1. Introduction
Although many inner ear diseases are common and significantly impact daily life, their pathophysiology remains largely unknown. Neurobiological research on the inner ear is challenging due to its complex tissue structure and anatomy. To date, cell, tissue, and organ cultures have been used in inner ear research. However, cell and tissue cultures have the disadvantage of being detached from their physiological environment. In contrast, organ cultures preserve the entire organ and maintain its polarized fluid environment.
While organ cultures of the inner ear had been performed previously, 3D organ culture was a significant challenge until 2008 when Hahn et al. succeeded using a rotating bioreactor system[
1,
2,
3]. This method preserved the three-dimensional structure and unique fluid conditions of the cochlea by opening the bony labyrinth at the basal and apical turns, allowing the preservation of inner and outer hair cells, stereocilia bundles, and the integrity of the reticular lamina[
3]. This created an in vitro model of the cochlea with conditions close to those in vivo.
Due to the accessibility and availability of rodent cochleae, various animal models have been established. However, whole organ cochlea culture has so far been limited to early postnatal animals[
3,
4,
5]. Previous studies using cell cultures have shown differences in regeneration and repair between early postnatal and adult mammalian cochleae[
6]. Therefore, research using whole organ cochlea cultures should extend beyond early postnatal organs.
As noted, whole organ cochlea cultures are challenging. Optimizing factors such as temperature, the speed of dissection, and the addition of neurotrophins may improve the process and enable the culturing of older animals. Currently, cochlea organ culture is performed at 37°C, which represents body temperature. However, studies have shown that mild hypothermia can provide otoprotection by slowing metabolism, reducing oxidative stress, and involving cold shock proteins[
7]. These findings could enhance the structure and survival of cochlea organ cultures.
Preparation time is also crucial since inner ear hair cells remain viable for only a short period under hypoxic conditions[
8]. Rapid cochlea dissection is essential to preserve hair cells in organ cultures. Additionally, modifying the culture medium, such as adding neurotrophins, could improve cochlea culture survival. Neurotrophins, which play roles in cell proliferation, differentiation, axonal outgrowth, and apoptosis, could reduce spiral ganglion cell degeneration, as demonstrated by Ramekers et al. in 2012[
9]. Specifically, NT-3 and BDNF are important in murine cochlea development, so their potential to improve culture conditions needs further investigation.
This study aimed to identify factors that enhance whole cochlea culture conditions, with a focus on preparation time, culture temperature, and neurotrophic factor concentrations.
2. Results
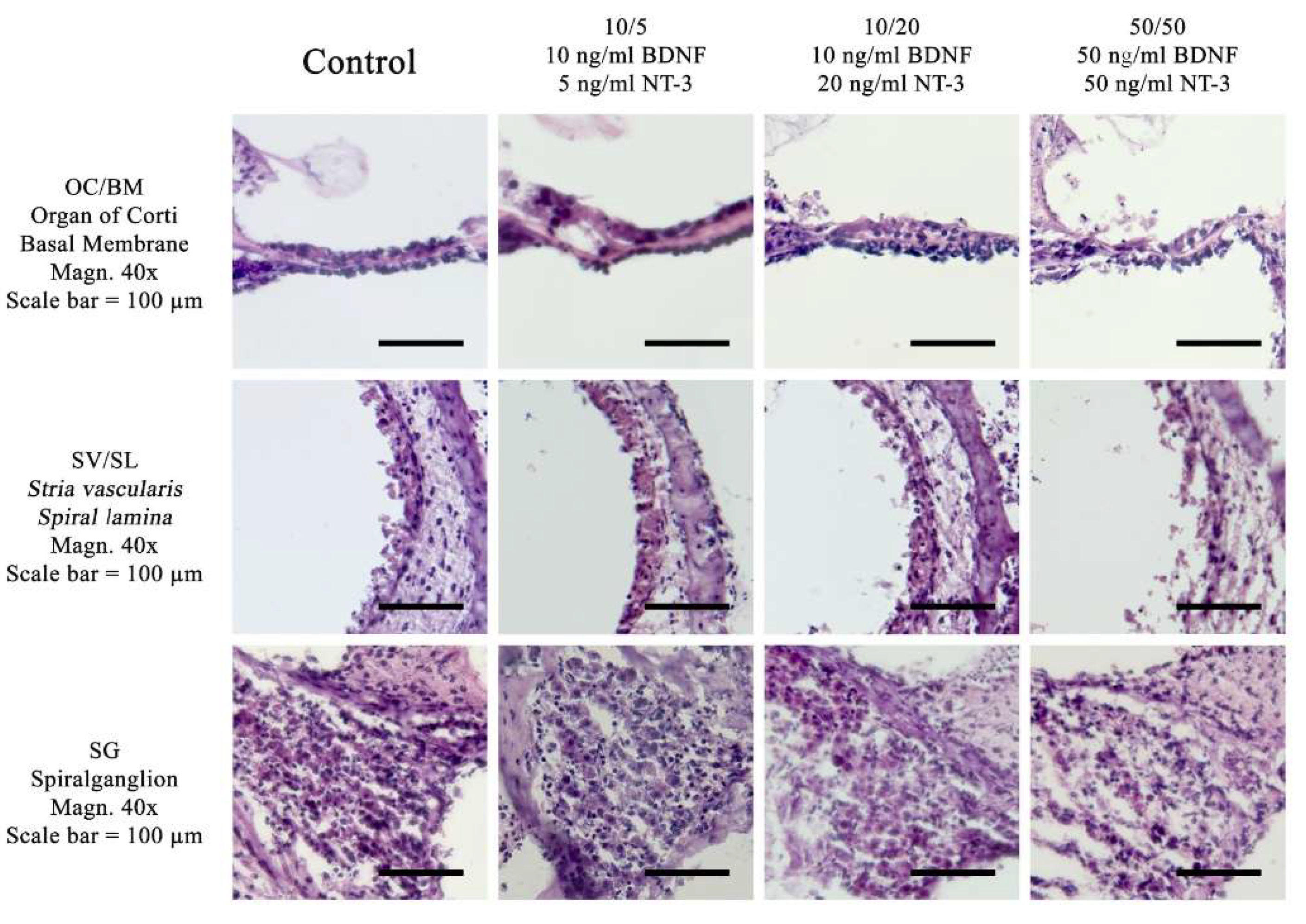
Cell and structural preservation of the cochlea in various culture conditions were evaluated. Cultures were performed in either normothermic conditions (37°C) or mild hypothermic conditions (32°C). Furthermore, a comparison of different culture media with different concentrations of added BDNF and NT-3 was performed. With the help of HE-stains
Figure 1 shows a comparison of the different culture media at 37°C and
Figure 2 at 32°C.
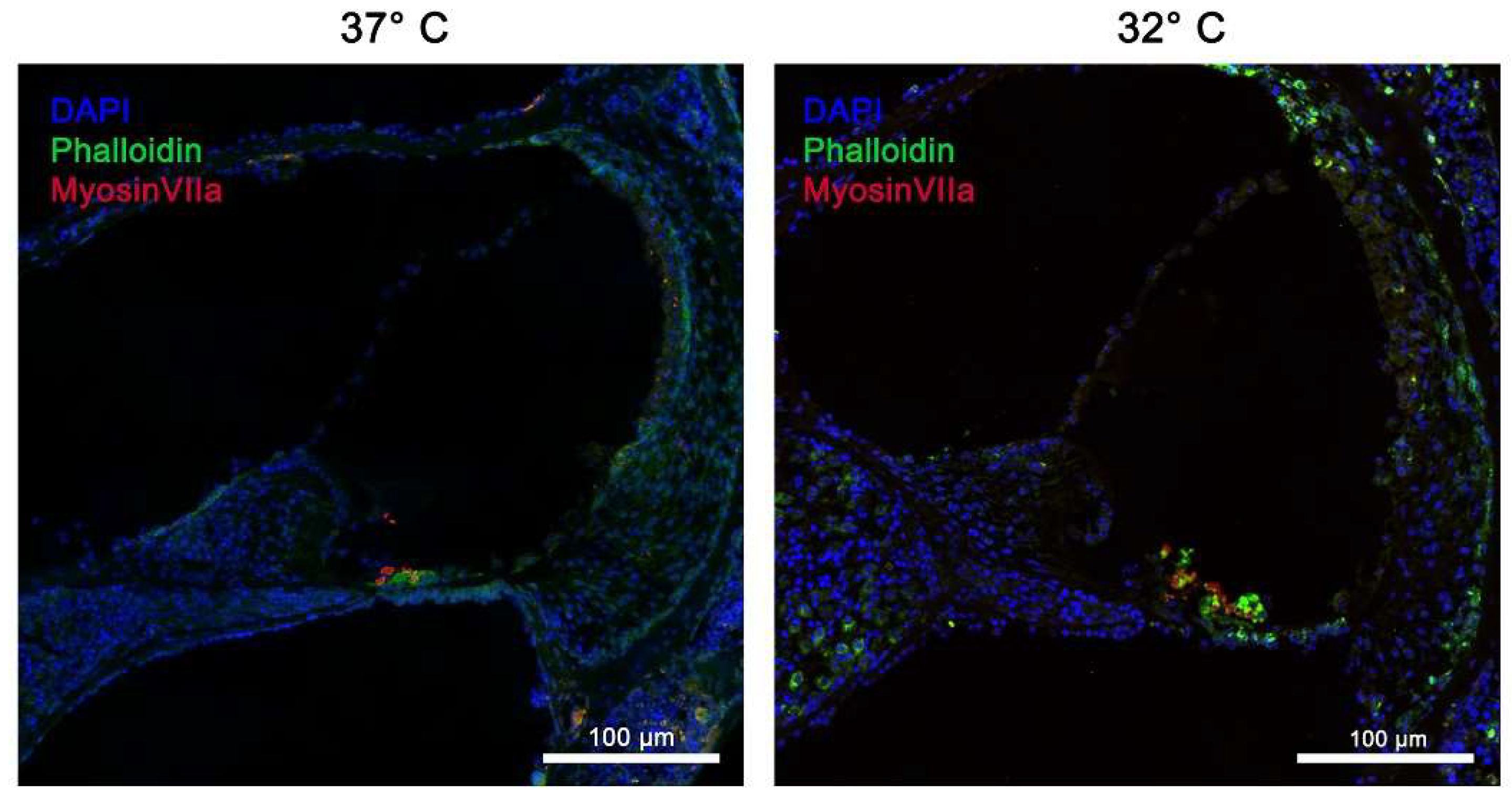
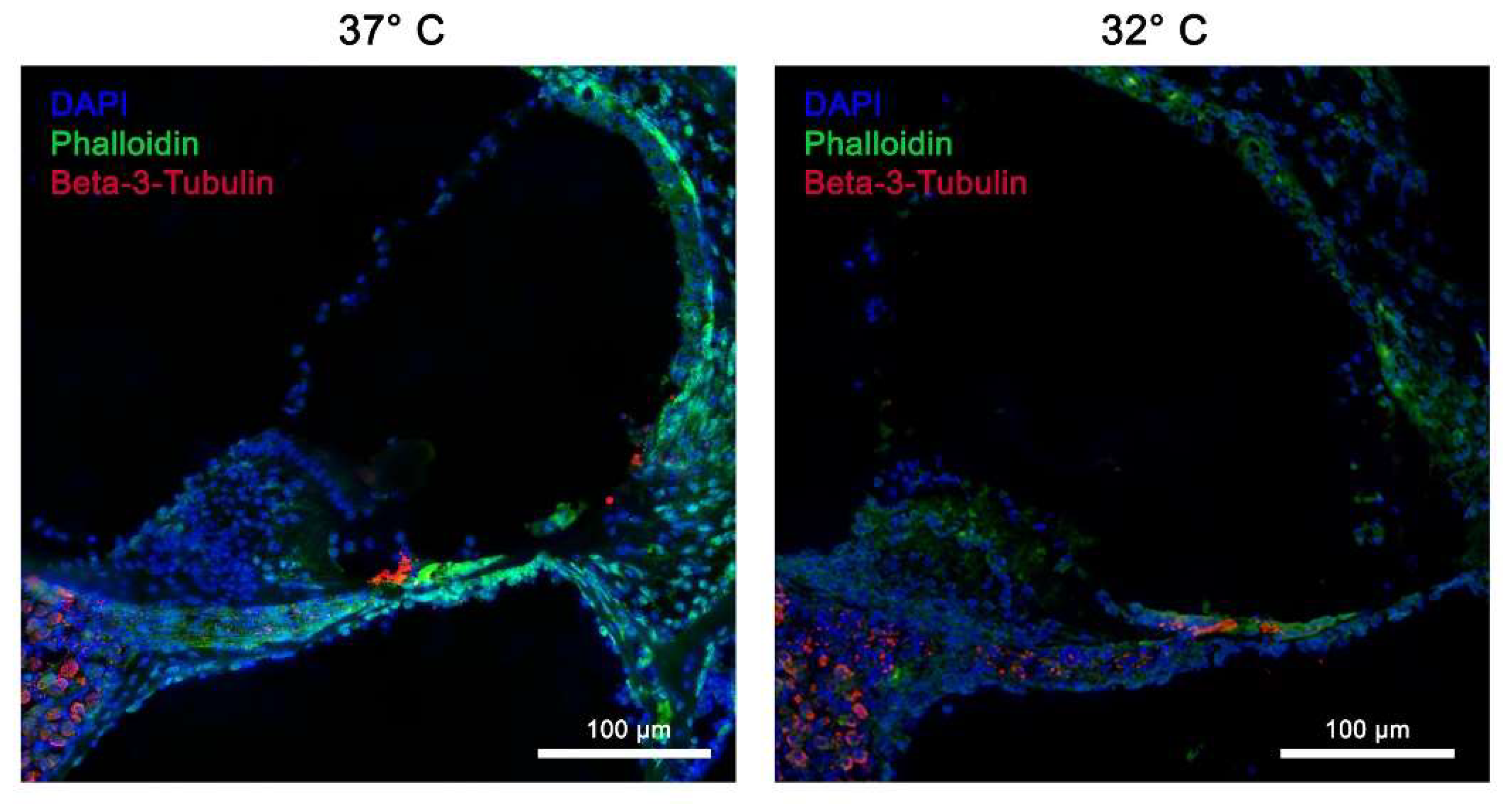
In addition to the HE-stains, fluorescent immunohistochemistry was performed to further examine the integrity of the cochleae. Immunofluorescence staining was performed to visualize Phalloidin, Myosin VIIa and ß-III-tubulin. In particular, cell cultures performed at 32°C showed increased fluorescence of Phalloidin and therefore a better-preserved organization of the cytoskeleton in contrast to the culture at 37°C. Myosin VIIa and ß-III-tubulin could be detected at both 32°C and 37°C. However, the intensified staining was seen at 32°C as seen in
Figure 3 and 4.
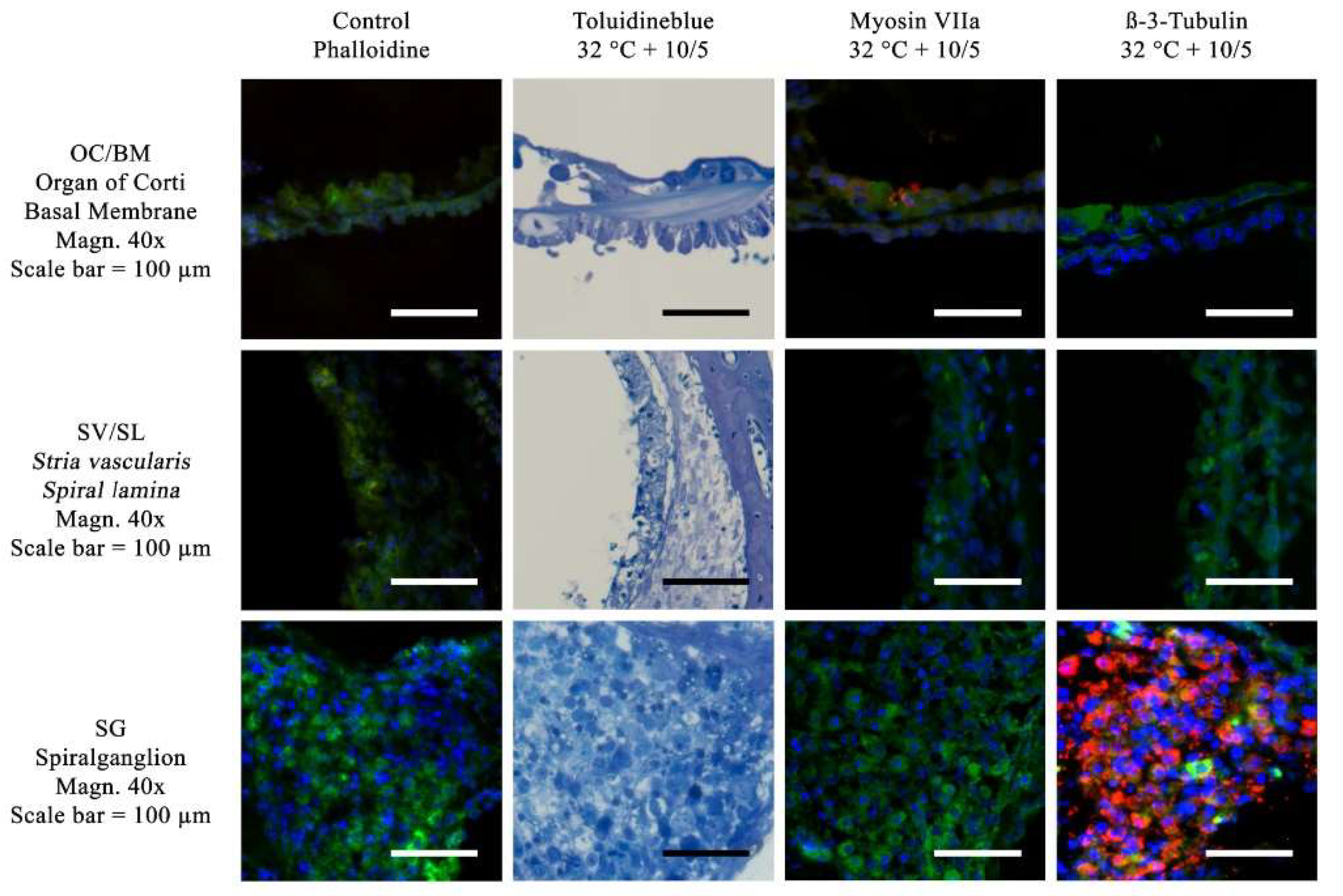
Figure 5 presents the results of immunofluorescence staining pictorially.
Figure 3.
Structural preservation of inner ear tissue using Myosin VIIa marker at simulated physiological body temperature at 37 °C vs. simulated mild hypothermia at 32 °C in Rotary Culture using a 10/5 concentration (10 ng/ml BDNF and 5 ng/ml NT-3). Inner hair cells (IHC) and outer hair cells (OHC) are well preserved and indicated in red; blue = Dapi stain of cell nuclei, green = Phalloidin (FITC) tissue fibers and connective tissue; Magnification = 40x, Scalebar = 100 μm.
Figure 3.
Structural preservation of inner ear tissue using Myosin VIIa marker at simulated physiological body temperature at 37 °C vs. simulated mild hypothermia at 32 °C in Rotary Culture using a 10/5 concentration (10 ng/ml BDNF and 5 ng/ml NT-3). Inner hair cells (IHC) and outer hair cells (OHC) are well preserved and indicated in red; blue = Dapi stain of cell nuclei, green = Phalloidin (FITC) tissue fibers and connective tissue; Magnification = 40x, Scalebar = 100 μm.
Figure 4.
Structural preservation of inner ear tissue using ß-III-tubulin marker at simulated physiological body temperature at 37 °C vs. simulated mild hypothermia at 32 °C in Rotary Culture using a 10/5 concentration (10 ng/ml BDNF and 5 ng/ml NT-3). Nerve fibers, stained with ß-IIItubulin are clearly visible and directly connected to the spiral ganglion; all nerve fibers and Ganglia cells are stained in red; blue = DAPI stain of cell nuclei, green = Phalloidin (FITC) tissue fibers and connective tissue; Inner hair cells (IHC) and outer hair cells (OHC) are well preserved and stained with Phalloidin; Magnification = 40x, Scalebar = 100 μm.
Figure 4.
Structural preservation of inner ear tissue using ß-III-tubulin marker at simulated physiological body temperature at 37 °C vs. simulated mild hypothermia at 32 °C in Rotary Culture using a 10/5 concentration (10 ng/ml BDNF and 5 ng/ml NT-3). Nerve fibers, stained with ß-IIItubulin are clearly visible and directly connected to the spiral ganglion; all nerve fibers and Ganglia cells are stained in red; blue = DAPI stain of cell nuclei, green = Phalloidin (FITC) tissue fibers and connective tissue; Inner hair cells (IHC) and outer hair cells (OHC) are well preserved and stained with Phalloidin; Magnification = 40x, Scalebar = 100 μm.
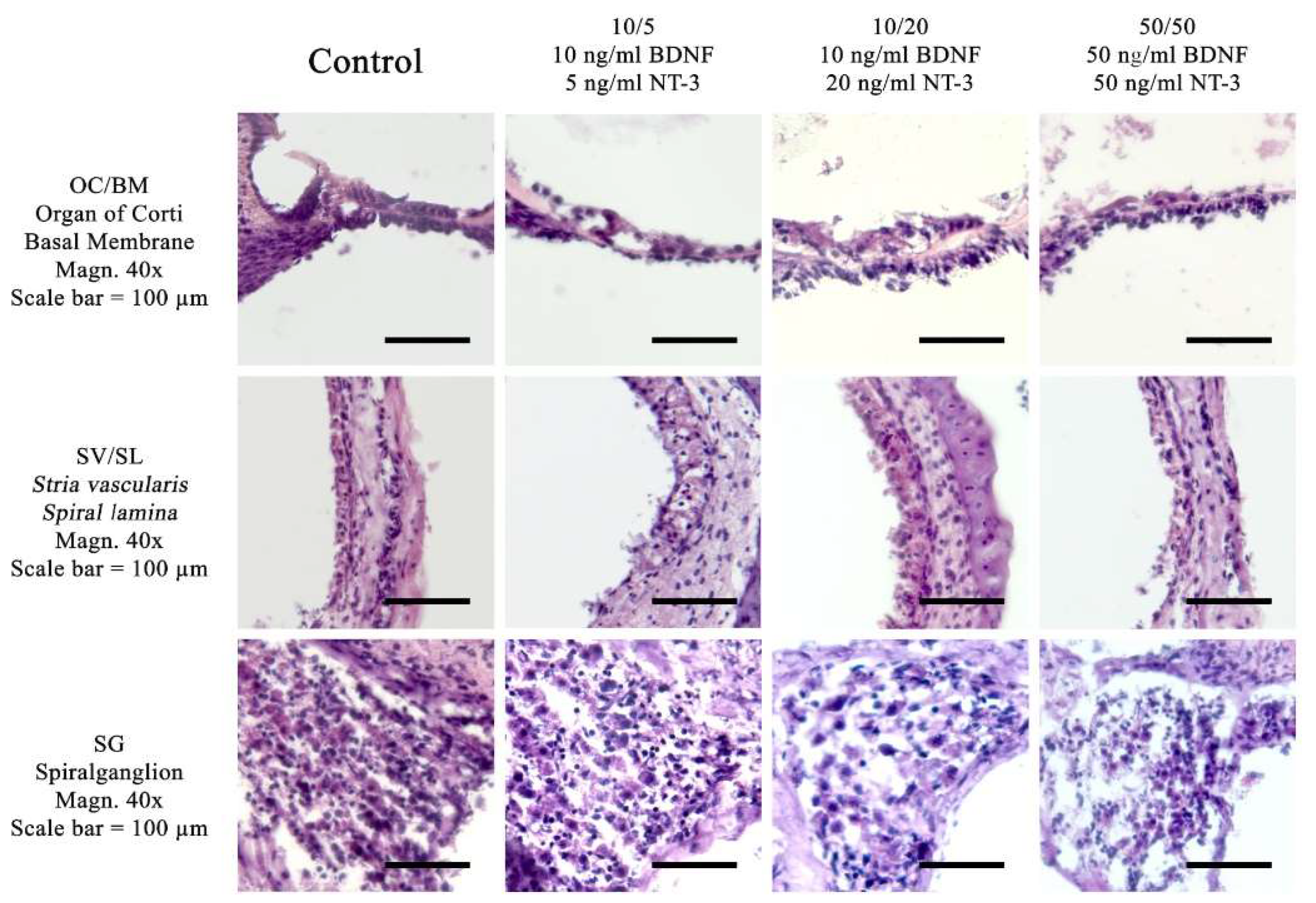
Figure 5.
Visualization of the Toluidine blue stain and immunofluorescence stains for ß-III-tubulin and Myosin VIIa. Toluidin blue and immunohistochemical stainings have been performed using a culture medium with 10ng/ml BDNF and 5 ng/ml NT-3. No suppliments have been added to the treated control group. The following three anatomical structures are compared: OC/BM (Organ of Corti and Basal Membrane), SV/SL (Stria vascularis and Spiral lamina) as well as SG (spiral ganglion), Magnification = 40x, Scalebar = 100 μm. The red Myosin VIIa labeling in the OC/BM slide indicates a survival of the hair cells. The ß-III-tubulin staining is positive in the spiral ganglion cells in the SG slide.
Figure 5.
Visualization of the Toluidine blue stain and immunofluorescence stains for ß-III-tubulin and Myosin VIIa. Toluidin blue and immunohistochemical stainings have been performed using a culture medium with 10ng/ml BDNF and 5 ng/ml NT-3. No suppliments have been added to the treated control group. The following three anatomical structures are compared: OC/BM (Organ of Corti and Basal Membrane), SV/SL (Stria vascularis and Spiral lamina) as well as SG (spiral ganglion), Magnification = 40x, Scalebar = 100 μm. The red Myosin VIIa labeling in the OC/BM slide indicates a survival of the hair cells. The ß-III-tubulin staining is positive in the spiral ganglion cells in the SG slide.
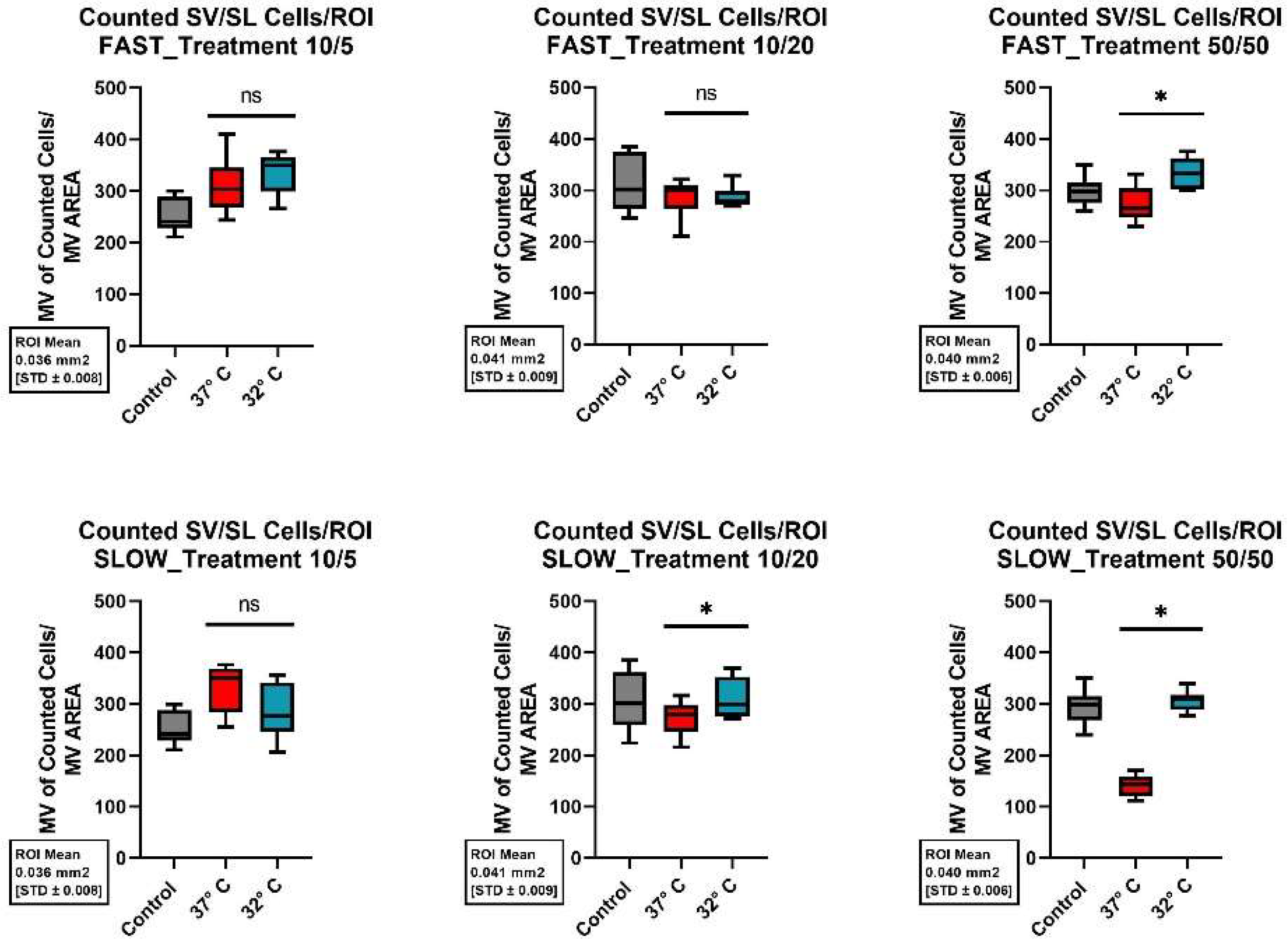
In addition, quantitative evaluation of cell survival sorted by different areas of the cochlea under different culture conditions was performed. The different areas were: SPG, OC, and SV/SL.
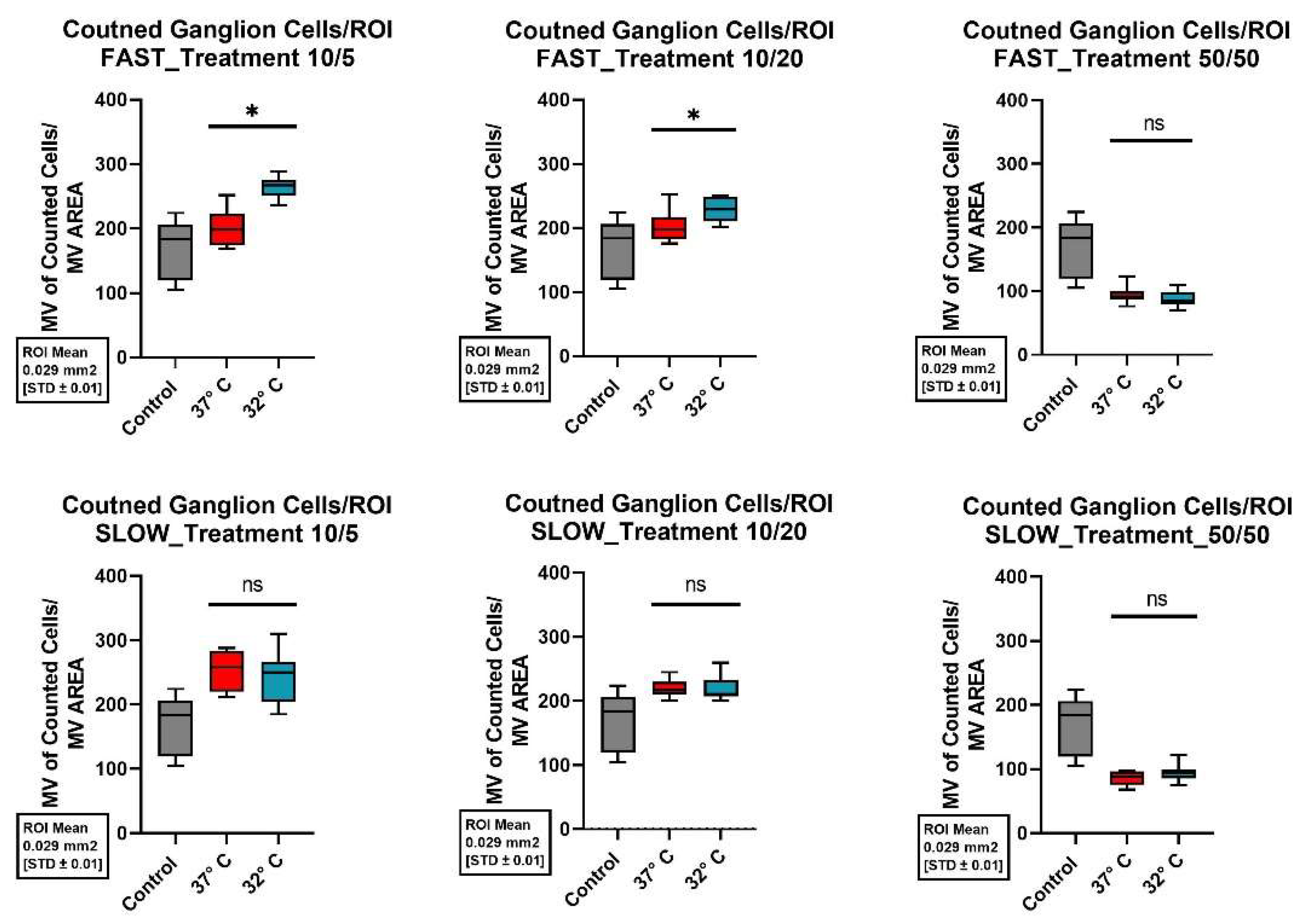
2.1. Spiral Ganglion
In the investigated area of the SPG, the control group (37°C, no neurotrophins) showed an average cell maintenance of 170.57 ±45.4 (Standard deviation, SD) cells per investigated unit. Compared with the control group, both the addition of neurotrophins at the 10/5 concentration (10ng/dL BDNF and 5 ng/dL NT-3) and the 10/20 concentration (10ng/ml BDNF and 20ng/ml NT-3) showed a statistically significant improvement in mean cell survival when combined with a fast dissected cochlea and mild hypothermic culture conditions at 32°C (p-values <0,001 and 0,005). Using these modifications of the culture a mean of 263.50 ±15.94 (SD) cells per unit was preserved for the 10/5 concentration and 227.78 ±18.57 (SD) cells per unit for the 10/20 concentration. ß-III-tubulin immunofluorescence staining in
Figure 4 already showed significantly improved structural preservation of spiral ganglion cells at 32°.
Regardless of the culture temperature and the speed of the cochlea dissection the addition of neurotrophins at the 50/50 concentration (50 ng/ml BDNF and 50ng/ml NT-3) to the culture medium consistently resulted in a major decrease of cell survival when compared to the control group.
Figure 6 shows corresponding boxplots of cell survival of the different treatments in the spiral ganglion.
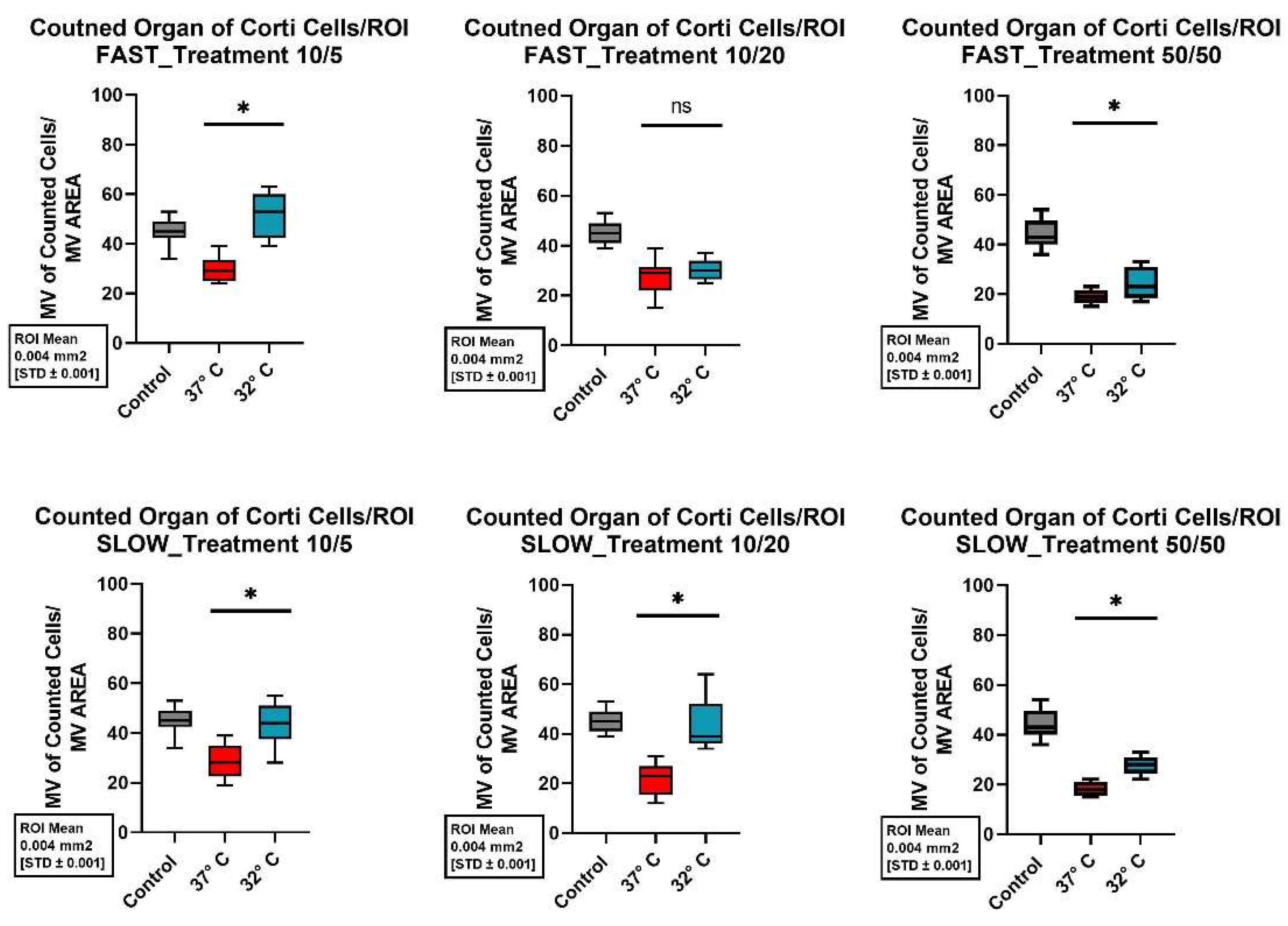
2.2. Organ of Corti
In the OC a mean of 45 ±5.41 (SD) cells per examinated unit was obtained in the control group (37°C, no neurotrophins). Only the combination of the fast dissected cochlea, the addition of 10ng/ml BDNF and 5ng/ml NT-3 and cultivation at 32°C resulted in a similarly good cell condition with preserving a mean of 51.89 ±8.92 (SD) cells per unit. Even if more cells were preserved with the corresponding modifications, there was no statistically significant difference.
Figure 7 shows an overview of the total cells obtained in the OC for the different cultures performed.
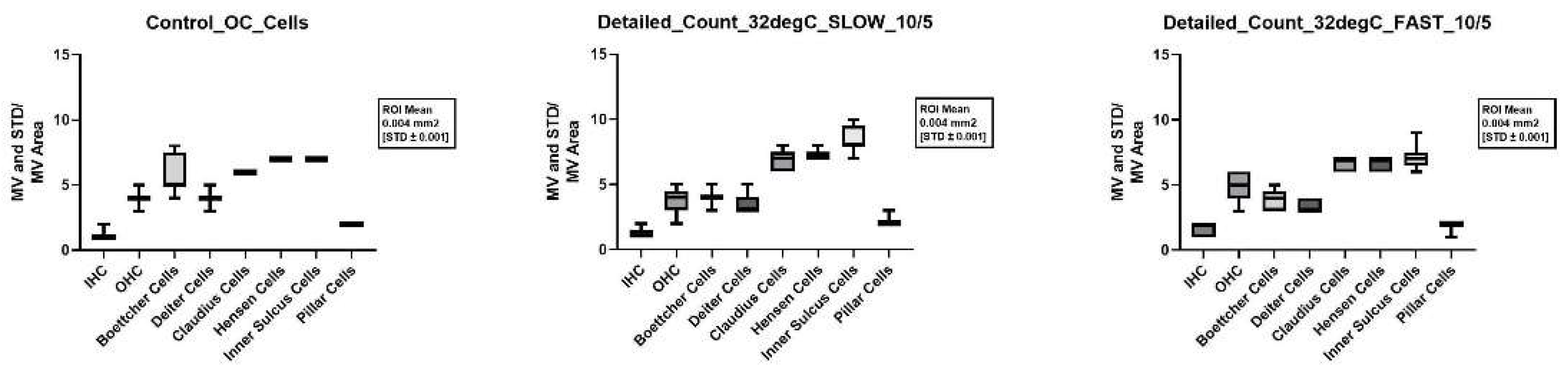
Nevertheless, when considering not only the total cells preserved, but the individual cell types, statistically significant better cell preservation of the inner and outer hair cells was seen when appropriately cultured with 10ng/ml BDNF and 5ng/ml NT-3 at 32°C after a fast cochlea dissection. The corresponding p-values in comparison with the control group were 0.015 for the inner hair cells and 0.042 for the outer hair cells.
Not only does the combination of all factors affect cell preservation of the hair cells, but the speed of the dissection alone also has an influence. Thus, with a fast dissection at the same neurotrophin concentration (10ng/ml BDNF and 5ng/ml NT-3), significantly more inner hair cells were preserved both at 37°C and at 32°C (p-value 0.036 and < 0.001). Fast dissection at 32°C with neurotrophins of the combination 10ng/ml BDNF plus 20ng/ml NT-3 and 50ng/ml BDNF plus 50ng/ml NT-3 showed a similar significance for the preservation of inner hair cells (p-value 0.001 and < 0.001). For the outer hair cells, the significance of a fast dissection was only observed when using neurotrophins at a concentration of 10ng/ml BDNF and 20ng/ml NT-3 and 32°C (p-value 0.004).
Figure 3 shows the better structural preservation of hair cells in an immunofluorescence staining. A hair cell marker for Myosin VIIa, which is exclusively found in hair cells was used.
2.3. Stria Vascularis/Spiral Ligament
For the cells of the SV/SL, the addition of BDNF and NT-3 at a concentration of 10/20 ng/ml and 50/50 ng/ml did not result in any significant change in cell preservation. However, the addition of 10ng/ml BDNF and 5ng/ml NT-3 generated statistically significantly better cell survival, especially when combined with mild hypothermia (32°C) and fast dissection of the cochlea (p-value <0.001).
Figure 8 graphically summarizes the changes in cell preservation due to the various culture modifications.
A comparison of the structural preservation in the different areas of the cochlea at different culture conditions showed that the combination of a fast cochlea dissection, adding 10 ng/ml BDNF and 5 ng/ml NT-3 to the culture medium, and culturing at 32°C achieved best cell survival in all areas of the cochlea. The combination of normothermia and a slow cochlea dissection results in worst structural preservation.
Figure 9 shows the results of the different factor combinations in image form.
3. Discussion
The combination of a fast dissection of the cochlea, hypothermic culture conditions at 32°C, and the addition of BDNF and NT-3 results in best structural preservation.
Rapid removal of the cochlea shows better structural preservation of the inner ear than a slow removal, although only a few minutes elapse in between. The death of the mouse terminates the blood flow towards the cochlea and the connection to the brain is divided. The resulting hypoxia starts the signal apoptosis cascade and progresses until the cochlea reaches the culture medium[
10]. The cellular changes due to hypoxia of the cochlea have been well studied[
10,
11,
12]. So, hypoxia leads to a decrease of inner and outer hair cells being equally sensitive. In addition to the lack of oxygen, the reduction of glucose has to be mentioned. The combination of glucose deprivation and oxygen deficiency further exacerbates the loss of inner and outer hair cells with hypoxia being the determining factor[
13]. This finding is in agreement with the results of this study. Under the same culture conditions, more hair cells could be preserved with fast dissection. Additionally, to hair cells, hypoxia initiates in a loss of spiral ganglia.
The extent of cellular changes are dependent on the duration of ischemia with longer ischemia leading to greater loss of structures[
10,
14]. Consequently, the shortest possible period from explantation, preparation and transfer to the culturing medium needs to be encouraged. The prompt removal of the cochlea has a positive impact on the microstructures of the inner ear and results in preservation of the ischemia sensitive hair cells and neuronal cells. This time is very dependent on the individual skill and routine of the examiner.
In addition to prevent ischemia, neurotrophins may contribute to the improved structural preservation. Neurotrophins have been studied extensively to prevent degeneration of auditory neurons in deafened animals and to promote the regrowth of nerve fibers after destruction: Various neurotrophic factors, such BDNF[
15,
16,
17,
18,
19,
20] and NT-3[
20,
21] have been investigated. In addition to BDNF and NT-3, other factors, such as NGF, Neurotrophin 4, Neurotrophin 5, ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) and members of the fibroblast growth factors (FGF) family are also known to play a role in the survival regulation of cochlear and vestibular cells[
22].
BDNF and NT-3 are both naturally expressed in the adult cochlea[
23,
24]. The cells in the basal turn are more susceptible to BDNF, while those in the apex are more susceptible to NT-3[
25]. This suggests the necessity of both neurotrophins to improve maintenance of the structures. Thus, both neurotrophins need be added. In the present study only the combined influence of BDNF and NT-3 were tested. Thus, results on the effects of the individual factors cannot be described. Vink et al. described the combination of BDNF and NT-3 to be superior in spiral ganglion cell preservation than treatment with BDNF or NT-3 alone[
26]. They also achieved better cell preservation of the spiral ganglion with BDNF therapy alone versus NT-3 therapy alone[
26].
The present study, evaluating the previously established whole cochlea organ culture procedure[
3], the addition of neurotrophic factors BDNF and NT-3 combined improved histopathological cell preservation. However, the dose of neurotrophins in the culture mediums seems to be crucial, as adding too high neurotrophin concentrations seem to have a negative effect on culture result. Significantly fewer structures were preserved under the addition of 50ng/ml BDNF and 50 ng/ml NT- 3 compared to cultures without neurotrophins. Although in other context, a negative effect due to overexpression of NT-3 has already been observed in previous studies[
27,
28]. Mild hypothermia was investigated as a third potentially culture improving factor. Previous studies have connected mild hypothermia to reduce the inflammatory immune response and decrease intracellular apoptosis. This mechanism could be linked with hearing preservation and improved hair cell survival after various ear traumas.[
7,
29,
30,
31,
32,
33]
The best protective effect of mild hypothermia during cultivation was reached in combination with a fast cochlea dissection and the addition of neurotrophins.
Considered individually a rapid dissection of the cochlea, the addition on neurotrophins and mild hypothermia each showed a positive effect on structural preservation of the inner ear organs. However, the combination of all three factors summed up to better structural preservation of all the different cell types of the inner ear in a 10 day old C57BL6/J model as shown in
Figure 5. Therefore, the combination of protective factors seems to be crucial. Since the combination of various positive factors with various control factors resulted in the same histopathological picture as shown in
Figure 9, it is not possible to say which one of these factors have a greater effect. An equivalence of the tested protective factors needs to be assumed.
The preparation time of the cochlea, the culture temperature, and the addition of neurotrophins to the culture medium show only a small effect in an organ culture of the cochlea when considered individually. The combination of these factors, however, represents a strong positive influence.
4. Materials and Methods
The study protocol was designed to optimize the prior described whole organ cochlea culture protocol[
3] regarding structure preservation and culturing age. It is aimed to optimize the culture conditions for 10-day old C57/BL6J mice. After explantation the cochleae were transferred into a culture medium. The cochleae were divided into a fast and slow preparation group. The fast group representing the first explanted cochlea and the slow group representing the second. A total of four different culture media were compared consisting different concentrations of neurotrophins. The exact concentrations are shown in
Table 1.
Finally, the organs were cultured as described below at either 37°C or at 32°C. After a 24-hour culturing period the cochleae were processed and stained using a hemotoxyl-eosin (HE) and toluidine blue staining. In addition, immunostaining for Myosin VIIa, Phalloidin and ß-IIItubulin was performed.
The structure preservation was determined in three different areas and interpreted in a blinded manner by two independent specialists (SJ, ASF). The areas were: Organ of Corti (OC), spiral ganglion (SPG) and Stria vascularis with Spiral ligament (SV/SL). For each experiment performed, three cochleae were studied.
4.1. Animals
For this study C57/BL6J mice were obtained from the Charles River Company in Freiburg, Germany. Mice were maintained at the Innsbruck animal facility with unlimited access to food and water. Housing, feeding, breeding, and handling of the mice were according to federal/institutional guidelines with the approval of the local government. Mice of each sex were used to this study. The dissection of the inner ear organs took place at postnatal day 10. Deeply anesthetized animals — intraperitoneal ketamine hydrochloride (Graeub®, Senden-Bösensell, Germany) (67.5 mg/kg body weight), xylazine hydrochloride (Bayer®, Leverkusen, Germany) (5.4 mg/kg body weight) and atropine sulfate (Nycomed®, Linz, Austria) (0.085 mg/kg)— were euthanized by rapid cervical dislocation.
4.2. Dissection of the Inner Ear Organs
The dissection was performed under sterile conditions. Juvenile mice (postnatal-day 10) were deeply anesthetized and sacrificed by decapitation. Starting at the foramen magnum the skull was cut at the sagittal plane, followed by the removal of the brain. Then the bone plates surrounding the cochlea were removed so the cochlea and vestibular organ could be harvested. Afterwards the oval and the round window were opened. Then the bone coverage of the apex got broken along the basal and middle turn of the cochlea. Finally, the bony shell of the scala tympani was opened and removed resulting in a 180° opening along the basal turn.
After the dissection the inner ear organs were finally placed on a fresh petri dish and carefully rinsed with neurobasal medium (GibcoTM, Thermofisher Scientific®, Germany, cat-nr. 21103049). Depending on the timepoint of preparation – fast or slow – the cochleae were cultured separately. The cochleae in the fast group were transferred into the culture medium approximately 5 minutes after decapitation, whereas those in the slow group were placed into the culture medium approximately 10 minutes after decapitation. Therefore, there was an average of 5 minutes between the fast and slowly harvested cochlea.
4.3. Culture Medium
Preparation of culturing medium was performed the day before the harvest of the inner ears. The base liquid used for the culturing medium was neurobasal medium. 5 mM L-Glutamin, 10 mM HEPES, 100 U/mL Penicillin G and B27 Supplement (GibcoTM, Thermo Fisher Scientific®, Germany) were added. Afterwards pH was adjusted to 7.4 by adding 1 M NaOH. Then different concentrations of NT- 3 (PeproTech® EC, ltd., London, United Kingdom) and BDNF (PeproTech® EC, ltd., London, United Kingdom) were added and tested as described below.
Four different culture mediums were included into the study as listed in
Table 1.
4.4. Culturing of the Dissected Cochlea
Culturing was performed at 37°C or at 32°C in a humidified 5% CO2/95% Water Jacketed Incubator (ScientificTM, Thermo Fisher Scientific®, Marietta, Ohio), which provided stable temperature gradients. After the dissection of the inner ear organs, they were placed into a 10ml disposable rotary vessel filled with warmed neurobasal medium. Then the vessels were mounted to the rotating bioreactor machine and the machine rotated the vessels clockwise at 50 – 52 rounds per minute (rpm) to simulate zero gravity. Depending on the size and weight of the cochlea different rounds per minute were used ensuring that the cochlea did not collide with the wall of the vessel while rotating.
4.5. Fixation, Decalcification and Freezing
After incubation the cochleae were transferred into 2% (weight/volume) paraformaldehyde (PFA), which was fold diluted with neurobasal medium at pH 7.4. Afterwards the cochlea got decalcified by using a 10 % ethylenediaminetetraacetic acid (EDTA) and phosphate-buffered saline (PBS 1x) at pH 7.4 at 37°C. The cochlea was placed in this mixture for four hours. Before and after the process of decalcification the cochleae were washed three times in PBS 1x. Afterwards the cochleae were frozen using a cryoprotected freezing method[
34]. For this purpose, the inner ears were rinsed with PBS and then shaken with 10% sucrose and 15% sucrose (dissolved in PBS 1x) at room temperature. Subsequently, the inner ears were placed in a mixture consisting of half 15% sucrose dissolved in PBS 1x and half OCT and shaken. They were frozen on a mixture of CO2 and EtOH. This was followed by cryocutting producing 5-7μm thick sections.
4.6. Staining
According to standard protocols a HE-stain and Toluidine blue stain were done. In addition, the cultured cochleae were immunostained using the Roche Ventana Discovery System (Roche Diagnostics®, Rotkreuz, Switzerland). The markers used were Phalloidin FITC (Sigma-Aldrich®, Vienna, Austria, cat-nr. P5282) for staining F-Actin of supporting cells, Anti-Myosin VIIa (Proteus Biosciences® Inc., Ramona, CA, United States, cat.-nr. 25-6790) to stain hair cells – indicating the condition of inner hair cells (IHC) and outer hair cells (OHC) - and Anti-ß-III-tubulin (Abcam® plc., Cambridge, United Kingdom, cat.-nr. ab52623) for staining nerve fibers and ganglia cells. A concentration of 1:100 for ß-III-tubulin antibody, 1:100 for Myosin VIIa antibody and a concentration of 1:20 for Phalloidin FITC was applied. Alexa Rabbit 594 (Life TechnologiesTM, InVitrogen® Inc., Darmstadt, Germany, ref. A21207, lot. 2313074) was used as secondary antibody in a concentration of 1:200.
The exact stain immunohistochemistry procedures have been described in previously publications[
23,
35].
4.7. Quantitative and Statistical Evaluation
To quantify the structural preservation of the cochlea, histological sections were investigated in detail by the help of TissueQuest (version 7.0.1.139, Tissuegnostics ltd., Vienna, Austria). We used DAPI to stain cell nuclei (Life TechnologiesTM, Invitrogen®, Inc., Darmstadt, Germany) on the master canal, Myosin VIIa as a marker for hair cell survival and ß-III-tubulin as marker for ganglion cell survival. TissueQuest then automatically detected different stained cells. We manually dissected the sample into three different regions of interest (ROIs), which were: Organ of Corti (OC), spiral ganglion (SG), and Stria vascularis with Spiral ligament (SV/SL). The number of cells per region was calculated in TissueQuest and also counted manually. Each count was based on three replicates with three repetitions.
For statistical evaluation the mean value and standard deviation was calculated using Graphpad Prism (version 9.4.1681 by Graphpad Software Inc., San Diego, CA, United States) and the "Statistical Package for the Social Sciences" version 26.0.0.0 from IBM (SPSS Statistics; IBM, Armonk, USA). Afterwards an unpaired T-Test was used to calculate possible significances between the different treatments. A significance level of α = 0.05 was set for the statistical calculations.
Author Contributions
Conceptualization, J.S. and A.S.; methodology, W.B., A.T.; A.S. and J.S.; software, W.B. and A.S.; validation, J.S. and A.S.; formal analysis, A.T., M.S. and W.B.; investigation, B.W., A.T., J.S., A.S.; resources, A.T.; data curation, A.T. , C.S., T.G. and M.S.; writing—original draft preparation, A.T. and J.S., C.S. and T.G. and M.S. ; writing—review and editing, J.S. and A.S.; visualization, W.B. and A.T. ; supervision, J.S. and A.S.; project administration, J.S. and A.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Extraction of tissue from euthanized animals conforms to the Austrian Federal act on Experiments of Living Animals (Tierversuchsgesetz 2012 – TVG 2012, §2) based on the EU Directive 2010/63/EU. Thus, ethical review and approval was not required for the resent animal study. No human studies are presented in this manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The author (J.S.) received research funds from the R&D Department of MED-El Elektromedizinische Geräte GmbH Innsbruck. In addition, this work was also supported by Austrian Science Fund (FWF) Grant No. I4147-B
Abbreviations
The following abbreviations are used in this manuscript:
| BDNF |
Brain-derived neurotrophic factor |
| HE |
Hemotoxyl-eosin |
| NT-3 |
Neurotrophin 3 |
| OC |
Organ of Corti |
| PBS 1x |
Phosphate-buffered saline |
| SD |
Standard deviation |
| SL |
Spiral ligament |
| SPG |
Spiral ganglion |
| SV |
Stria vascularis |
References
- H. Staecker, W. Liu, B. Malgrange, P.P. Lefebvre, T.R. Van De Water, Vector-mediated delivery of bcl-2 prevents degeneration of auditory hair cells and neurons after injury, ORL J Otorhinolaryngol Relat Spec 69(1) (2007) 43-50. [CrossRef]
- C.T. Dinh, S. Haake, S. Chen, K. Hoang, E. Nong, A.A. Eshraghi, T.J. Balkany, T.R. Van De Water, Dexamethasone protects organ of corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes, Neuroscience 157(2) (2008) 405-13. [CrossRef]
- H. Hahn, M. Müller, H. Löwenheim, Whole organ culture of the postnatal sensory inner ear in simulated microgravity, J Neurosci Methods 171(1) (2008) 60-71. [CrossRef]
- Maniu, M. Perde-Schrepler, M. Cosgarea, Protective effect of L-N-acetylcysteine against gentamycin ototoxicity in the organ cultures of the rat cochlea, Rom J Morphol Embryol 52(1) (2011) 159-64.
- D.T. Chang, R. Chai, R. DiMarco, S.C. Heilshorn, A.G. Cheng, Protein-engineered hydrogel encapsulation for 3-D culture of murine cochlea, Otol Neurotol 36(3) (2015) 531-8. [CrossRef]
- X. Lou, Y. Dong, J. Xie, X. Wang, L. Yang, M. Tokuda, Y. Zhang, Comparing the cultivated cochlear cells derived from neonatal and adult mouse, J Transl Med 12 (2014) 150. [CrossRef]
- Spankovich, B.J. Walters, Mild Therapeutic Hypothermia and Putative Mechanisms of Hair Cell Survival in the Cochlea, Antioxid Redox Signal 36(16-18) (2021) 1203-14. [CrossRef]
- J.M. Ogier, R.A. Burt, H.R. Drury, R. Lim, B.A. Nayagam, Organotypic Culture of Neonatal Murine Inner Ear Explants, Front Cell Neurosci 13 (2019) 170. [CrossRef]
- Ramekers, H. Versnel, W. Grolman, S.F. Klis, Neurotrophins and their role in the cochlea, Hear Res 288(1-2) (2012) 19-33. [CrossRef]
- C.D. Lin, I.H. Wei, M.H. Tsai, M.C. Kao, C.H. Lai, C.J. Hsu, T. Oshima, Changes in guinea pig cochlea after transient cochlear ischemia, Neuroreport 21(15) (2010) 968-75. [CrossRef]
- M. Shirane, R.V. Harrison, The effects of hypoxia on sensory cells of the cochlea in chinchilla, Scanning Microsc 1(3) (1987) 1175-83.
- Olivetto, E. Simoni, V. Guaran, L. Astolfi, A. Martini, Sensorineural hearing loss and ischemic injury: Development of animal models to assess vascular and oxidative effects, Hear Res 327 (2015) 58-68. [CrossRef]
- B. Mazurek, E. Winter, J. Fuchs, H. Haupt, J. Gross, Susceptibility of the hair cells of the newborn rat cochlea to hypoxia and ischemia, Hear Res 182(1-2) (2003) 2-8. [CrossRef]
- J. Wu, J. Ye, W. Kong, S. Zhang, Y. Zheng, Programmed cell death pathways in hearing loss: A review of apoptosis, autophagy and programmed necrosis, Cell Prolif 53(11) (2020) e12915. [CrossRef]
- H. Staecker, R. Kopke, B. Malgrange, P. Lefebvre, T.R. Van de Water, NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells, Neuroreport 7(4) (1996) 889-94. [CrossRef]
- J.M. Miller, D.H. Chi, L.J. O’Keeffe, P. Kruszka, Y. Raphael, R.A. Altschuler, Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss, Int J Dev Neurosci 15(4-5) (1997) 631-43. [CrossRef]
- T. Shinohara, G. Bredberg, M. Ulfendahl, I. Pyykkö, N.P. Olivius, R. Kaksonen, B. Lindström, R. Altschuler, J.M. Miller, Neurotrophic factor intervention restores auditory function in deafened animals, Proc Natl Acad Sci U S A 99(3) (2002) 1657-60. [CrossRef]
- L.N. Gillespie, G.M. Clark, P.F. Bartlett, P.L. Marzella, BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects, J Neurosci Res 71(6) (2003) 785-90. [CrossRef]
- S.L. McGuinness, R.K. Shepherd, Exogenous BDNF rescues rat spiral ganglion neurons in vivo, Otol Neurotol 26(5) (2005) 1064-72. [CrossRef]
- R. Glueckert, M. Bitsche, J.M. Miller, Y. Zhu, D.M. Prieskorn, R.A. Altschuler, A. Schrott-Fischer, Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor, J Comp Neurol 507(4) (2008) 1602-21. [CrossRef]
- P. Ernfors, M.L. Duan, W.M. ElShamy, B. Canlon, Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3, Nat Med 2(4) (1996) 463-7. [CrossRef]
- B. Fritzsch, I. Silos-Santiago, L.M. Bianchi, I. Fariñas, The role of neurotrophic factors in regulating the development of inner ear innervation, Trends Neurosci 20(4) (1997) 159-64.
- L. Johnson Chacko, M.J.F. Blumer, E. Pechriggl, H. Rask-Andersen, W. Dietl, A. Haim, H. Fritsch, R. Glueckert, J. Dudas, A. Schrott-Fischer, Role of BDNF and neurotrophic receptors in human inner ear development, Cell Tissue Res 370(3) (2017) 347-363.
- J. Schulze, H. Staecker, D. Wedekind, T. Lenarz, A. Warnecke, Expression pattern of brain-derived neurotrophic factor and its associated receptors: Implications for exogenous neurotrophin application, Hear Res 413 (2022) 108098. [CrossRef]
- R.L. Davis, Gradients of neurotrophins, ion channels, and tuning in the cochlea, Neuroscientist 9(5) (2003) 311-6.
- H.A. Vink, D. Ramekers, H. Thomeer, H. Versnel, Combined brain-derived neurotrophic factor and neurotrophin-3 treatment is preferred over either one separately in the preservation of the auditory nerve in deafened guinea pigs, Front Mol Neurosci 15 (2022) 935111. [CrossRef]
- M.Y. Lee, T. Kurioka, M.M. Nelson, D.M. Prieskorn, D.L. Swiderski, Y. Takada, L.A. Beyer, Y. Raphael, Viral-mediated Ntf3 overexpression disrupts innervation and hearing in nondeafened guinea pig cochleae, Mol Ther Methods Clin Dev 3 (2016) 16052. [CrossRef]
- K. Hashimoto, T.T. Hickman, J. Suzuki, L. Ji, D.C. Kohrman, G. Corfas, M.C. Liberman, Protection from noise-induced cochlear synaptopathy by virally mediated overexpression of NT3, Sci Rep 9(1) (2019) 15362. [CrossRef]
- K.R. Henry, R.A. Chole, Hypothermia protects the cochlea from noise damage, Hear Res 16(3) (1984) 225-30. [CrossRef]
- Watanabe, K. Koga, N. Hakuba, K. Gyo, Hypothermia prevents hearing loss and progressive hair cell loss after transient cochlear ischemia in gerbils, Neuroscience 102(3) (2001) 639-45. [CrossRef]
- K.R. Henry, Hyperthermia exacerbates and hypothermia protects from noise-induced threshold elevation of the cochlear nerve envelope response in the C57BL/6J mouse, Hear Res 179(1-2) (2003) 88-96. [CrossRef]
- A.A. Eshraghi, T.R. Van de Water, Cochlear implantation trauma and noise-induced hearing loss: Apoptosis and therapeutic strategies, Anat Rec A Discov Mol Cell Evol Biol 288(4) (2006) 473-81.
- Tamames, C. King, E. Bas, W.D. Dietrich, F. Telischi, S.M. Rajguru, A cool approach to reducing electrode-induced trauma: Localized therapeutic hypothermia conserves residual hearing in cochlear implantation, Hear Res 339 (2016) 32-9. [CrossRef]
- H. Spoendlin, A. Schrott, The block surface method for evaluation of human inner ears, Acta Otolaryngol Suppl 436 (1987) 25-36. [CrossRef]
- L. Johnson Chacko, E.J. Pechriggl, H. Fritsch, H. Rask-Andersen, M.J. Blumer, A. Schrott-Fischer, R. Glueckert, Neurosensory Differentiation and Innervation Patterning in the Human Fetal Vestibular End Organs between the Gestational Weeks 8-12, Front Neuroanat 10 (2016) 111.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).