Background
Industrial animal agriculture is a leading contributor to critical environmental problems such as land degradation and water scarcity and is responsible for up to 26 to 34% of anthropogenic greenhouse gas emissions (Crippa et al., 2021; Poore and Nemecek, 2018). This raises concerns about sustainability, food safety and security, worker safety, public health, and the ethical treatment of animals (FAO 2006). Cultivated meat and seafood products may supply animal-based protein while offering the potential to enhance global food security and provide benefits to human health, the environment, and animal welfare (Sinke et al., 2023). Safety demonstration is a critical aspect of cultivated meat and seafood commercialization and large-scale consumer acceptance.
Culture media, made primarily of water and basic nutrients such as amino acids, carbohydrates, salts, and vitamins, is used throughout the cultivated meat and seafood manufacturing process to support survival, growth, and differentiation of cells. Most culture media is removed from the cells after collection from the bioreactors. However, substances in the media have the potential to accumulate in or bind to the cells, remaining as residues in the final cultivated food product. Safety of individual culture media substances is typically evaluated using information demonstrating that the substances can be safely consumed at the estimated or measured concentration in the final product, or by demonstration that the substance is absent from the final product. Inputs used in the manufacturing process are assessed by manufacturers and during regulatory review to determine whether they are safe for use in food or considered ‘food safe’. Critically, there is no established definition of ‘food safe’ culture media, leaving producers to discern how to demonstrate that their media formulations are ‘food safe’ to food safety authorities.
Additionally, most companies hold their customized media formulations as trade secrets, a common practice throughout the food manufacturing industry. This customisation is required to match the specific metabolic requirements of unique cell lines employed by different companies. Formulations with different substances are regularly developed and iterated to reduce production costs or improve the efficiency of cell growth. As a result, the composition and safety of each formulation is evaluated on a case-by-case basis, a time-consuming effort for both companies and regulatory reviewers.
Creating a risk assessment framework and Safety-Assessed Media Ingredient (SAMI) list resource for cultivated meat and seafood media components aims to streamline the safety assessment and regulatory evaluation of cultivated meat and seafood products.
The overall goal of the SAMI list and assessment approach framework proposed in this paper is to create a methodology for cataloging media components commonly used in cultivated meat and seafood production and to develop an internationally accepted resource for conducting assessments of commonly used components with proposed associated SAMI use levels. The list is intended as a reference to reduce the level of effort and improve the efficiency of assessing the safety of media inputs for cultivated meat and seafood and concluding the components to be ‘food safe’ for their intended use level. Note that the use levels are intentionally conservative for screening purposes and are not intended to be restrictive limits; exceeding the SAMI use level does not indicate a safety concern, rather it indicates a need for more detailed safety analysis.
Approach to Developing the SAMI List
To develop the SAMI list:
The list of media components was developed and refined with industry and regulatory stakeholder feedback. A short list of 56 substances was selected for Phase 1.
Components were organized in a categorization framework considering their history of safe consumption and the availability of dietary safety information.
A safe level for each component was derived from available safety information
Stakeholders were engaged in workshops, and feedback was incorporated into the proposed SAMI methodology and list.
Development of the SAMI List
As cultivated meat is an emerging industry, and companies culture media formulations are protected as proprietary information, no publicly available list of commercially relevant media substances currently exists. Therefore, an initial draft list of 41 media substances was generated by the authors as a starting point. Substances were included if identified in literature or by expert opinion as common to cultivated meat manufacture.
The results from a targeted online survey of cultivated meat manufacturers, with whom the draft list was shared, were then used to develop an expanded Phase 1 list and improve its commercial relevance (with 17 unique cultivated meat-producing or servicing company respondents from a variety of countries). Seventeen companies replied to a survey sent with the initial list. Companies were asked to comment on whether each of the 41 media substances should remain in, or be removed from, the SAMI list and to suggest the inclusion of any additional substances. Substances were included in the final list if the majority of companies surveyed were in favor of their inclusion of the component on the list, resulting in 56 components for this Phase 1 list (see
Table 2 for the Phase 1 list).
Companies were invited to voluntarily provide information on the stage of manufacturing the substance is employed, its concentration, the presence of any residues in harvested cells, and whether the substances are species- or cell line-specific. Most companies declined to provide these data; therefore, the list does not consider these attributes. Of the voluntary information provided, it was confirmed that most substances were not species- or cell line-specific, and therefore the SAMI list would be widely applicable to many cultivated meat and seafood production processes. All information was shared by companies on the requirement that it only be used in a de-identified and aggregated format.
Categorization Approach
A categorization approach aims to classify media components in support of more efficient risk assessments. Some companies and regulatory jurisdictions propose the categorization of media components according to their regulatory status, history of safe consumption by humans, and/or safety assessment (e.g., UPSIDE Foods U.S Food and Drug Administration [FDA] submission (CCC002), GOOD Meat FDA submission (CCC001)). As the approach for developing the SAMI list is intended to be internationally acceptable and flexible for use across differing jurisdictions, categorization relies on available safety information rather than regulatory status.
The initial focus was on media components with established histories of safe use in food, or those that have been evaluated and confirmed as safe by regulatory or safety experts. These components are organized into two categories (Category 1 and Category 2). Category 1 substances are recognized as safe for food consumption without requiring defined upper intake limits, whereas Category 2 substances, although widely agreed to be safe, have established tolerable upper intake levels (UL) or toxicological thresholds (
Table 1). Future work will extend this framework to include additional substances for which safety data are less comprehensive.
Category 1
Category 1 culture media components have an established history of safe consumption or use in food as per relevant regulatory or legislative documents. These substances have been reviewed by a panel of experts (e.g., Food Chemicals Codex (FCC), Joint FAO/WHO Expert Committee on Food Additives (JECFA), European Food Safety Authority (EFSA), Food and Drug Administration (FDA), or other recognized expert body) and/or are common components of conventional food with a history of safe consumption. These components may have a health-based guidance value (HBGV) of ‘not specified’, meaning the food substance has very low toxicity which, on the basis of the available data (chemical, biochemical, toxicological and other), does not represent a hazard to health when consumed in food. These substances have been established to be safe for use without the need to establish Tolerable Upper Intake Levels (ULs) or toxicological thresholds. Toxicological studies may have been conducted for category 1 inputs where the highest concentration tested did not result in any adverse effects leading to the No Observed Adverse Effect Level (NOAEL) value from the study being equal to or greater than the highest concentration tested in the study. Category 1 substances may have a Dietary Reference Intake (DRI) value, but do not have an associated UL value.
Category 2
Category 2 culture media ingredients also have a history of safe consumption and/or use in food as per relevant regulatory or legislative documents, but also have established ULs or toxicological thresholds in addition to HBGV. These substances have been reviewed by a panel of experts (e.g., FCC, JECFA, EFSA, FDA, or other recognized expert body) and/or are common components of conventional food with a history of safe consumption. Nonetheless, they also have established upper limits for safe dietary exposure. The UL or dietary toxicological threshold values may be established by a recognized expert body or derived from peer-reviewed scientific safety data. Dietary toxicological thresholds include peer-reviewed dietary health base guidance values such as NOAELs, provided the established NOAEL is not equal to or greater than the maximum dose tested in the study (where the highest dose did not result in any adverse effects) or any other experimentally derived value that is associated with toxicological effects with suitable margin of exposure values.
Table 1.
Summary of Category 1 and 2 inclusion criteria for culture media inputs.
Table 1.
Summary of Category 1 and 2 inclusion criteria for culture media inputs.
| Category |
Inclusion Criteria |
| Category 1 |
1. Substance DOES have a history of safe consumption or HAS been evaluated by a panel of experts.
2. Substance DOES have a health-based guidance value that is ‘not specified’ and DOES NOT have an established toxicological threshold.
3. Substance is NOT made using a new manufacturing method
4. Substance is NOT a functionalized form of a safe substance |
| Category 2 |
1. Substance DOES have a history of safe consumption or HAS been evaluated by a panel of experts.
2. Substance DOES have a specified health-based guidance value or DOES have an established toxicological threshold.
3. Substance is NOT made using a new manufacturing method
4. Substance is NOT a functionalized form of a safe substance |
Derivation of SAMI Use Levels
The proposed SAMI Phase 1 list culture media substances are Category 1 or 2 substances. The SAMI use levels in the SAMI list represent final product/residue levels in the final cultivated meat or seafood products that have been derived from established DRIs, concentrations of the substance in conventional foods, and/or developed from established NOAELs. The SAMI use levels represent non-restrictive conservative safe use levels to streamline risk assessment. The presence of a substance above the SAMI use level does not imply that it is not safe for use; rather, it indicates that further risk assessment is required by the assessor. SAMI use levels are provided in units of mg of component consumed per day by an individual eating a cultivated meat or seafood product to be inclusive of the differing intended serving sizes across cultivated meat and seafood products and to be flexible for use across all jurisdictions.
The DRI values were obtained from those established by the Food and Agriculture Organization/World Health Organization (FAO/WHO) or other authoritative expert groups. Where available, SAMI use levels are equivalent to the established DRI value in mg/day.
Concentrations of components in conventional meat and seafood (beef, pork, chicken, and salmon), or other foods were obtained from government food composition databases and/or peer-reviewed literature. SAMI use levels are established at a 10% increase over the maximum concentration of the component in one serving of the conventional food, accounting for variability across products.
Category 1
Category 1 substances include those with a history of safe consumption. This includes some carbohydrates, inorganic salts, water-soluble vitamins, fatty acids, and nucleic acid-related compounds. Category 1 substances may either be either present in conventional food, used in conventional food processing, and/or have been evaluated by a panel of experts that has reached a conclusion of safety for use in food, with “not specified” HBGV or dietary toxicological threshold values. For salts that dissociate in aqueous solution, the assessment is conducted on the dissociated aqueous constituent ions.
The SAMI use levels are derived from:
- a)
Established DRIs; or
- b)
Levels in conventional meat and seafood; or
- c)
Levels in other conventional foods with a history of safe consumption.
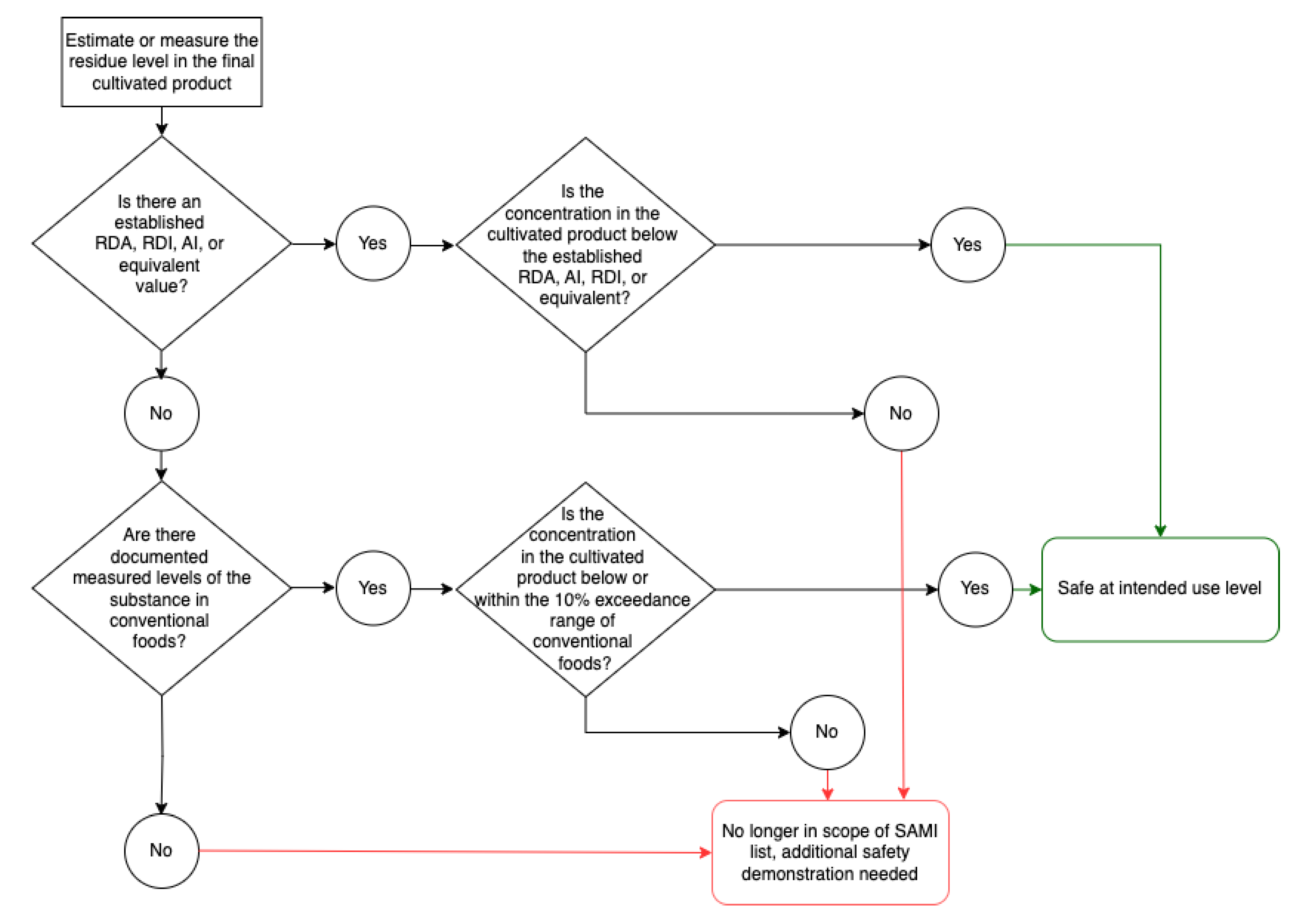
Figure 1 is a flowchart to visualise the risk assessment strategy for Category 1 components (
Figure 1).
Where a DRI exists, the SAMI use level for Category 1 components is equal to an established DRI (e.g., Acceptable Daily Intake (ADI), Adequate Intake (AI), Recommended Dietary Intake (RDI), Recommended Dietary Allowance (RDA), or equivalent value), expressed in mg/day. Although users of this framework may consider incorporating background exposure, such adjustments have not been included in this current general framework due to wide variation in different jurisdictions.
Category 1 components present in final cultivated meat and seafood products at concentrations equal to or below the DRI values satisfy the SAMI use level criteria and are concluded to be at safe levels for use in cultivated meat and seafood.
For substances with no DRI, the concentration of the substance in conventional meat and seafood or other foods (if they are not present in conventional meat and seafood) forms the basis for the SAMI use level. These concentrations are collected from the following food composition databases: USDA FoodData Central, FSANZ Australian Food Composition Database – Release 2.0, and MEXT – Standard Tables of Food Composition in Japan - 2015 - (Seventh Revised Version). Values from beef, pork, and chicken are used to represent meat and poultry values. Salmon was chosen to represent seafood because of the availability of composition data and because salmon aquaculture is the fastest-growing food production system in the world (WWF 2024). Values were included only if the concentrations of the substance were measured in conventional meat or seafood, while estimates of concentrations were excluded. If the food composition databases lacked data, a literature search was conducted for any reported concentrations of the substance in meat, seafood, or other conventional foods.
The maximum measured concentration of the substance in conventional food was identified. A 10% exceedance of this maximum concentration was calculated (see Equation 1). A 110% threshold value is applied to account for biological variability. In the absence of an internationally harmonized threshold for biological variation, a 10% exceedance is adopted for this framework. The concept of substantial equivalence – commonly used to compare genetically modified products with their conventional counterparts – supports this approach. For example, regulatory agencies have employed a 10% threshold to determine whether nutritional differences between cloned salmon or pigs and their natural comparators were biologically significant (FDA 2008; FDA 2015). Furthermore, studies on variability in amino acids, vitamins, and minerals in conventional livestock often report values exceeding 10% due to difference in genetics, breed, species, and environment (Kahmouh et al., 2024). Therefore, a 10% threshold was adopted for this general framework. Additional explanation for the uncertainties represented by the 10% exceedance of the maximum concentration in conventional meat and seafood are provided in the Uncertainties and Limitations of the SAMI list section of the paper.
Assuming that cultivated meat and seafood is intended to serve as a substitute for conventional meat, an appropriate serving size is established considering recommended serving sizes. As a representative case, the SAMI use level (expressed in units of mg/day) assumes a daily consumption of 90g of cultivated meat and seafood, equivalent to one suggested serving size of meat in Singapore (HealthHub 2022). These baseline values may be adjusted to reflect geographical variations in dietary patterns. In cases where direct measurements for a substance in meat are unavailable (such as for substances lacking published data, or for substances that are not typically present in meat), values from other conventional food sources are used. When the comparator is derived from other conventional foods, the assumed serving size is aligned with that of the reported conventional food. For example, the concentration of sulphate is not reported in meat, therefore values reported in brussels sprouts are used, with a corresponding standard serving size of 100 g for vegetables (HealthHub 2022).
Equation 1. SAMI use level, derived from concentration in conventional foods
If a Category 1 component is estimated or measured in the product above the SAMI use level, additional safety demonstration is required to conclude whether that it is safe under the conditions of intended use. However, the exceedance of a SAMI use level in a product does not indicate the level is unsafe, only that further safety demonstration is necessary to reach a safety conclusion.
Media components must also meet regional requirements, including the specified standards for food-grade substances (e.g., those set by the FCC or JECFA) or similar, where applicable.
Category 2
Category 2 media components include substances with a history of safe consumption, including some amino acids, water-soluble vitamins, fatty acids, inorganic salts, nucleic acid-related compounds, and organic substances with established ULs or dietary toxicological threshold values. Category 2 substances may either be present in conventional food; are used in conventional food processing; and/or an evaluation conducted by a panel of experts has concluded their safe use in food if below ULs, or established dietary toxicological thresholds. For salts that dissociate in aqueous solution, the assessment is conducted on the constituent ions.
Category 2 SAMI use levels are derived from:
- a)
Established Dietary Reference Intakes (DRIs); or
- b)
Levels in conventional meat and seafood; or
- c)
Levels in other types of conventional foods with a history of safe consumption; or
- d)
Peer reviewed published toxicological thresholds.
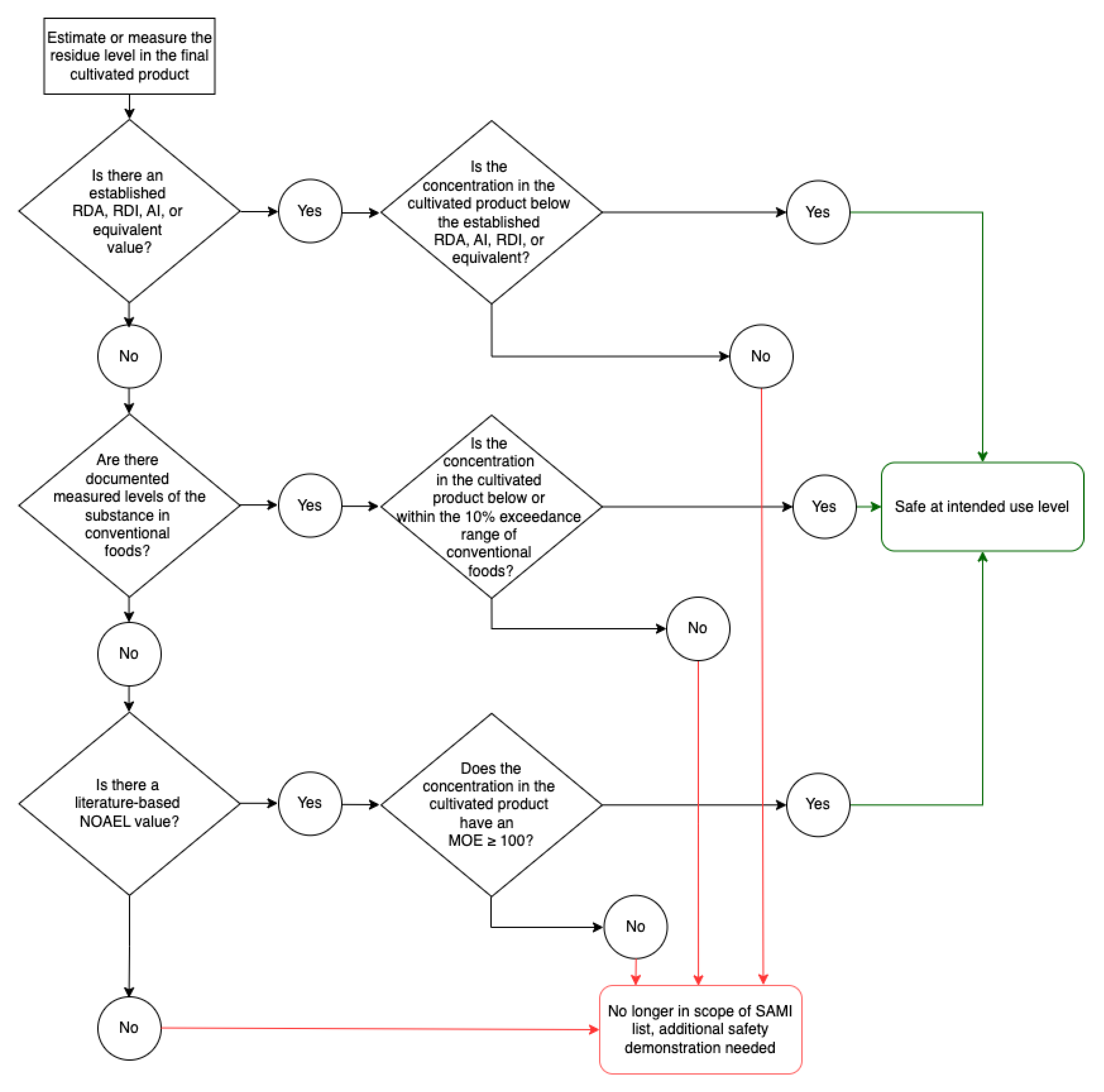
A flowchart has been developed to visualise the risk assessment strategy for Category 2 components (
Figure 2).
If a DRI exists, the SAMI use level for Category 2 components is equivalent to an established DRI (e.g., ADI, AI, RDI, RDA, etc.). Recommended or adequate intake values (e.g., ADI, AI, RDI, RDA), were selected as the benchmarks for safety assessment because they provide a more conservative approach than using the UL. Category 2 media components present at levels equal to or below the DRI values satisfy the SAMI use level criteria and are safe for use in cultivated meat and seafood.
For substances with no established DRI, the concentrations of the substance in conventional meat and seafood or other foods (if they are not present in conventional meat and seafood) are used to establish SAMI use levels. The use level is derived by calculating 10% above the reported concentrations in conventional meat and seafood or other conventional foods, as previously described.
For substances without DRIs or reported concentrations in meat, seafood, or other foods, the SAMI use levels for Category 2 components are derived from a Margin of Exposure (MOE) calculation using an established NOAEL value, according to the principles described by FAO/WHO (2009). NOAELs are established from relevant and scientifically sound animal or human dietary studies. Appropriate uncertainty factors are applied to account for interspecies differences, intraspecies variability, and other uncertainties in the data. For example, an uncertainty factor of 100 is used to convert the NOAEL from a study in experimental animals (e.g., repeated dose 90-day oral toxicity study in rats) into a mg/kg SAMI level. The mg/kg SAMI level is then multiplied by a conservative adult body weight value of 60 kg to reach the mg/day SAMI level. A body weight of 60 kg is used as a conservative body weight for most populations (FAO/WHO, 2020). For the initial SAMI list, DRIs or concentrations in meat, seafood, or other foods exist for all components; therefore, derivation of a safe-use level based on a NOAEL was not required.
If a Category 2 component is estimated or measured in the cultivated meat or seafood product at a concentration above the SAMI use level, this is not an indication the level is unsafe, only that additional safety demonstration is required to reach a conclusion of safety under the conditions of intended use.
Media components must also meet regional requirements, including the specified standards for food-grade substances (e.g., those set by the FCC or JECFA) or similar, where applicable.
Engagement with Stakeholders
Two workshops were held to present the SAMI list, categorization approach, and SAMI use level calculations to cultivated meat and seafood stakeholders and receive feedback on the SAMI framework approach. The stakeholders included representatives of cultivated meat companies, culture media companies, academia, governmental, non-governmental, and other organizations interested in the field of cultivated meat and seafood. A brief overview of the SAMI list methodology including the media categorization approach and high-level explanation of the use level calculations was presented alongside the media components currently included on the SAMI list and their respective use levels. The first workshop was held in Amsterdam, Netherlands on October 24, 2024 as a part of the Regulating the Future of Food Conference was attended by 20 individuals. The second workshop was held at the Marina Bay Sands Conference Center, Singapore on November 18, 2024 as a part of the Roundtable on Novel Food Regulations organised by the Singapore Food Agency during Singapore International Agri-Food Week 2024 included around 120 participants.
Many participants provided feedback on the SAMI framework, including its overall concept, the categorization of media components, the methodology to derive the SAMI use levels, and additional considerations for its implementation. They also highlighted needs associated with assessing media components beyond Category 1 and 2 substances. Overall, there was consensus that the framework could enhance regulatory and industry efficiency while maintaining safety. However, participants emphasized the need for flexibility to accommodate jurisdictional difference in factors such as body weight, serving sizes, and DRIs. Some participants noted that listing final residue level poses practical challenges for producers compared to specifying allowable concentrations in manufacturing. However, from a safety assessment perspective, the proposed approach is more practical as it accounts for variations in manufacturing processes—where the same substance concentration can result in different residue levels depending on the process used. The SAMI approach remains aligned with regulatory frameworks such as maximum residue limits for agricultural residues. There was discussion on the appropriate unit for reporting exposure levels. While some participants suggested using mg/kg, expressing limits in mg/day was identified to be a more suitable approach, as it accounts for variations in serving sizes across different products. There was a strong interest in expanding the SAMI framework to include additional substances, particularly those beyond Category 1 and 2. The collected feedback has been incorporated into the methodology and informed next steps outlined in this paper.
SAMI List Use Levels
The proposed SAMI use levels are expressed in milligrams (mg) per day, facilitating an exposure-based level in cultivated meat and seafood products. The SAMI use levels are presented in mg/day to provide cultivated meat and seafood companies flexibility in product development. Units of mg/day allow companies to account for custom serving sizes for products as long as the concentration present in the anticipated consumed volume of their product in one day (generally assumed as one serving) does not exceed the SAMI use level. Providing the SAMI use levels in other units, such as concentration in the product (mg/kg), does not consider serving sizes for various types of cells (e.g., muscle, fat) across different products (e.g., chicken breast vs. sausage). The SAMI use levels in mg/day also takes into account variation in recommended serving sizes across different regions. This approach provides all jurisdictions with the flexibility to adapt SAMI use levels with consideration to local consumption patterns, DRI values, background exposure levels and average body weights. Additionally, providing the SAMI use levels as concentration in media (mg/L) was not considered as it does not account for the differences that occur in cultivated meat and seafood manufacturing and culturing processes, nor does it provide final product safety demonstration. Therefore, units of mg/day for SAMI use levels allow companies and regulatory reviewers to identify the serving size for products and demonstrate that daily consumption of the substance in a cultivated meat or seafood product does not pose a safety hazard. For cultivated meat and seafood products that are consumed in smaller volumes, a higher concentration of the component in the final product may be acceptable, and conversely, lower concentrations may be warranted for cultivated products consumed in larger quantities.
Risk assessors may use the SAMI use level as follows: The concentration of a specific culture media component is estimated or measured in the final cultivated meat and seafood product (mg/kg) and multiplied by a daily serving size (kg/day), resulting in a daily intake value (mg/day). The daily intake value is compared to the SAMI use level. This list is intended to assist risk assessors in their evaluation of culture media inputs; however, the SAMI use levels may be tailored to adhere to the requirements of different jurisdictions. Culture media components lower than the reported SAMI use level are considered to be safe for their intended use in cultivated meat and seafood products. Conversely, higher concentrations do not signify that the final product is unsafe; rather, they indicate that further safety assessment is necessary to conclude safety of the component under the intended usage conditions.
Equation 2: Calculation of daily intake
SAMI use levels for the components included in the Phase 1 list have been derived using Equation 2 and summarized in
Table 2. The Chemical Abstract Service (CAS) registry numbers for the components originate from the specifications of relevant salt forms and isomers (for example, amino acid isomers) as listed in the FCC and JECFA specifications. Future versions of SAMI list may be expanded to include CAS numbers of additional salt derivatives or isomers that currently lack specifications.
Table 2.
Phase 1 SAMI list Components with Use Levels.
Table 2.
Phase 1 SAMI list Components with Use Levels.
| Component |
CAS Number |
Type of Component |
Category |
FCC/ JECFA Spec |
Derivation of SAMI Use level |
SAMI use level (mg/day) |
| Glycine |
56-40-6 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
1600 |
| L-Alanine |
56-41-7
302-72-7 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1400 |
| L-Arginine hydrochloride |
74-79-3
1119-34-2 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1400 |
| L-Asparagine monohydrate |
70-47-3
5794-13-8 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
3300 |
| L-Aspartic Acid |
56-84-8
617-45-8 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
2600 |
| L-Cysteine |
52-89-1
7048-04-6 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
270 |
| L-Glutamic Acid |
56-86-0
138-15-8 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
3500 |
| L-Glutamine |
56-85-9 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
3500 |
| L-Histidine hydrochloride monohydrate |
5934-29-2
71-00-1 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
1100 |
| L-Isoleucine |
73-32-5
443-79-8 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
1100 |
| L-Leucine |
61-90-5
328-39-2 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1800 |
| L-Lysine hydrochloride |
657-27-2 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
2100 |
| L-Methionine |
63-68-3
59-51-8 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
710 |
| L-Phenylalanine |
63-91-2
150-30-1 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
900 |
| L-Proline |
147-85-3 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1100 |
| L-Serine |
56-45-1
302-84-1 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
900 |
| L-Threonine |
72-19-5 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1100 |
| L-Tryptophan |
73-22-3
54-12-6 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
280 |
| L-Tyrosine sodium salt dihydrate |
60-18-4 |
Amino Acid |
2 |
Yes |
Concentration in meat and seafood |
1000 |
| L-Valine |
72-18-4 |
Amino Acid |
1 |
Yes |
Concentration in meat and seafood |
1200 |
| Linoleic acid |
60-33-3 |
Fatty acid |
1 |
Yes |
DRI |
2200 |
| Lipoic acid |
1077-28-7 |
Fatty acid |
2 |
No |
Concentration in meat and seafood |
0.099 |
| Myristic acid |
544-63-8 |
Fatty acid |
1 |
Yes |
Concentration in meat and seafood |
2600 |
| Oleic acid |
112-80-1
143-19-1 |
Fatty acid |
1 |
Yes |
Concentration in meat and seafood |
26000 |
| Palmitic acid |
57-10-3 |
Fatty acid |
1 |
Yes |
Concentration in meat and seafood |
15000 |
| Stearic acid |
57-11-4
1592-23-0 |
Fatty acid |
1 |
Yes |
DRI |
5700 |
| Calcium chloride |
10043-52-4
10035-04-8 |
Inorganic salt |
2 |
Yes |
DRI |
Calcium - 1000 |
| DRI |
Chloride - 3100 |
| Cupric Sulphate |
7758-98-7
7758-99-8 |
Inorganic salt |
2 |
Yes |
DRI |
Copper – 0.9 |
| Concentration in conventional food |
Sulphate - 100 |
| Ferric Ammonium Citrate |
1185-57-5 |
Inorganic salt |
2 |
Yes |
DRI |
Iron - 8 |
| Concentration in meat and seafood |
Ammonium - 18 |
| Concentration in conventional food |
Citrate - 3000 |
| Ferric Nitrate |
7782-61-8 |
Inorganic salt |
2 |
No |
DRI |
Iron - 8 |
| DRI |
Nitrate - 220 |
| Ferric Sulphate |
7782-63-0
7720-78-7 |
Inorganic salt |
2 |
Yes |
DRI |
Iron - 8 |
| Concentration in conventional food |
Sulphate - 100 |
| Magnesium Chloride |
7791-18-6 |
Inorganic salt |
2 |
Yes |
DRI |
Magnesium - 320 |
| DRI |
Chloride - 3100 |
| Magnesium Sulphate |
14168-73-1
10034-99-8
15244-36-7 |
Inorganic salt |
2 |
Yes |
DRI |
Magnesium - 320 |
| Concentration in conventional food |
Sulphate - 100 |
| Potassium Chloride |
7447-40-7 |
Inorganic salt |
1 |
Yes |
DRI |
Potassium - 2600 |
| DRI |
Chloride - 3100 |
| Sodium Bicarbonate |
144-55-8 |
Inorganic salt |
1 |
Yes |
DRI |
Sodium - 2000 |
| Concentration in conventional food |
Carbonate - 6600 |
| Sodium Chloride |
7647-14-5 |
Inorganic salt |
1 |
Yes |
DRI |
Sodium - 2000 |
| DRI |
Chloride - 3100 |
| Sodium Phosphate Dibasic |
7558-79-4
10028-24-7 |
Inorganic salt |
2 |
Yes |
DRI |
Sodium - 2000 |
| DRI |
Phosphorus - 800 |
| Sodium Phosphate Monobasic |
7558-80-7
10049-21-5 |
Inorganic salt |
2 |
Yes |
DRI |
Sodium - 2000 |
| DRI |
Phosphorus - 800 |
| Sodium pyruvate |
113-24-6 |
Inorganic salt |
1 |
No |
DRI |
Sodium - 2000 |
| Concentration in conventional food |
Pyruvate – 4.3 |
| Sodium Selenite |
10102-18-8
13410-01-0 |
Inorganic salt |
2 |
Partial (selenate) |
DRI |
Sodium - 2000 |
| DRI |
Selenium – 0.055 |
| Zinc Sulphate |
7446-20-0
7446-19-7 |
Inorganic salt |
2 |
Yes |
DRI |
Zinc - 8 |
| Concentration in conventional food |
Sulphate - 120 |
| Biotin (Vitamin B7) |
58-85-5 |
Vitamin |
1 |
Yes |
DRI |
0.03 |
| Cobalamin (Vitamin B12) |
68-19-9 |
Vitamin |
1 |
Yes |
DRI |
2.4 |
| Choline Chloride (Vitamin B4) |
67-48-1 |
Vitamin |
2 |
Yes |
DRI |
430 |
| D-Calcium pantothenate (Vitamin B5) |
137-08-6
6381-63-1
6363-38-8 |
Vitamin |
1 |
Yes |
DRI |
5 |
| Folic Acid (Vitamin B9) |
59-30-3 |
Vitamin |
2 |
Yes |
DRI |
0.4 |
| Niacinamide (Vitamin B3) |
98-92-0 |
Vitamin |
2 |
Yes |
DRI |
14 |
| Pyridoxine hydrochloride (Vitamin B6) |
58-56-0 |
Vitamin |
2 |
Yes |
DRI |
1.3 |
| Riboflavin (Vitamin B2) |
83-88-5
130-40-5 |
Vitamin |
1 |
Yes |
DRI |
1.1 |
| Thiamine Hydrochloride (Vitamin B1) |
67-03-8
532-43-4 |
Vitamin |
1 |
Yes |
DRI |
1.1 |
| Vitamin A Acetate (Retinyl acetate) |
68-26-8 |
Vitamin |
2 |
Yes |
DRI |
0.7 |
| Vitamin C (Ascorbic Acid) |
50-81-7
134-03-2 |
Vitamin |
2 |
Yes |
DRI |
75 |
| D-Glucose |
50-99-7|58367-01-4|50-99-7 |
Organic compound |
1 |
Yes |
DRI |
50000 |
| Hypoxanthine sodium salt |
45738-97-4 |
Organic compound |
1 |
No |
Concentration in meat and seafood |
130 |
| i-inositol |
87-89-8 |
Organic compound |
1 |
Yes |
Concentration in meat and seafood |
42 |
| Putrescine dihydrochloride |
333-93-7 |
Organic compound |
2 |
No |
Concentration in meat and seafood |
38 |
Derivation of SAMI Use Levels for Category 1 Components
This section explains the approaches employed in establishing SAMI use levels for the subcategories within Category 1 components.
Amino Acids
Category 1 amino acids include L-glutamic acid, L-leucine, L-lysine hydrochloride, L-threonine, L-valine, L-alanine, L-arginine hydrochloride, L-proline, L-serine. These amino acids have a history of safe consumption, have been reviewed as ingredients by panels of experts, and do not have established upper toxicity thresholds. None of these amino acids have established internationally recognized DRIs. There are reported concentrations in conventional meat and seafood. Therefore, the SAMI use level was derived from a 10% exceedance of the reported amino acid concentration in one 90 g serving of conventional meat and seafood.
Fatty Acids
All listed fatty acids (except lipoic acid) are classified as Category 1 components. These fatty acids have a history of safe consumption as a component of food, and do not have established upper toxicity thresholds. Stearic acid and linoleic acid have established ADIs or RDIs which were used to derive the SAMI use level. The remaining fatty acids (myristic acid, oleic acid, and palmitic acid) have reported concentrations in conventional meat and seafood, and SAMI use levels were derived using a 10% exceedance of the reported fatty acid concentration in one 90 g serving of conventional meat and seafood.
Inorganic Salts
Potassium chloride, sodium bicarbonate, and sodium chloride are classified as Category 1. These inorganic salts have a history of safe consumption, have been reviewed for safety by experts, and their respective dissociated ions do not have established upper toxicity thresholds. The SAMI use level for each inorganic salt was derived from the established DRI for the aqueously dissociated ions of the inorganic salt.
Vitamins
Biotin, D-calcium pantothenate, riboflavin, thiamine hydrochloride, and cobalamin are classified as Category 1 components. These vitamins have a history of safe consumption, have been reviewed by expert panels, and do not have established upper toxicity thresholds. The SAMI use level for each vitamin was derived from an established DRI.
Organic Substances
Five other organic substances are included on the SAMI list: D-glucose, i-inositol, hypoxanthine
sodium salt, and putrescine dihydrochloride. Except for putrescine dihydrochloride, these substances were classified as Category 1 components. These organic substances have a history of safe consumption as components of food, and do not have established upper toxicity thresholds. The SAMI use level for D-glucose was derived from the established daily upper intake recommendation level. None of the other organic substances had a DRI value. I-inositol and hypoxanthine sodium salt have reported concentrations in conventional meat and seafood, therefore, the established SAMI use level for these components was derived from a 10% exceedance of the reported concentration of the substance in one 90 g serving of conventional meat and seafood.
Category 1 Case Study – L-Alanine
L-alanine is an amino acid with a long history of safe consumption and has been reviewed by several expert panels and concluded to be safe (FAO/WHO 2005, EFSA 2010). Oral toxicity studies were conducted for L-alanine. A four-week oral toxicity study in Sprague-Dawley rats observed that a repeated oral dose of 2000 mg/kg bw/day of L-alanine did not result in any adverse effects (Aoki et al., 2014). The highest concentration administered in the study was 2000 mg/kg. Therefore, a NOAEL could not be established for L-alanine. Additionally, no established DRIs exist for L-alanine or any of the other amino acids. The concentration of L-alanine in conventional meat and seafood is reported in government food composition databases. The concentrations of L-alanine in beef, pork, chicken, and salmon were obtained from USDA FoodData Central, FSANZ Australian Food Composition Database - Release 2.0, and MEXT - Standard Tables of Food Composition in Japan - 2015 - (Seventh Revised Version). Values from beef, pork, and chicken are used to represent meat and poultry values. Salmon was chosen to represent seafood because of the availability of composition data and because salmon aquaculture is the fastest-growing food production system in the world (WWF 2024).
The L-alanine concentration ranges for beef, pork, chicken, and salmon were 620-1290 mg/100 g, 750-1200 mg/100 g, 984-1300 mg/100 g, and 1200-1390 mg/100 g, respectively. This results in a range of 620-1390 mg/100 g for L-alanine in conventional meat and seafood. The calculated 10% exceedance for that range is 558-1529 mg/100 g. The 10% exceedance of the maximum concentration of L-alanine in conventional meat and seafood was adjusted from a recommended single serving size of 90 g of conventional meat and seafood to result in the derived SAMI safety limit of 1400 mg/day for L-alanine for one serving of cultivated meat and seafood. Final cultivated meat and seafood products containing L-alanine in concentrations equal to or less than 1400 mg/day are similar to levels in conventional food and do not pose a food safety concern. Cultivated meat and seafood products containing concentrations of L-alanine that result in consumption of greater than 1400 mg per day of L-alanine fall outside of the scope of the SAMI list and require additional justification effort to demonstrate safety at the intended use levels. Note than an exceedance of the SAMI use level indicates only that additional analysis is required, not that the product is not safe for consumption.
Derivation of SAMI Use Levels for Category 2 Components
This section describes the approach used in establishing SAMI use levels for the subcategories within Category 2 components.
Amino Acids
The Category 2 amino acids include glycine, L-cysteine, L-isoleucine, L-methionine, L-phenylalanine, L-tryptophan, L-asparagine monohydrate, L-aspartic acid, L-glutamine, L-histidine monohydrate, and L-tyrosine sodium salt dihydrate. These amino acids: have a history of safe consumption; have been reviewed by expert panels; and have established NOAEL values. These amino acids do not have internationally recognized established DRI values. However, they did have reported concentrations in conventional meat and seafood. Therefore, the established SAMI use level was derived from a 10% exceedance of the reported amino acid concentration in a 90 g serving of conventional meat and seafood.
Fatty Acids
Lipoic acid is classified as a Category 2 component. Lipoic acid has a history of safe consumption as a component of food and has an established toxicological threshold (a NOAEL value). There is not an established DRI value for lipoic acid. However, there are reported concentrations of lipoic acid in conventional meat and seafood. Therefore, the established SAMI use level for lipoic acid was derived from a 10% exceedance of the reported concentration of the fatty acid in a 90 g serving of conventional meat and seafood.
Inorganic Salts
Calcium chloride, cupric sulphate, ferric ammonium citrate, ferric nitrate, ferric sulphate, magnesium chloride, magnesium sulphate, sodium phosphate dibasic, sodium phosphate monobasic, sodium pyruvate, sodium selenite, and zinc sulphate are classified as Category 2 components. Their respective dissociated ions have a history of safe consumption and have been reviewed by expert panels, but also have established UL values. The SAMI use level for each inorganic salt was calculated from the established DRI value for the dissociated ions of each inorganic salt.
Vitamins
Choline chloride, folic acid, niacinamide, pyridoxine hydrochloride, retinyl acetate, and ascorbic acid are classified as Category 2 components. The vitamins have a history of safe consumption and have been reviewed by expert panels, with have established UL values. The SAMI use level for each vitamin was calculated using the established DRI value.
Other Organic Substances
Putrescine dihydrochloride is classified as Category 2 due to a history of safe consumption as a component of food, but has an established toxicological threshold (a NOAEL value). Putrescine does not have an established DRI value. However, there are reports of putrescine concentrations in conventional meat and seafood available in the literature. Putrescine is present in fermented sausages at levels of up to 1550 mg/kg and in fish and fish products at levels of up to 337 mg/kg (EFSA 2011; del Rio et al., 2019). Therefore, the established SAMI use level for putrescine dihydrochloride was derived from a 10% exceedance of the reported concentration in a 90 g serving of conventional meat and seafood.
Category 2 Case Study - Sodium Phosphate Dibasic
Sodium phosphate dibasic is an inorganic salt that dissociates into sodium and phosphate ions in aqueous solution. Sodium and phosphorus have long histories of safe consumption as they are present in conventional foods. In addition, sodium phosphate dibasic has a long history of use in food processing (Gélinas 2022). The FAO/WHO recommends a maximum of 2000 mg/day sodium in adults (FAO/WHO 2014). No ULs have been established for sodium. The FAO/WHO established an RDA of 800 mg/day and a UL of 4000 mg/day for phosphorus (FAO/WHO 2019). Due to the establishment of a UL for the dissociated ions of phosphorus, sodium phosphate dibasic is classified as a Category 2 substance. Sodium and phosphorus have established DRI values, and these were used to derive the SAMI use levels. The SAMI use levels for the dissociated ions of sodium and phosphorus are equal to their established DRI values, 2000 mg/day and 800 mg/day, respectively. Final cultivated meat and seafood products containing sodium in concentrations equal to or less than 2000 mg per day and phosphorus in concentrations equal to or less than 800 mg per day are within the scope of the SAMI list and do not pose a food safety concern at the intended use levels. Cultivated meat and seafood products containing concentrations of sodium greater than 2000 mg/day or concentrations of phosphorus greater than 800 mg/day are outside of the scope of the SAMI list. While this does indicate inherent safety concerns, additional evidence is required to establish safety at the intended use levels.
Uncertainties and Limitations of the SAMI List
The SAMI list is intended to be a non-restrictive list of common culture media inputs and their respective levels of safety in cultivated meat and seafood products. It is intended to be a flexible approach that can be adapted and modified to encompass the differing regional requirements across international jurisdictions. The SAMI list presented here aimed to incorporate available internationally accepted values, or in absence of such values, standard values established by jurisdictions (e.g. average body weight, serving size). The assumptions and data used to create the SAMI list are detailed below for clarification and to assist in the adaptation of the framework to be applicable in other jurisdictions.
The DRI values used to calculate the SAMI use levels were adopted from internationally recognized bodies such as FAO/WHO, however, some jurisdictions may establish their own DRI values; the SAMI use levels may requirement adjustment of DRI-derived levels to reflect the jurisdiction of interest. Additionally, the DRI values chosen are generally applicable to healthy, adult (19+ years of age) individuals. The SAMI use levels do not encompass how the safety levels may change for sensitive subpopulations such as immuno-compromised, hyper-sensitive, or chronically ill individuals. Further, background exposure levels have not been specifically incorporated beyond consideration of conventional food comparators.
The SAMI use levels derived from conventional concentrations were obtained from a 10% exceedance of the reported measured concentration range for comparable meat products. There is a large range of variation in the concentration of substances in meat products due to species variation, variation in the animal itself across the different cuts of meat, and different breeds of animals in the same species (Yaranoğlu et al., 2023; Jiménez-Colmenero et al., 2010; Marshall 1994). Variations in an animal's diet, environmental conditions, and management practices can significantly influence its physiological composition and the levels of micro- and macro-nutrients in conventional meat (Średnicka-Tober et al., 2016). The values collected from government databases only represented one measured datapoint of a specific food item, not an average compositional assessment obtained from measurements across multiple meat and seafood samples. Additionally, the US, Australia, and Japan were the only regions represented in the collected data due to the availability of information for the components of interest. When concentrations of the component of interest were not reported in the governmental composition databases, the levels were obtained from peer-reviewed literature that fully described the methods of concentration analysis (Wu et al., 2016; McLean et al., 2024; FoodData Central 2020; FoodData Central 2019a; Kopec et al., 2020; FoodData Central 2019b; Kaminska and Chwatko, 2020; Mattulat and Baltes, 1992; Young 1982; Kaneko et al., 2014; Clements and Darnell, 1980; del Rio et al., 2019; Schirone et al., 2022; Florin et al., 1993; Sun Yoo and Pike, 2001; Rudman et al., 1973; Granchi et al., 2019; Haleblian et al., 2008; National University of Singapore 2021; Oregon Department of Human Services 1998; HealthHub 2022).
Next Steps
This paper is intended to serve as a starting point for discussion toward a more efficient safety assessment methodology of the inputs to cultivated meat and seafood products. The ultimate aim of the SAMI framework is for the categorisation framework and SAMI list to be a guide employed by companies for their use of media components and development of safety dossiers for regulatory approval, and for regulatory bodies across jurisdictions to support the development of a harmonised international approach to the safety assessment of media components.
Feedback from stakeholders plays a vital role in enhancing the SAMI risk assessment framework and the SAMI list, as we aim to ensure that the methods used align with international food risk assessment standards, providing value to the industry, regulators, and other involved parties. Additionally, this project may encourage non-governmental bodies such as the FCC or JECFA to develop additional specifications for the SAMI culture media inputs currently lacking specifications or create culture media specific specifications for some components.
The next step is to continue gathering stakeholder feedback on the categorization approach and SAMI list. The vision is to expand the list to include additional Category 1 and 2 components commonly used in cultivated meat and seafood production, include other subcategories of substances such as metabolites of the culturing process and allergens, and, as more data is developed, eventually include substances that may not have an established history of safe use in food, such as recombinant growth factors. The SAMI list may also expand to include categorization and assessment of more complex macromolecules that may be used in the cultivated meat and seafood process such as scaffolds, microcarriers, animal sera, and complex extracts from biological sources.
Collecting stakeholder feedback on the current version of the SAMI list helped identify outstanding questions. Future stakeholder engagement is intended to gather feedback from more non-governmental bodies such as the FCC, JECFA, and the Organisation for Economic Co-operation and Development (OECD). Further outreach will assist in optimizing the SAMI list to incorporate additional substances and be flexible enough to be an approach that is adopted across all jurisdictions.
As the SAMI framework continues to be developed and expanded, new substances such as substances without a history of safe use in food may require the development of new analytical methods for measuring components in the final cultivated meat and seafood products or to measure the stability of components in cultivated meat and seafood after cooking and digestion.
References
- Aoki, M., Mochizuki, M., Okamura, T., Hatayama, K., Nakamura, A., & Morishita, K. (2014). A 4-week oral toxicity study of L-alanine in rats with a recovery period of 2 weeks. Fundamental Toxicological Sciences, 1(2), 63-72. [CrossRef]
- Clements Jr, R. S., & Darnell, B. (1980). Myo-inositol content of common foods: development of a high-myo-inositol diet. The American Journal of Clinical Nutrition, 33(9), 1954-1967. [CrossRef]
- Crippa, M., Solazzo, E., Guizzardi, D., Monforti-Ferrario, F., Tubiello, F. N., & Leip, A. J. N. F. (2021). Food systems are responsible for a third of global anthropogenic GHG emissions. Nature food, 2(3), 198-209. [CrossRef]
- Del Rio, B., Redruello, B., Linares, D. M., Ladero, V., Ruas-Madiedo, P., Fernandez, M.,... & Alvarez, M. A. (2019). The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Scientific reports, 9(1), 120. [CrossRef]
- EFSA. (2010). Amino acids from chemical group 34 Flavouring Group Evaluation 26, Revision 1-Scientific opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC). EFSA Journal, 6(8), 790. [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). (2011). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Journal, 9(10), 2393. [CrossRef]
- FAO/WHO. (2005). Evaluation of certain food additives. WHO. https://iris.who.int/bitstream/handle/10665/43141/WHO_TRS_928.pdf;jsessionid=8A30DFA0B27BD6DC71FAE86097287D00?sequence=1.
- FAO. (2006). Livestock’s Long Shadow. FAO. https://openknowledge.fao.org/server/api/core/bitstreams/36ade937-4641-46ed-aac4-6162717d8a7f/content.
- FAO/WHO. (2009). Principles and Methods for the Risk Assessment of Chemicals in Food. WHO. https://iris.who.int/bitstream/handle/10665/44065/WHO_EHC_240_eng.pdf.
- FAO/WHO. (2014). Guideline: Sodium intake for adults and children. WHO. https://iris.who.int/bitstream/handle/10665/77985/9789241504836_eng.pdf.
- FAO/WHO. (2019). Codex nutrient reference values. FAO. https://openknowledge.fao.org/server/api/core/bitstreams/2033128c-4d26-47d0-8f67-2f736c4a1d29/content.
- FAO/WHO. (2020). Dietary Exposure Assessment for Chemicals in Food. In Environmental Health Criteria 240: Principles and Methods for the Risk Assessment of Chemicals in Food (2nd ed., Chapter 6). Retrieved from https://www.who.int/docs/default-source/chemical-safety/ehc240-chapter6-edited(4-1).pdf.
- FDA. (2008). Animal cloning: A risk assessment. FDA. https://public4.pagefreezer.com/browse/FDA/06-04-2023T20:24/https://www.fda.gov/media/75280/download.
- FDA. (2015). Freedom of information summary. Original new animal drug application, NADA 141-454. opAFP-GHc2 rDNA construct in EO-1α lineage Atlantic salmon (AquAdvantage Salmon). FDA. https://www.fda.gov/files/animal%20&%20veterinary/published/AquAdvantage-Salmon-FOI-Summary.pdf.
- Florin, T. H., Neale, G., Goretski, S., & Cummings, J. H. (1993). The sulfate content of foods and beverages. Journal of food composition and analysis, 6(2), 140-151. [CrossRef]
- FoodData Central. (2019a). Fish, bass, striped, raw. FDC. Retrieved from https://fdc.nal.usda.gov/food-details/171948/nutrients. Accessed February 15, 2025.
- FoodData Central. (2019b). Chicken, broilers or fryers, meat and skin, cooked, roasted. FDC. Retrieved from https://fdc.nal.usda.gov/food-details/171450/nutrients. Accessed February 15, 2025.
- FoodData Central. (2020). [HISTORICAL RECORD]: 6/8 OZ CO V/P REDFISH. FDC. Retrieved from https://fdc.nal.usda.gov/food-details/763384/nutrients. Accessed February 15, 2025.
- FSANZ. (n.d.). Australian Food Composition Database - Release 2.0. Retrieved from https://afcd.foodstandards.gov.au/. Accessed February 15, 2025.
- Gélinas, P. (2022). Inventions on phosphates for chemical leavening. International Journal of Food Science & Technology, 57(5), 2840–2861. [CrossRef]
- Granchi, D., Baldini, N., Ulivieri, F. M., & Caudarella, R. (2019). Role of citrate in pathophysiology and medical management of bone diseases. Nutrients, 11(11), 2576. [CrossRef]
- Haleblian, G. E., Leitao, V. A., Pierre, S. A., Robinson, M. R., Albala, D. M., Ribeiro, A. A., & Preminger, G. M. (2008). Assessment of citrate concentrations in citrus fruit-based juices and beverages: implications for management of hypocitraturic nephrolithiasis. Journal of endourology, 22(6), 1359-1366. [CrossRef]
- HealthHub. (2022). Know Your Servings: Photo Guide. Retrieved from https://www.healthhub.sg/live-healthy/know-your-servings-photo-guide. Accessed February 15, 2025.
- Jiménez-Colmenero, F., Pintado, T., Cofrades, S., Ruiz-Capillas, C., & Bastida, S. (2010). Production variations of nutritional composition of commercial meat products. Food Research International, 43(10), 2378-2384. [CrossRef]
- Kamińska, A., & Chwatko, G. (2020). Estimation of lipoyllysine content in meat and its antioxidative capacity. Journal of Agricultural and Food Chemistry, 68(39), 10992-10999. [CrossRef]
- Kaneko, K., Aoyagi, Y., Fukuuchi, T., Inazawa, K., & Yamaoka, N. (2014). Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biological and Pharmaceutical Bulletin, 37(5), 709-721. [CrossRef]
- Kopec, W., Jamroz, D., Wiliczkiewicz, A., Biazik, E., Pudlo, A., Korzeniowska, M.,... & Skiba, T. (2020). Antioxidative characteristics of chicken breast meat and blood after diet supplementation with carnosine, L-histidine, and β-alanine. Antioxidants, 9(11), 1093. [CrossRef]
- Marshall, D. M. (1994). Breed differences and genetic parameters for body composition traits in beef cattle. Journal of animal science, 72(10), 2745-2755. [CrossRef]
- Mattulat, A., & Baltes, W. (1992). Determination of lipoic acid in meat of commercial quality. Zeitschrift fur Lebensmittel-untersuchung und-forschung, 194(4), 326-329. [CrossRef]
- McLean, E., Alfrey, K. B., Gatlin III, D. M., Gaylord, T. G., & Barrows, F. T. (2024). Muscle amino acid profiles of eleven species of aquacultured animals and their potential value in feed formulation. Aquaculture and Fisheries, 9(4), 642-652. [CrossRef]
- MEXT. (2015). STANDARD TABLES OF FOOD COMPOSITION IN JAPAN - 2015 - (Seventh Revised Version). Retrieved from https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm. Accessed February 15, 2025.
- National University of Singapore. (2021). Maintaining a healthy weight doesn't have to be a struggle. Retrieved from https://www.nus.edu.sg/uhc/articles/details/fruits-and-veggies. Accessed February 15, 2025.
- Oregon Department of Human Services. (1998). HEALTH EFFECTS INFORMATION. https://www.oregon.gov/oha/ph/healthyenvironments/drinkingwater/monitoring/documents/health/caco3.pdf.
- Poore, J., & Nemecek, T. (2018). Reducing food’s environmental impacts through producers and consumers. Science, 360(6392), 987-992. [CrossRef]
- Rudman, D., Smith III, R. B., Salam, A. A., Warren, W. D., Galambos, J. T., & Wenger, J. (1973). Ammonia content of food. The American Journal of Clinical Nutrition, 26(5), 487-490. [CrossRef]
- Schirone, M., Esposito, L., D’Onofrio, F., Visciano, P., Martuscelli, M., Mastrocola, D., & Paparella, A. (2022). Biogenic amines in meat and meat products: a review of the science and future perspectives. Foods, 11(6), 788. [CrossRef]
- Schmeisser, S., Miccoli, A., von Bergen, M., Berggren, E., Braeuning, A., Busch, W.,... & Tralau, T. (2023). New approach methodologies in human regulatory toxicology–Not if, but how and when! Environment International, 178, 108082. [CrossRef]
- SFA. (2023). Requirements for the safety assessment of novel foods and novel food ingredients. SFA. https://www.sfa.gov.sg/docs/default-source/regulatory-standards-frameworks-and-guidelines/requirements-for-the-safety-assessment-of-novel-foods-and-novel-food-ingredients.pdf.
- Sinke, P., Swartz, E., Sanctorum, H., van der Giesen, C., & Odegard, I. (2023). Ex-ante life cycle assessment of commercial-scale cultivated meat production in 2030. International Journal of Life Cycle Assessment, 28(3), 234–254. [CrossRef]
- Średnicka-Tober, D., Barański, M., Seal, C., Sanderson, R., Benbrook, C., Steinshamn, H.,... & Leifert, C. (2016). Composition differences between organic and conventional meat: a systematic literature review and meta-analysis. British Journal of Nutrition, 115(6), 994-1011. [CrossRef]
- USDA. (n.d.). FoodData Central. Retrieved from https://fdc.nal.usda.gov/. Accessed on February 15, 2025.
- Wu, G., Cross, H. R., Gehring, K. B., Savell, J. W., Arnold, A. N., & McNeill, S. H. (2016). Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts. Journal of animal science, 94(6), 2603-2613. [CrossRef]
- WWF. (2004). Farmed Salmon. Retrieved from https://www.worldwildlife.org/industries/farmed-salmon. Accessed February 15, 2025.
- Yaranoğlu, B., Zengin, M., Gökçe, M., Avcılar, Ö. V., Postacı, B. B., Erdoğan, Ç., & Odabaş, E. (2023). Chemical composition of meat from different species of animals. International Journal of Agriculture Environment and Food Sciences, 7(3), 581-587. [CrossRef]
- Yoo, K. S., & Pike, L. M. (1999). Development of an automated system for pyruvic acid analysis in onion breeding. Scientia Horticulturae, 82(3-4), 193-201. [CrossRef]
- Young, L. L. (1982). Purine content of raw and roasted chicken broiler meat. Journal of Food Science, 47(4), 1374-1375. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).