J.W.; Luk, K.C.; Fleming, S.M.; Sortwell, C.E.; Bernstein, A.I. Developmental exposure to the organochlorine pesticide dieldrin causes male-specific exacerbation of alpha-synuclein-preformed fibril-induced toxicity and motor deficits. Neurobiol Dis. 2020, 141, 104947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kochmanski, J.; VanOeveren, S.E.; Patterson, J.R.; Bernstein, A.I. Developmental Dieldrin Exposure Alters DNA Methylation at Genes Related to Dopaminergic Neuron Development and Parkinson’s Disease in Mouse Midbrain. Toxicological Sciences. 2019, 169, 593–607. [Google Scholar] [CrossRef]
- Boyd, S.L.; Kuhn, N.C.; Patterson, J.R.; Stoll, A.C.; Zimmerman, S.A.; Kolanowski, M.R.; Neubecker, J.J.; Luk, K.C.; Ramsson, E.S.; Sortwell, C.E.; Bernstein, A.I. Developmental exposure to the Parkinson’s disease-associated organochlorine pesticide dieldrin alters dopamine neurotransmission in α-synuclein pre-formed fibril (PFF)-injected mice. Toxicological Sciences. 2023, 196, 99–111. [Google Scholar] [CrossRef]
- Kochmanski, J.; Virani, M.; Kuhn, N.C.; Boyd, S.L.; Becker, K.; Adams, M.; Bernstein, A.I. Developmental origins of Parkinson’s disease risk: Perinatal exposure to the organochlorine pesticide dieldrin leads to sex-specific DNA modifications in critical neurodevelopmental pathways in the mouse midbrain. Toxicological Sciences. 2024, 201, 263–281. [Google Scholar] [CrossRef]
- Paul, K.C.; Cockburn, M.; Gong, Y.; Bronstein, J.; Ritz, B. Agricultural paraquat dichloride use and Parkinson's disease in California's Central Valley. Int J Epidemiol. 2024, 53. [Google Scholar] [CrossRef] [PubMed]
- Konthonbut, P.; Kongtip, P.; Nankongnab, N.; Tipayamongkholgul, M.; Yoosook, W.; Woskie, S. Paraquat Exposure of Pregnant Women and Neonates in Agricultural Areas in Thailand. International Journal of Environmental Research and Public Health. 2018, 15, 1163. [Google Scholar] [CrossRef] [PubMed]
- Trakulsrichai, S.; Paisanrodjanarat, B.; Sriapha, C.; Tongpoo, A.; Udomsubpayakul, U.; Wananukul, W. Clinical outcome of paraquat poisoning during pregnancy. Clinical Toxicology. 2019, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Ait-Bali, Y.; Ba-M’hamed, S.; Bennis, M. Prenatal Paraquat exposure induces neurobehavioral and cognitive changes in mice offspring. Environmental Toxicology and Pharmacology. 2016, 48, 53–62. [Google Scholar] [CrossRef]
- Zuo, Z.; Li, J.; Zhang, B.; Hang, A.; Wang, Q.; Xiong, G.; Tang, L.; Zhou, Z.; Chang, X. Early-Life Exposure to Paraquat Aggravates Sex-Specific and Progressive Abnormal Non-Motor Neurobehavior in Aged Mice. Toxics. 2023, 11, 842. [Google Scholar] [CrossRef]
- Mittra, N.; Chauhan, A.K.; Singh, G.; Patel, D.K.; Singh, C. Postnatal zinc or paraquat administration increases paraquat or zinc-induced loss of dopaminergic neurons: Insight into augmented neurodegeneration. Molecular and cellular biochemistry. 2020, 467, 27–43. [Google Scholar]
- Colle, D.; Santos, D.B.; Naime, A.A.; Gonçalves, C.L.; Ghizoni, H.; Hort, M.A.; Farina, M. Early postnatal exposure to paraquat and maneb in mice increases nigrostriatal dopaminergic susceptibility to a Re-challenge with the same pesticides at adulthood: Implications for Parkinson’s disease. Neurotoxicity Research. 2020, 37, 210–26. [Google Scholar] [CrossRef]
- Aschengrau, A.; Janulewicz, P.A.; White, R.F.; Vieira, V.M.; Gallagher, L.G.; Getz, K.D.; Webster, T.F.; Ozonoff, D.M. Long-term Neurotoxic Effects of Early-life Exposure to Tetrachloroethylene-contaminated Drinking Water. Annals of Global Health. 2016, 82, 169–179. [Google Scholar] [CrossRef]
- Aschengrau, A.; Weinberg, J.M.; Janulewicz, P.A.; Romano, M.E.; Gallagher, L.G.; Winter, M.R.; Martin, B.R.; Vieira, V.M.; Webster, T.F.; White, R.F.; Ozonoff, D.M. Occurrence of mental illness following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: A retrospective cohort study. Environ Health. 2012, 11, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aschengrau, A.; Winter, M.R.; Vieira, V.M.; Webster, T.F.; Janulewicz, P.A.; Gallagher, L.G.; Weinberg, J.; Ozonoff, D.M. Long-term health effects of early life exposure to tetrachloroethylene (PCE)-contaminated drinking water: A retrospective cohort study. Environmental Health. 2015, 14, 36. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; Comyns, K.; Richards, M.B.; Meng, C.; Priestley, B.; Fernandez, H.H.; Cambi, F.; Umbach, D.M.; Blair, A.; Sandler, D.P.; Langston, J.W. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress. 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Pires, J.M.; Foresti, M.L.; Silva, C.S.; Rêgo, D.B.; Calió, M.L.; Mosini, A.C.; Nakamura, T.K.E.; Leslie, A.T.F.; Mello, L.E. Lipopolysaccharide-Induced Systemic Inflammation in the Neonatal Period Increases Microglial Density and Oxidative Stress in the Cerebellum of Adult Rats. Frontiers in Cellular Neuroscience. 2020, 14. [Google Scholar] [CrossRef]
- Fan, L.-W.; Tien, L.-T.; Lin, R.C.; Simpson, K.L.; Rhodes, P.G.; Cai, Z. Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiology of Disease. 2011, 44, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Fan, L.-W.; Kaizaki, A.; Tien, L.-T.; Ma, T.; Pang, Y.; Lin, S.; Lin, R.C.S.; Simpson, K.L. Neonatal Systemic Exposure to Lipopolysaccharide Enhances Susceptibility of Nigrostriatal Dopaminergic Neurons to Rotenone Neurotoxicity in Later Life. Developmental Neuroscience. 2013, 35, 155–71. [Google Scholar] [CrossRef] [PubMed]
- Tien, L.-T.; Kaizaki, A.; Pang, Y.; Cai, Z.; Bhatt, A.J.; Fan, L.-W. Neonatal exposure to lipopolysaccharide enhances accumulation of α-synuclein aggregation and dopamine transporter protein expression in the substantia nigra in responses to rotenone challenge in later life. Toxicology. 2013, 308, 96–103. [Google Scholar] [CrossRef]
- Ling, Z.; Chang, Q.A.; Tong, C.W.; Leurgans, S.E.; Lipton, J.W.; Carvey, P.M. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Experimental Neurology. 2004, 190, 373–383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




