Submitted:
07 March 2025
Posted:
10 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
| Mean (n=170) | Male (n=76) | Female (n=94) | p-value | |
|---|---|---|---|---|
| Age a | 70.5 (+/-9.224) | 71.61(+/-8.343) | 69.59(+/-9.83) | p>0.05 |
| Years of study a | 13.135(+/-7.156) | 13.22(+/-6.005) | 13.064(+/-7.997) | p>0.05 |
| CI b | 71 (41.76) | 52 (73%) | 47 (66%) | p<0.01** |
| NCI-SMC b | 99 (58.24) | 24 (24%) | 47 (47%) |
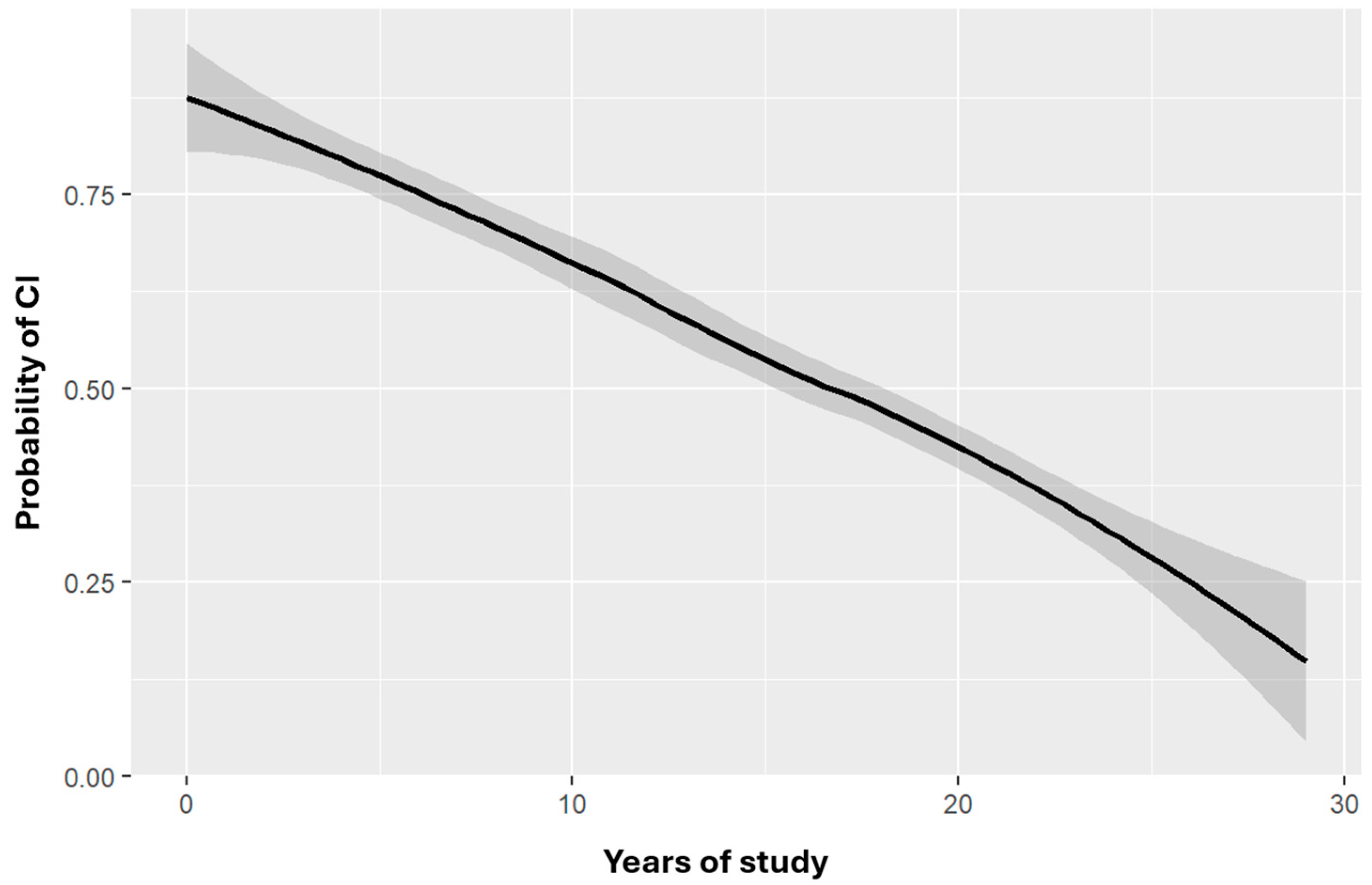
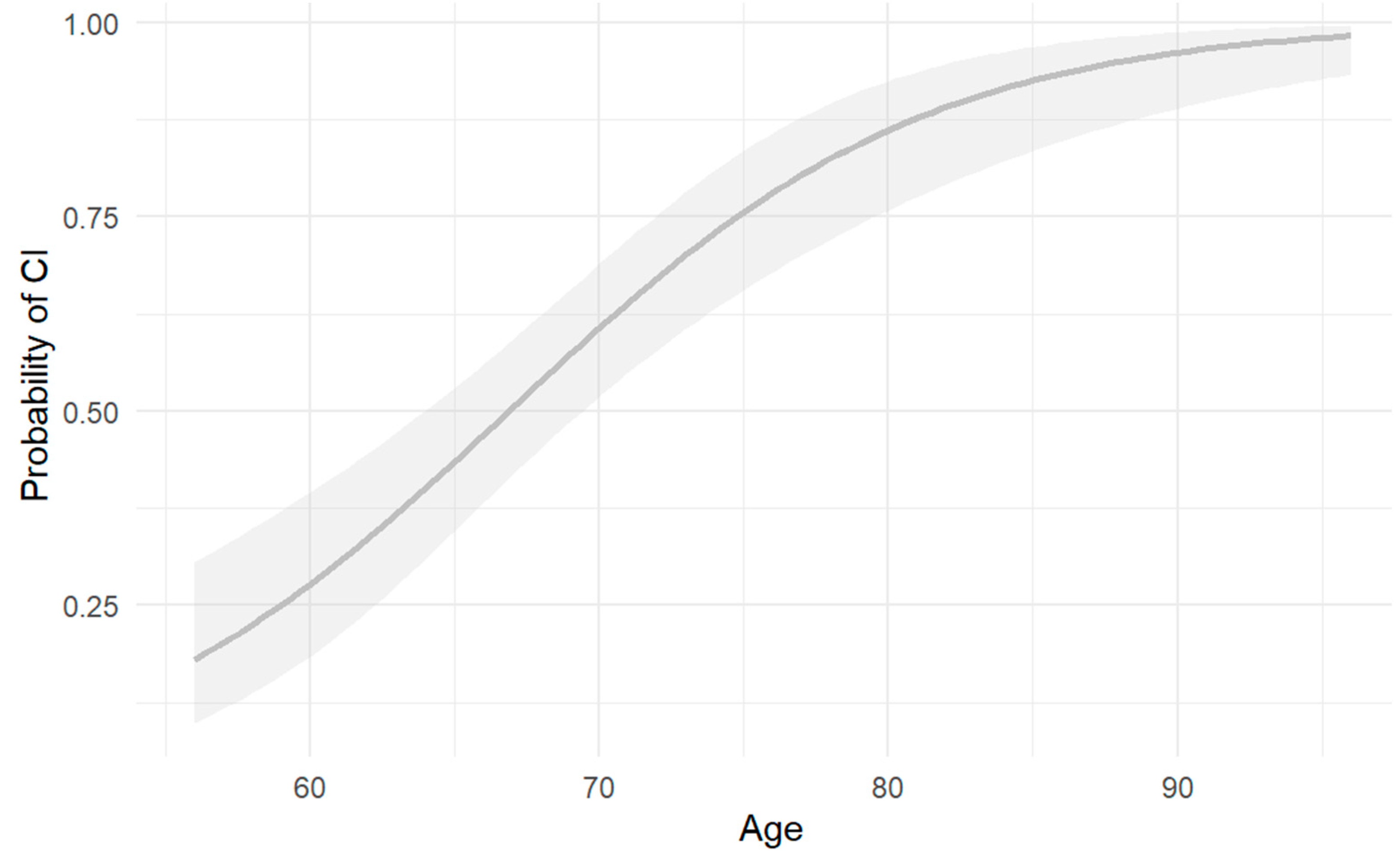
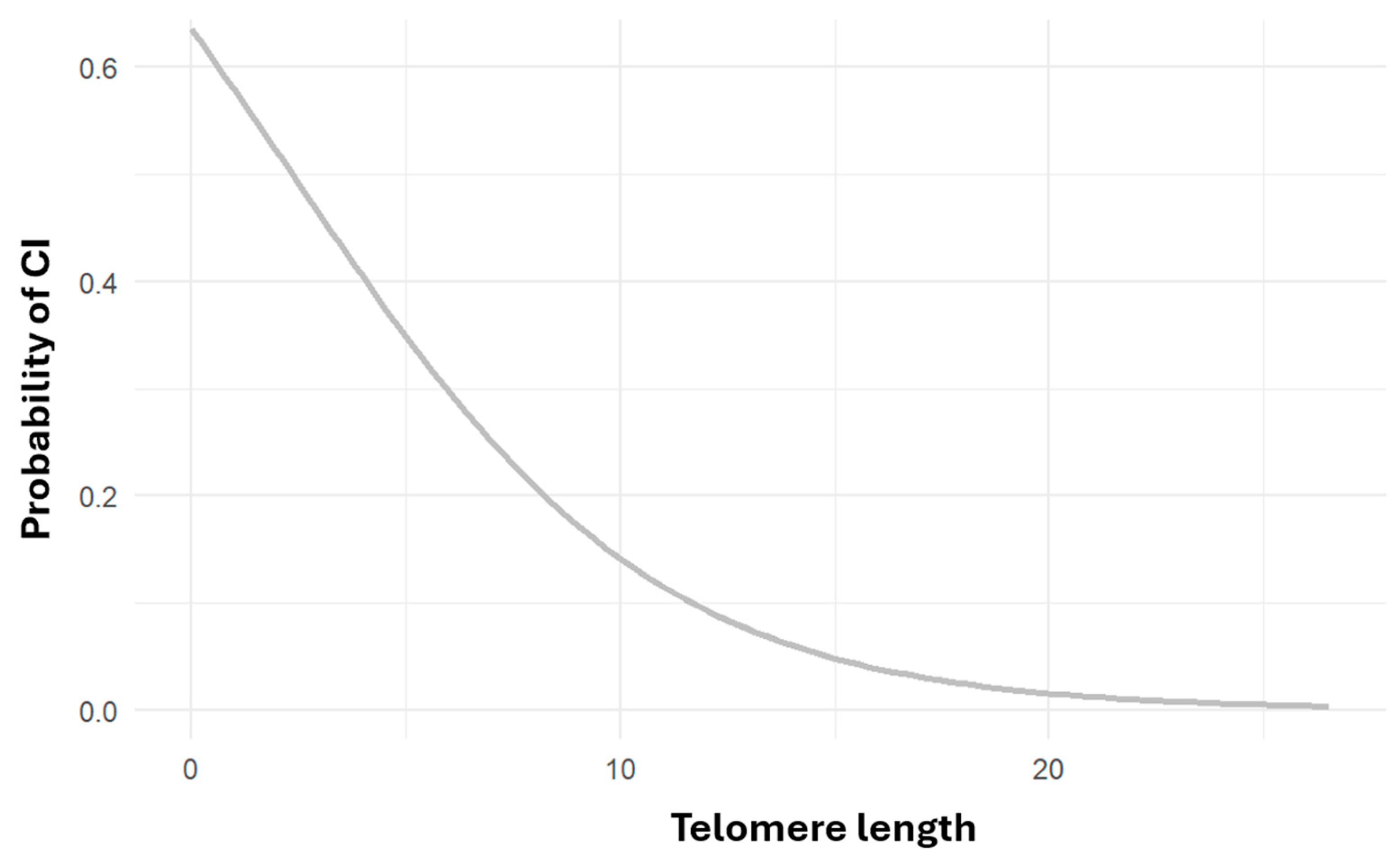
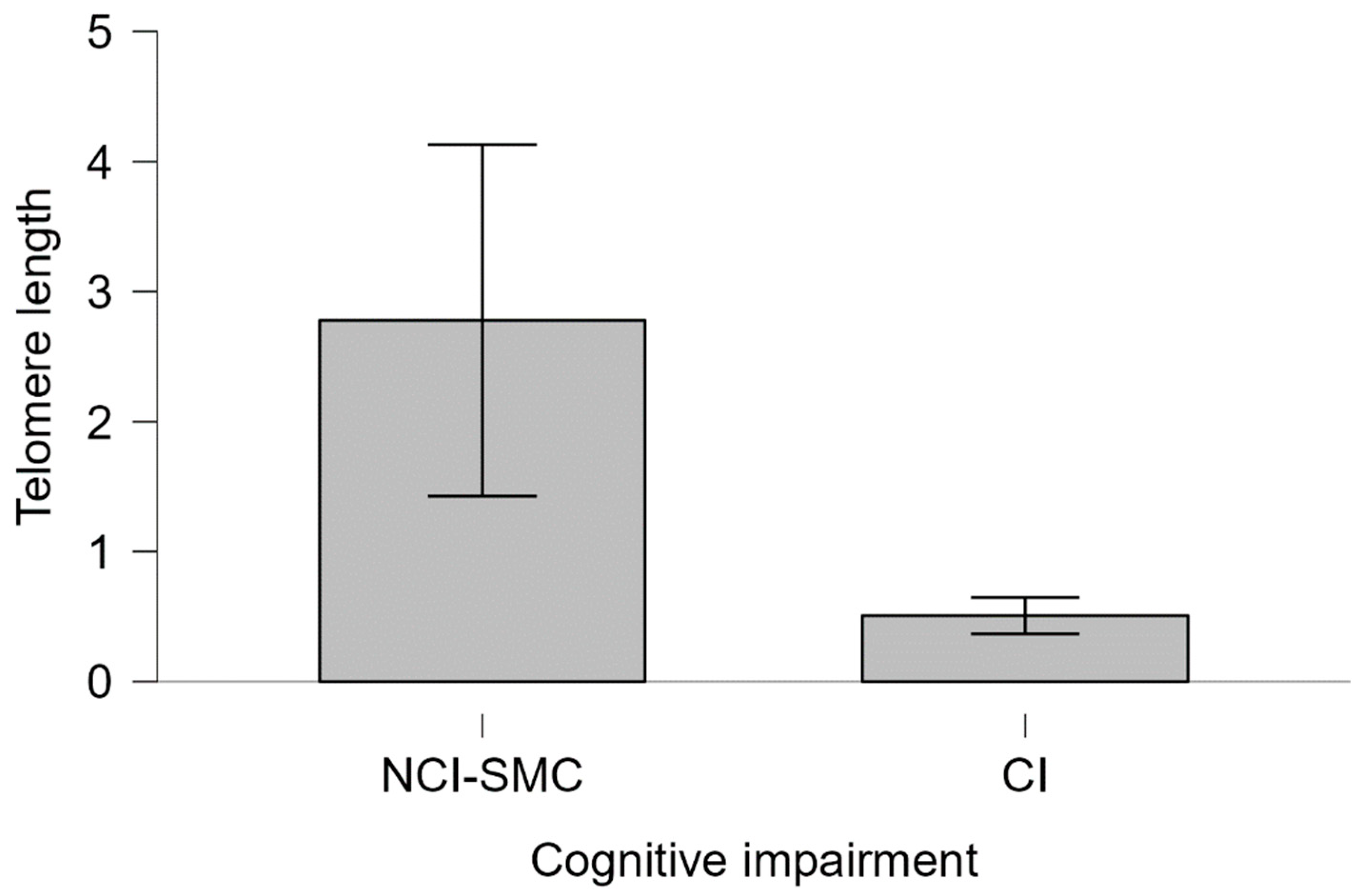
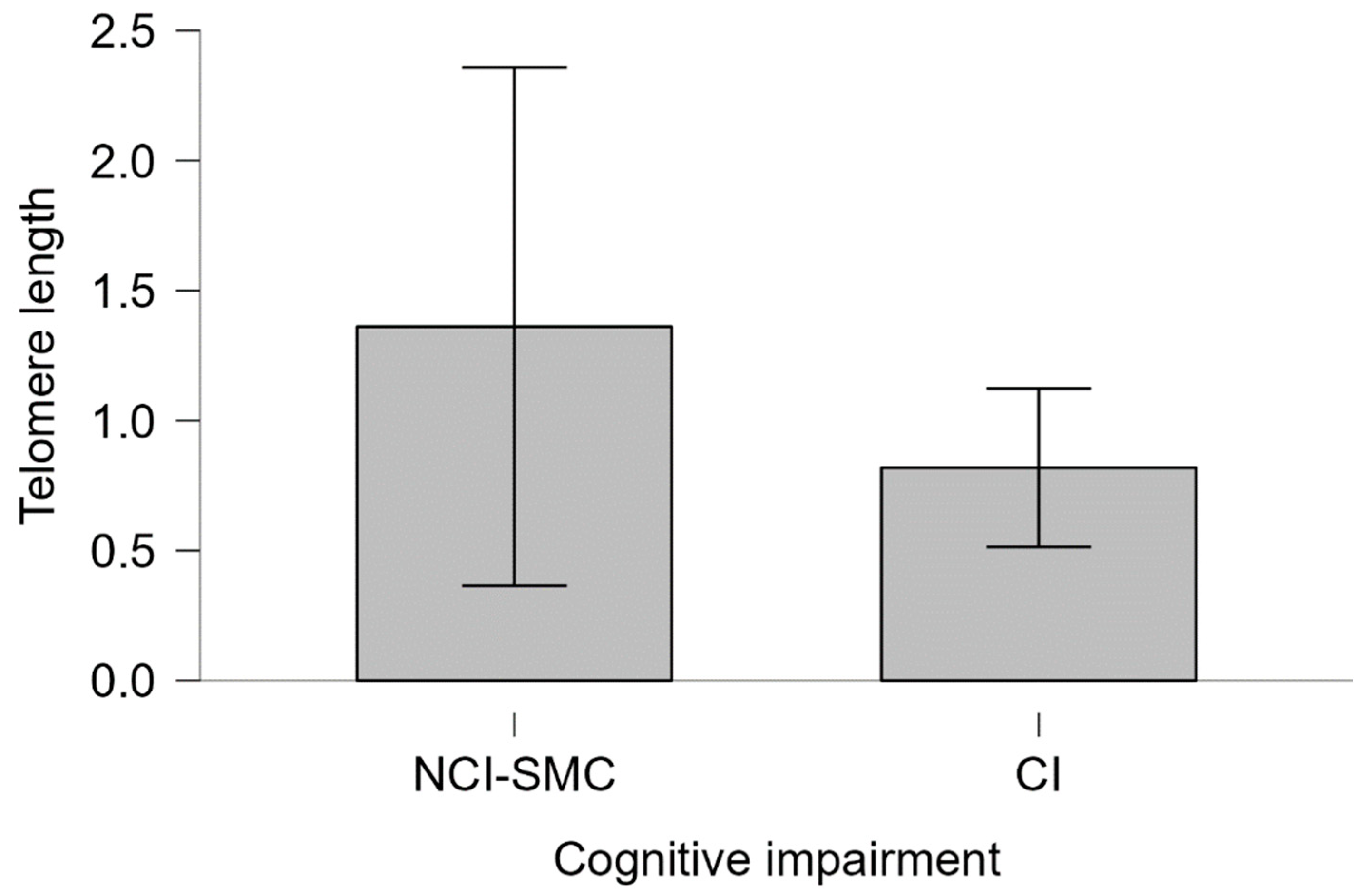
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing.
- Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology, 10(11), 634-642. [CrossRef]
- Castro-Chavira, S. A., Fernandez, T., Nicolini, H., Diaz-Cintra, S., & Prado-Alcala, R. A. (2015). Genetic markers in biological fluids for aging-related major neurocognitive disorder. Current Alzheimer research, 12(3), 200–209. [CrossRef]
- Choreño-Parra, J. A., De la Rosa-Arredondo, T., & Guadarrama-Ortíz, P. (2020). Abordaje diagnóstico del paciente con deterioro cognitivo en el primer nivel de atención. Medicina Interna de México, 36(6). [CrossRef]
- Instituto Nacional de Estadística y Geografía. (2023, 6 de julio). Encuesta Nacional sobre Salud y Envejecimiento en México (ENASEM) 2018. Encuesta Nacional sobre Salud y Envejecimiento en México (ENASEM) y Encuesta de Evaluación Cognitiva, 2021. INEGI. https://www.inegi.org.mx/app/saladeprensa/noticia.html?id=8294.
- Organización Mundial de la Salud. (2024, 1 de octubre). Envejecimiento y salud. https://www.who.int.
- Kim, J., Basak, J. M., & Holtzman, D. M. (2009). The role of apolipoprotein E in Alzheimer's disease. Neuron, 63(3), 287–303. [CrossRef]
- Martínez, S., Ochoa, B., Pérez, M. R., Torrico, F., García, I., & Garcia, C. C. (2022). Apolipoprotein E polymorphisms in adults over 60 years of age with mild cognitive impairment and Alzheimer’s disease in different Venezuelan populations. Polimorfismos del gen de la apolipoproteína E en adultos mayores de 60 años con disminución de la memoria cognitiva y enfermedad de Alzheimer en diferentes poblaciones venezolanas. Biomedica: revista del Instituto Nacional de Salud, 42(Sp. 1), 116–129. [CrossRef]
- Andrews, S. J., Fulton-Howard, B., & Goate, A. (2020). Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. The Lancet. Neurology, 19(4), 326–335. [CrossRef]
- Lambert, J. C., Ibrahim-Verbaas, C. A., Harold, D., Naj, A. C., Sims, R., Bellenguez, C., DeStafano, A. L., Bis, J. C., Beecham, G. W., Grenier-Boley, B., Russo, G., Thorton-Wells, T. A., Jones, N., Smith, A. V., Chouraki, V., Thomas, C., Ikram, M. A., Zelenika, D., Vardarajan, B. N., Kamatani, Y., … Amouyel, P. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature genetics, 45(12), 1452–1458. [CrossRef]
- Namba, Y., Tomonaga, M., Kawasaki, H., Otomo, E., & Ikeda, K. (1991). Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain research, 541(1), 163–166. [CrossRef]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977-81. doi: 10.1073/pnas.90.5.1977. PMID: 8446617; PMCID: PMC46003. Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, Meschi T. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging. 2018 Aug 29;13:1497-1511. PMID: 30214170; PMCID: PMC6120508. [CrossRef]
- Pimenova AA, Raj T, Goate AM. Untangling Genetic Risk for Alzheimer's Disease. Biol Psychiatry. 2018 Feb 15;83(4):300-310. Epub 2017 May 22. PMID: 28666525; PMCID: PMC5699970. [CrossRef]
- Coon, K. D., Myers, A. J., Craig, D. W., Webster, J. A., Pearson, J. V., Lince, D. H., Zismann, V. L., Beach, T. G., Leung, D., Bryden, L., Halperin, R. F., Marlowe, L., Kaleem, M., Walker, D. G., Ravid, R., Heward, C. B., Rogers, J., Papassotiropoulos, A., Reiman, E. M., Hardy, J., … Stephan, D. A. (2007). A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. The Journal of clinical psychiatry, 68(4), 613–618. [CrossRef]
- Raghavan, N., & Tosto, G. (2017). Genetics of Alzheimer's Disease: the Importance of Polygenic and Epistatic Components. Current neurology and neuroscience reports, 17(10), 78. [CrossRef]
- Ska, B., & Joanette, Y. (2006). Vieillissement normal et cognition [Normal aging and cognition]. Medecine sciences: M/S, 22(3), 284–287. [CrossRef]
- Dziechciaż, M., & Filip, R. (2014). Biological psychological and social determinants of old age: bio-psycho-social aspects of human aging. Annals of agricultural and environmental medicine : AAEM, 21(4), 835–838. [CrossRef]
- 18. Anisimova AS, Alexandrov AI, Makarova NE, Gladyshev VN, Dmitriev SE. Protein synthesis and quality control in aging. Aging (Albany NY). 2018 Dec 18;10(12):4269-4288. PMID: 30562164; PMCID: PMC6326689. [CrossRef]
- da Silva, P. F. L., & Schumacher, B. (2021). Principles of the Molecular and Cellular Mechanisms of Aging. The Journal of investigative dermatology, 141(4S), 951–960. [CrossRef]
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278. [CrossRef]
- Hernández Fernández, R. A. (2000). Telómeros y telomerasas. Revista Cubana de Investigaciones Biomédicas, 18(2), 121-129. Recuperado de http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-03001999000200009&lng=es&tlng=es.
- Scarabino, D., Broggio, E., Gambina, G., & Corbo, R. M. (2017). Leukocyte telomere length in mild cognitive impairment and Alzheimer's disease patients. Experimental gerontology, 98, 143–147. [CrossRef]
- Gampawar, P., Schmidt, R., & Schmidt, H. (2022). Telomere length and brain aging: A systematic review and meta-analysis. Ageing Research Reviews, 80, 101679. [CrossRef]
- Suchy-Dicey, A. M., Muller, C. J., Madhyastha, T. M., Shibata, D., Cole, S. A., Zhao, J., Longstreth, W. T., Jr, & Buchwald, D. (2018). Telomere Length and Magnetic Resonance Imaging Findings of Vascular Brain Injury and Central Brain Atrophy: The Strong Heart Study. American journal of epidemiology, 187(6), 1231–1239. [CrossRef]
- King, K. S., Kozlitina, J., Rosenberg, R. N., Peshock, R. M., McColl, R. W., & Garcia, C. K. (2014). Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA neurology, 71(10), 1247–1254. [CrossRef]
- Rodríguez-Fernández, B., Vilor-Tejedor, N., Arenaza-Urquijo, E. M., Sánchez-Benavides, G., Suárez-Calvet, M., Operto, G., Minguillón, C., Fauria, K., Kollmorgen, G., Suridjan, I., de Moura, M. C., Piñeyro, D., Esteller, M., Blennow, K., Zetterberg, H., De Vivo, I., Molinuevo, J. L., Navarro, A., Gispert, J. D., Sala-Vila, A., & Crous-Bou, M.; ALFA study. (2022). Genetically predicted telomere length and Alzheimer's disease endophenotypes: A Mendelian randomization study. Translational Psychiatry, 12(1), 9. [CrossRef]
- Hackenhaar, F. S., Josefsson, M., Adolfsson, A. N., Landfors, M., Kauppi, K., Hultdin, M., Adolfsson, R., Degerman, S., & Pudas, S. (2021). Short leukocyte telomeres predict 25-year Alzheimer's disease incidence in non-APOE ε4-carriers. Alzheimer's research & therapy, 13(1), 130. [CrossRef]
- Wagner, K. H., Cameron-Smith, D., Wessner, B., & Franzke, B. (2016). Biomarkers of Aging: From Function to Molecular Biology. Nutrients, 8(6), 338. [CrossRef]
- Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews. Neurology, 9(2), 106–118. [CrossRef]
- Ciesielska, N., Sokołowski, R., Mazur, E., Podhorecka, M., Polak-Szabela, A., & Kędziora-Kornatowska, K. (2016). Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Czy test Montreal Cognitive Assessment (MoCA) może być skuteczniejszy od powszechnie stosowanego Mini-Mental State Examination (MMSE) w wykrywaniu łagodnych zaburzeń funkcji poznawczych u osób po 60. roku życia? Metaanaliza. Psychiatria polska, 50(5), 1039–1052. [CrossRef]
- Delgado, C., Araneda, A., & Behrens, M. I. (2019). Validation of the Spanish-language version of the Montreal Cognitive Assessment test in adults older than 60 years. Validación del instrumento Montreal Cognitive Assessment en español en adultos mayores de 60 años. Neurologia, 34(6), 376–385. [CrossRef]
- Au, B., Dale-McGrath, S., & Tierney, M. C. (2017). Sex differences in the prevalence and incidence of mild cognitive impairment: A meta-analysis. Ageing research reviews, 35, 176–199. [CrossRef]
- Mutchie, H. L., Albrecht, J. S., Orwig, D. L., Huang, Y., Boscardin, W. J., Hochberg, M. C., Magaziner, J. S., & Gruber-Baldini, A. L. (2022). Differential misclassification of cognitive impairment by sex among hip fracture patients. Journal of the American Geriatrics Society, 70(3), 838–845. https://doi.org/. [CrossRef]
- Katabathula, S., Davis, P. B., Xu, R., & Alzheimer’s Disease Neuroimaging Initiative (2023). Sex-Specific Heterogeneity of Mild Cognitive Impairment Identified Based on Multi-Modal Data Analysis. Journal of Alzheimer's disease : JAD, 91(1), 233–243. [CrossRef]
- Calatayud, E., Marcén-Román, Y., Rodríguez-Roca, B., Salavera, C., Gasch-Gallen, A., & Gómez-Soria, I. (2023). Sex differences on anxiety and depression in older adults and their relationship with cognitive impairment. Semergen, 49(4), 101923. [CrossRef]
- Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., Ewers, M., Franzmeier, N., Kempermann, G., Kremen, W. S., Okonkwo, O., Scarmeas, N., Soldan, A., Udeh-Momoh, C., Valenzuela, M., Vemuri, P., Vuoksimaa, E., & the Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's & dementia : the journal of the Alzheimer's Association, 16(9), 1305–1311. [CrossRef]
- Nelson, M. E., Jester, D. J., Petkus, A. J., & Andel, R. (2021). Cognitive Reserve, Alzheimer's Neuropathology, and Risk of Dementia: A Systematic Review and Meta-Analysis. Neuropsychology review, 31(2), 233–250. [CrossRef]
- Soldan, A., Pettigrew, C., Cai, Q., Wang, J., Wang, M. C., Moghekar, A., Miller, M. I., Albert, M., & BIOCARD Research Team (2017). Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer's disease. Neurobiology of aging, 60, 164–172. [CrossRef]
- Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F., & Aguinaga-Ontoso, I. (2017). Prevalence and incidence of Alzheimer's disease in Europe: A meta-analysis. Prevalencia e incidencia de la enfermedad de Alzheimer en Europa: metaanálisis. Neurologia (Barcelona, Spain), 32(8), 523–532. [CrossRef]
- Baum, L., Chen, L., Ng, H. K., & Pang, C. P. (2000). Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology. Microscopy research and technique, 50(4), 278–281. https://doi.org/10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T.
- Molero, A. E., Pino-Ramírez, G., & Maestre, G. E. (2001). Modulación por edad y género del riesgo de enfermedad de Alzheimer y demencia vascular asociada al alelo de la apolipoproteína E-ε4 en latinoamericanos: Hallazgos del Estudio de Envejecimiento de Maracaibo. Cartas de Neurociencia, 307(1), 5.
- Li, Y., Li, J., Li, J., Li, X., & Li, Y. (2018). Association between apolipoprotein E polymorphisms and Parkinson's disease risk: A meta-analysis. Frontiers in Aging Neuroscience, 10, 1-9. [CrossRef]
- Campos, M., Edland, S. D., & Peavy, G. M. (2013). Exploratory study of apolipoprotein E ε4 genotype and risk of Alzheimer's disease in Mexican Hispanics. Journal of the American Geriatrics Society, 61(6), 1038–1040. [CrossRef]
- Villalpando-Berumen, J. M., Mejía-Arango, S., Aguilar-Salinas, C. A., Ordoñez-Sánchez, M. L., & Gutiérrez-Robledo, L. M. (2008). Apolipoprotein E epsilon4, Alzheimer's disease, and cognitive performance in elderly Mexican Mestizos. Journal of the American Geriatrics Society, 56(4), 677–682. [CrossRef]
- Piyush, G., Schmidt, R., & Schmidt, H. (2020). Leukocyte telomere length is related to brain parenchymal fraction and attention/speed in the elderly: Results of the Austrian Stroke Prevention Study. Frontiers in Psychiatry, 11, 1-9. [CrossRef]
- Crocco, P., De Rango, F., Dato, S., La Grotta, R., Maletta, R., Bruni, A. C., Passarino, G., & Rose, G. (2023). The shortening of leukocyte telomere length contributes to Alzheimer's disease: Further evidence from late-onset familial and sporadic cases. Biology (Basel), 12(10), 1286. [CrossRef]
- Fani, L., Hilal, S., Sedaghat, S., Broer, L., Licher, S., Arp, P. P., van Meurs, J. B. J., Ikram, M. K., & Ikram, M. A. (2020). Telomere length and the risk of Alzheimer's disease: The Rotterdam Study. Journal of Alzheimer's Disease, 73(2), 707-714. [CrossRef]
| APOE ε4 | |||
|---|---|---|---|
| With | Without | p-value | |
| Cognitive impairment | 17 (10) | 83 (48) | p<0.05* |
| Age b | 74.118 (+/-8.49) | 74.195 (+/-9.29) | |
| Years of study b | 7.941 (+/-5.55) | 9.964 (+/-6.01) | |
| NCI-Subjetive memory complaint | 21 (12.35) | 49 (28.8) | |
| Age b | 63.238 (+/-5.04) | 66.26 (+/-6.56) | |
| Años de estudio b | 18.047(+/-5.8) | 18.204 (+/-5.53) | |
| Total | 38 (22.35) | 132 (77.65) | |
| Age | 68.105 (+/-8.67) | 71.189 (+/-9.3) | p=0.966 |
| Years of study | 13.52 (+/-7.58) | 13.023 (+/-7.05) | p=0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





