Submitted:
10 March 2025
Posted:
11 March 2025
You are already at the latest version
Abstract
Gallbladder cancer (GBC) is an aggressive malignancy with poor prognosis. Due to the generally asymptomatic course of the disease in its early stages, patients are most often diagnosed incidentally (iGBC), following cholecystectomy for presumed benign disease. Thorough staging to predict oncologic and technical resectability is mandatory. Robotic surgery has emerged as a promising approach for the treatment of complex hepatic malignancies, and it can be performed in almost all types of liver resections. However, due to limited data, the minimally invasive approach in GBC remains controversial. Positive regional lymph nodes (LNs) and therefore the quality of lymphadenectomy have been proven to be significant predictors of survival after surgery. This study aims to report our approach for regional lymphadenectomy and robotic liver resection (RLR) in patients with GBC or iGBC diagnosed after laparoscopic cholecystectomy (LC), focusing on the surgical technique, adequacy of regional lymphadenectomy and outcomes.
Keywords:
1. Introduction
2. Description of Surgical Techniques
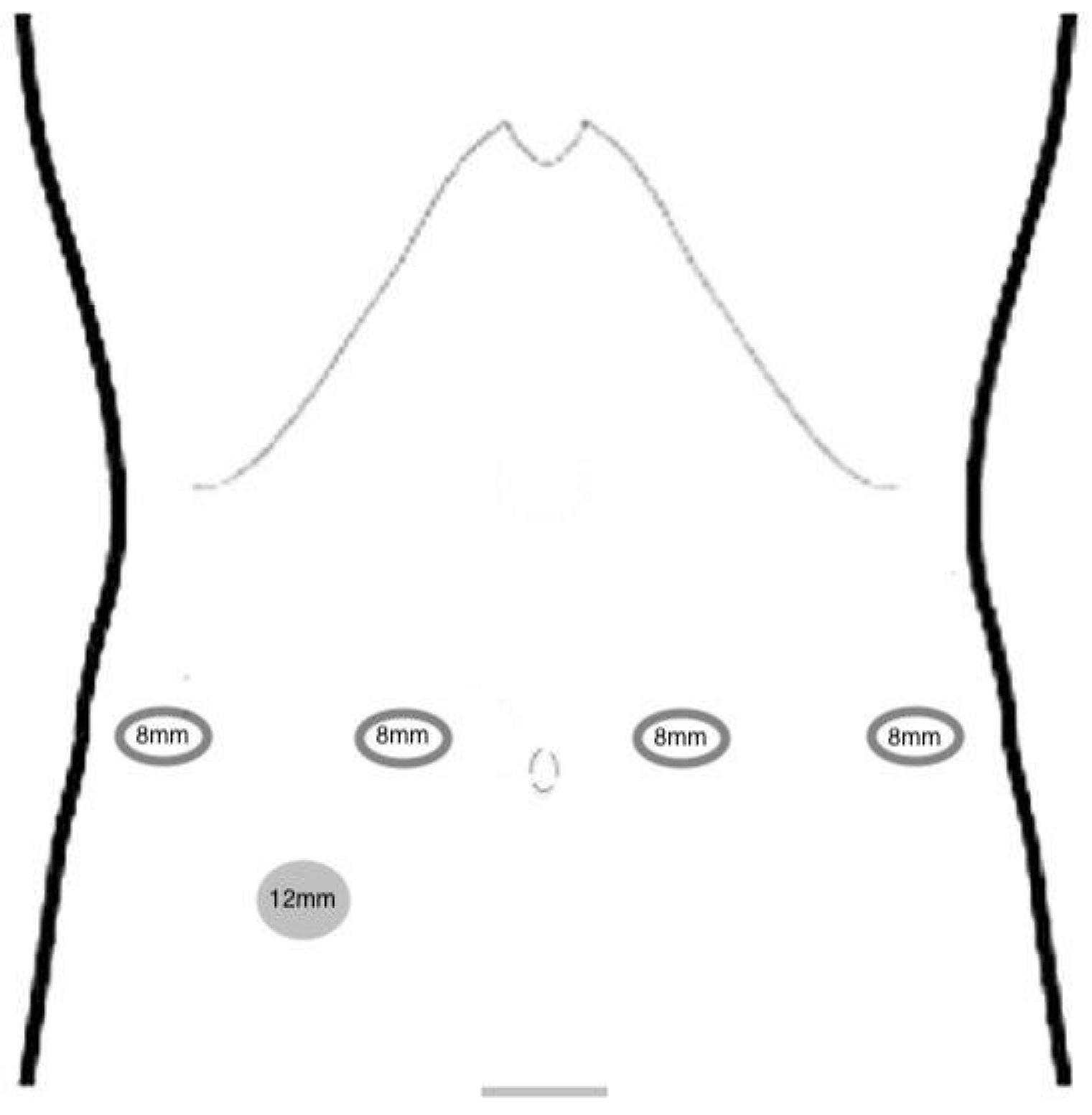
Patient Positioning and Port Placement
Robotic Instrumentation
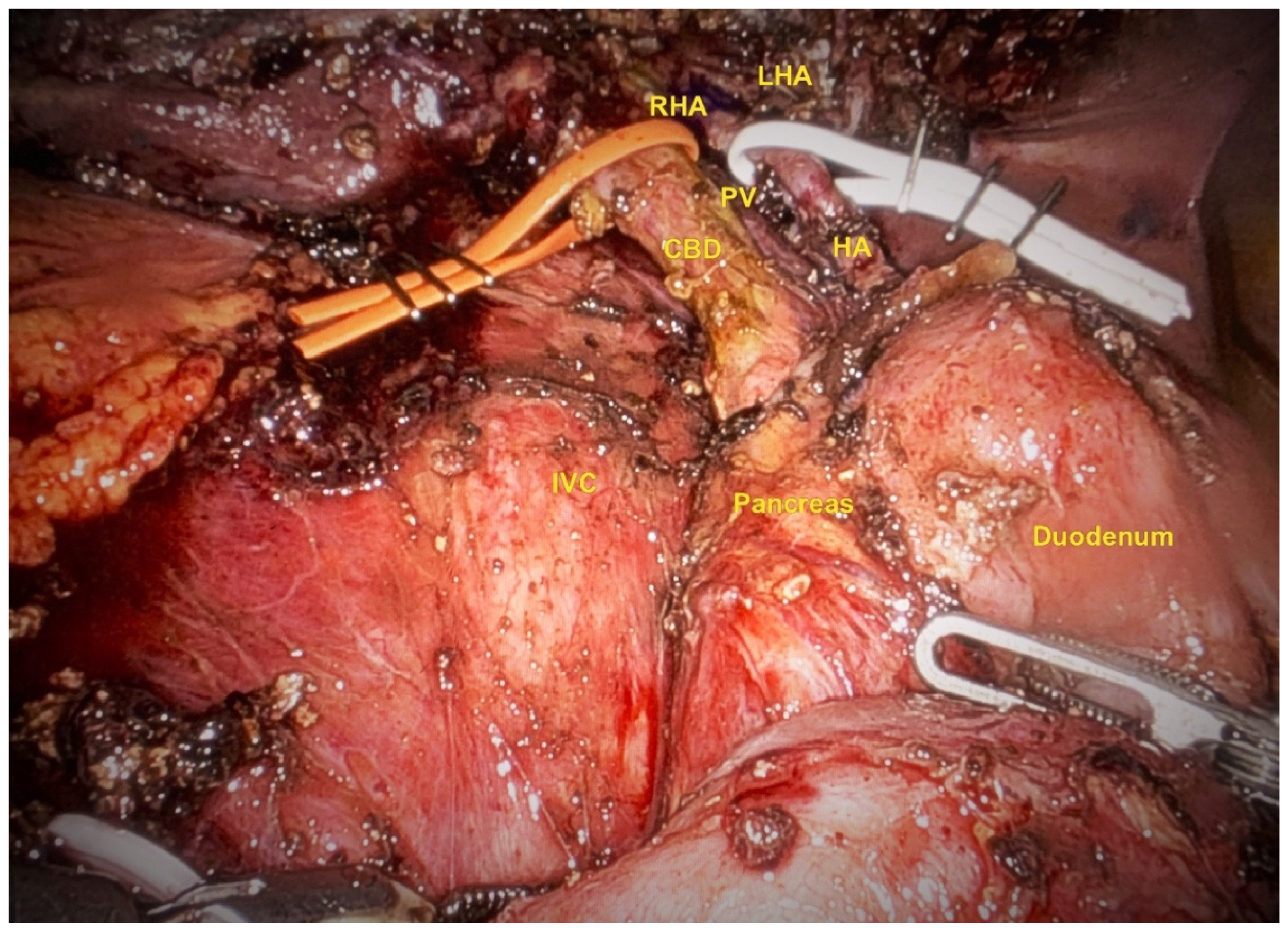
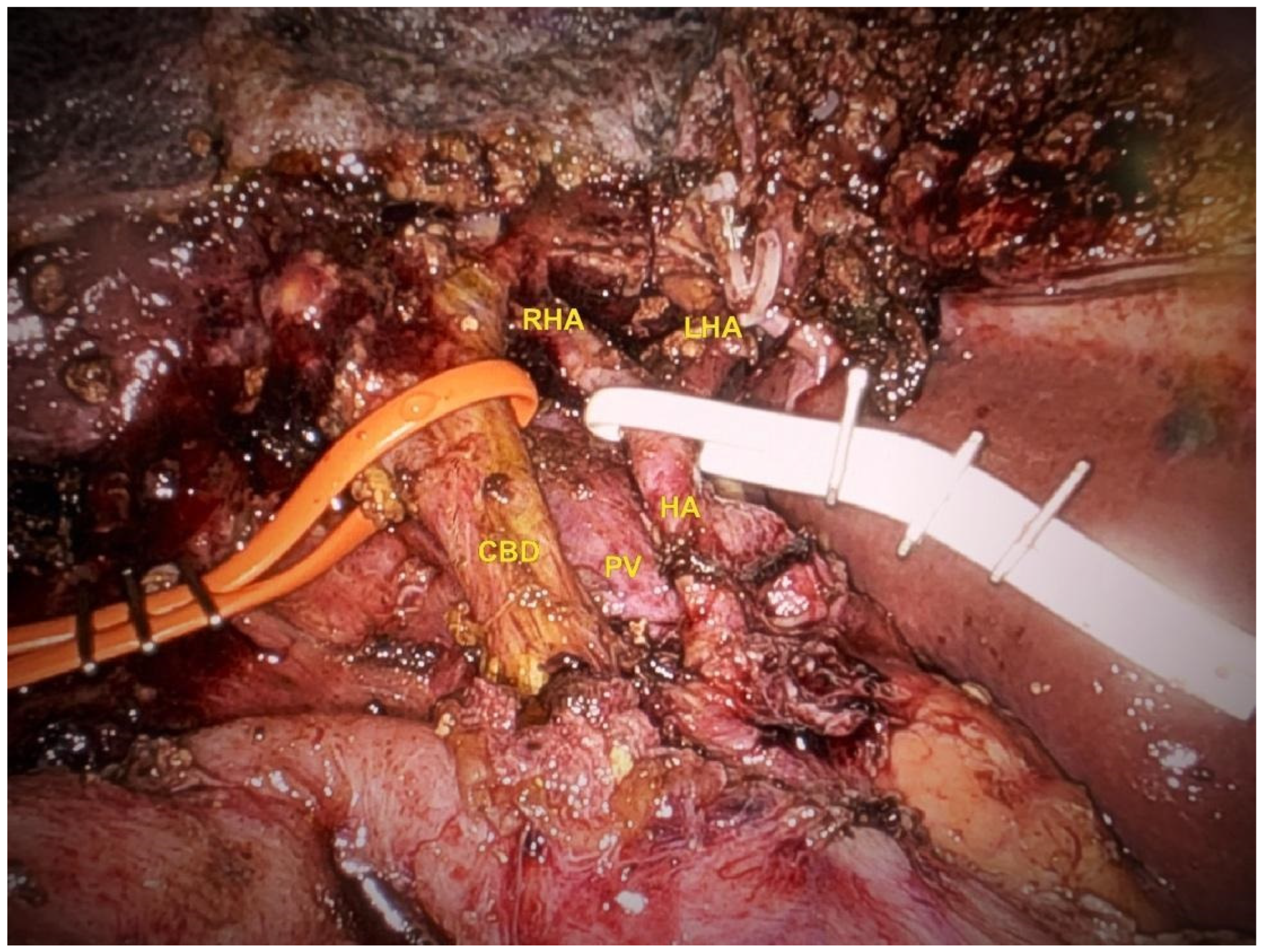
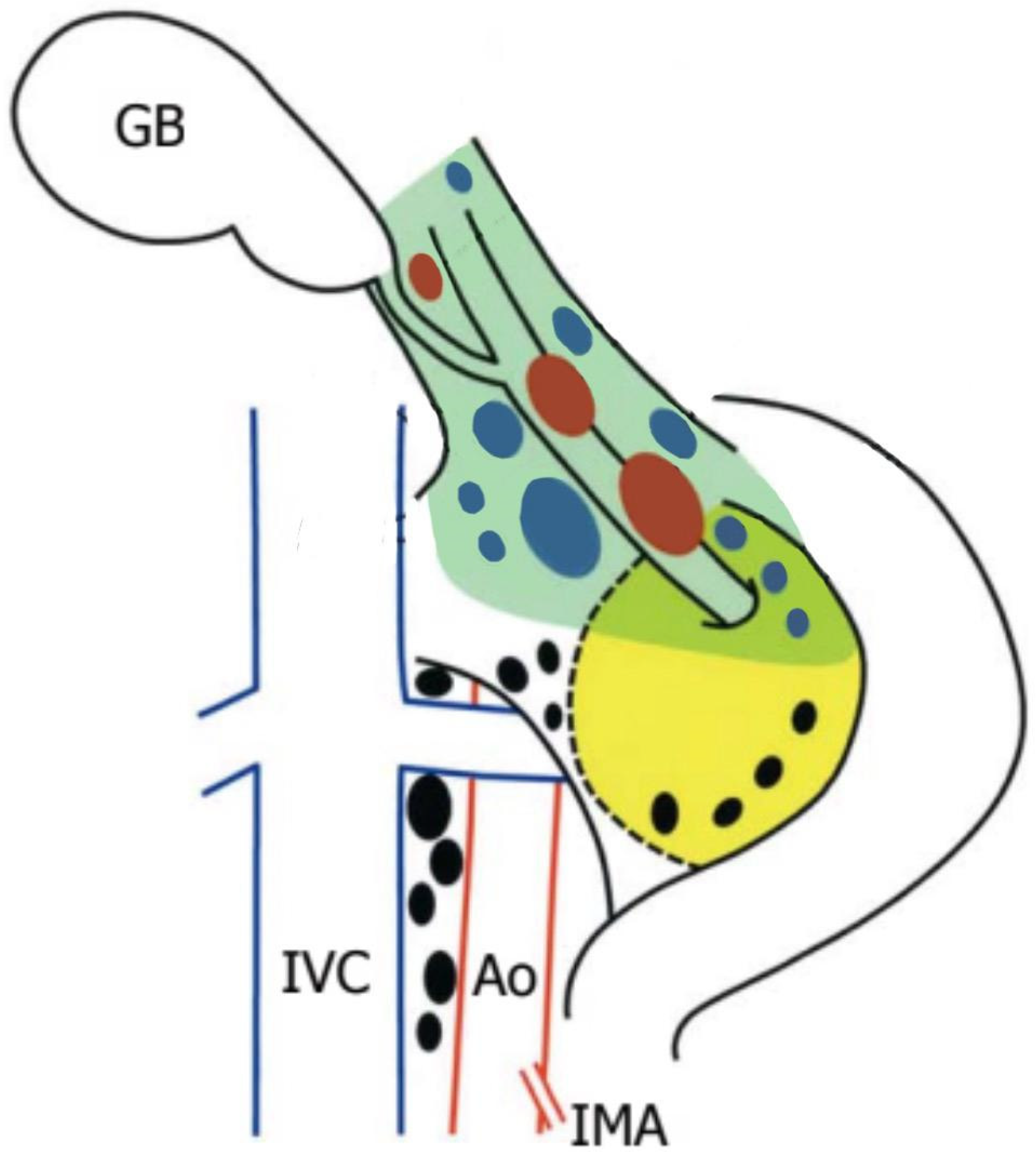
Lymphadenectomy and Hilar Dissection
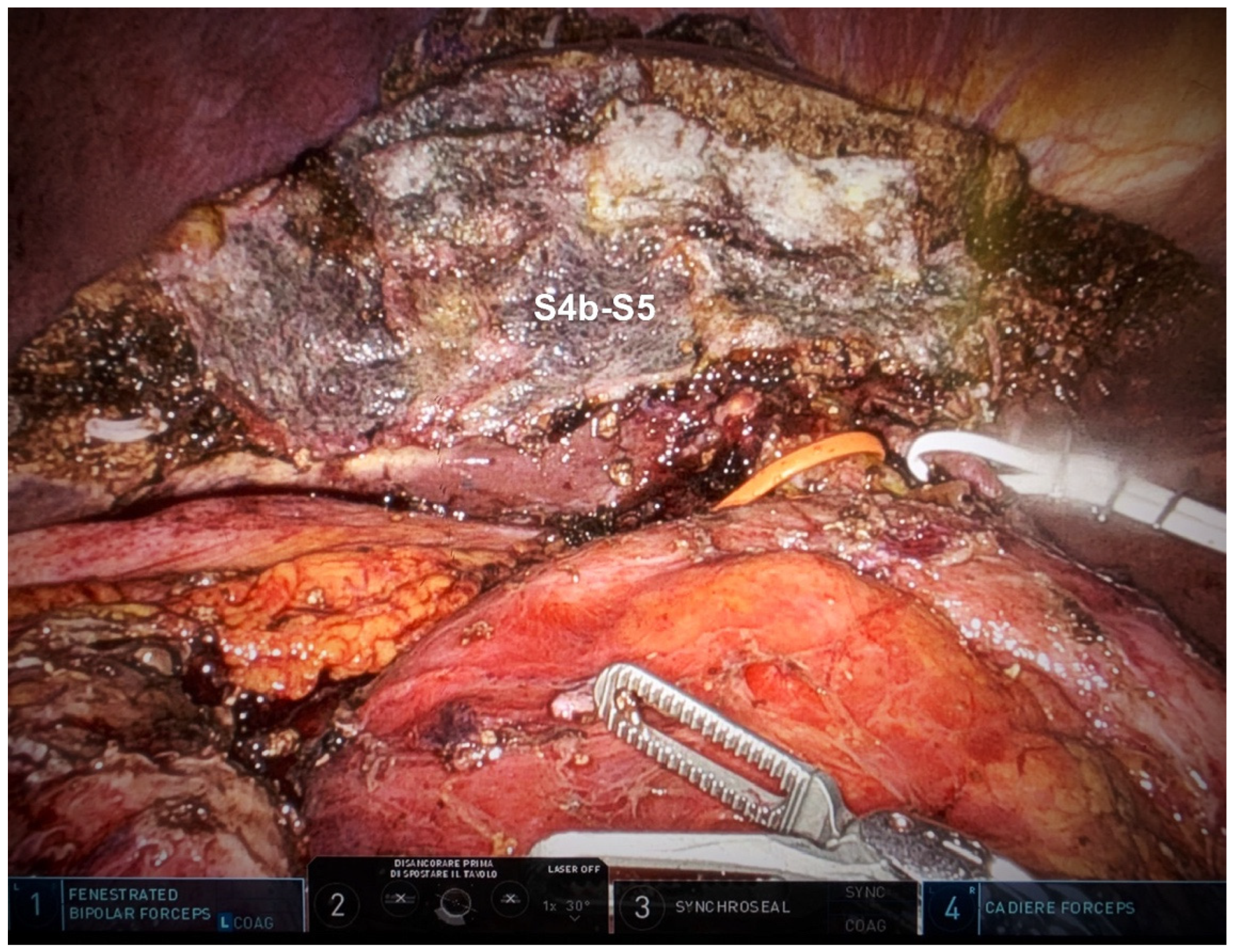
Hepatic Bisegmentectomy (S4b + S5)
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GBC | gallbladder cancer; |
| iGBC | incidentally gallbladder cancer; |
| RLR | robotic liver resection; |
| OLR | open liver resection; |
| LLR | laparoscopic liver resection; |
| LNs | lymph nodes; |
| LC | laparoscopic cholecystectomy; |
| CBD | common bile duct; |
| CHD | common hepatic duct. |
References
- Ielpo B, Vittoria d'Addetta M, Cremona S, Podda M, Di Martino M, Di Franco G et al. IRON: A retrospective international multicenter study on robotic versus laparoscopic versus open approach in gallbladder cancer. Surgery. 2024 Oct;176(4):1008-1015. [CrossRef]
- Singh BP, Khan WF, Rathore YS, Pol MM. Incidental Carcinoma Gallbladder: Incidence, Risk Factors, and Factors Affecting Survival-5-Year Experience from a Tertiary Care Institute. J Gastrointest Cancer. 2020 Sep;51(3):980-987. [CrossRef]
- de Savornin Lohman EAJ, van der Geest LG, de Bitter TJJ, Nagtegaal ID, van Laarhoven CJHM, van den Boezem P et al. Re-resection in Incidental Gallbladder Cancer: Survival and the Incidence of Residual Disease. Ann Surg Oncol. 2020 Apr;27(4):1132-1142. [CrossRef]
- Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015 Aug;17(8):681-90. [CrossRef]
- Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019 Mar 28;25(12):1432-1444. [CrossRef]
- Stewart C, Wong P, Warner S, Raoof M, Singh G, Fong Y, Melstrom L. Robotic minor hepatectomy: optimizing outcomes and cost of care. HPB (Oxford). 2021 May;23(5):700-706. [CrossRef]
- Sucandy I, Luberice K, Lippert T, Castro M, Krill E, Ross S, Rosemurgy A. Robotic Major Hepatectomy: An Institutional Experience and Clinical Outcomes. Ann Surg Oncol. 2020 Dec;27(13):4970-4979. [CrossRef]
- Sucandy I, Jacoby H, Crespo K, Syblis C, App S, Ignatius J et al. A Single Institution's Experience With Robotic Minor and Major Hepatectomy. Am Surg. 2023 May;89(5):1387-1391. [CrossRef]
- Broering DC, Elsheikh Y, Alnemary Y, Zidan A, Elsarawy A, Saleh Y et al. Robotic Versus Open Right Lobe Donor Hepatectomy for Adult Living Donor Liver Transplantation: A Propensity Score-Matched Analysis. Liver Transpl. 2020 Nov;26(11):1455-1464. [CrossRef]
- Rojas AE, Paterakos P, Choi SH. Robotic Central Bisectionectomy for Centrally Located Hepatic Malignant Tumor. Ann Surg Oncol. 2022 Mar 31. [CrossRef]
- Liu R, Abu Hilal M, Wakabayashi G, Han HS, Palanivelu C, Boggi U et al. International experts consensus guidelines on robotic liver resection in 2023. World J Gastroenterol. 2023 Aug 28;29(32):4815-4830. [CrossRef]
- Tran TB, Nissen NN. Surgery for gallbladder cancer in the US: a need for greater lymph node clearance. J Gastrointest Oncol. 2015 Oct;6(5):452-8. [CrossRef]
- Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008 Sep;207(3):371-82. [CrossRef]
- Widmann B, Warschkow R, Beutner U, Weitzendorfer M, Ukegjini K, Schmied BM et al. Effect of lymphadenectomy in curative gallbladder cancer treatment: a systematic review and meta-analysis. Langenbecks Arch Surg. 2020 Aug;405(5):573-584. [CrossRef]
- Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg. 2010 Apr;14(4):668-78. [CrossRef]
- Vega EA, Newhook TE, Mellado S, Ruzzenente A, Okuno M, De Bellis M et al. Benchmarks and Geographic Differences in Gallbladder Cancer Surgery: An International Multicenter Study. Ann Surg Oncol. 2023 Aug;30(8):4904-4911. [CrossRef]
- Chun, Y.S., Pawlik, T.M. & Vauthey, JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 25, 845–847 (2018). [CrossRef]
- Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012 Jun 14;18(22):2775-83. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




