Submitted:
11 March 2025
Posted:
12 March 2025
You are already at the latest version
Abstract
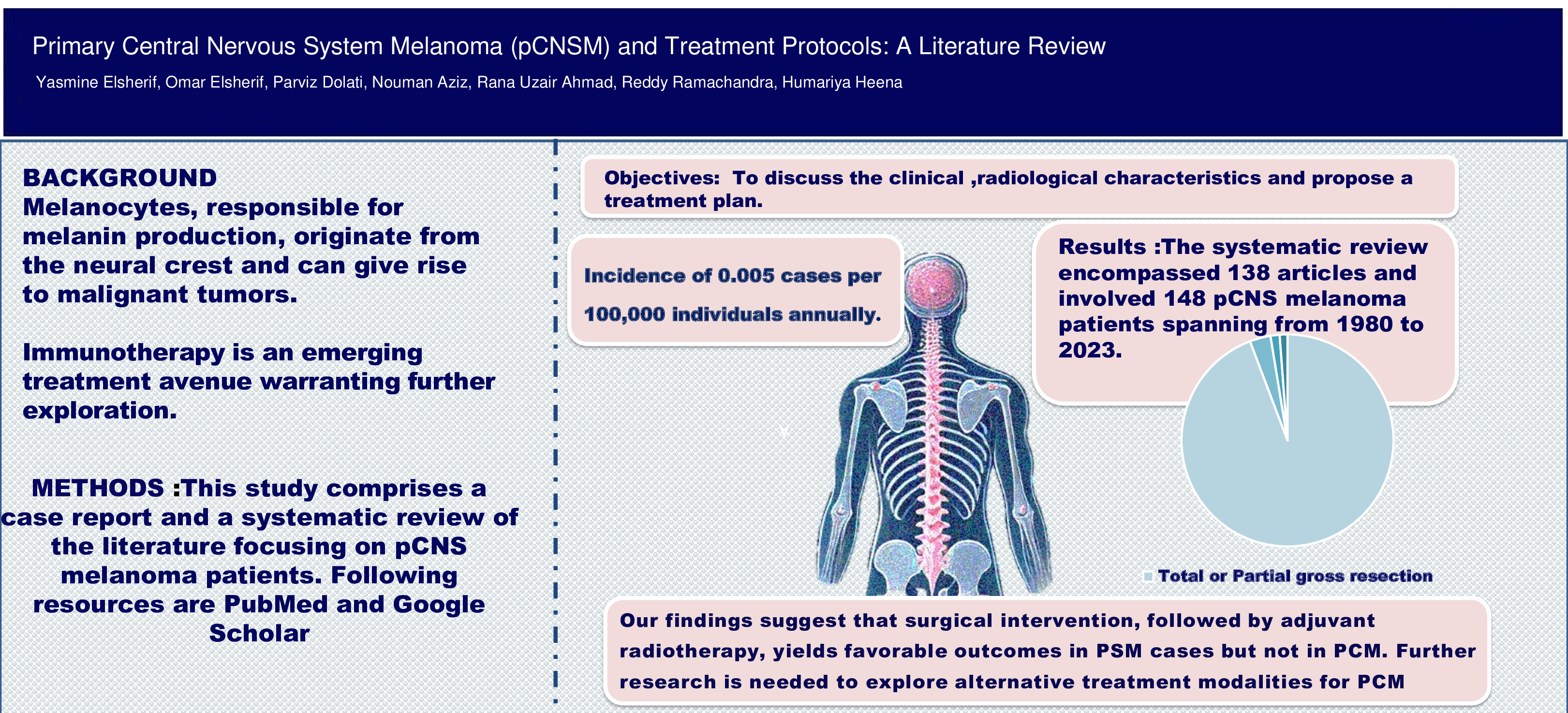
Background: Melanocytes, responsible for melanin production, originate from the neural crest and can give rise to malignant tumors known as melanoma. Primary Central Nervous System Melanoma (pCNSM) is rare, with an incidence of 0.005 cases per 100,000 individuals annually. Considering its rare occurrence and the variations in tumor biology, a comprehensive treatment strategy has not yet been introduced. Immunotherapy is an emerging treatment avenue warranting further exploration. Objectives: In this review, we conducted an extensive literature review on patients with pCNSM, including the brain and spine, to determine the characteristics and most successful treatment protocol for pCNSM. Materials and Methods: A review of all published articles from 1980 to 2023 was done including PubMed and Google Scholar articles, multiple published case studies, and the Archives of the American Institute for Radiologic Pathology. Results: During our review we found 138 articles with 148 pCNSMs (77 cases of brain and 71 cases of spinal primary melanoma) spanning from 1980 to 2023. The vast majority of patients (95%) underwent therapeutic gross total or partial resection of the tumors followed by radiotherapy. Only two cases with brain melanomas underwent immunotherapy. The treatment strategies of primary brain melanomas were similar to those of primary spinal melanoma (PSM) but exhibited better outcomes in PSM cases. Conclusion: Our findings suggest surgical intervention, followed by adjuvant radiotherapy + immunotherapy, yields favorable outcomes in PSM cases but not in primary brain melanomas. Further multicentric studies are needed to explore alternative, more effective treatment modalities.
Keywords:
Introduction
Methods
Results
| Primary intracranial malignant melanoma | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | N0.(%) | Mean Age |
Gender M(n.)/F(n.) |
Major clinical manifestation | Imaging | Treatment modality: Number (%) | |||||||||
| Treatment (without surgery )* | Adjuvant therapy | Surgical intervention only | |||||||||||||
| CTx** | RT | IMT | Combined therapy | ||||||||||||
| Temporal lobe | 9(12) | 48.2 | M(5),F(5) | Headache | MRI :Hyperintense T1, hypointense T2,CT:Hyperdense | _ | _ | 1 | _ | 4 | 4 | ||||
| Frontal lobe | 15(19.5) | 41.73333 | M(10),F(5) | Headache and Blurring of vision | MRI :Hyperintense T1, hypointense T2,CT:Hyperdense | _ | _ | 6 | 1 | 4 | 4 | ||||
| Parietal lobe | 8(10.4) | 28.14 | M(6),F(2) | Headache and vomiting | MRI:Iso/hyperintinse T1,Hypo /Hyperintinse T2,CT:Hyperdense | _ | _ | 4 | _ | _ | 4 | ||||
| Occipital lobe | 3(3.9) | 40.66667 | M(2),F(1) | Diminished vision bilaterally | MRI:Hyperintense T1 , Iso /hypointense T2 | _ | _ | 1 | _ | _ | 2 | ||||
| Front-parietal lobe | 1(1.3) | 66 | M(1),F(0) | Clouding of consciousness | MRI :Hyperintense T1, hypointense T2 | _ | _ | _ | _ | _ | 1 | ||||
| Parieto-temporal lobe*** | 1(1.3) | 67 | M(0),F(1) | Psychomotor agitation, altered consciousness | CT-angiography mass measured (6 cm × 5 cm) with intralesional calcifications | _ | _ | _ | _ | _ | 1 | ||||
| Temporoparitetal lobe (Insular) | 2(2.6) | 28.5 | M(2),F(0) | Impaired vision ,contralateral paresis | MRI:Hyperintense T1 , hypointense T2 | _ | 1 | _ | _ | 1 | _ | ||||
| Cerebellopontine angle | 13(16.9) | 40.30769 | M(4),F(9) | Hearing loss | MRI :Hyperintense T1, hypointense T2 | _ | _ | 7 | _ | 1 | 5 | ||||
| Cerebellum | 3(3.9) | 52 | M(1),F(2) | Dizziness and gait disturbance | MRI :Hyperintense T1, hypointense T2 | _ | _ | 1 | _ | 2 | _ | ||||
| Pons | 1(1.3) | 10 | M(0),F(1) | Lethargy | MRI:Hyperintense T2 | _ | _ | _ | _ | 1 | _ | ||||
| Forniceal callosum | 1(1.3) | 51 | M(0),F(1) | Headache | MRI :Hyperintense T1, hypointense T2 | _ | _ | _ | _ | 1 | _ | ||||
| Cavernous sinus | 1(1.3) | 36 | M(0),F(1) | Incomplete ipsilateral paralysis | MRI :Hyperintense T1, hypointense T2 | 1 | _ | _ | _ | _ | _ | ||||
| Pineal gland | 8(10.4) | 58.66667 | M(5),F(4) | Gait disturbances | MRI:Hyperintense T1 , Iso /hypointense T2 | 2 | 1 | 5 | _ | _ | 1 | ||||
| Intra and suprasellar | 2(2.6) | 37 | M(0),F(2) | Deterioration of vision | MRI :Signal heterogeneity of the lesion | _ | _ | 1 | _ | 1 | _ | ||||
| Medulla oblongata | 1(1.3) | 40 | M(1),F(0) | Vertigo and headache | MRI :Hyperintense T1, hypointense T2 | _ | _ | _ | _ | _ | 1 | ||||
| Others**** | 5(6) | 45 | M(3),F(2) | Swelling in the head | MRI :Hyperintense T1, hypointense T2,CT: mixed iso/hyperdensity | _ | _ | 1 | _ | 1 | 3 | ||||
| More than one Structure | 3(4) | 33.33 | M(0),F(3) | Vomiting | Nonspecific | 1 | _ | _ | _ | 1 | 1 | ||||
| Total | 77(100) | 42.24 | M(40),F(37) | Headache ,Blurring of vision, Vomiting and Gait disturbances | MRI: Hyperintense T1, hypointense T2, CT: mixed iso/hyperdensity | 4(5%) | 2(3%) | 27(34.6%) | 1(1%) | 17(21.8%) | 27(34.6%) | ||||
| Primary Spinal melanoma | |||||||||||||||
| Site | N0.(%) | Mean Age |
Gender M(n.)/F(n.) |
Major clinical manifestation | Imaging | Adjuvant Treatment modality: Number (%) | |||||||||
| Treatment (Without surgery) | Adjuvant Therapy | Surgical intervention only | |||||||||||||
| CTx | RT | IMT | Combined Therapy | ||||||||||||
| Cervical vertebrae and CMJ | 24(33.8%) | 46 | M(15),F(9) | Neck pain, quadriparesis and Shoulder pain | MRI :Hyperintense T1, hypointense T2 | _ | 2(8.3%) | 6(25) | _ | 2(8.3%) | 14(58.4%) | ||||
| Thoracic vertebrae | 26(36.6%) | 50 | M(14),F(12) | Paresthesia and paraparesis of lower limb | MRI :Hyperintense T1, hypointense T2, Spinal Myelography : Complete block of the contrast material | _ | 3(11.53%) | 10(38.4%) | _ | 6(23.07) | 7(27%) | ||||
| Lumber vertebrae | 8(11.3%) | 60 | M(3),F(5) | Chronic back pain | MRI:Iso,hyperintense T1 , hypointense T2CT: Hyperdense | _ | _ | 1(12.5%) | _ | 2(25%) | 5(62.5%) | ||||
| Sacral vertebrae | 2(2.8%) | 22.5 | M(2),F(0) | Left posterior hip pain ,Schwannoma or ependymoma | MRI :Hyperintinse T1,heterogenous T2 | _ | 1(50%) | 1(50%) | _ | _ | _ | ||||
| Multiple vertebras | 11(15.5%) | 52 | M(6),F(5) | Quadriplegia | MRI: Hyperintense T1, iso/hypointense T2, Whole-body FDG PET/CT: demonstrated abnormal activity in the cauda equina. 16.2 mCi of FDG was used, and this lesion measured an average standard uptake value of 4.4 | _ | _ | 3(27.2%) | _ | 4(36.4%) | 4(36.4%) | ||||
| Total | 71(100%) | 49.49 | M(40),F(31) | Back neck and shoulder pain, Paresthesia and paraparesis of lower limb | MRI:Hyperintense T1 , hypointense T2 CT: Hyperdense |
_ | 6(8.45%) | 21(29.6%) | _ | 14(19.7%) | 30(42.25%) | ||||
| Differential diagnosis | Age range | Gender | Type | Pathology (Microscopic) | Imaging | Treatment | |
|---|---|---|---|---|---|---|---|
| MRI | CT | ||||||
| Melanotic meningioma | 40-60 | Benign affect twice female ,Malignant equal. | Majority Benign | Mild Swirly shape cells, sand bodies easily visible. Positive for EMA ,negative for HMB-45 and Melan-A | T1: iso- or hyperintense ,T2: hypo- or hyperintense | Isodense | Surgical and adjuvant radiotherapy |
| Malignant melanotic nerve sheath tumours (Melanotic shwannoma ) | 20-60 | Equal | Potentially malignant | Mild and sorted out in a palisade shape, negative for HMB-45 and Melan-A | T1: hyperintense ,T2:hypointinse | Isodense or slightly hyperdense | Complete surgical resection |
| Meningeal melanocytoma | 30-50 | Equal | Benign | Uniform cell, small atypia, no hemorrhage and necrosis, mitosis 0 to 1/10HPF, no infiltration in adjacent tissues | T1: isointense or hyperintense T2: isointense or hypointense | Isodense to hyperdense | Complete surgical resection |
| Primary melanoma | 40-50 | Male | Malignant | T1:hyperintinse ,T2:hypointinse | Hyderdense | Limited data | |
| Metastatic melanoma (cutaneous origin ) | 55-65 | < 50 Female | Malignant | Atypia is prominent, necrosis is and the BRAF, NRAS, and KIT gene variants | T1:hyperintinse ,T2:hypointinse | NECT: single to multiple nodules of increased attenuation | Combination of regional/systemic chemotherapy with associated immunotherapy and/or radiation therapy |
| 65-80 Male | CECT: well-enhanced lesions | ||||||
| Note: CPA: Cerebropointine angle, NECT: non contrast enhanced computed tomography, CECT: contrast enhanced computed tomography Reference 1.Lacruz, César R., and Eugenio Leonardo. "Primary CNS Melanocytic Neoplasms." Central Nervous System Tumors: Diagnostic Pathology (2024): 321-329. | |||||||
Discussion
Conclusion
Acknowledgment
Ethical consideration
Declaration of competing interest
References
- Hayward RD. Malignant melanoma and the central nervous system. A guide for classification based on the clinical findings. J Neurol Neurosurg Psychiatry. 1976;39(6):526-530. [CrossRef]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97-109. Erratum in: Acta Neuropathol. 2007 Nov;114(5):547. [CrossRef] [PubMed] [PubMed Central]
- Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: radiologic-pathologic correlation. Radiographics. 2009;29(5):1503-1524. [CrossRef]
- Virchow. Pigment und diffuse melanose der arachnoides Virchows Archiv, 16 (1859), pp. 180-182.
- Ogle. Sarcoma of pineal body with diffused melanotic sarcoma of the surface of cerebrum. Tr Pathol Soc Land, 1 (1899), pp. 4-6.
- Foot NC, Zeek P. Two Cases of Melanoma of the Meninges with Autopsy. Am J Pathol. 1931;7(6):605-618.5.
- Chang, J. W., Yeh, K. Y., Chao, J., & Hsiao, C. H. (2016). Primary Central Nervous System Melanoma: A Case Report and Literature Review. Oncology Letters, 11(3), 2329–2333. [CrossRef]
- Suranagi VV, Maste P, Malur PR. Primary intracranial malignant melanoma: A rare case with review of literature. Asian J Neurosurg. 2015;10(1):39-41. [CrossRef]
- Somers KE, Almast J, Biemiller RA, Silberstein HJ, Johnson MD, Mohile NA. Diagnosis of primary CNS melanoma with neuroimaging. J Clin Oncol. 2013;31(1): e9-e11. [CrossRef]
- Quillo-Olvera J, Uribe-Olalde JS, Alcántara-Gómez LA, Rejón-Pérez JD, Palomera-Gómez HG. Melanoma maligno primario del sistema nervioso central: un reto diagnóstico [Primary malignant melanoma of the central nervous system: A diagnostic challenge]. Cir Cir. 2015;83(2):129-134. [CrossRef]
- Yamane K, Shima T, Okada Y, et al. Primary pineal melanoma with long-term survival: case report. Surg Neurol. 1994;42(5):433-437. [CrossRef]
- Willis AJ, Huang AH, Carroll P. Primary melanoma of the bladder: a case report and review. J Urol. 1980;123(2):278-281. [CrossRef]
- Troya-Castilla M, Rocha-Romero S, Chocrón-González Y, Márquez-Rivas FJ. Primary cerebral malignant melanoma in insular region with extracranial metastasis: case report and review literature. World J Surg Oncol. 2016;14(1):235. Published 2016 Sep 1. [CrossRef]
- Tang K, Kong X, Mao G, et al. Primary cerebral malignant melanoma: A case report with literature review. Medicine (Baltimore). 2017;96(4): e5805. [CrossRef]
- Savitz MH. Meningeal melanocytoma. J Neurosurg. 1998;89(1):172. [CrossRef]
- Arai N, Kagami H, Mine Y, Ishii T, Inaba M. Primary Solitary Intracranial Malignant Melanoma: A Systematic Review of Literature. World Neurosurg. 2018; 117:386-393. [CrossRef]
- Chen X, Zhimao J, Yuzhuo H, et al. Congenital melanocytic nevi with primary cerebral melanoma: a rarity.APSP J Case Rep. 2013;4(3):43.
- Wadasadawala T, Trivedi S, Gupta T, Epari S, Jalali R. The diagnostic dilemma of primary central nervous system melanoma. J Clin Neurosci. 2010;17(8):1014-1017. [CrossRef]
- Balakrishnan R, Porag R, Asif DS, Satter AM, Taufiq M, Gaddam SS. Primary Intracranial Melanoma with Early Leptomeningeal Spread: A Case Report and Treatment Options Available. Case Rep Oncol Med. 2015; 2015:293802. [CrossRef]
- Pan Z, Yang G, Wang Y, Yuan T, Gao Y, Dong L. Leptomeningeal metastases from a primary central nervous system melanoma: a case report and literature review. World J Surg Oncol. 2014; 12:265. Published 2014 Aug 20. [CrossRef]
- Gajaria PK, Shenoy AS, Goel NA. Melanoma of the central nervous system: A report of three cases. Indian J Pathol Microbiol. 2021;64(3):535-540. [CrossRef]
- Rodriguez y Baena R, Gaetani P, Danova M, Bosi F, Zappoli F. Primary solitary intracranial melanoma: case report and review of the literature. Surg Neurol. 1992;38(1):26-37. [CrossRef]
- Kashiwagi N, Hirabuki N, Morino H, Taki T, Yoshida W, Nakamura H. Primary solitary intracranial melanoma in the Sylvian fissure: MR demonstration. Eur Radiol. 2002;12 Suppl 3: S7-S10. [CrossRef]
- Qazi SS, Shah SMI, Baqai MWS, Enam SA. Primary leptomeningeal melanoma in association with neurocutaneous melanosis: A case report. Surg Neurol Int. 2022; 13:547. Published 2022 Nov 25. [CrossRef]
- Tanoue N, Ummah FC, Hanada T, et al. Incidentally Found Primary Cerebral Malignant Melanoma Associated with Ota Nevus—Wide Dissemination after an Initial Phase of Slow Growth. Hiroshima Journal of Medical Sciences. 2018;67(1):21-29. [CrossRef]
- AL-Zaidi R. Primary CNS melanoma: A case report with review of the literature. Saudi Journal of Pathology and Microbiology. 2020;5(10):414-419. [CrossRef]
- Otero-Soto GA, Vidal-Anaya V, Labat EJ. Primary Brain Melanoma in a Pediatric Patient: A Case Report. Am J Case Rep. 2021;22: e926749. Published 2021 Mar 9. [CrossRef]
- Sarmast AH, Mujtaba B, Bhat AR, Kirmani AR, Tanki HN. A Unique Case of Primary Intracranial Melanoma. Asian J Neurosurg. 2018;13(1):168-171. [CrossRef]
- Suranagi VV, Maste P, Malur PR. Primary intracranial malignant melanoma: A rare casewith review of literature. Asian J Neurosurg. 2015;10(1):39-41. [CrossRef]
- Jaiswal S, Vij M, Tungria A, Jaiswal AK, Srivastava AK, Behari S. Primary melanocytic tumors of the central nervous system: a neuroradiological and clinicopathological study of five cases and brief review of literature. Neurol India. 2011;59(3):413-419. [CrossRef]
- Gempt J, Buchmann N, Grams AE, et al. Black brain: transformation of a melanocytoma with diffuse melanocytosis into a primary cerebral melanoma. J Neurooncol. 2011;102(2):323-328. [CrossRef]
- Xie ZY, Hsieh KL, Tsang YM, Cheung WK, Hsieh CH. Primary leptomeningeal melanoma. J Clin Neurosci. 2014;21(6):1051-1052. [CrossRef]
- Lim MJ, Tan EE, Wong RX, et al. Paediatric primary intracranial malignant melanoma-case report and literature review [published online ahead of print, 2023 Jun 14]. Pediatr Neurosurg. 2023;10.1159/000531544. [CrossRef]
- Son YJ, Wang KC, Kim SK, Cho BK, Chi JG, Kim YM. Primary intracranial malignant melanoma evolving from leptomeningeal melanosis. Med Pediatr Oncol. 2003;40(3):201-204. [CrossRef]
- Gu S, Pant MC, Husain N, Sundar S, Singh PK. Primary intraparenchymal brain melanoma: a case report. J Radiosurg SBRT. 2012;1(4):339-341.
- Said S, Alkhateeb H, Cooper CJ, et al. Primary central nervous system amelanotic melanoma in a Hispanic male: Case report. Pol J Radiol. 2014;79:199-202. Published 2014 Jul 10. [CrossRef]
- Dinesh SM, Suneetha B, Sen A. A rare case of primary malignant melanoma of clivus with extensive skeletal metastasis demonstrated on 18F-FDG PET/CT. Indian J Nucl Med. 2013;28(4):234-236. [CrossRef]
- Lee P-H, Wang L-C, Lee E-J. Primary intracranial melanoma. Journal of Cancer Research and Practice. 2017;4(1):23-26. [CrossRef]
- Lanka K, Kumari N, Sireesha A, Kumar O. A rare case of primary malignant melanoma in CNS. International Surgery Journal. 2015;2(1):95. [CrossRef]
- Bhople L, Kharosekar HU, Naik H, Velho V. Primary intracranial malignant melanoma mimicking meningioma—report of two cases. Indian Journal of Neurosurgery. 2021;11(01):093-098. [CrossRef]
- Cougo Samueli P, Nunes Rabelo N, Honorato Pereira VH, Nunes Rabelo N, Araujo Dias Junior LA, Pereira CU. Unusual intracranial melanoma: A case report. Revista Chilena de Neurocirugía. 2019;45(2):179-183. [CrossRef]
- Azar M, Kazemi F, Bahrami E, et al. Meningeal melanomas associated with transforming Ota nevus to malignant melanoma: a case report. Medical Journal of The Islamic Republic of Iran (MJIRI). 2010;24(3):163-168. Accessed September 13, 2023. https://mjiri.iums.ac.ir/browse.php?a_code=A-10-1-121&sid=1&slc_lang=en.
- Yuen M-H, Kwok N-F, Chiu H-M, Ho L-C. Rare tumour mimicking meninigioma: A case of primary intracranial amelanotic malignant melanoma with negative staining of S100 and HMB-45. Surgical Practice. 2016;20(4):171-174. [CrossRef]
- Naik H, Kharosekar H, Velho V. Black brain caused by primary intracranial malignant melanoma. Neurol India. 2016;64(1):193-194. [CrossRef]
- Sivaraju L, Ghosal N, Mahadevan A, Uday Krishna AS, Rao S, Hegde AS. Aggressive Primary Pediatric Intracranial Malignant Melanoma: Sphinx of the Tissue Diagnosis. Asian J Neurosurg. 2019;14(1):275-279. [CrossRef]
- Iizuka H, Nakamura T, Kurauchi M. Primary intracranial melanoma--case report. Neurol Med Chir (Tokyo). 1990;30(9):698-702. [CrossRef]
- Pal S, Mondal S, Pradhan R, Bhattacharya S, Banerjee A, Bhattacharyya D. Primary intracranial malignant melanoma in an adolescent girl: A case report. Clinical Cancer Investigation Journal. 2016;5(6):551. [CrossRef]
- Shankar D, Singh DK, Chand VK, Kaif M. Primary intracranial malignant melanoma: A case report and review of literature. Published online 2021. [CrossRef]
- Troya-Castilla M, Rocha-Romero S, Chocrón-González Y, Márquez-Rivas FJ. Primary cerebral malignant melanoma in insular region with extracranial metastasis: case report and review literature. World J Surg Oncol. 2016;14(1):235. Published 2016 Sep 1. [CrossRef]
- Rodriguez-Zuniga MJ, Effio-Iman PH. Primary cerebellopontine angle melanoma: Case report and systematic review. Polish Annals of Medicine. 2017;24(2):268-275. [CrossRef]
- Ponni A, Jagannatha A, Gururajachar J, et al. Primary Cerebello-Pontine Angle Melanoma: A case report. International Journal of Cancer Therapy and Oncology. 2014;2(3):020315. [CrossRef]
- H R, D K, V K, MK P, M S. Primary melanocytic tumor of the cerebellopontine angle mimicking acoustic schwannoma: Case report. Journal of Medical and Scientific Research. 2017;5(3):106-110. [CrossRef]
- Brackmann DE, Doherty JK. CPA melanoma: diagnosis and management. Otol Neurotol. 2007;28(4):529-537. [CrossRef]
- Whinney D, Kitchen N, Revesz T, Brookes G. Primary malignant melanoma of the cerebellopontine angle. Otol Neurotol. 2001;22(2):218-222. [CrossRef]
- Oluigbo CO, Cooke SR, Flynn PA, Choudhari KA. Primary malignant melanoma of the cerebellopontine angle: a diagnostic dilemma: case report. Neurosurgery. 2006;59(6):E1336. [CrossRef]
- Braga FM, Tella Júnior OI, Ferreira A, Jordy CF. Malignant melanoma of the cerebello-pontine angle region. Arq Neuropsiquiatr. 1989;47(4):496-500. [CrossRef]
- Sinai Khandeparkar SG, Fegade LA, Gogate BP, Talathi N. Primary malignant melanoma of the cerebellopontine angle: A rare entity. Indian J Cancer. 2021;58(4):621-624. [CrossRef]
- Kan P, Shelton C, Townsend J, Jensen R. Primary Malignant Cerebellopontine Angle Melanoma Presenting as a Presumed Meningioma: Case Report and Review of the Literature. Skull Base. 2003;13(3):159-166. [CrossRef]
- Desai K, Dindorkar K, Goel A, Shenoy A. Primary cerebello-pontine angle malignant melanoma: a case report. Neurol India. 2001;49(2):200-202.
- Bhandari L, Alapatt J, Govindan A, Sreekumar T. Primary cerebellopontine angle melanoma: a case report and review. Turk Neurosurg. 2012;22(4):469-474. [CrossRef]
- Wong TF, Chen YS, Zhang XH, et al. Longest survival with primary intracranial malignant melanoma: A case report and literature review. World J Clin Cases. 2022;10(30):11162-11171. [CrossRef]
- Long WL, Chen FY, Huang XL, Lu JX, Xu YN. Imaging and pathological diagnosis of primary intracranial malignant melanoma: A case report and literature review. Medicine (Baltimore). 2023;102(5):e32767. [CrossRef]
- El Ouazzani H, Oudghiri MY, Abbas S, et al. Diagnostic challenge: primary leptomeningeal melanoma with melanomatosis, illustrative case report. J Surg Case Rep. 2023;2023(6):rjad323. Published 2023 Jun 12. [CrossRef]
- Aaroe AE, Glitza Oliva IC, Al-Zubidi N, et al. Pearls & Oy-sters: Primary Pineal Melanoma With Leptomeningeal Carcinomatosis. Neurology. 2021;97(5):248-250. [CrossRef]
- Wendel C, Kaech DL, Woodtli M. Primary Malignant Melanoma in the Pineal Region: Case Report and Literature Review. J Neurol Surg A Cent Eur Neurosurg. 2018;79(4):344-352. [CrossRef]
- Cedeño Diaz OM, Leal RG, La Cruz Pelea C. Primary pineal malignant melanoma. Clin Pract. 2011;1(2):e31. Published 2011 Jun 21. [CrossRef]
- Park JH, Hong YK. Primary malignant melanoma in the pineal region. J Korean Neurosurg Soc. 2014;56(6):504-508. [CrossRef]
- Rubino GJ, King WA, Quinn B, Marroquin CE, Verity MA. Primary pineal melanoma: case report. Neurosurgery. 1993;33(3):511-515. [CrossRef]
- Barron J, Morris-Larkin C, Finch T, Maroun F, Hache N, Yousef GM. Long survival of primary pineal melanoma with radiation treatment only. Can J Neurol Sci. 2007;34(2):251-253. [CrossRef]
- Singh RK, Leekha N, Varghese BT, Anila KR. Primary Melanoma of Infra Temporal Fossa: A Case Report. Indian J Surg Oncol. 2015;6(3):276-279. [CrossRef]
- Ma J, Zhang Z, Li S, Chen X, Wang S. Intracranial amelanotic melanoma: a case report with literature review. World J Surg Oncol. 2015;13:182. Published 2015 May 12. [CrossRef]
- Naeem A, Mubarak F. Primary malignant melanoma of brainstem medulla mimicking as cavernoma – case report. International Neuropsychiatric Disease Journal. Published online 2019:1-5. [CrossRef]
- Al-Hadithy N, Al-Nakib K, McGurk S, Quaba A. Primary intracranial melanoma in a child with a giant congenital melanocytic naevus and normal MRI. BMJ Case Rep. 2013;2013:bcr2013009276. Published 2013 Apr 29. [CrossRef]
- Garge S, Mani S, Inbaraj A, Rajshekhar V, Mohapatra P. Cavernous sinus melanoma: A rare tumor. Indian J Radiol Imaging. 2017;27(1):43-45. [CrossRef]
- Costanzo R, Parmar V, Marrone S, et al. Differential diagnosis between primary intracranial melanoma and cerebral cavernoma in crohn’s disease: A case report and literature review. Oncologie. 2022;24(4):937-942. [CrossRef]
- Park J-H, Hong Y-K. Primary malignant melanoma in the pineal region. Journal of Korean Neurosurgical Society. 2014;56(6):504. [CrossRef]
- Tüttenberg J, Fink W, Back W, Wenz F, Schadendorf D, Thomé C. A rare primary sellar melanoma. Case report. J Neurosurg. 2004;100(5):931-934. [CrossRef]
- Copeland DD, Sink JD, Seigler HF. Primary intracranial melanoma presenting as a suprasellar tumor. Neurosurgery. 1980;6(5):542-545. [CrossRef]
- Cao Y, Wang YB, Tan XY, Cui YH, Zhao G. Multifocal primary amelanotic meningeal melanomas mimicking lymphoma: a case report and literature review [published online ahead of print, 2020 Oct 15]. Br J Neurosurg. 2020;1-5. [CrossRef]
- Hung PC, Chang YC, Hsieh MY, Wu CT. Primary intracranial meningeal melanoma mimicking chronic meningitis: A case report. Pediatr Neonatol. 2019;60(5):589-591. [CrossRef]
- Liu QY, Liu AM, Li HG, Guan YB. Primary spinal melanoma of extramedullary origin: a report of three cases and systematic review of the literature [published correction appears in Spinal Cord Ser Cases. 2016 Jul 21;2:16019]. Spinal Cord Ser Cases. 2015;1:15003. Published 2015 Jul 9. [CrossRef]
- Salpietro FM, Alafaci C, Gervasio O, et al. Primary cervical melanoma with brain metastases. Case report and review of the literature. J Neurosurg. 1998;89(4):659-666. [CrossRef]
- Yu J, Zhao DD, Chen S, Zhang JM, Xu J. Primary melanoma of the cervical spine with cerebral metastases: case report and review of the literature. J Int Med Res. 2012;40(3):1207-1215. [CrossRef]
- Kumar KS. Primary cervical intramedullary spinal cord melanoma. The Journal of Spinal Surgery. 2014;1(3):128-131. [CrossRef]
- Vij M, Jaiswal S, Jaiswal AK, Behari S. Primary spinal melanoma of the cervical leptomeninges: report of a case with brief review of literature. Neurol India. 2010;58(5):781-783. [CrossRef]
- Iga T, Iwanami A, Funakoshi T, et al. Multifocal primary melanoma of the cervical spinal cord successfully treated by tumorectomy: a case report. Spinal Cord Ser Cases. 2018;4:24. Published 2018 Mar 21. [CrossRef]
- Fuld AD, Speck ME, Harris BT, et al. Primary melanoma of the spinal cord: a case report, molecular footprint, and review of the literature. J Clin Oncol. 2011;29(17):e499-e502. [CrossRef]
- Kwon SC, Rhim SC, Lee DH, Roh SW, Kang SK. Primary malignant melanoma of the cervical spinal nerve root. Yonsei Med J. 2004;45(2):345-348. [CrossRef]
- Marx S, Fleck SK, Manwaring J, Vogelgesang S, Langner S, Schroeder HW. Primary leptomeningeal melanoma of the cervical spine mimicking a meningioma-a case report. J Neurol Surg Rep. 2014;75(1):e93-e97. [CrossRef]
- Ozden B, Barlas O, Hacihanefioğlu U. Primary dural melanomas: report of two cases and review of the literature. Neurosurgery. 1984;15(1):104-107. [CrossRef]
- Shi YF, Chen YQ, Chen HF, Hu X. An atypical primary malignant melanoma arising from the cervical nerve root: A case report and review of literture. World J Clin Cases. 2022;10(1):381-387. [CrossRef]
- Lee CH, Moon KY, Chung CK, et al. Primary intradural extramedullary melanoma of the cervical spinal cord: case report. Spine (Phila Pa 1976). 2010;35(8):E303-E307. [CrossRef]
- Valenzuela F, Desai S. Intradural Extramedullary Primary Central Nervous System Melanoma of the Craniovertebral Junction during Pregnancy: Observations and Outcomes. Surg Neurol Int. 2021;12:198. Published 2021 May 3. [CrossRef]
- Beul H, Skomorac R, Jusi A, et al. A rare case of primary extramedullary intradural and extradural malignant melanoma of cervical spine. Medeniyet Medical Journal. Published online 2015. [CrossRef]
- Mahajan A, Jalali R. Primary Cervicomedullary Junction Melanocytic Melanoma: An Illustrated Case Report. J Glob Oncol. 2016;3(2):177-179. Published 2016 Jul 6. [CrossRef]
- Kanatas AN, Bullock MD, Pal D, Chakrabarty A, Chumas P. Intradural extramedullary primary malignant melanoma radiographically mimicking a neurofibroma. Br J Neurosurg. 2007;21(1):39-40. [CrossRef]
- Bidziński J, Kroh H, Leszczyk C, Bojarski P. Primary intraspinal cervical melanoma. Acta Neurochir (Wien). 2000;142(9):1069-1070. [CrossRef]
- Lee NK, Lee BH, Hwang YJ, et al. Findings from CT, MRI, and PET/CT of a primary malignant melanoma arising in a spinal nerve root. Eur Spine J. 2010;19 Suppl 2(Suppl 2):S174-S178. [CrossRef]
- Skarli SO, Wolf AL, Kristt DA, Numaguchi Y. Melanoma arising in a cervical spinal nerve root: report of a case with a benign course and malignant features. Neurosurgery. 1994;34(3):533-637. [CrossRef]
- Chatterjee R, Nascimento FA, Heck KA, Ropper AE, Sabichi AL. Primary Spinal Cord Melanoma - An Uncommon Entity. Can J Neurol Sci. 2019;46(3):348-350. [CrossRef]
- Kounin GK, Romansky KV, Traykov LD, Shotekov PM, Stoilova DZ. Primary spinal melanoma with bilateral papilledema. Clin Neurol Neurosurg. 2005;107(6):525-527. [CrossRef]
- Kim MS, Yoon DH, Shin DA. Primary spinal cord melanoma. J Korean Neurosurg Soc. 2010;48(2):157-161. [CrossRef]
- Vaquero J, de Prado F, Pedrosa M. Primary intraparenchymatous spinal cord melanoma. Spinal Cord. 1998;36(5):363-365. [CrossRef]
- Wuerdeman M, Douglass S, Abda RB, Krasnokutsky M. A rare case of primary spinal cord melanoma. Radiol Case Rep. 2018;13(2):424-426. Published 2018 Feb 20. [CrossRef]
- Tuz Zahra F, Ajmal Z, Qian J, Wrzesinski S. Primary Intramedullary Spinal Melanoma: A Rare Disease of the Spinal Cord. Cureus. 2021;13(7):e16194. Published 2021 Jul 5. [CrossRef]
- Saleem N, Saleem R, Asghar H, Zubair M, Farooque U. Primary Spinal Melanoma With Intra- and Extradural Extensions: A Rare Case. Cureus. 2021;13(1):e12855. Published 2021 Jan 22. [CrossRef]
- Jeong DH, Lee CK, You NK, Kim SH, Cho KH. Primary spinal cord melanoma in thoracic spine with leptomeningeal dissemination and presenting hydrocephalus. Brain Tumor Res Treat. 2013;1(2):116-120. [CrossRef]
- Kolasa M, Jesionek-Kupnicka D, Kordek R, Kolasa P. Primary spinal cord melanoma - a case report. Folia Neuropathol. 2010;48(3):212-216.
- Corrêa DG, Dos Santos RQ, Hygino da Cruz LC Jr. Primary intramedullary malignant melanoma: can imaging lead to the correct diagnosis?. J Int Med Res. 2020;48(10):300060520966152. [CrossRef]
- Li YP, Zhang HZ, She L, et al. Primary extramedullary spinal melanoma mimicking spinal meningioma: A case report and literature review. Oncol Lett. 2014;8(1):339-344. [CrossRef]
- Jo KW, Kim SR, Kim SD, Park IS. Primary thoracic epidural melanoma : a case report. Asian Spine J. 2010;4(1):48-51. [CrossRef]
- Huang X, Pan X, Huang H, Zhan R. Multiple spinal cord melanoma: case report with emphasis on the difficult preoperative diagnosis. Turk Neurosurg. 2013;23(4):534-538. [CrossRef]
- Yadav YR, Tandon JK, Kriplani TC, Kapoor JP, Arora BM, Chandrakar S. Primary extradural spinal melanoma. Neurol India. 1995;43(3):161-164.
- Albastaki A, Ahmed S, Khan A, Farhan A, Almayman T. Malignant Melanoma Presenting as Spinal Cord and Pleural Lesions. Case Rep Oncol Med. 2023;2023:9647892. Published 2023 Feb 21. [CrossRef]
- Mallick S, Roy S, Joshi NP, Julka PK. Primary spinal melanoma treated with adjuvant radiotherapy and concurrent temozolomide: A case report and review of literature. J Cancer Res Ther. 2015;11(4):1027. [CrossRef]
- Hering K, Bresch A, Lobsien D, Mueller W, Kortmann RD, Seidel C. Primary Intradural Extramedullary Spinal Melanoma in the Lower Thoracic Spine. Case Rep Oncol Med. 2016;2016:3815280. [CrossRef]
- Katalinic D, Anic B, Stern-Padovan R, et al. Low back pain as the presenting sign in a patient with primary extradural melanoma of the thoracic spine--a metastatic disease 17 years after complete surgical resection. World J Surg Oncol. 2011;9:150. Published 2011 Nov 17. [CrossRef]
- Kawanabe Y, Ueda S, Sasaki N, Hoshimaru M. Incidental Detection of Primary Spinal Malignant Melanoma before Central Nervous System Dissemination. NMC Case Rep J. 2014;1(1):24-27. Published 2014 Jul 4. [CrossRef]
- Chance A, Liu JJ, Raskin JS, Zherebitskiy V, Gultekin SH, Raslan AM. Thoracic primary central nervous system melanoma and lumbar schwannoma of complex neurocristopathy: case report. J Neurosurg Spine. 2015;23(6):780-783. [CrossRef]
- Haberfellner E, Elbaroody M, Alkhamees AF, et al. Primary Spinal Melanoma: Case Report and Systematic Review [published online ahead of print, 2021 Apr 17]. Clin Neurol Neurosurg. 2021;205:106649. [CrossRef]
- Salame K, Merimsky O, Yosipov J, Reider-Groswasser I, Chaitchik S, Ouaknine GE. Primary intramedullary spinal melanoma: diagnostic and treatment problems. J Neurooncol. 1998;36(1):79-83. [CrossRef]
- Yamasaki T, Kikuchi H, Yamashita J, Asato R, Fujita M. Primary spinal intramedullary malignant melanoma: case report. Neurosurgery. 1989;25(1):117-121. [CrossRef]
- Majeed K, Hussain I, Pisapia D, et al. Intradural Intramedullary Primary Spinal Melanoma: A Case Report and Review of the Literature- Scientifi c Journal of Neurology & Neurosurgery. Scientifi c Journal of Neurology & Neurosurgery SCIRES Literature. 2019;5. Accessed September 13, 2023. https://www.sciresliterature.org/Neurology/SJNN-ID37.pdf.
- Chen F, Sun Y, Chen J, Chen B, Chen D. Case Report Intraspinal primary melanoma of intermediate grade: a case report and literature review. Int J Clin Exp Med. 2018;11(7):7471-7476. Accessed September 13, 2023. https://e-century.us/files/ijcem/11/7/ijcem0073850.pdf.
- Ganiüsmen O, Özer FD, Mete M, Özdemir N, Bayol Ü. Slow progression and benign course of a primary malign melanoma of a lumbar nerve root. Clin Neurol Neurosurg. 2012;114(2):166-168. [CrossRef]
- Nogueira RM, Cardoso LS, Fonseca L, et al. An uncommon intramedullary tumor: Primary medullary cone melanoma. Surg Neurol Int. 2020;11:200. Published 2020 Jul 18. [CrossRef]
- Yan L, Chang Z, Liu Y, He BR, Hao DJ. Primary spinal melanoma: a case report and literature review. Chin Med J (Engl). 2012;125(22):4138-4141.
- Naing A, Messina JL, Vrionis FR, Daud AI. Uncommon manifestations of common malignancies: case 3. Malignant melanoma arising from a spinal nerve root. J Clin Oncol. 2004;22(15):3194-3195. [CrossRef]
- Sharma A, Sinha VD. Primary Spinal Cord Melanoma of Intradural Extramedullary Origin. J Neurosci Rural Pract. 2019;10(3):522-525. [CrossRef]
- Sinha R, Rizvi TH, Chakraborti S, Ballal CK, Kumar A. Primary melanoma of the spinal cord: a case report. J Clin Diagn Res. 2013;7(6):1148-1149. [CrossRef]
- Cicuendez M, Paredes I, Munarriz PM, Hilario A, Cabello A, Lagares A. Primary melanoma of the cauda equina: Case report and review of the literature. Neurocirugia (Astur). 2012;23(3):112-115. [CrossRef]
- Chang W, Scarano A, Berg L. Primary leptomeningeal melanoma in an adolescent: Case report and review of the literature. Radiol Case Rep. 2015;8(3):857. Published 2015 Nov 6. [CrossRef]
- Sharma AD, Poddar J, Kunikullaya US, Neema JP. Primary malignant melanoma of the spinal cord: A case report. Journal of Cancer Metastasis and Treatment. 2016;2(4):144. [CrossRef]
- Golden MJ, Wheir WH, Katzman J, Turk ZA, Hartshorne MF. A case of primary leptomeningeal melanoma evaluated on FDG PET/CT. Clin Nucl Med. 2011;36(10):919-921. [CrossRef]
- Mohapatra A, Choudhury P. An Uncommon Case of Primary Leptomeningeal Melanoma in a 66-Year-Old White Caucasian Male. Cureus. 2020;12(10):e10793. Published 2020 Oct 4. [CrossRef]
- Zou C, Cheng W, Zhu C, Guo Q, Wu A. Primary Extradural Melanoma Arising in Cervical Spinal Nerve Root. World Neurosurg. 2018;111:211-215. [CrossRef]
- Wu L, Xu Y. Primary spinal intramedullary malignant melanoma involving the medulla oblongata. Spine J. 2016;16(8):e499-e500. [CrossRef]
- Cetinalp NE, Yildirim AE, Divanlioglu D, Belen D. An uncommon intramedullary tumor: primary spinal cord melanoma. Asian Spine J. 2014;8(4):512-515. [CrossRef]
- Farrokh D, Fransen P, Faverly D. MR findings of a primary intramedullary malignant melanoma: case report and literature review. AJNR Am J Neuroradiol. 2001;22(10):1864-1866.
- Sun LD, Chu X, Xu L, Fan XZ, Qian Y, Zuo DM. Primary intramedullary melanoma of lumbar spinal cord: A case report. World J Clin Cases. 2021;9(10):2352-2356. [CrossRef]
- Nakamae T, Kamei N, Tanaka N, et al. Primary Spinal Cord Melanoma: A Two-Case Report and Literature Review. Spine Surg Relat Res. 2022;6(6):717-720. Published 2022 Apr 12. [CrossRef]
- Trinh V, Medina-Flores R, Taylor CL, Yonas H, Chohan MO. Primary melanocytic tumors of the central nervous system: Report of two cases and review of literature. Surg Neurol Int. 2014;5:147. Published 2014 Oct 13. [CrossRef]
- House H, Archer J, Bradbury J. Primary spinal melanoma: illustrative case. J Neurosurg Case Lessons. 2021;2(20):CASE21542. Published 2021 Nov 15. [CrossRef]
- Bergdahl L, Boquist L, Liliequist B, Thulin CA, Tovi D. Primary malignant melanoma of the central nervous system. A report of 10 cases. Acta Neurochir (Wien). 1972;26(2):139-149. [CrossRef]
- Koenigsmann M, Jautzke G, Unger M, Théallier-Janko A, Wiegel T, Stoltenburg-Didinger G. June 2002: 57-year-old male with leptomeningeal and liver tumors. Brain Pathol. 2002;12(4):519-521. [CrossRef]
- Puyana C, Denyer S, Burch T, et al. Primary Malignant Melanoma of the Brain: A Population-Based Study. World Neurosurg. 2019;130: e1091-e1097. [CrossRef]
- Larson TC 3rd, Houser OW, Onofrio BM, Piepgras DG. Primary spinal melanoma. J Neurosurg. 1987;66(1):47-49. [CrossRef]
- Kim DH, Choi CY, Lee CH, Joo M. Primary Intracranial Leptomeningeal Melanomatosis. J Korean Neurosurg Soc. 2015;58(6):554-556. [CrossRef]
- Sinha, R., Rizvi, T. H., Chakraborti, S., Ballal, C. K., & Kumar, A. (2013). Primary melanoma of the spinal cord: a case report. Journal of clinical and diagnostic research: JCDR, 7(6), 1148–1149. [CrossRef]
- Ali Y, Rahme R, Moussa R, Abadjian G, Menassa-Moussa L, Samaha E. Multifocal meningeal melanocytoma: a new pathological entity or the result of leptomeningeal seeding? J Neurosurg. 2009;111(3):488-491. [CrossRef]
- Little J, Braniff K. Endometrial metastasis of primary malignant melanoma of the brain. BMJ Case Rep. 2018;2018: bcr2018224435. Published 2018 May 30. [CrossRef]
- Del Fiore P, Rastrelli M, Dall'Olmo L, Cavallin F, Cappellesso R, Vecchiato A, Buja A, Spina R, Parisi A, Mazzarotto R, Ferrazzi B, Grego A, Rotondi A, Benna C, Tropea S, Russano F, Filoni A, Bassetto F, Tos APD, Alaibac M, Rossi CR, Pigozzo J, Sileni VC, Mocellin S. Melanoma of Unknown Primary: Evaluation of the Characteristics, Treatment Strategies, Prognostic Factors in a Monocentric Retrospective Study. Front Oncol. 2021 Mar 5;11:627527. Erratum in: Front Oncol. 2021 Apr 14;11:686051. doi: 10.3389/fonc.2021.686051. [CrossRef] [PubMed] [PubMed Central]
- Long WL, Chen FY, Huang XL, Lu JX, Xu YN. Imaging and pathological diagnosis of primary intracranial malignant melanoma: A case report and literature review. Medicine (Baltimore). 2023 Feb 3;102(5):e32767. [CrossRef] [PubMed] [PubMed Central]
- Damadian R, Zaner K, Hor D, DiMaio T. Human tumors detected by nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1974;71(4):1471-1473. [CrossRef]
- Gomori JM, Grossman RI, Shields JA, Augsburger JJ, Joseph PM, DeSimeone D. Choroidal melanomas: correlation of NMR spectroscopy and MR imaging. Radiology. 1986;158(2):443-445. [CrossRef]
- Otero-Soto GA, Vidal-Anaya V, Labat EJ. Primary Brain Melanoma in a Pediatric Patient: A Case Report. Am J Case Rep. 2021;22: e926749. Published 2021 Mar 9. [CrossRef]
- Khanna A, Venteicher AS, Walcott BP, Kahle KT, Mordes DA, William CM,GhogawalaZ, Ogilvy CS. Glioblastoma mimicking an arteriovenous malformation. Front Neurol. 2013 Sep 30; 4:144. [CrossRef] [PubMed] [PubMed Central]
- Hoover JM, Bledsoe JM, Giannini C, Krauss WE. Intramedullary melanotic schwannoma. Rare Tumors. 2012 Jan 2;4(1):e3. [CrossRef] [PubMed] [PubMed Central]
- Khoo M, Pressney I, Hargunani R, Tirabosco R. Melanotic schwannoma: an 11-year case series. Skeletal Radiol. 2016 Jan;45(1):29-34. [CrossRef] [PubMed]
- Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long-term survival results on metastatic melanoma treatment. J Immunother Cancer. 2020 Oct;8(2):e000948. [CrossRef]
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P,Bedikian A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011 Apr 15;117(8):1687-96. [CrossRef] [PubMed]
- Ibáez J, Weil B, Ayala A, Jimenez A, Acedo C, Rodrigo I. Meningeal melanocytoma: case report and review of the literature. Histopathology. 1997;30(6):576-581. [CrossRef]
- Bittencourt FV, Marghoob AA, Kopf AW, Koenig KL, Bart RS. Large congenital melanocytic nevi and the risk for development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics. 2000;106(4):736-741. [CrossRef]
- Hoffman HJ, Freeman A. Primary malignant leptomeningeal melanoma in association with giant hairy nevi. J Neurosurg. 1967;26(1):62-71. [CrossRef]
- Zaal LH, Mooi WJ, Klip H, van der Horst CM. Risk of malignant transformation of congenital melanocytic nevi: a retrospective nationwide study from The Netherlands. Plast Reconstr Surg. 2005;116(7):1902-1909. [CrossRef]
- Arlant PA, Grunnet ML, Heilbrun MP. Primary malignant melanoma of the pineal region. Surg Neurol. 1977;7(3):121-123.
- Nishihara M, Sasayama T, Kondoh T, Tanaka K, Kohmura E, Kudo H. Long-term survival after surgical resection of primary spinal malignant melanoma. Neurol Med Chir (Tokyo). 2009;49(11):546-548. [CrossRef]
- Huang YM, Yeh KY, Chen PY, et al. Primary intracranial malignant melanomas in solitary type: a tertiary center experience. J Clin Neurosci. 2022; 101:37-46. [CrossRef]
- Madureira P, de Mello RA. BRAF and MEK gene rearrangements in melanoma: implications for targeted therapy. Mol Diagn Ther. 2014;18(3):285-291. [CrossRef]
- Martin-Liberal J, Márquez-Rodas I, Cerezuela-Fuentes P, Soria A, Garicano F, Medina J, García Galindo R, Oramas J, Luis Manzano J, Delgado M, Valdivia J, Sanchez P. Challenges and perspectives in the management of BRAF-mutated metastatic melanoma: Systemic treatment sequencing and brain metastases. Cancer Treat Rev. 2025 Jan 23;133:102886. Epub ahead of print. [CrossRef] [PubMed]
- Man W, Wang G. Incidence, Outcomes and Predictors of Primary Central Nervous System Melanoma: A SEER-Based Study. World Neurosurg. 2019 Sep;129:e782-e790. [CrossRef] [PubMed]
- Guo P, Wei X, Guo Z, Wu D. Clinicopathological features, current status, and progress of primary central nervous system melanoma diagnosis and treatment. Pigment Cell Melanoma Res. 2024 Mar;37(2):265-275. [CrossRef] [PubMed]
- Internò, V., Sergi, M. C., Metta, M. E., Guida, M., Trerotoli, P., Strippoli, S., Circelli, S., Porta, C., & Tucci, M. (2023). Melanoma Brain Metastases: A Retrospective Analysis of Prognostic Factors and Efficacy of Multimodal Therapies. Cancers, 15(5), 1542. [CrossRef]
- Amer MH. Bone metastases in melanoma: an analysis of 110 cases. Melanoma Res. 2014;24(1):48-51. [CrossRef] [PubMed]
- Fife Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978 Aug;42(2):660-8. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





