Submitted:
09 April 2025
Posted:
09 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
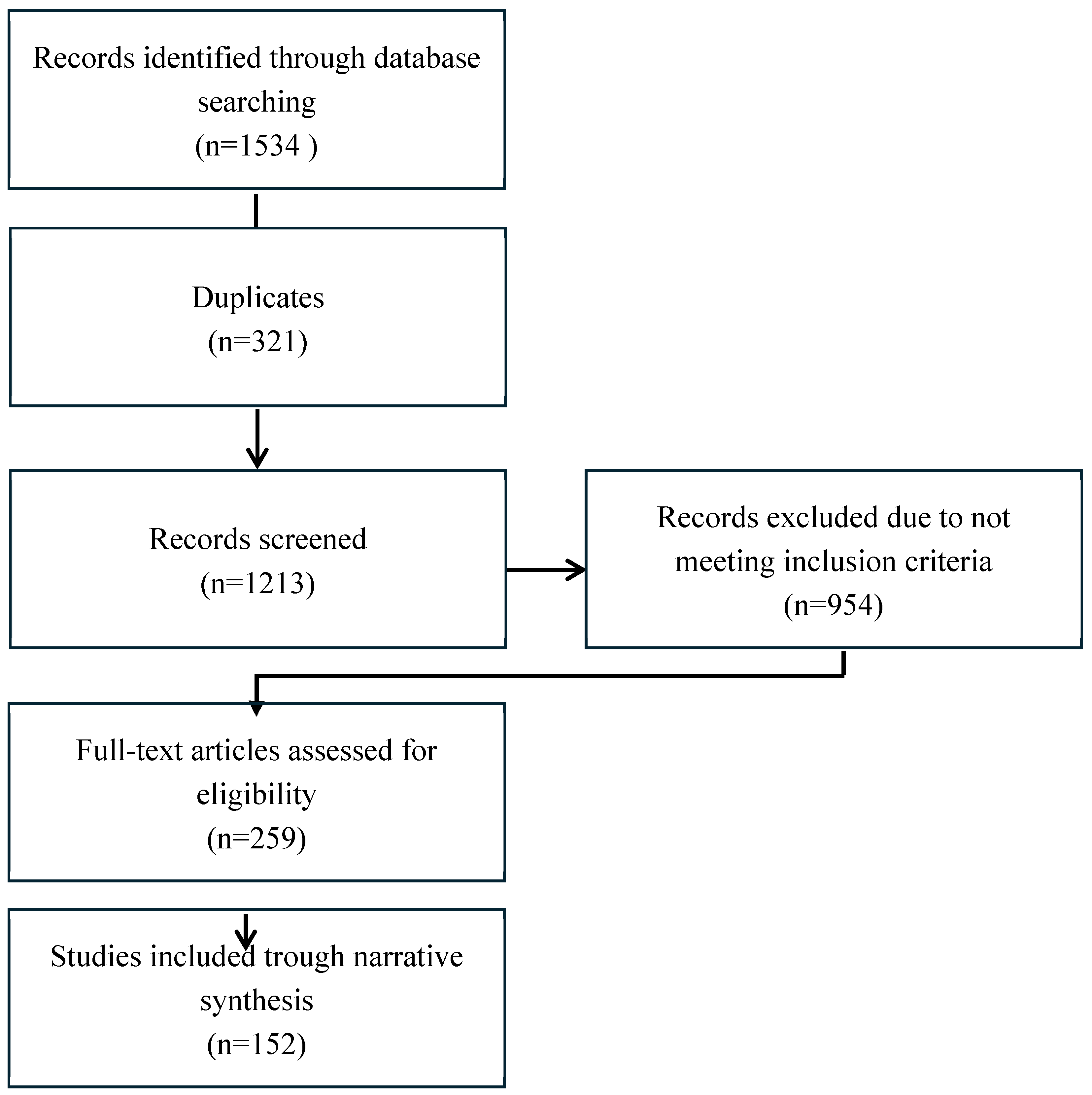
2. Material and Methods
3. Results
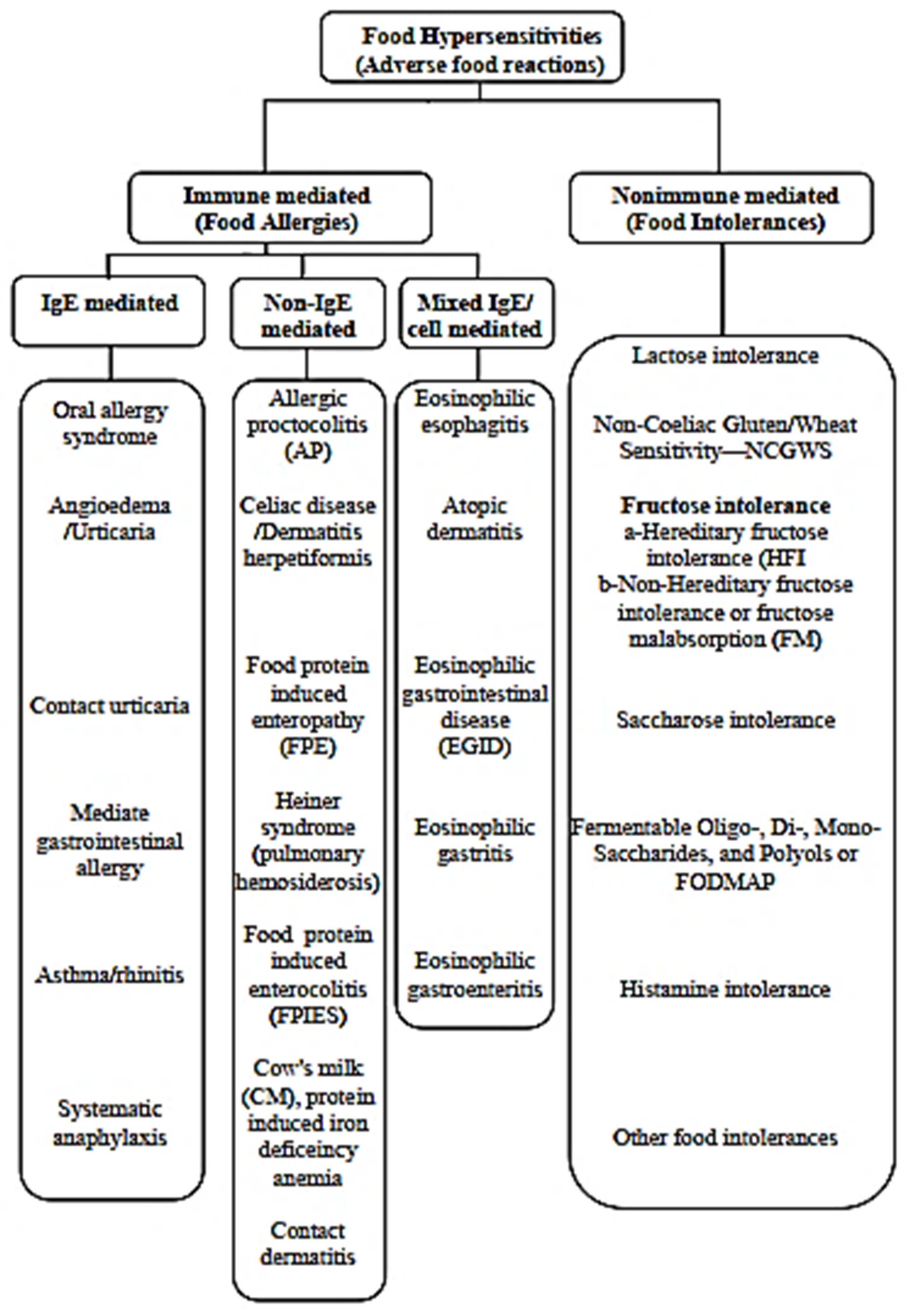
3.1. Food Allergies
| Author | Year | Study Type | Country | Sample Size | Duration of Intervention | Summary of Findings |
| Vassilopoulou et al. [10] | 2022 | Retrospective, observational, multicenter case-control study | Greece | 96 mothers of infants with and 141 mothers of infants without a history of Food Protein-Induced Allergic Proctocolitis (FPIAP). |
From May 2018 to November 2020 | Identified cow milk (83%), eggs (7.3%), wheat (6.4%), and beef (6.4%) as the main triggers for allergic proctocolitis (AP) in infants through the maternal diet. |
| Ruffner et al. [11] | 2013 | Retrospective chart review | USA | 462 cases were identified from the hospital patients | From 2007 until 2012 | Food Protein-Induced Enterocolitis Syndrome (FPIES) reactions were observed more frequently than previously reported, though their presentation and clinical characteristics remained consistent with earlier findings. Milk- and soy-induced FPIES were prevalent, with 43.5% of patients who reacted to milk also experiencing a reaction to soy. |
| Pinto-Sánchez et al. [12] | 2021 | Prospective study | Canada | prospective study of 50 patients with Irritable Bowel Syndrome (IBS) (ROME III, all subtypes), with and without serologic reactivity to gluten (antigliadin IgG and IgA), and 25 healthy subjects (controls) | Between 2012 and 2016 | Evaluated the effectiveness of a gluten-free diet in achieving mucosal healing for celiac patients. |
| Ford et al. [13] | 2014 | Cross Sectional | Canada | 4224 patients recruited | Between January 2008and December 2014 | Functional bowel disorders (FBDs) showed significant demographic and psychological differences among patients. The Rome III classification system did not clearly distinguish between different FBD subtypes. There was considerable symptom overlap among irritable Bowel syndrome (IBS), functional diarrhea, and chronic idiopathic constipation (CIC). The findings suggest a need for improved diagnostic criteria to differentiate FBDs more effectively. |
| Schink et al. [14] | 2018 | Cross- sectional observational study | Germany | 64 participants 8 with histamine intolerance (HIT), 25 with food hypersensitivity (FH), 21 with food allergy and 10 healthy controls (HC) |
12 months | Suggested dietary modifications and DAO supplements for histamine intolerance. |
| Halmos et al. [15] | 2014 | Randomized, controlled, cross-over trial | Australia | 30 patients with IBS and 8 healthy individuals (controls, matched for demographics and diet) |
Between April 2009 and June 2011 | Confirmed the efficacy of the low FODMAP diet in IBS symptom reduction. |
| Nwaru et al. [16] | 2014 | Systematic review and meta-analysis | Europe | Not Applicable | Between 1 January 2000 and 30 September 2012 | Highlighted that early introduction of allergenic foods may reduce the risk of developing IgE-mediated food allergies. |
| West et al. [17] | 2014 | Observational population-based study | UK | 57 million | Between 1990 and 2011 | Found that the incidence of celiac disease is increasing, estimating 19.1 per 100,000 cases annually. |
3.1.1. Definition
3.1.2. Symptoms
3.1.3. IgE-Mediated Food Allergies
3.1.4. Mixed IgE/Non IgE Mediated Food Allergies
3.2. Food Intolerances
3.2.1. Lactose Intolerance
3.2.2. Non-Coeliac Gluten/Wheat Sensitivity (NCGWS)
3.2.3. Fructose Intolerance
3.2.4. Saccharose Intolerance
3.2.5. Histamine Intolerance
3.2.6. FODMAP
3.3. Irritable Bowel Syndrome IBS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.J.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.C.; Hoest, A.; Lack, G.; et al. EAACI Food Allergy and Anaphylaxis Guidelines. Primary prevention of food allergy. Allergy 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. Journal of Allergy and Clinical Immunology 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.; Swagerty, J.; Walling, A.D.; Klein, R.M. Lactose Intolerance. afp 2002, 65, 1845–1851. [Google Scholar]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Tedner, S.G.; Asarnoj, A.; Thulin, H.; Westman, M.; Konradsen, J.R.; Nilsson, C. Food allergy and hypersensitivity reactions in children and adults—A review. Journal of Internal Medicine 2022, 291, 283–302. [Google Scholar] [CrossRef]
- Connors, L.; O’Keefe, A.; Rosenfield, L.; Kim, H. Non-IgE-mediated food hypersensitivity. Allergy, Asthma & Clinical Immunology 2018, 14, 56. [Google Scholar] [CrossRef]
- Zingone, F.; Bertin, L.; Maniero, D.; Palo, M.; Lorenzon, G.; Barberio, B.; Ciacci, C.; Savarino, E.V. Myths and Facts about Food Intolerance: A Narrative Review. Nutrients 2023, 15, 4969. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Konstantinou, G.N.; Zekakos Xypolias, D.; Valianatou, M.; Petrodimopoulou, M.; Vourga, V.; Tasios, I.; Papadopoulos, N.G. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front Nutr 2022, 9. [Google Scholar] [CrossRef]
- Ruffner, M.A.; Ruymann, K.; Barni, S.; Cianferoni, A.; Brown-Whitehorn, T.; Spergel, J.M. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract 2013, 1, 343–349. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; De Palma, G.; Calo, N.C.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-Free Diet Reduces Symptoms, Particularly Diarrhea, in Patients With Irritable Bowel Syndrome and Antigliadin IgG. Clin Gastroenterol Hepatol 2021, 19, 2343–2352.e8. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Bercik, P.; Morgan, D.G.; Bolino, C.; Pintos-Sanchez, M.I.; Moayyedi, P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther 2014, 39, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Schink, M.; Konturek, P.C.; Tietz, E.; Dieterich, W.; Pinzer, T.C.; Wirtz, S.; Neurath, M.F.; Zopf, Y. Microbial patterns in patients with histamine intolerance. J Physiol Pharmacol 2018, 69. [Google Scholar] [CrossRef]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Muraro, A.; Werfel, T.; Cardona, V.; Dubois, A.E.J.; Halken, S.; Hoffmann-Sommergruber, K.; Poulsen, L.K.; et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 2014, 69, 62–75. [Google Scholar] [CrossRef]
- West, J.; Fleming, K.M.; Tata, L.J.; Card, T.R.; Crooks, C.J. Incidence and Prevalence of Celiac Disease and Dermatitis Herpetiformis in the UK Over Two Decades: Population-Based Study. Am J Gastroenterol 2014, 109, 757–768. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. Journal of Allergy and Clinical Immunology 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Waserman, S.; Bégin, P.; Watson, W. IgE-mediated food allergy. Allergy, Asthma & Clinical Immunology 2018, 14, 55. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-Mediated Food Allergy. Clinic Rev Allerg Immunol 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Patel, B.Y.; Volcheck, G.W. Food Allergy: Common Causes, Diagnosis, and Treatment. Mayo Clinic Proceedings 2015, 90, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol 2014, 134, 1016–1025.e43. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J Allergy Clin Immunol Pract 2016, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test – European standards. Clin Transl Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test – European standards. Clinical and Translational Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Samolinski, B.; Demoly, P.; Baena-Cagnani, C.E.; Bachert, C.; Bonini, S.; Boulet, L.P.; Bousquet, P.J.; Brozek, J.L.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 2012, 130, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J 2020, 13, 100080. [Google Scholar] [CrossRef]
- Wood, R.A.; Kim, J.S.; Lindblad, R.; Nadeau, K.; Henning, A.K.; Dawson, P.; Plaut, M.; Sampson, H.A. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016, 137, 1103–1110.e11. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol 2016, 27 Suppl 23, 1–250. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Ewan, P.W.; Dugué, P.; Mirakian, R.; Dixon, T.A.; Harper, J.N.; Nasser, S.M. BSACI guidelines for the investigation of suspected anaphylaxis during general anaesthesia. Clinical & Experimental Allergy 2010, 40, 15–31. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014, 133, 291–307. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Thiele, L.; Tang, M.L.; Ponsonby, A.-L.; Dharmage, S.C.; Allen, K.J.; HealthNuts Study Investigators. The HealthNuts population-based study of paediatric food allergy: validity, safety and acceptability. Clin Exp Allergy 2010, 40, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Falk, G.W. Eosinophilic Esophagitis: A Review. JAMA 2021, 326, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Jensen, E.T.; Martin, C.F.; Shaheen, N.J.; Kappelman, M.D. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol 2014, 12, 589–596.e1. [Google Scholar] [CrossRef]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. Journal of Allergy and Clinical Immunology 2011, 128, 3–20.e6. [Google Scholar] [CrossRef]
- Hirano, I.; Pandolfino, J.E.; Boeckxstaens, G.E. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol 2017, 15, 325–334. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Arias, Á.; Molina-Infante, J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2016, 14, 13–22.e1. [Google Scholar] [CrossRef]
- Barni, S.; Mori, F.; Giovannini, M.; Liotti, L.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; Arasi, S.; Caminiti, L.; et al. Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic–Therapeutic Algorithm. Life (Basel) 2023, 13, 1824. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Blanco-Pérez, F.; Infante, S.; Zubeldia, J.M.; Pérez-Gordo, M. Non-IgE-Mediated Gastrointestinal Food Protein-Induced Allergic Disorders. Clinical Perspectives and Analytical Approaches. Clinical Perspectives and Analytical Approaches. Foods 2021, 10. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Seay, H.; Hickey, A.; Ndahayo, R.; Rosow, R.; Southwick, C.; Elkort, M.; Gupta, B.; Kramer, E.; et al. PROSPECTIVE ASSESSMENT OF PEDIATRICIAN-DIAGNOSED FOOD-PROTEIN INDUCED ALLERGIC PROCTOCOLITIS BY GROSS OR OCCULT BLOOD. J Allergy Clin Immunol Pract 2020, 8, 1692–1699.e1. [Google Scholar] [CrossRef] [PubMed]
- Elizur, A.; Cohen, M.; Goldberg, M.R.; Rajuan, N.; Cohen, A.; Leshno, M.; Katz, Y. Cow’s milk associated rectal bleeding: a population based prospective study. Pediatr Allergy Immunol 2012, 23, 766–770. [Google Scholar] [CrossRef]
- Mennini, M.; Fiocchi, A.G.; Cafarotti, A.; Montesano, M.; Mauro, A.; Villa, M.P.; Di Nardo, G. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organ J 2020, 13, 100471. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Warren, C.M.; Brown-Whitehorn, T.; Cianferoni, A.; Schultz-Matney, F.; Gupta, R.S. Food protein-induced enterocolitis syndrome in the US population-based study. J Allergy Clin Immunol 2019, 144, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.-C.; Szajewska, H.; Shamir, R.; Nowak-Węgrzyn, A. Non-IgE-mediated gastrointestinal food allergies in children. Pediatr Allergy Immunol 2017, 28, 6–17. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Alkalay, M.J. Nutrition in Patients with Lactose Malabsorption, Celiac Disease, and Related Disorders. Nutrients 2021, 14, 2. [Google Scholar] [CrossRef]
- Hadithi, M.; von Blomberg, B.M.E.; Crusius, J.B.A.; Bloemena, E.; Kostense, P.J.; Meijer, J.W.R.; Mulder, C.J.J.; Stehouwer, C.D.A.; Peña, A.S. Accuracy of Serologic Tests and HLA-DQ Typing for Diagnosing Celiac Disease. Ann Intern Med 2007, 147, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Murray, J.A. Epidemiology of Celiac Disease. Gastroenterol Clin North Am 2019, 48, 1–18. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Official journal of the American College of Gastroenterology | ACG 2023, 118, 59. [Google Scholar] [CrossRef]
- Salmi, T.; Hervonen, K. Current Concepts of Dermatitis Herpetiformis. Acta Derm Venereol 2020, 100, 5664. [Google Scholar] [CrossRef]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Annals of Medicine 2017, 49, 23–31. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Kim, S.-J. Dermatitis Herpetiformis: An Update on Diagnosis, Disease Monitoring, and Management. Medicina (Kaunas) 2021, 57, 843. [Google Scholar] [CrossRef]
- Antiga, E.; Maglie, R.; Quintarelli, L.; Verdelli, A.; Bonciani, D.; Bonciolini, V.; Caproni, M. Dermatitis Herpetiformis: Novel Perspectives. Front Immunol 2019, 10, 1290. [Google Scholar] [CrossRef]
- Reunala, T.; Hervonen, K.; Salmi, T. Dermatitis Herpetiformis: An Update on Diagnosis and Management. Am J Clin Dermatol 2021, 22, 329–338. [Google Scholar] [CrossRef]
- Mansikka, E.; Hervonen, K.; Kaukinen, K.; Collin, P.; Huhtala, H.; Reunala, T.; Salmi, T. Prognosis of Dermatitis Herpetiformis Patients with and without Villous Atrophy at Diagnosis. Nutrients 2018, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med 2017, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Antiga, E.; Melani, L.; Fabbri, P.; Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol 2009, 23, 633–638. [Google Scholar] [CrossRef]
- Feuille, E.; Nowak-Węgrzyn, A. Food Protein-Induced Enterocolitis Syndrome, Allergic Proctocolitis, and Enteropathy. Curr Allergy Asthma Rep 2015, 15, 50. [Google Scholar] [CrossRef]
- Jenkins, H.R.; Pincott, J.R.; Soothill, J.F.; Milla, P.J.; Harries, J.T. Food allergy: the major cause of infantile colitis. Arch Dis Child 1984, 59, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.C.; Ford, L.S.; Sickles, L.; Järvinen, K.M.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol 2014, 134, 382–389. [Google Scholar] [CrossRef]
- Fernandes, B.N.; Boyle, R.J.; Gore, C.; Simpson, A.; Custovic, A. Food protein-induced enterocolitis syndrome can occur in adults. J Allergy Clin Immunol 2012, 130, 1199–1200. [Google Scholar] [CrossRef]
- Koc, A.S.; Sucu, A.; Celik, U. A different clinical presentation of Heiner syndrome: The case of diffuse alveolar hemorrhage causing massive hemoptysis and hematemesis. Respir Med Case Rep 2019, 26, 206–208. [Google Scholar] [CrossRef]
- Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Barni, S.; Caminiti, L.; Castagnoli, R.; Liotti, L.; Saretta, F.; et al. Heiner Syndrome and Milk Hypersensitivity: An Updated Overview on the Current Evidence. Nutrients 2021, 13, 1710. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, M.; Jung, J.H.; Kim, S.Y.; Kim, Y.H.; Hahn, S.M.; Kim, S.; Lee, M.-J.; Shim, H.S.; Sohn, M.H.; et al. Children with Heiner Syndrome: A Single-Center Experience. Children (Basel) 2021, 8, 1110. [Google Scholar] [CrossRef]
- Moissidis, I.; Chaidaroon, D.; Vichyanond, P.; Bahna, S.L. Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatric Allergy and Immunology 2005, 16, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, M.; Jung, J.H.; Kim, S.Y.; Kim, Y.H.; Hahn, S.M.; Kim, S.; Lee, M.-J.; Shim, H.S.; Sohn, M.H.; et al. Children with Heiner Syndrome: A Single-Center Experience. Children (Basel) 2021, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Barni, S.; Caminiti, L.; Castagnoli, R.; Liotti, L.; Saretta, F.; et al. Heiner Syndrome and Milk Hypersensitivity: An Updated Overview on the Current Evidence. Nutrients 2021, 13, 1710. [Google Scholar] [CrossRef]
- Exl, B.M.; Vandenplas, Y.; Blecker, U. Role of hydrolyzed formulas in nutritional allergy prevention in infants. South Med J 1997, 90, 1170–1175. [Google Scholar] [CrossRef]
- Labrosse, R.; Graham, F.; Caubet, J.-C. Non-IgE-Mediated Gastrointestinal Food Allergies in Children: An Update. Nutrients 2020, 12, 2086. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Bianchi, A.; D’Auria, E.; Cardinale, F.; Votto, M.; Martelli, A.; Tosca, M.; Chiappini, E.; Brambilla, I.; et al. Update on Food protein-induced enterocolitis syndrome (FPIES). Acta Biomed 2021, 92, e2021518. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: Executive summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. Journal of Allergy and Clinical Immunology 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef]
- Mehr, S.; Frith, K.; Campbell, D.E. Epidemiology of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol 2014, 14, 208–216. [Google Scholar] [CrossRef]
- Wang, K.Y.; Lee, J.; Cianferoni, A.; Ruffner, M.A.; Dean, A.; Molleston, J.M.; Pawlowski, N.A.; Heimall, J.; Saltzman, R.W.; Ram, G.S.; et al. Food Protein–Induced Enterocolitis Syndrome Food Challenges: Experience from a Large Referral Center. The Journal of Allergy and Clinical Immunology: In Practice 2019, 7, 444–450. [Google Scholar] [CrossRef]
- Venter, C.; Mazzocchi, A.; Maslin, K.; Agostoni, C. Impact of elimination diets on nutrition and growth in children with multiple food allergies. Curr Opin Allergy Clin Immunol 2017, 17, 220–226. [Google Scholar] [CrossRef]
- Taico Oliva, C.; Musa, I.; Kopulos, D.; Ardalani, F.; Maskey, A.; Wilson, A.; Yang, N.; Li, X.-M. The gut microbiome and cross-reactivity of food allergens: current understanding, insights, and future directions. Front Allergy 2025, 5. [Google Scholar] [CrossRef]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose intolerance: An update on its pathogenesis, diagnosis, and treatment. Nutrition Research 2021, 89, 23–34. [Google Scholar] [CrossRef]
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-persistence and Lactose Intolerance. Curr Gastroenterol Rep 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a variant associated with adult-type hypolactasia. Nat Genet 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Stotzer, P. Use and abuse of hydrogen breath tests. Gut 2006, 55, 297–303. [Google Scholar] [CrossRef]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef]
- Jo, I.H.; Paik, C.-N.; Kim, Y.-J.; Lee, J.M.; Choi, S.Y.; Hong, K.P. Lactase Deficiency Diagnosed by Endoscopic Biopsy-based Method is Associated With Positivity to Glucose Breath Test. J Neurogastroenterol Motil 2023, 29, 85–93. [Google Scholar] [CrossRef]
- Swallow, D.M. Genetics of lactase persistence and lactose intolerance. Annu Rev Genet 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010, 152, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, A.; Pawlicka, M.; Mroczek, A.; Bałabuszek, K.; Nieradko-Iwanicka, B. Non-Celiac Gluten Sensitivity: A Review. Medicina (Kaunas) 2019, 55, 222. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-Celiac Wheat Sensitivity Diagnosed by Double-Blind Placebo-Controlled Challenge: Exploring a New Clinical Entity. Official journal of the American College of Gastroenterology | ACG 2012, 107, 1898. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Ensari, A.; Marsh, M.N. Diagnosing celiac disease: A critical overview. Turk J Gastroenterol 2019, 30, 389–397. [Google Scholar] [CrossRef]
- Singh, S.K.; Sarma, M.S. Hereditary fructose intolerance: A comprehensive review. World J Clin Pediatr 2022, 11, 321–329. [Google Scholar] [CrossRef]
- Debray, F.-G.; Seyssel, K.; Fadeur, M.; Tappy, L.; Paquot, N.; Tran, C. Effect of a high fructose diet on metabolic parameters in carriers for hereditary fructose intolerance. Clinical Nutrition 2021, 40, 4246–4254. [Google Scholar] [CrossRef]
- Adamowicz, M.; Płoski, R.; Rokicki, D.; Morava, E.; Giżewska, M.; Mierzewska, H.; Pollak, A.; Lefeber, D.J.; Wevers, R.A.; Pronicka, E. Transferrin hypoglycosylation in hereditary fructose intolerance: Using the clues and avoiding the pitfalls. Journal of Inherited Metabolic Disease 2007, 30, 407. [Google Scholar] [CrossRef]
- Ali, M.; Rellos, P.; Cox, T.M. Hereditary fructose intolerance. J Med Genet 1998, 35, 353–365. [Google Scholar] [CrossRef]
- Tolan, D.R.; Brooks, C.C. Molecular analysis of common aldolase B alleles for hereditary fructose intolerance in North Americans. Biochem Med Metab Biol 1992, 48, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ebert, K.; Witt, H. Fructose malabsorption. Mol Cell Pediatr 2016, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.F.; Fox, M.R.; Keller, J.; Salvatore, S.; Basilisco, G.; Hammer, J.; Lopetuso, L.; Benninga, M.; Borrelli, O.; Dumitrascu, D.; et al. European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Neurogastroenterology and Motility, and European Society for Paediatric Gastroenterology Hepatology and Nutrition consensus. United European Gastroenterol J 2021, 10, 15–40. [Google Scholar] [CrossRef]
- Komericki, P.; Akkilic-Materna, M.; Strimitzer, T.; Weyermair, K.; Hammer, H.F.; Aberer, W. Oral xylose isomerase decreases breath hydrogen excretion and improves gastrointestinal symptoms in fructose malabsorption – a double-blind, placebo-controlled study. Alimentary Pharmacology & Therapeutics 2012, 36, 980–987. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. Journal of Gastroenterology and Hepatology 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- Frissora, C.L.; Rao, S.S.C. Sucrose intolerance in adults with common functional gastrointestinal symptoms. Proc (Bayl Univ Med Cent) 2022, 35, 790–793. [Google Scholar] [CrossRef]
- Treem, W.R.; McAdams, L.; Stanford, L.; Kastoff, G.; Justinich, C.; Hyams, J. Sacrosidase therapy for congenital sucrase-isomaltase deficiency. J Pediatr Gastroenterol Nutr 1999, 28, 137–142. [Google Scholar] [CrossRef]
- Robayo-Torres, C.C.; Opekun, A.R.; Quezada-Calvillo, R.; Villa, X.; Smith, E.O.; Navarrete, M.; Baker, S.S.; Nichols, B.L. 13C-breath tests for sucrose digestion in congenital sucrase isomaltase-deficient and sacrosidase-supplemented patients. J Pediatr Gastroenterol Nutr 2009, 48, 412–418. [Google Scholar] [CrossRef]
- Aljaaly, E.A.; Khatib, M.A. Exploring the Prevalence of Functional Gastrointestinal Diseases and the Accompanied Differences in Dietary and Lifestyle Patterns: A Two-Generational Study. Diagnostics 2024, 14, 1630. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef]
- Puntis, J.W.L.; Zamvar, V. Congenital sucrase–isomaltase deficiency: diagnostic challenges and response to enzyme replacement therapy. ( 2015. [CrossRef] [PubMed]
- Marcadier, J.L.; Boland, M.; Scott, C.R.; Issa, K.; Wu, Z.; McIntyre, A.D.; Hegele, R.A.; Geraghty, M.T.; Lines, M.A. Congenital sucrase–isomaltase deficiency: identification of a common Inuit founder mutation. CMAJ 2015, 187, 102–107. [Google Scholar] [CrossRef]
- Latorre-Moratalla, M.L.; Comas-Basté, O.; Bover-Cid, S.; Vidal-Carou, M.C. Tyramine and histamine risk assessment related to consumption of dry fermented sausages by the Spanish population. Food and Chemical Toxicology 2017, 99, 78–85. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou M del, C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Schwelberger, H.G.; Feurle, J.; Houen, G. Mapping of the binding sites of human diamine oxidase (DAO) monoclonal antibodies. Inflamm Res 2018, 67, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou M del, C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Holasek, S.J.; Mangge, H. Evaluation of symptoms and symptom combinations in histamine intolerance. Intest Res 2019, 17, 427–433. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Mangge, H.; Holasek, S.J. Non-celiac gluten sensitivity: people without celiac disease avoiding gluten—is it due to histamine intolerance? ( 2017. [CrossRef]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Dölle-Bierke, S.; Kleine-Tebbe, J.; Klimek, L.; Lämmel, S.; Lepp, U.; Saloga, J.; Schäfer, C.; et al. Guideline on management of suspected adverse reactions to ingested histamine: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergology and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA) as well as the Swiss Society for Allergology and Immunology (SGAI) and the Austrian Society for Allergology and Immunology (ÖGAI). Allergologie Select 2021, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol Immunopathol (Madr) 2016, 44, 475–483. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil 2022, 34, e14339. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci Biotechnol 2019, 28, 1779–1784. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am J Clin Nutr 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Jochum, C. Histamine Intolerance: Symptoms, Diagnosis, and Beyond. Nutrients 2024, 16, 1219. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Duelo, A.; Veciana-Nogués, M.T.; Berlanga, M.; Vidal-Carou, M.C.; Latorre-Moratalla, M.L. The dietary treatment of histamine intolerance reduces the abundance of some histamine-secreting bacteria of the gut microbiota in histamine intolerant women. A pilot study. A pilot study. Front Nutr 2022, 9. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. FODMAP modulation as a dietary therapy for IBS: Scientific and market perspective. Comprehensive Reviews in Food Science and Food Safety 2022, 21, 1491–1516. [Google Scholar] [CrossRef]
- Morariu, I.-D.; Avasilcai, L.; Vieriu, M.; Lupu, V.V.; Morariu, B.-A.; Lupu, A.; Morariu, P.-C.; Pop, O.-L.; Starcea, I.M.; Trandafir, L. Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review. Nutrients 2023, 15, 2295. [Google Scholar] [CrossRef]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv Nutr 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet Into Gastroenterological Practice. J Neurogastroenterol Motil 2022, 28, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Nagy, A.G.; Pancetti, A.; Costa, F.; Ricchiuti, A.; de Bortoli, N.; Mosca, M.; Marchi, S.; Rossi, A. Low FODMAP Diet: Evidence, Doubts, and Hopes. Nutrients 2020, 12, 148. [Google Scholar] [CrossRef]
- Gwioździk, W.; Krupa-Kotara, K.; Całyniuk, B.; Helisz, P.; Grajek, M.; Głogowska-Ligus, J. Traditional, Vegetarian, or Low FODMAP Diets and Their Relation to Symptoms of Eating Disorders: A Cross-Sectional Study among Young Women in Poland. Nutrients 2022, 14, 4125. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Loughman, A.; Staudacher, H.M. Effects of a low FODMAP diet on the colonic microbiome in irritable bowel syndrome: a systematic review with meta-analysis. Am J Clin Nutr 2022, 116, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M.; et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. The Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Ford, A.C.; Forman, D.; Bailey, A.G.; Axon, A.T.R.; Moayyedi, P. Irritable bowel syndrome: a 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol 2008, 103, 1229–1239. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Nasseri-Moghaddam, S.; Mostajabi, P.; Malekzadeh, R. Overlapping gastroesophageal reflux disease and irritable bowel syndrome: Increased dysfunctional symptoms. World J Gastroenterol 2010, 16, 1232–1238. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Quigley, E.M.M.; Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol quiz S27. 2014, 109 Suppl 1, S2–26. [Google Scholar] [CrossRef] [PubMed]
- Matheis, A.; Martens, U.; Kruse, J.; Enck, P. Irritable bowel syndrome and chronic pelvic pain: A singular or two different clinical syndrome? World J Gastroenterol 2007, 13, 3446–3455. [Google Scholar] [CrossRef]
- Barsky, A.J. Assessing the New DSM-5 Diagnosis of Somatic Symptom Disorder. Psychosom Med 2016, 78, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.A.; Jones, M.; Weltman, M.; Kalantar, J.; Bone, C.; Gowryshankar, A.; Walker, M.M.; Talley, N.J. Identification of early environmental risk factors for irritable bowel syndrome and dyspepsia. Neurogastroenterol Motil 2015, 27, 1317–1325. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e1. [Google Scholar] [CrossRef] [PubMed]
- Czaja-Bulsa, G. Non coeliac gluten sensitivity - A new disease with gluten intolerance. Clin Nutr 2015, 34, 189–194. [Google Scholar] [CrossRef]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016, 2, 16014. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Drossman, D.A.; Tack, J.; Ford, A.C.; Szigethy, E.; Törnblom, H.; Van Oudenhove, L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology 2018, 154, 1140–1171.e1. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Pasta, A.; Formisano, E.; Calabrese, F.; Plaz Torres, M.C.; Bodini, G.; Marabotto, E.; Pisciotta, L.; Giannini, E.G.; Furnari, M. Food Intolerances, Food Allergies and IBS: Lights and Shadows. Nutrients 2024, 16, 265. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prebiotics and Probiotics in Digestive Health. Clin Gastroenterol Hepatol 2019, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
| Pathology | Disorder | Key features | Most common causal foods |
| IgE-mediated (acute onset) | Acute urticaria/angioedema | Food commonly causes acute (20%) but rarely chronic urticaria | Cow milk, gluten, eggs, wheat, beans, soybean, nuts, and seafood |
| Contact urticaria | Direct skin contact results in lesions. Histamine release, in rare cases, can cause urticaria. |
Multiple | |
| Anaphylaxis | Rapidly progressive, multiple organs system reactions can include cardiovascular collapse | Any but more commonly peanut, Tree nuts, shellfish, fish, milk, and egg | |
| Food-associated, exercise-induced anaphylaxis | Food triggers anaphylaxis only if ingestion is followed temporally by exercise | Wheat, shellfish, and celery are most often described | |
| Oral allergy syndrome (pollen-associated food allergy syndrome) | Pruritus and mild edema are confined to the oral cavity and uncommonly progress beyond the mouth (w7%) and rarely to anaphylaxis (1% to 2%). It might increase after the pollen season. | Raw fruit/vegetables; cooked forms tolerated; examples of relationships: birch (apple, peach, pear, carrot), ragweed (melons) | |
| Immediate gastrointestinal hypersensitivity | Immediate vomiting, pain | Cow milk, gluten, eggs, wheat, beans, soybean, nuts, and seafood | |
| Combined IgE and cell-mediated (delayed onset/chronic) | Atopic dermatitis | Associated with food allergy in 35% of children with moderate-to-severe rash | Major allergens, particularly egg, milk |
| Eosinophilic esophagitis | Symptoms might include feeding disorders, reflux symptoms, vomiting, dysphagia, and food impaction. | Multiple | |
| Eosinophilic gastroenteritis | Vary on site(s)/degree of eosinophilic inflammation; might include ascites, weight loss, edema, obstruction | Multiple | |
| Cell-mediated (delayed onset/chronic) | Food protein-induced enterocolitis syndrome Cow’s milk, soy, rice, oat, meat | Primarily affects infants; chronic exposure: emesis, diarrhea, poor growth, lethargy; re-exposure after restriction: emesis, diarrhea, hypotension (15%) 2 hours after ingestion | Cow’s milk, soy, rice, oat, meat |
| Food protein induced allergic proctocolitis | Mucus-laden, bloody stools in infants Milk (through breast-feeding) | Milk (through breast-feeding) | |
| Allergic contact dermatitis | Often occupational because of chemical moieties, oleoresins. Systemic contact dermatitis is a rare variant because of ingestion | Spices, fruits, vegetables | |
| Heiner syndrome | Pulmonary infiltrates, failure to thrive, iron deficiency anemia | Cow’s milk |
| Celiac Disease | NCGWS | Wheat Allergy | |
| Prevalence | 0.5–1.7% | 0.6-10% | 0.5–9% in children |
| Pathogenesis | Autoimmune | Non-specific immune response | IgE mediated response |
| DQ2-DQ8 HLA haplotypes | Positive in 95% cases | Positive in 50% cases | Negative |
| Serological markers | IgA anti-EMA, IgA anti-tTG, IgG anti-DGP, IgA anti-gliadin | IgA/IgG anti-gliadin in 50% cases | specific IgE antibodies against wheat and gliadin |
| Duodenal biopsy * | Marsh I to IV with domination of Marsh III and IV | Marsh 0-II, but according to some experts Marsh III might also be in NCGS | Marsh 0-II |
| Duodenal villi atrophy | Present | Absent | Might be present or absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).