Introduction
Leishmaniasis is an infectious disease caused by intracellular parasites of the genus
Leishmania and is transmitted by sandflies of the genera
Phlebotomus (
Old World) and
Lutzomyia (
New World). When infecting humans, parasites belonging to this genus multiply intracellularly and produce 4 types of syndromes: Visceral Leishmaniasis (VL, also known as Kala-Azar—black fever, the most severe form, fatal if not treated), Post Kala-Azar Dermal Leishmaniasis (PKDL), Cutaneous Leishmaniasis (CL, the most common form) and Mucocutaneous Leishmaniasis (ML, affecting the oral, nasal and pharyngeal cavity) [
1,
2]. Leishmaniasis is widespread in 98 countries, in temperate and tropical areas, with an incidence of over 1.5 million cases / year, of which 0.7-1.2 million cases/year are CL, while VL occurs in 200,000–400,000 cases / year. The spread of the disease depends on the distribution of sand flies, but cases of human leishmaniasis are increasing globally [
2]. In most endemic areas, asymptomatic forms are more frequent than clinical forms. VL may be caused by
L. donovani and
L. infantum (synonym
L. chagasi).
L. infantum is widespread in the Mediterranean area (Spain, France, Greece) and mainly affects children under 10 years of age and immunocompromised adults (70% of VL adult cases are associated with HIV infection). The tropism of this species derives from the lower virulence of this parasite compared to other species, the immaturity of the immune system of children and the lack of previous exposure and development of specific immunity; other risk factors include injection drug use and immunosuppression (e.g., organ transplant patients [
2,
3]. The incubation period is between two and 6 months, but can also vary from several weeks to several years [
4].
Case Presentation
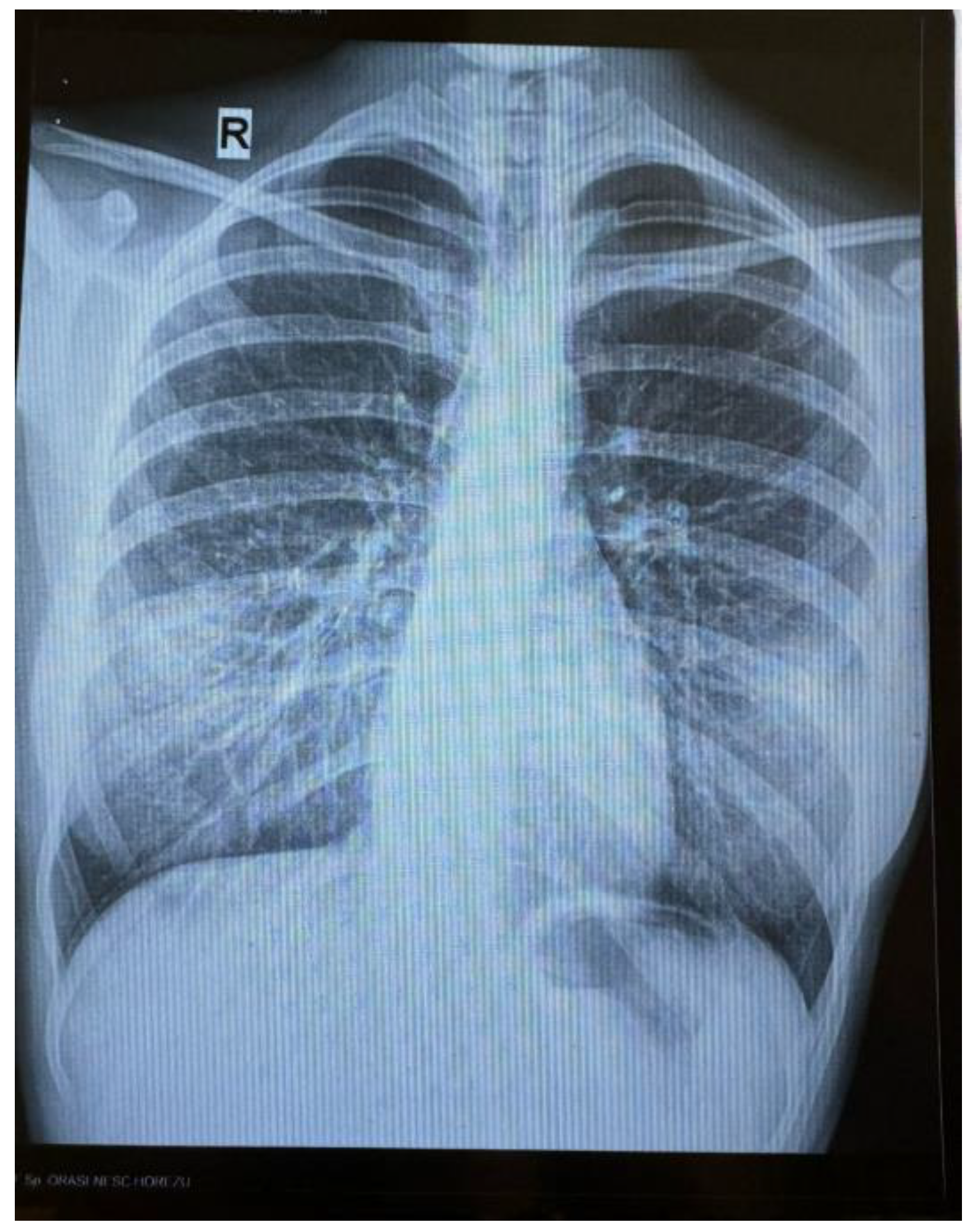
An 18-year-old female patient with no significant personal pathological history, is admitted in a hospital unit for symptoms consisting of continuous evening fever (maximum temperature of 40.3 °C), chills, night sweats, arthromyalgias, non-selective loss of appetite, involuntary decrease in weight (7 kg) and physical asthenia, with the onset approximately 8 months before (October 2023). During this period of time, the patient also manifested 3 respiratory complications: in November 2023—tonsillitis, for which she received macrolide treatment for 5 days; in January 2024—pneumonia, for which treatment was initiated with lincosamide for 7 days, which was later replaced, due to lack of clinical response, with tetracycline and second-generation cephalosporin for 5 days; in February 2024—influenza with influenza B virus, for which she received treatment with Oseltamivir. The patient underwent Gastroenterology and Internal Medicine consultations, after which symptomatic treatment was administered, without any improvement of the general condition. A radiographic aspect of bronchiectasis, completed with native chest CT, was identified in March (
Figure 1), with no detection of an acute infectious process. In June 2024, the abdominal—pelvic ultrasound was normal, with no indication of hepatomegaly, splenomegaly or regional adenopathies. The patient also reported one to two episodes per week of nausea and vomiting since one and a half year ago, which she associated with the mental stress of returning to Romania, after only having lived in Spain. Last trip abroad was to Valencia, in October 2023. The patient denied any use of intravenous drugs or risky sexual behaviour.
At the time of consultation, the clinical examination was normal. Laboratory analysis revealed normal blood count, absence of biological inflammatory syndrome, negative results for anti-HCV and anti-HBc antibodies (Ab), negative HIV serology, no liver cytolysis or cholestasis syndromes, normal coagulogram, protein electrophoresis within normal limits, normal cell distribution of the peripheral blood smear, no nitrogen retention syndrome, urine examination within normal limits, negative urine culture, normal thyroid hormone profile, autoimmunity markers (rheumatoid factor, antinuclear Ab, anti-dsDNA Ab, anti-CCP Ab) within normal limits, unmodified oncogenic markers (CEA, CA125, CA 19-9, CA15-3, proGRP, CYFRA 21-1). Laboratory tests targeting serological markers for infections with Toxoplasma gondii, Toxocara canis, Coxiella burnetii, Leishmania spp. and QuantiFERON TB GOLD were performed, as part of the differential diagnosis. The results were negative, except for the Western-blot test for anti-Leishmania IgG Ab, which revealed the L. infantum specific antigenic band profile (the presence of 14 and 16 kDa antigenic bands).
Transthoracic cardiac ultrasound was performed in the context of prolonged febrile syndrome suggesting the differential diagnosis of infective endocarditis. The results showed no indication of significant valvopathies or intracardiac formations suggestive for vegetations.
The case was interpreted as an acute infection with L. infantum and treatment with Amphotericin B, 3 mg/kg of bodyweight on days 1–5, 14 and 21, up to a total dose of 21 mg/ of bodyweight, was initiated. At the start of the treatment, the patient reported feeling dizziness, paraesthesia in the upper limbs and the sensation of suffocation, without desaturation or decrease in blood pressure values. It was difficult to differentiate between a panic attack and a hypersensitivity reaction. Therefore, treatment with Amphotericin B was restarted, taking into consideration the recommendations of the allergist regarding the administration of adrenergic premedication, steroidal anti-inflammatory drugs and antihistamines.
Discussions
Mediterranean VL, an endemic disease caused by infection with
L. infantum, has mainly a canine reservoir. Prior to the increase of cases of HIV infection, it was mainly identified in children. In Mediterranean Europe, 70% of adult VL cases are associated with HIV infection, a combination that, untreated, leads to a 100% death rate due to the cumulative impact on the immune system [
2].
The largest leishmaniasis outbreak recorded in Europe was in 2009, in the South-West region of Madrid, in the city of Fuenlabrada. 446 cases of VL and CL were reported between 2009 and 2012, with an increase of up to 758 cases in January 2018. A meta-analysis of seroprevalence studies carried out between 1985 and 2019 in Spain indicated a ≥ 17% prevalence of canine infections with
L. infantum in the region of Valencia, placing the city in the hyperendemic, high-risk area [
5,
6]. The patient’s report of traveling for several days to this region 4 weeks prior to the onset of symptoms, was indicative as epidemiological link and possible source of infection.
Most patients infected with
L. infantum develop an effective immune response, controlling the infection without resulting to symptomatic disease. Conversely, immunocompetent children and immunosuppressed patients (e.g., by HIV infection, organ transplant, etc.) may develop a clinical syndrome characterized by moderate to high fever with sudden onset, persisting for several weeks and decreasing in intensity to afebrile status, with resumption of febrile seizures accompanied by chills, weight loss, physical asthenia, haemorrhagic syndrome caused by thrombocytopenia, hepatosplenomegaly, lymphadenopathy, secondary infections like pneumonia, tuberculosis, rubella, measles, gastroenteritis,
Herpes Zoster, scabies. The biological parameters reflect pancytopenia, increased transaminases and bilirubin, polyclonal increase of immunoglobulins [
2]. Most of these manifestations coincide with those described by the patient—prolonged fever, successive respiratory complications, physical asthenia—without manifesting, however, associated changes at the reticuloendothelial system level—normal hemogram and immunogram outside of infectious episodes, normal abdominal ultrasound profile. This suggests an ineffective immune response to
L. infantum infection, with no clear causes of immunosuppression identified by subsequent investigations (negative HIV serology, negative QuantiFERON TB GOLD test result, negative test results for the presence of autoimmune disease markers, no history of organ transplantation or administration of immunosuppressive therapy, patient’s statement of no intravenous drug use or risky sexual behaviour). In this context, the chronic stress associated with the change in the living environment, as stated by the patient, could be speculated as a possible cause of disease.
VL may be diagnosed by: 1) parasitological methods, through the gold standard method consisting of amastigotes identification in biopsy segments from the bone marrow, adenopathy, spleen or liver, 2) immunological methods like Enzyme-Linked Immunosorbent Assay (ELISA), Indirect Immunofluorescence test (IFAT), rapid immunochromatographic test with recombinant antigen (ICT rK39), Western blot assays, 3) nucleic acid (kinetoplast DNA—kDNA) identification at the species level—PCR (qualitative), Real-Time-PCR (quantitative), PCR-RFLP, 4) protein-based methods using monoclonal antibodies, MALDI-TOF MS, Katex [
2,
7]. For laboratory diagnosis of the case presented in this work, the Western blot immunological technique was chosen for its high level of sensitivity and specificity (the method can detect 0.1 ng/mm
2 of the proteins specific to a pathogen) [
8].
The differential diagnosis includes malaria, typhoid fever, tuberculosis, brucellosis, schistosomiasis and histoplasmosis [
2]. In this case, in addition to infectious aetiologies, it was chosen to extend the laboratory testing by also targeting autoimmune, rheumatological and oncological markers.
The treatment may be initiated with pentavalent antimonic (Sbv—sodium stibogluconate and meglumine antimonate), Amphotericin B (AmB—deoxycholate and liposomal), Paromomycin and Miltefosine. In the Mediterranean area, treatment with liposomal AmB, which ensures definitive healing, without relapses, in immunocompetent patients, is preferred. Response to treatment is generally assessed clinically, with resolution of fever after one to two weeks of therapy, reduction in spleen size one month after initiation, and weight gain. Serological tests are not useful in confirming infection resolution, as they may remain positive for months to years after the end of treatment. Patients should be clinically monitored for 12 months and reassessed if symptoms recur [
2,
9]. The improvement of this patient’s clinical condition was observed shortly after the initiation of the treatment and the clinical and biological evaluation performed 3 months after the completion of AmB therapy showed parameters within normal limits. Serologic testing was repeated. Serological testing was repeated at the same laboratory with a positive Western blot result and a negative PCR result, showing the effectiveness of the treatment. The patient was discharged with the recommendation to return for re-evaluation and the prospect of marrow puncture—aspiration, in case of recurrence of symptoms.
Conclusions
The peculiarity of this case is represented by the serological identification of L. infantum infection in a patient without characteristic risk factors like childhood age or immunodepression, without a biopsy being performed as part of the differential diagnosis of the febrile syndrome. The long period of time that passed from the onset of the symptoms to diagnosis (8 months) should also be noted. During this time, the patient underwent multiple medical consultations and paraclinical investigations that failed to reveal the real cause of the disease. Also, in the current context of expansion of the circulation areas of various pathogens as a result of globalization, travel and climate change, close collaboration with infectious disease specialists and inclusion of tropical diseases in the differential diagnosis of febrile syndromes, depending on the clinical and epidemiological context of the patient, have become critical.
Author Contributions
Conceptualization, C.-M.P. and M.-C.C.; methodology, C.-M.P., M.-C.C., P.-R.R., D.L.-D.M. and A.-G.B.; investigation, C.-M.P., M.-C.C. and A.-G.B.; writing—original draft preparation, C.-M.P. and M.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from subject involved in the article.
Acknowledgments
Publication of this paper was supported by the Cantacuzino National Military Medical Institute for Research and Development, Bucharest, Romania.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Brito RCF, Aguiar-Soares RD de O, Cardoso JM de O, Coura-Vital W, Roatt BM, Reis AB. Recent advances and new strategies in Leishmaniasis diagnosis. Vol. 104, Applied Microbiology and Biotechnology. Springer; 2020. p. 8105–16. [CrossRef]
- Dennis, L. Kasper ASF. Dennis L. Kasper (editor)_ Anthony S. Fauci (editor) - Harrison’s infectious diseases (2017). 2017;3.
- Bern, C. Visceral leishmaniasis: Epidemiology and control [Internet]. 2024 [cited 2024 Oct 3]. Available from: https://www.uptodate.com.
- Bern C. Visceral leishmaniasis: Clinical manifestations and diagnosis [Internet]. 2024 [cited 2024 Oct 3]. Available from: https://www.uptodate.com.
- Ibarra-Meneses AV, Carrillo E, Nieto J, Sánchez C, Ortega S, Estirado A, et al. Prevalence of asymptomatic leishmania infection and associated risk factors, after an outbreak in the Southwestern Madrid Region, Spain, 2015. Eurosurveillance. 2019 May 30;24(22). [CrossRef]
- Gálvez R, Montoya A, Cruz I, Fernández C, Martín O, Checa R, et al. Latest trends in Leishmania infantum infection in dogs in Spain, Part I: Mapped seroprevalence and sand fly distributions. Parasit Vectors. 2020 Apr 21;13(1). [CrossRef]
- Reimão JQ, Coser EM, Lee MR, Coelho AC. Laboratory diagnosis of cutaneous and visceral leishmaniasis: Current and future methods. Vol. 8, Microorganisms. MDPI AG; 2020. p. 1–30.
- Heidari S, Gharechahi J, Mohebali M, Akhoundi B, Mirshahvaladi S, Azarian B, et al. Western Blot Analysis of Leishmania infantum Antigens in Sera of Patients with Visceral Leishmaniasis [Internet]. Vol. 14. Available from: http://ijpa.tums.ac.ir.
- Bern, C. Visceral leishmaniasis: Treatment [Internet]. 2024 [cited 2024 Oct 3]. Available from: https://www.uptodate.com.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).