1. Introduction
Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in the biosynthesis of serotonin. TPH catalyzes the biopterin-dependent monooxygenation of tryptophan to 5-hydroxytryptophan, which is subsequently decarboxylated by aromatic amino acid decarboxylase to form serotonin (5-hydroxytryptamine; 5-HT).
Since the discovery of the two isotypes of TPH (TPH1 and TPH2) [
1], it has become widely accepted that the central and peripheral 5-HT systems are functionally separate. The spatial separation is attributable to the inability of 5-HT to cross the blood-brain barrier (BBB); thus, 5-HT is biosynthesized centrally by TPH2 and peripherally by TPH1 [
1,
2].
TPH1 is mainly expressed in the gut and in other peripheral tissues and supplies platelets in the circulation with 5-HT [
1,
2]. High expression of TPH1 was recently reported in the human pituitary [
2], and polymorphisms of Tph1 may be associated with an elevated risk for suicide behavior [
3] and schizophrenia [
4] in humans. The roles of TPH1 in brain 5-HT synthesis as well as in tryptophan and its metabolites, however, remain unclear.
In the present study, we demonstrated alterations in tryptophan and its metabolites in the plasma and brain of young (8-week-old) and older (7-month-old) Tph1 mutant mice compared with age-matched wild-type mice. We also revealed age-related alterations in body weight, food intake, and blood glucose levels in single-housed Tph1 mutants.
2. Results
2.1.
Plasma 5-HT levels were remarkably decreased in Tph1 mutants to 2.5% of the levels in age-matched wild-type mice (
Table 1a). Significant decreases were also observed in the plasma levels of l-tryptophan (Trp), 5-hydroxy indoleacetic acid (5-HIAA), kynurenine (KYN), xanthurenic acid (XA), indole-3-propionic acid (IPA), indole-3-acetic acid (IAA), and kynurenic acid (KYNA) levels in 8-week-old Tph1 mutant mice compared with age-matched wild-type mice (
Table 1a). These findings suggest that Tph1 upregulates not only peripheral 5-HT synthesis but also Trp and its metabolites involved in the KYN and IPA pathways in 8-week-old mice.
Plasma levels of 5-HT and 5-HIAA were significantly decreased in 7-month-old Tph1 mutants compared with age-matched wild-type mice, whereas no significant differences were detected in the plasma levels of Trp, KYN, XA, and IPA between 7-month-old Tph1 mutants and age-matched wild-type mice (
Table 1a). Rather, plasma IAA levels were significantly increased in 7-month-old Tph1 mutants compared with age-matched wild-type mice (
Table 1a). Thus, Tph1 mutants exhibited age-related changes in the plasma levels of Trp and its metabolites involved in the KYN and IPA pathways.
In the brain, Trp, 5-HT, 5-HIAA, XA, IPA, and IAA levels were significantly decreased in 8-week-old Tph1 mutants compared with age-matched wild-type mice (
Table 1b). Brain levels of Trp, 5-HT, 5-HIAA, KYN, and IPA were significantly decreased in 7-month-old Tph1 mutants compared with age-matched wild-type mice (
Table 1b), whereas there were no significant differences in brain levels of XA and IAA (
Table 1b). These results suggest that Tph1 is involved in the upregulation of brain Trp, 5-HT, KYN, and IPA, but the upregulation is likely to be smaller in older mice than in young mice.
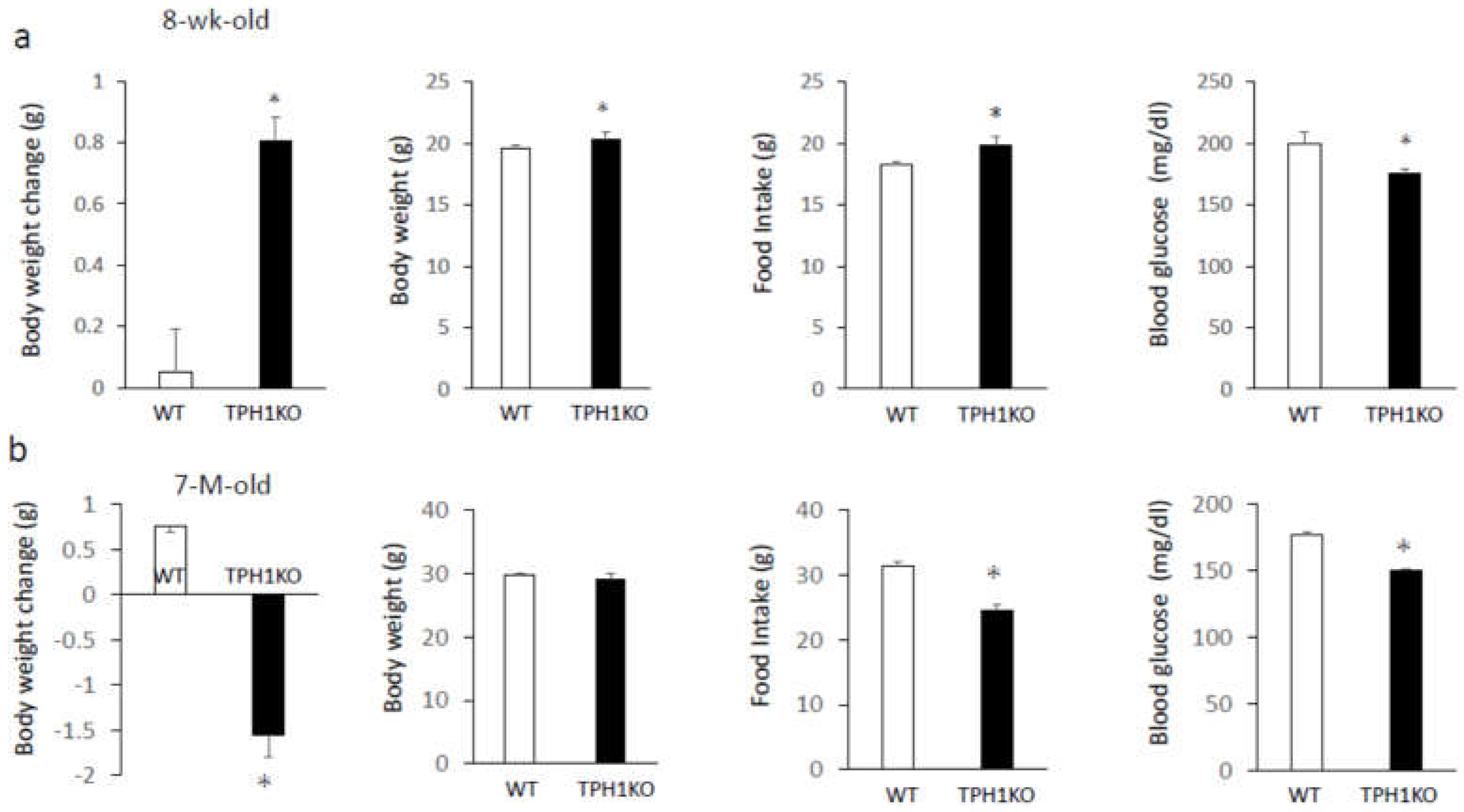
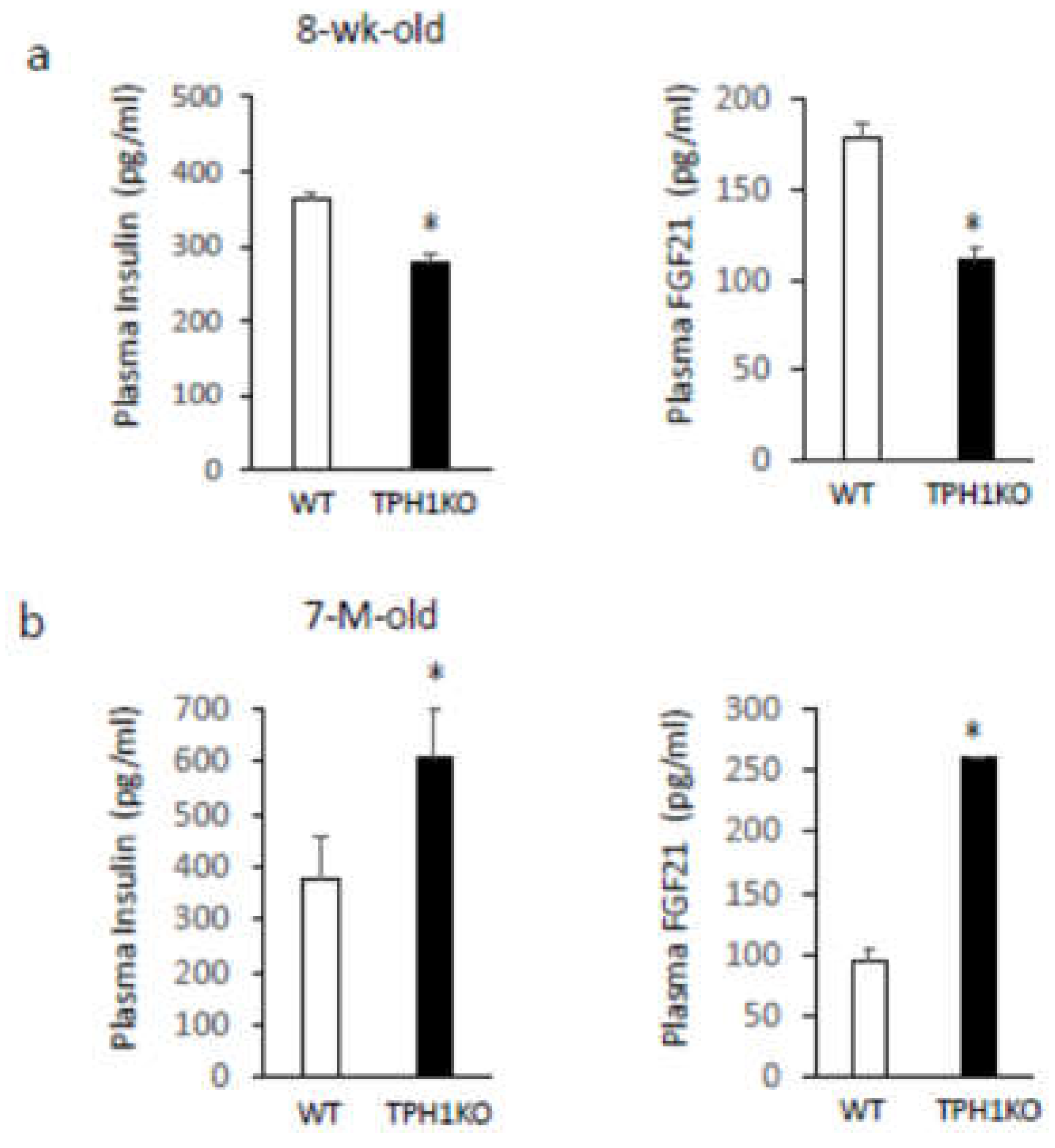
Body weight and total food intake for 6 days were significantly increased in single-housed 8-week-old Tph1 mutants compared with age-matched wild-type mice, whereas blood glucose, plasma FGF21, and plasma insulin levels were significantly decreased (
Figure 1a). On the other hand, age-related differences were observed in older mice. Body weight, total food intake for 6 days, and blood glucose levels were significantly decreased in single-housed 7-month-old Tph1 mutants compared with age-matched wild-type mice, and plasma FGF21 and insulin levels were significantly increased (
Figure 1b). Thus, despite the age-related alterations in body weight, food intake, and plasma FGF21 and insulin levels in single-housed Tph1 mutants, blood glucose levels were lower in both young and older Tph1 mutants compared with wild-type mice.
3. Discussion
The results of the present study revealed that Tph1 is required for maintaining not only the plasma levels of 5-HT but also the plasma levels of Trp and its metabolites, including KYN, XA, IPA, and IAA, in young mice. Although Trp is an essential amino acid that cannot be synthesized in the body and must be obtained from the diet, our results revealed that Tph1 partially regulates Trp levels in the plasma and brain. Circulating Trp can enter the brain through the BBB, and thus the decreased brain Trp levels in Tph1 mutants may result from a decrease in the plasma Trp levels. The decreased plasma and brain metabolite levels in the KYN and IPA pathways in Tph1 mutants may result from the decrease in Trp.
In addition, our results revealed that Tph1 is involved in brain 5-HT synthesis in young and older mice. Because peripheral 5-HT cannot cross the BBB, brain Tph1 may directly contribute to brain 5-HT synthesis. The decreases in plasma and brain Trp levels might also contribute to the decrease in brain 5-HT in Tph1 mutants.
Moreover, our results revealed that aging can affect Trp and its metabolites in the plasma and brain. Plasma Trp and its metabolites outside the 5-HT pathway tended to be lower in older wild-type mice than in young wild-type mice, but these age-related differences were attenuated in Tph1 mutants. Rather, metabolites in the plasma IPA pathway tended to increase in older Tph1 mutants compared with the age-matched wild-type mice.
Similarly, brain Trp and its metabolites tended to be lower in older wild-type mice than young wild-type mice; the differences in Trp and its metabolites between wild-type mice and Tph1 mutants were smaller in older mice than young mice. The decreases in brain Trp and its metabolites in older wild-type mice might result from the decreases in plasma Trp in older wild-type mice.
Our results support our previous findings that food intake and body weight are increased in association with decreases in hepatic FGF21 expression and plasma FGF21 levels in young Tph1 mutants
5, suggesting that 5-HT upregulates hepatic FGF21 production. The present results, however, also revealed that food intake and body weight are decreased in association with increases in plasma levels of FGF21 and insulin in single-housed older Tph1 mutants. The age-related alterations in plasma FGF21 might be related to age-related changes in plasma IAA levels. FGF21 is implicated in the regulation of glucose uptake and metabolism [
6,
7,
8]. Despite the age-related alterations in food intake, body weight, and plasma levels of FGF21 and insulin, blood glucose levels were lower in both young and older Tph1 mutants than in age-matched wild-type mice. These results suggest that 5-HT synthesis via Tph1 contributes to blood glucose homeostasis independently of feeding, body weight, insulin, and FGF21.
In summary, these findings suggest that Tph1 is required for the regulation of Trp and its metabolites involved in the KYN and IPA pathways, in plasma and brain, and that Tph1 is required for blood glucose homeostasis.
4. Materials and Methods
4.1. Tph1 Mutant Mice
Homozygous mutant males bearing a null mutation of the Tph1 gene (congenic on a C57BL/6N background) and age-matched wild-type mice were used. The line has been maintained through mating of females heterozygous for the Tph1 gene with heterozygous males obtained from Cyagen Biosciences Inc. Genomic DNA was extracted from tails of littermates using TaKaRa MiniBEST Universal Genomic DNA Extraction kit (Ver.5.0_Code No.9765). Genotypes were confirmed by PCR-LabChip (PerkinElmer LabChip GX Touch HT) analysis using the forward primer F1: 5’-ACATCAGCCTTCTGCTCTGTTTC-3’ and the reverse primer R1: 5’-TCACTGAGAGCATCAAGCCCAG-3’ and R2: 5’-ATTTCCGGGACTCGATGTGTAAC-3’. Tph1 mutant and wild-type alleles correspond to the 611- and 489-bp fragments, respectively.
In the wild-type allele, the forward primer F1 and the reverse primer R1 span 14449 bp of the Tph1 gene on mouse chromosome 7, prior to the deletion of exons in the Tph1 gene. Due to the deletion of exons in the Tph1 gene in the mutant allele, the distance between the forward primer F1 and the reverse primer R1 is 611 bp.
Animals were all housed (3–5 mice per cage) with free access to water and chow pellets in a light- (12 h on/12 h off; lights off at 2000 h) and temperature-(20–22 oC) controlled environment. The animals were singly housed in cages for 1 week before the experiment. The animal studies were conducted in accordance with the institutional guidelines for animal experiments at Tohoku University Graduate School of Medicine and all experimental protocols were approved by the institutional committee at Tohoku University.
4.2. Blood Chemistry
Whole blood was mixed with EDTA-2Na (2 mg/ml) and aprotinin (500 kIU/ml) to determine the plasma levels of FGF21 and insulin. Plasma levels of FGF21 and insulin were measured by enzyme-linked immunosorbent assay (rat/mouse FGF21 ELISA Kit, R&D Systems, Tokyo, Japan; and a mouse Insulin ELISA Kit [TMB], AKRIN-011T, Shibayagi, Gunma, Japan, respectively) as described previously [
5,
9]. Blood glucose levels were measured using glucose strips (blood glucose monitoring system; Accu-Check, Roche Diagnostics, Tokyo, Japan).
4.3. Tryptophan and Its Metabolites Analysis
Tryptophan and its metabolites were subjected to analysis by LSI Medience Corporation, a contracted laboratory based in Tokyo, Japan as described previously [
9]. Briefly, brain and plasma were introduced into sample disruptor tubes provided by Yasui Kikai (Osaka, Japan). Subsequently, these tubes were agitated with iron cones that had been pre-cooled in liquid nitrogen. The resulting sample powders were suspended in 500 µL of methanol and vigorously shaken for 15 minutes, after which centrifugation was carried out at 20,000 × g for 3 minutes. The supernatants, constituting 40 µL, were meticulously transferred to 2 mL microtubes. Internal standards were then introduced and combined with the supernatants, followed by the addition of 1,000 µL of a 2% formic acid solution to induce protein precipitation. Afterward, we purified the analytes from the supernatant using solid-phase extraction (OASIS MCX, Waters, Milford, MA) and analyzed them using liquid chromatography-tandem mass spectrometry (Ultivo, Agilent, Santa Clara, CA) with a reverse-phase LC column (ACQUITY UPLC HSS T3, 1.8 µm, 2.1 mm × 50 mm, Waters, Milford, MA). The data were processed with Mass Hunter software (Agilent, Santa Clara, CA). We normalized the peak areas using internal standards and determined the concentration of each analyte using a standard curve.
4.4. Statistical Methods
Data are presented as mean ± SEM (n= 6). Comparisons between two groups were performed using Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Author Contributions
K.N. designed the study, performed the experiments, interpreted all analyses, generated all figures and tables, and wrote the manuscript. T.K. performed the experiments and interpreted all analysis.
Funding
This work was supported by a Grant in-Aid for Scientific Research.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by a Grant in-Aid for Scientific Research.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.; Lee, J.; Lee, J.Y.; Kim, H.; Lee, S.; Oh, C-M. A Systems Biology Approach to Investigating the Interaction between Serotonin Synthesis by Tryptophan Hydroxylase and the Metabolic Homeostasis. Int. J. Mol. Sci. 2021, 22, 2452. [CrossRef] [PubMed]
- Genis-Mendoza, A.D.; Hernández-Díaz, Y.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Castillo-Avila, R.G.; López-Narváez, M.L.; Ramos-Méndez, M.Á.; Nicolini, H. Association between TPH1 polymorphisms and the risk of suicide behavior: An updated meta-analysis of 18,398 individuals. Front. Psychiatry. 2022, 13, 932135. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.C.; Bagade, S.; McQueen, M.B.; Ioannidis, J.P.; Kavvoura, F.K.; Khoury, M.J.; Tanzi, R.E.; Bertram, L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat. Genet. 2008, 40, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Kaji, T. Whey protein isolate inhibits hepatic FGF21 production, which precedes weight gain, hyperinsulinemia and hyperglycemia in mice fed a high-fat diet. Sci. Rep. 2020, 10, 15784. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; DiMarchi, R. FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends. Endocrinol. Metab. 2015, 26, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Kaji, T. The GLP-1 Receptor agonist liraglutide decreases primary bile acids and serotonin in the colon independently of feeding in mice. Int. J. Mol. Sci. 2024, 25, 7784. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).