Submitted:
17 March 2025
Posted:
18 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
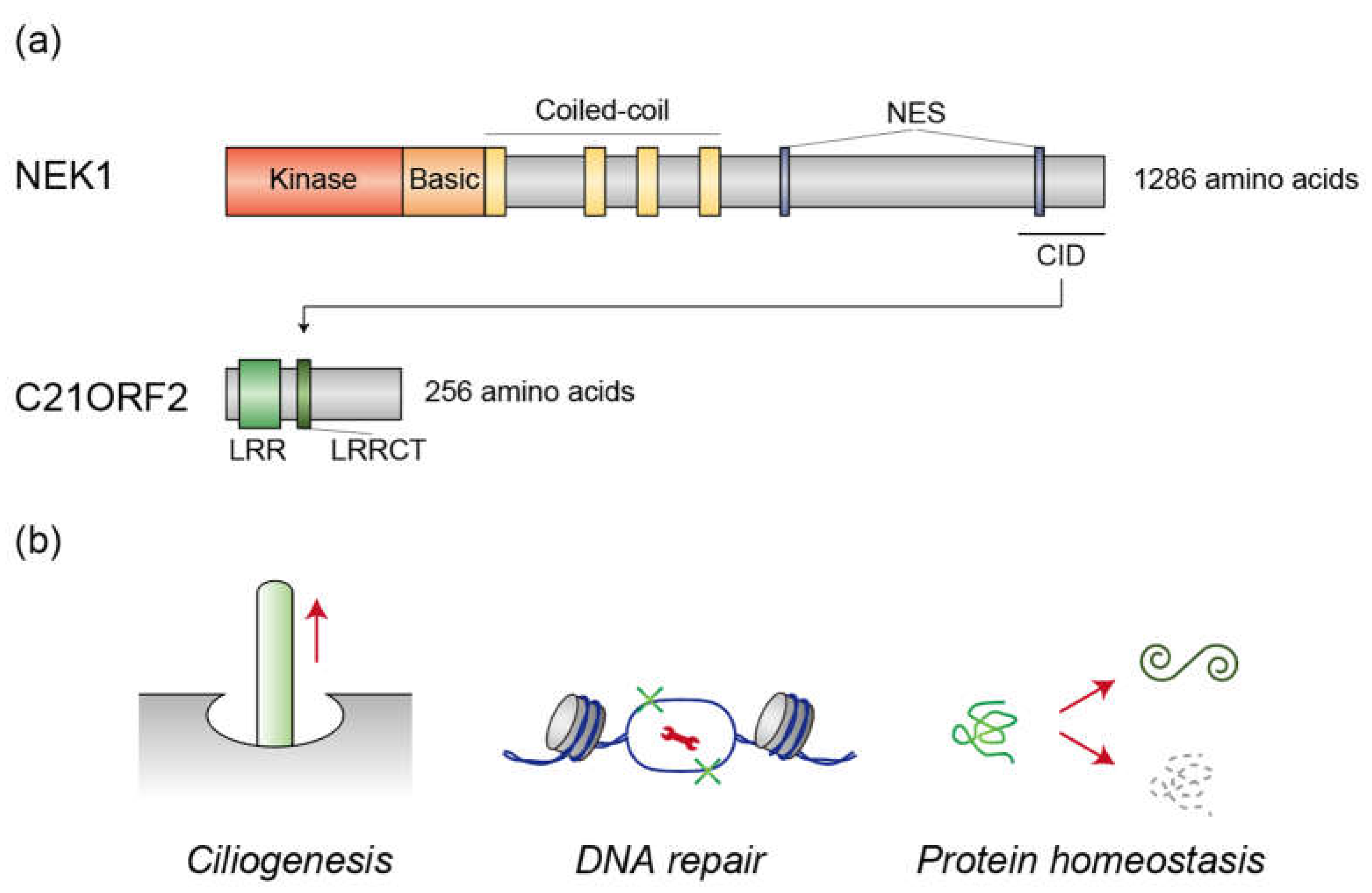
2. NEK1 and C21ORF2
2.1. Human Genetics
2.2. Ciliogenesis
2.3. DNA Repair
2.4. Protein Homeostasis
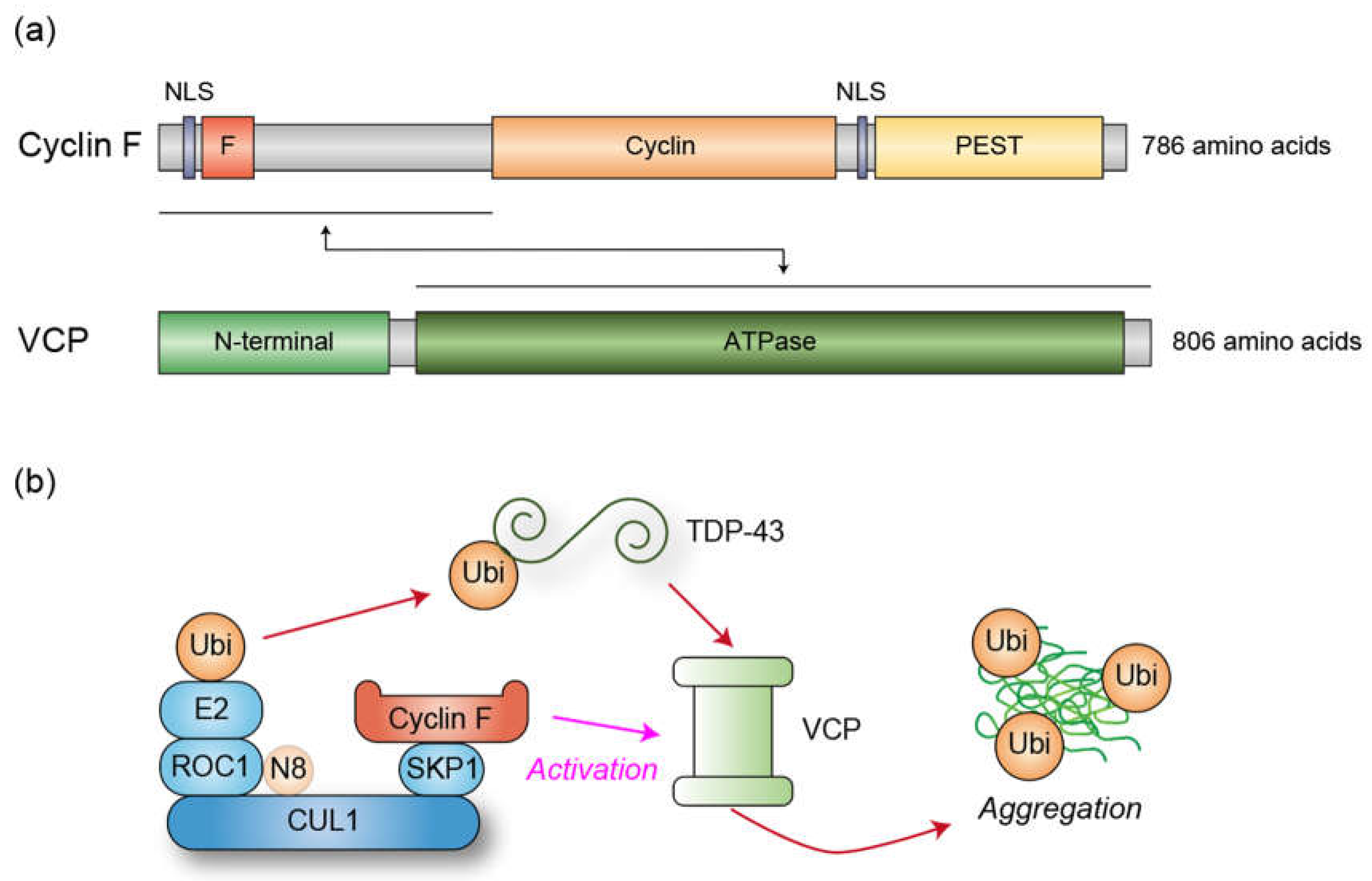
3. Cyclin F and VCP
3.1. Human Genetics
3.2. VCP Activation by Cyciln F
3.3. Cyclin F as an Ubiquitin Ligase for ALS-Associated Proteins
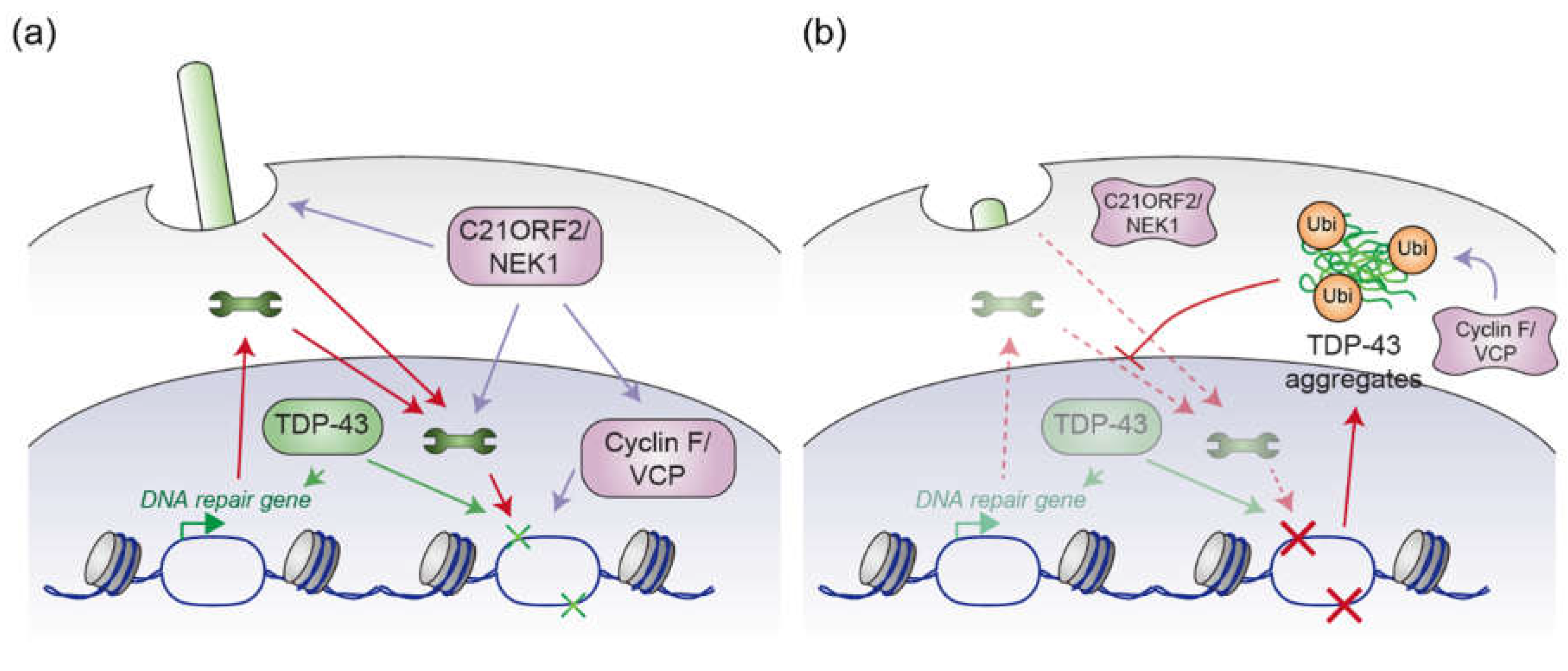
4. Possible Convergent Mechanisms of ALS
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.H. and A. Al-Chalabi, Amyotrophic Lateral Sclerosis. N Engl J Med, 2017. 377(2): p. 162-172.
- Wang, H., L. Guan, and M. Deng, Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front Neurosci, 2023. 17: p. 1170996.
- Mead, R.J. , et al., Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov, 2023. 22(3): p. 185-212.
- Soto, C. and S. Pritzkow, Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci, 2018. 21(10): p. 1332-1340.
- Moda, F. , et al., Secondary Protein Aggregates in Neurodegenerative Diseases: Almost the Rule Rather than the Exception. Front Biosci (Landmark Ed), 2023. 28(10): p. 255.
- Menzies, F.M. , et al., Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis. Brain, 2002. 125(Pt 7): p. 1522-33.
- Pedrini, S. , et al., ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet, 2010. 19(15): p. 2974-86.
- Richardson, K. , et al., The effect of SOD1 mutation on cellular bioenergetic profile and viability in response to oxidative stress and influence of mutation-type. PLoS One, 2013. 8(6): p. e68256.
- Ash, P.E. , et al., Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron, 2013. 77(4): p. 639-46.
- Lee, K.H. , et al., C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles. Cell, 2016. 167(3): p. 774-788.e17.
- Ryan, S. , et al., C9orf72 dipeptides disrupt the nucleocytoplasmic transport machinery and cause TDP-43 mislocalisation to the cytoplasm. Sci Rep, 2022. 12(1): p. 4799.
- Johnson, B.S. , et al., TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem, 2009. 284(30): p. 20329-39.
- Nonaka, T. , et al., Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet, 2009. 18(18): p. 3353-64.
- Dormann, D. , et al., ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. Embo j, 2010. 29(16): p. 2841-57.
- Kino, Y. , et al., Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res, 2011. 39(7): p. 2781-98.
- Ederle, H. and D. Dormann, TDP-43 and FUS en route from the nucleus to the cytoplasm. FEBS Lett, 2017. 591(11): p. 1489-1507.
- Chen, H.J. , et al., The heat shock response plays an important role in TDP-43 clearance: evidence for dysfunction in amyotrophic lateral sclerosis. Brain, 2016. 139(Pt 5): p. 1417-32.
- Riemenschneider, H. , et al., Gel-like inclusions of C-terminal fragments of TDP-43 sequester stalled proteasomes in neurons. EMBO Rep, 2022. 23(6): p. e53890.
- Zhao, M. , et al., RNA-Binding Proteins in Amyotrophic Lateral Sclerosis. Mol Cells, 2018. 41(9): p. 818-829.
- Xue, Y.C. , et al., Dysregulation of RNA-Binding Proteins in Amyotrophic Lateral Sclerosis. Front Mol Neurosci, 2020. 13: p. 78.
- Sareen, D. , et al., Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med, 2013. 5(208): p. 208ra149.
- Lagier-Tourenne, C. , et al., Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A, 2013. 110(47): p. E4530-9.
- Zu, T. , et al., RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A, 2013. 110(51): p. E4968-77.
- Tran, H. , et al., Differential Toxicity of Nuclear RNA Foci versus Dipeptide Repeat Proteins in a Drosophila Model of C9ORF72 FTD/ALS. Neuron, 2015. 87(6): p. 1207-1214.
- Sun, Y. , et al., The role of DNA damage response in amyotrophic lateral sclerosis. Essays Biochem, 2020. 64(5): p. 847-861.
- Farg, M.A. , et al., The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum Mol Genet, 2017. 26(15): p. 2882-2896.
- Kim, B.W. , et al., DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol Commun, 2020. 8(1): p. 7.
- Fang, M. , et al., Loss of TDP-43 function contributes to genomic instability in amyotrophic lateral sclerosis. Front Neurosci, 2023. 17: p. 1251228.
- Wang, H. , et al., Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat Commun, 2018. 9(1): p. 3683.
- Mitra, J. , et al., Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc Natl Acad Sci U S A, 2019. 116(10): p. 4696-4705.
- Reddy, K. , et al., The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem, 2013. 288(14): p. 9860-9866.
- Haeusler, A.R. , et al., C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature, 2014. 507(7491): p. 195-200.
- Crossley, M.P., M. Bocek, and K.A. Cimprich, R-Loops as Cellular Regulators and Genomic Threats. Mol Cell, 2019. 73(3): p. 398-411.
- Andrade, N.S. , et al., Dipeptide repeat proteins inhibit homology-directed DNA double strand break repair in C9ORF72 ALS/FTD. Mol Neurodegener, 2020. 15(1): p. 13.
- Thiel, C. , et al., NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet, 2011. 88(1): p. 106-14.
- Wang, Z. , et al., Axial spondylometaphyseal dysplasia is also caused by NEK1 mutations. J Hum Genet, 2017. 62(4): p. 503-506.
- Nguyen, H.P. , et al., NEK1 genetic variability in a Belgian cohort of ALS and ALS-FTD patients. Neurobiol Aging, 2018. 61: p. 255.e1-255.e7.
- Brenner, D. , et al., NEK1 mutations in familial amyotrophic lateral sclerosis, in Brain. 2016: England. p. e28.
- Kenna, K.P. , et al., NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat Genet, 2016. 48(9): p. 1037-42.
- Gratten, J. , et al., Whole-exome sequencing in amyotrophic lateral sclerosis suggests NEK1 is a risk gene in Chinese. Genome Med, 2017. 9(1): p. 97.
- Yao, L. , et al., NEK1 mutations and the risk of amyotrophic lateral sclerosis (ALS): a meta-analysis. Neurol Sci, 2021. 42(4): p. 1277-1285.
- Wang, Z. , et al., Axial Spondylometaphyseal Dysplasia Is Caused by C21orf2 Mutations. PLoS One, 2016. 11(3): p. e0150555.
- van Rheenen, W. , et al., Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat Genet, 2016. 48(9): p. 1043-8.
- Khan, A.O. , et al., C21orf2 is mutated in recessive early-onset retinal dystrophy with macular staphyloma and encodes a protein that localises to the photoreceptor primary cilium. Br J Ophthalmol, 2015. 99(12): p. 1725-31.
- Suga, A. , et al., Identification of Novel Mutations in the LRR-Cap Domain of C21orf2 in Japanese Patients With Retinitis Pigmentosa and Cone-Rod Dystrophy. Invest Ophthalmol Vis Sci, 2016. 57(10): p. 4255-63.
- Wheway, G. , et al., An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat Cell Biol, 2015. 17(8): p. 1074-1087.
- Reiter, J.F. and M.R. Leroux, Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol, 2017. 18(9): p. 533-547.
- Gregorczyk, M. , et al., Functional characterization of C21ORF2 association with the NEK1 kinase mutated in human in diseases. Life Sci Alliance, 2023. 6(7).
- Watanabe, Y. , et al., An Amyotrophic Lateral Sclerosis-Associated Mutant of C21ORF2 Is Stabilized by NEK1-Mediated Hyperphosphorylation and the Inability to Bind FBXO3. iScience, 2020. 23(9): p. 101491.
- Mahjoub, M.R., M. L. Trapp, and L.M. Quarmby, NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol, 2005. 16(12): p. 3485-9.
- White, M.C. and L.M. Quarmby, The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol, 2008. 9: p. 29.
- De Decker, M. , et al., C21ORF2 mutations point towards primary cilia dysfunction in amyotrophic lateral sclerosis. Brain, 2025. 148(3): p. 803-816.
- Delint-Ramirez, I. and R. Madabhushi, DNA damage and its links to neuronal aging and degeneration. Neuron, 2025. 113(1): p. 7-28.
- Wang, H. , et al., DNA Damage and Repair Deficiency in ALS/FTD-Associated Neurodegeneration: From Molecular Mechanisms to Therapeutic Implication. Front Mol Neurosci, 2021. 14: p. 784361.
- Polci, R. , et al., NIMA-related protein kinase 1 is involved early in the ionizing radiation-induced DNA damage response. Cancer Res, 2004. 64(24): p. 8800-3.
- Liu, S. , et al., Nek1 kinase associates with ATR-ATRIP and primes ATR for efficient DNA damage signaling. Proc Natl Acad Sci U S A, 2013. 110(6): p. 2175-80.
- Patil, M. , et al., Nek1 interacts with Ku80 to assist chromatin loading of replication factors and S-phase progression. Cell Cycle, 2013. 12(16): p. 2608-16.
- Spies, J. , et al., Nek1 Regulates Rad54 to Orchestrate Homologous Recombination and Replication Fork Stability. Mol Cell, 2016. 62(6): p. 903-917.
- Pelegrini, A.L. , et al., Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest. Mutagenesis, 2010. 25(5): p. 447-54.
- Melo-Hanchuk, T.D. , et al., NEK1 kinase domain structure and its dynamic protein interactome after exposure to Cisplatin. Sci Rep, 2017. 7(1): p. 5445.
- Martins, M.B. , et al., NEK1 deficiency affects mitochondrial functions and the transcriptome of key DNA repair pathways. Mutagenesis, 2021. 36(3): p. 223-236.
- Higelin, J. , et al., NEK1 loss-of-function mutation induces DNA damage accumulation in ALS patient-derived motoneurons. Stem Cell Res, 2018. 30: p. 150-162.
- Santangelo, S. , et al., NEK1 haploinsufficiency worsens DNA damage, but not defective ciliogenesis, in C9ORF72 patient-derived iPSC-motoneurons. Hum Mol Genet, 2024. 33(21): p. 1900-1907.
- Fang, X. , et al., The NEK1 interactor, C21ORF2, is required for efficient DNA damage repair. Acta Biochim Biophys Sin (Shanghai), 2015. 47(10): p. 834-41.
- Peixoto, E. , et al., Cholangiocytes’ Primary Cilia Regulate DNA Damage Response and Repair. bioRxiv, 2025.
- Patil, M. , et al., Nek1 phosphorylates Von Hippel-Lindau tumor suppressor to promote its proteasomal degradation and ciliary destabilization. Cell Cycle, 2013. 12(1): p. 166-71.
- Mann, J.R. , et al., Loss of function of the ALS-associated NEK1 kinase disrupts microtubule homeostasis and nuclear import. Sci Adv, 2023. 9(33): p. eadi5548.
- Rifai, O.M. , et al., Clinicopathological analysis of NEK1 variants in amyotrophic lateral sclerosis. Brain Pathol, 2025. 35(1): p. e13287.
- Williams, K.L. , et al., CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat Commun, 2016. 7: p. 11253.
- Zelong, Y. , et al., Increased expression of Cyclin F in liver cancer predicts poor prognosis: A study based on TCGA database. Medicine (Baltimore), 2021. 100(31): p. e26623.
- Kwiatkowski, M. , et al., Overexpression of cyclin F/CCNF as an independent prognostic factor for poor survival in clear cell renal cell carcinoma. Sci Rep, 2024. 14(1): p. 9280.
- Li, Y. , et al., Cyclin F and KIF20A, FOXM1 target genes, increase proliferation and invasion of ovarian cancer cells. Exp Cell Res, 2020. 395(2): p. 112212.
- Liu, Y. , et al., Systematic analysis of the expression and prognosis relevance of FBXO family reveals the significance of FBXO1 in human breast cancer. Cancer Cell Int, 2021. 21(1): p. 130.
- Watts, G.D. , et al., Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet, 2004. 36(4): p. 377-81.
- Al-Obeidi, E. , et al., Genotype-phenotype study in patients with valosin-containing protein mutations associated with multisystem proteinopathy. Clin Genet, 2018. 93(1): p. 119-125.
- Gonzalez, M.A. , et al., A novel mutation in VCP causes Charcot-Marie-Tooth Type 2 disease. Brain, 2014. 137(Pt 11): p. 2897-902.
- Chan, N. , et al., Valosin-containing protein mutation and Parkinson’s disease, in Parkinsonism Relat Disord. 2012: England. p. 107-9.
- Majounie, E. , et al., Mutational analysis of the VCP gene in Parkinson’s disease. Neurobiol Aging, 2012. 33(1): p. 209.e1-2.
- van de Warrenburg, B.P. , et al., Clinical exome sequencing for cerebellar ataxia and spastic paraplegia uncovers novel gene-disease associations and unanticipated rare disorders. Eur J Hum Genet, 2016. 24(10): p. 1460-6.
- Johnson, J.O. , et al., Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron, 2010. 68(5): p. 857-64.
- Rayner, S.L. , et al., Cyclin F, Neurodegeneration, and the Pathogenesis of ALS/FTD. Neuroscientist, 2024. 30(2): p. 214-228.
- Scarian, E. , et al., The Role of VCP Mutations in the Spectrum of Amyotrophic Lateral Sclerosis-Frontotemporal Dementia. Front Neurol, 2022. 13: p. 841394.
- Yu, Y. , et al., Pathogenic mutations in the ALS gene CCNF cause cytoplasmic mislocalization of Cyclin F and elevated VCP ATPase activity. Hum Mol Genet, 2019. 28(20): p. 3486-3497.
- Wang, Q., C. Song, and C.C. Li, Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol, 2004. 146(1-2): p. 44-57.
- Braxton, J.R. and D.R. Southworth, Structural insights of the p97/VCP AAA+ ATPase: How adapter interactions coordinate diverse cellular functionality. J Biol Chem, 2023. 299(11): p. 105182.
- Manno, A. , et al., Enhanced ATPase activities as a primary defect of mutant valosin-containing proteins that cause inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Genes Cells, 2010. 15(8): p. 911-22.
- Rijal, R. , et al., Mutant p97 exhibits species-specific changes of its ATPase activity and compromises the UBXD9-mediated monomerisation of p97 hexamers. Eur J Cell Biol, 2016. 95(6-7): p. 195-207.
- van den Boom, J. and H. Meyer, VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol Cell, 2018. 69(2): p. 182-194.
- Ayyadevara, S. , et al., Intrinsically disordered proteins identified in the aggregate proteome serve as biomarkers of neurodegeneration. Metab Brain Dis, 2022. 37(1): p. 147-152.
- Ling, S.C., M. Polymenidou, and D.W. Cleveland, Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron, 2013. 79(3): p. 416-38.
- Kitamura, A., N. Iwasaki, and M. Kinjo, Molecular chaperone HSP70 prevents formation of inclusion bodies of the 25-kDa C-terminal fragment of TDP-43 by preventing aggregate accumulation. Cell Stress Chaperones, 2018. 23(6): p. 1177-1183.
- Lin, L.T. , et al., Hsp90 and its co-chaperone Sti1 control TDP-43 misfolding and toxicity. Faseb j, 2021. 35(5): p. e21594.
- Lam, A.Y.W. , et al., DNAJA2 and Hero11 mediate similar conformational extension and aggregation suppression of TDP-43. Rna, 2024. 30(11): p. 1422-1436.
- van Hummel, A. , et al., TDP-43 pathology and functional deficits in wild-type and ALS/FTD mutant cyclin F mouse models. Neuropathol Appl Neurobiol, 2023. 49(2): p. e12902.
- Bai, C. , et al., SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell, 1996. 86(2): p. 263-74.
- D’Angiolella, V. , et al., Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell, 2012. 149(5): p. 1023-34.
- Rayner, S.L. , et al., TDP-43 is a ubiquitylation substrate of the SCF(cyclin F) complex. Neurobiol Dis, 2022. 167: p. 105673.
- Rayner, S.L. , et al., Cyclin F can alter the turnover of TDP-43. Neurobiol Dis, 2024. 192: p. 106421.
- Swatek, K.N. and D. Komander, Ubiquitin modifications. Cell Res, 2016. 26(4): p. 399-422.
- Davidson, J.M. , et al., The E3 Ubiquitin Ligase SCF Cyclin F Promotes Sequestosome-1/p62 Insolubility and Foci Formation and is Dysregulated in ALS and FTD Pathogenesis. Mol Neurobiol, 2023. 60(9): p. 5034-5054.
- Foster, A.D. , et al., p62 overexpression induces TDP-43 cytoplasmic mislocalisation, aggregation and cleavage and neuronal death. Sci Rep, 2021. 11(1): p. 11474.
- Choudhury, R. , et al., The E3 Ubiquitin Ligase SCF(Cyclin F) Transmits AKT Signaling to the Cell-Cycle Machinery. Cell Rep, 2017. 20(13): p. 3212-3222.
- van de Kooij, B. , et al., Comprehensive substrate specificity profiling of the human Nek kinome reveals unexpected signaling outputs. Elife, 2019. 8.
- Cascella, R. , et al., Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies. J Biol Chem, 2016. 291(37): p. 19437-48.
- Kim, G. , et al., ALS Genetics: Gains, Losses, and Implications for Future Therapies. Neuron, 2020. 108(5): p. 822-842.
- Chou, C.C. , et al., TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci, 2018. 21(2): p. 228-239.
- Tsekrekou, M. , et al., Protein aggregation and therapeutic strategies in SOD1- and TDP-43- linked ALS. Front Mol Biosci, 2024. 11: p. 1383453.
- Provasek, V.E. , et al., TDP43 interacts with MLH1 and MSH6 proteins in a DNA damage-inducible manner. Mol Brain, 2024. 17(1): p. 32.
- Provasek, V.E. , et al., RNA/DNA Binding Protein TDP43 Regulates DNA Mismatch Repair Genes with Implications for Genome Stability. bioRxiv, 2024.
- Guerrero, E.N. , et al., Amyotrophic lateral sclerosis-associated TDP-43 mutation Q331K prevents nuclear translocation of XRCC4-DNA ligase 4 complex and is linked to genome damage-mediated neuronal apoptosis. Hum Mol Genet, 2019. 28(15): p. 2459-2476.
- Yuan, R. , et al., Cyclin F-dependent degradation of E2F7 is critical for DNA repair and G2-phase progression. Embo j, 2019. 38(20): p. e101430.
- Jiang, N. , et al., Valosin-containing protein regulates the proteasome-mediated degradation of DNA-PKcs in glioma cells. Cell Death Dis, 2013. 4(5): p. e647.
- He, J. , et al., Valosin-containing Protein (VCP)/p97 Segregase Mediates Proteolytic Processing of Cockayne Syndrome Group B (CSB) in Damaged Chromatin. J Biol Chem, 2016. 291(14): p. 7396-408.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




