Submitted:
04 March 2025
Posted:
05 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Categories
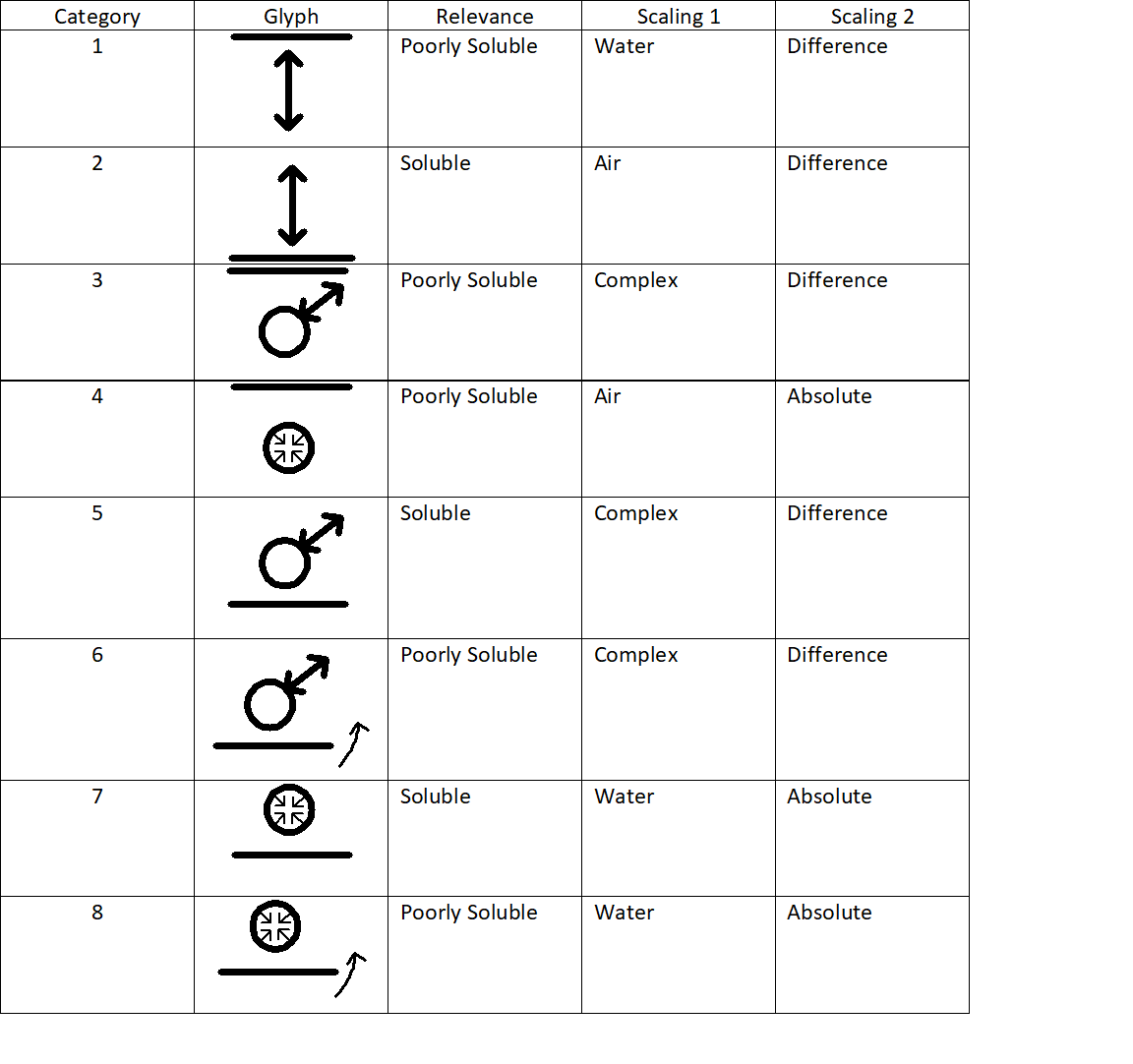
3.1.1. Category 1
3.1.2. Category 2
3.1.3. Category 3
3.1.4. Category 4
3.1.5. Category 5
3.1.6. Category 6
3.1.7. Category 7
3.1.8. Category 8
3.2. Scaling Formulae
3.2.1. Category 1
3.2.2. Category 2
3.2.3. Category 3
3.2.4. Category 4
3.2.5. Category 5
3.2.6. Category 6
3.2.7. Categories 7 and 8
3.3. Quantification
3.3.1. Popular Parameterizations for Poorly Soluble Gases
3.3.2. Parametrizations for Soluble Gases
3.3.3. Quantification of Bubble Fluxes
3.3.4. Quantification of Spray-Mediated Fluxes
3.3.5. Constraint by Evaporative Fluxes
- When the water is supersaturated, a high ejection rate will be interpreted as a positive anomaly in transfer velocity;
- When the water is undersaturated, a high ejection rate will be interpreted as a negative anomaly in transfer velocity;
- Ejection rates are proportional to the absolute concentration, while the direct flux is proportional to the difference in concentration, implying a relatively large effect of ejection on calculated transfer velocity for near-saturation conditions.
3.3.6. Constraint by Sea Spray Generation Fluxes
4. Discussion
5. Conclusions
- Carbon dioxide;
- Highly soluble gases
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COARE | Coupled Ocean-Atmosphere Response Experiment |
| IUPAC | International Union of Pure and Applied Chemistry |
| SSGF | Sea Spray Generation Function |
References
- Whitman, W.G. The two-film theory of gas absorption. Chem. Metall. Eng. 1923, 29, 146–148. [Google Scholar] [CrossRef]
- Liss, P.S.; Slater, P.G. Flux of gases across the air-sea interface. Nature 1974, 247, 181–184. [Google Scholar] [CrossRef]
- Deike, L. Mass transfer at the ocean-atmosphere interface: The role of wave breaking, droplets, and bubbles. Annu. Rev. Fluid Mech. 2022, 54, 191–224. [Google Scholar] [CrossRef]
- Woolf, D.K.; Thorpe, S.A. Bubbles and the air-sea exchange of gases in near-saturation conditions. J. Mar. Res. 1991, 49, 435–466. [Google Scholar] [CrossRef]
- Leighton, T.G.; Coles, D.G.H.; Srokosz, M.; White, P.; Woolf, D.K. Asymmetric transfer of CO2 across a broken sea surface. Scientific Reports 2018, 8, 8301. [Google Scholar] [CrossRef]
- Ulseth, A.J.; Hall, R.O.; Canadell, M.B.; Madinger, H.L.; Niayifar, A.; Battin, T.J. Distinct air-water gas exchange regimes in low-and high-energy streams. Nat. Geosci. 2019, 12, 259–263. [Google Scholar] [CrossRef]
- Andreas, E.L.; Vlahos, P.; Monahan, E.C. Spray-mediated air-sea gas exchange: The governing time scales. J. Mar. Sci. Eng. 2017, 5, 60. [Google Scholar] [CrossRef]
- Staniec, A.; Vlahos, P.; Monahan, E.C. The role of sea spray in atmosphere-ocean gas exchange. Nat. Geosci. 2021, 14, 593–598. [Google Scholar] [CrossRef]
- Bolin, B. On the exchange of carbon dioxide between the atmosphere and the sea. Tellus 1960, 12, 274–281. [Google Scholar] [CrossRef]
- Fairall, C.W.; Hare, J.E.; Edson, J.B.; McGillis, W.R. Measurement and parameterization of the air-sea gas transfer. Bound.-Layer Meteor. 2000, 96, 63–105. [Google Scholar] [CrossRef]
- Woolf, D.K.; Land, P.E.; Shutler, J.D.; Goddijn-Murphy, L.M.; Donlon, C.J. On the calculation of air-sea fluxes of CO2 in the presence of temperature and salinity gradients. J. Geophys. Res. Oceans 2026, 121, 1229–1248. [Google Scholar] [CrossRef]
- Woolf, D.K. Bubbles and the air-sea transfer velocity of gases. Atmosphere-Ocean 1993, 31, 517–540. [Google Scholar] [CrossRef]
- Bell, T.G.; Landwehr, S.; Miller, S.D.; de Bruyn, W.J.; Callaghan, A.H.; Scanlon, B.; Ward, B.; Yang, M.; Saltzman, E.S. Estimation of bubble-mediated air-sea gas exchange from concurrent DMS and CO2 transfer velocities at intermediate-high wind speeds. Atmos. Chem. Phys. 2017, 17, 9019–9033. [Google Scholar] [CrossRef]
- Liang, J.-H.; Deutsch, C.; McWilliams, J.C.; Baschek, B.; Sullivan, P.P.; Chiba, D. Parameterizing bubble-mediated air-sea gas exchange and its effect on ventilation. Global Biogeochem. Cycles 2013, 27, 894–905. [Google Scholar] [CrossRef]
- Woolf, D.K. Gas transfer in energetic conditions. Geophys. Monogr. Ser. 2002, 127, 205–211. [Google Scholar] [CrossRef]
- Harb, C.; Foroutan, H. Experimental development of a lake spray source function and its model implementation for Great Lakes surface emissions. Atmos. Chem. Phys. 2022, 22, 11759–11779. [Google Scholar] [CrossRef]
- Sander, R.; Acree, W.E.; de Visscher, A.; Schwartz, S.E.; Wallington, T.J. Henry’s law constants (IUPAC Recommendations 2021). Pure Appl. Chem. 2021, 94, 71–85. [Google Scholar] [CrossRef]
- Wright, J.; Colling, A. Seawater: Its Composition, Properties and Behaviour, 2nd ed.; Elsevier, 1995; pp. 94.
- Andreas, E.L. Time constants for the evolution of sea spray droplets. Tellus 1990, 42B, 481–497. [Google Scholar] [CrossRef]
- Fairall, C.W.; Yang, M.; Bariteau, L.; Edson, J.B.; Helmig, D.; McGillis, W.; Pezoa, S.; Hare, J.E.; Huebert, B.; Blomquist, B. Implementation of the Coupled Ocean-Atmosphere Response Experiment flux algorithm with CO2, dimethyl sulfide and O3. J. Geophys. Res. Oceans 2011, 116, C00F09. [Google Scholar] [CrossRef]
- Ford, D.J.; Shutler, J.D.; Blanco-Sacristán, J.; Corrigan, S.; Bell, T.G.; Yang, M.; Kitidis, V.; Nightingale, P.D.; Brown, I.; Wimmer, W.; Woolf, D.K.; Casal, T.; Donlon, C.; Tilstone, G.H.; Ashton, I. Enhanced ocean CO2 uptake due to near-surface temperature gradients. Nat. Geosci. 2024, 17, 1135–1140. [Google Scholar] [CrossRef]
- Nightingale, P.D.; Malin, G.; Law, C.S.; Watson, A.J.; Liss, P.S.; Liddicoat, M.J.; Boutin, J.; Upstill-Goddard, R.C. In situ evaluation of air-sea gas exchange using novel conservative and volatile tracers. Global Biogeochem. Cycles 2000, 14, 373–387. [Google Scholar] [CrossRef]
- Ho, D.T.; Wanninkhof, R.; Schlosser, P.; Ullman, D.S.; Hebert, D.; Sullivan, K.F. Toward a universal relationship between wind speed and gas exchange: Gas transfer velocities measured with 3He/SF6 during the Southern Ocean Gas Exchange Experiment. J. Geophys. Res. Atmospheres 2011, 116, C00F04. [Google Scholar] [CrossRef]
- Yang, M.; Moffat, D.; Dong, Y.; Bidlot, J.-R. Deciphering the variability in air-sea gas transfer due to sea state and wind history. PNAS Nexus 2024, 3, pgae389. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean revisited. Limnol. Oceanogr. Methods 2014, 12, 351–362. [Google Scholar] [CrossRef]
- Goddijn-Murphy, L.; Woolf, D.K.; Marandino, C. Space-based retrievals of air-sea gas transfer velocities using altimeters: Calibration for dimethyl sulfide. J. Geophys. Res. Oceans 2012, 117, C007535. [Google Scholar] [CrossRef]
- Andreas, E.L. Fallacies of the enthalpy transfer coefficient over the ocean in high winds. J. Atmos Sci. 2011, 68, 1435–1445. [Google Scholar] [CrossRef]
- Johnson, M. A numerical scheme to calculate temperature and salinity dependent air-water transfer velocities for any gas. Ocean Sci. 2010, 6, 913–932. [Google Scholar] [CrossRef]
- Yang, M.; Blomquist, B.W.; Nightingale, P.D. Air-sea exchange of methanol and acetone during HiWinGS; Estimation of air phase, water phase gas transfer velocities. J. Geophys. Res. Oceans 2014, 119, 7308–7323. [Google Scholar] [CrossRef]
- Yang, M.; Bell, T.G.; Blomquist, B.W.; Fairall, C.W.; Brooks, I.M.; Nightingale, P.D. Air-sea transfer of gas phase controlled compounds. IOP Conf. Series: Earth and Environmental Science 2016, 35, 012011. [Google Scholar] [CrossRef]
- Graham, A.; Woolf, D.K.; Hall, A.J. Aeration due to breaking waves. Part I: Bubble populations. J. Phys. Oceanogr. 2004, 34, 989–1007. [Google Scholar] [CrossRef]
- Czerski, H.; Brooks, I.M.; Gunn, S.; Pascal, R.; Matei, A.; Blomquist, B. Ocean bubbles under high wind conditions – Part 1: Bubble distribution and development. Ocean Sci. 2022, 18, 565–586. [Google Scholar] [CrossRef]
- Czerski, H.; Brooks, I.M.; Gunn, S.; Pascal, R.; Matei, A.; Blomquist, B. Ocean bubbles under high wind conditions - Part 2: Bubble size distributions and implications for models of bubble dynamics. Ocean Sci. 2022, 18, 587–608. [Google Scholar] [CrossRef]
- Spitzer, W.S.; Jenkins, W.J. Rates of vertical mixing, gas exchange and new production: Estimates from seasonal gas cycles in the upper ocean near Bermuda. J. Mar. Res. 1989, 47, 169–196. [Google Scholar] [CrossRef]
- Guttiérrez-Loza, L.; Nilsson, E.; Wallin, M.B.; Sahlée, E.; Rutgersson, A. 2022. On physical mechanisms enhancing air-sea CO2 exchange. Biogeosciences 2022, 19, 5645–5665. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Smith, L.; Qian, T.; Dai, A.; Fasullo, J. Estimates of the global water budget and its annual cycle using observational and model data. J. Hydrometeorol. 2007, 8, 758–769. [Google Scholar] [CrossRef]
- Deike, L.; Reichl, B.G.; Paulot, F. A mechanistic sea spray generation function based on the sea state and the physics of bubble bursting. AGU Advances 2022, 3, e2022AV000750. [Google Scholar] [CrossRef]
- Veron, F. Ocean spray. Annu. Rev. Fluid Mech. 2015, 47, 507–538. [Google Scholar] [CrossRef]
- de Leeuw, G.; Andreas, E.L.; Anguelova, M.D.; Fairall, C.W.; Lewis, E.R.; O’Dowd, C.; Schulz, M.; Schwartz, S.E. Production flux of sea spray aerosol. Rev. Geophys. 2011, 49, RG2001. [Google Scholar] [CrossRef]
- Grythe, H.; Ström, J.; Krejci, R.; Quinn, P.; Stohl, A. A review of sea-spray aerosol source functions using a large global set of sea salt aerosol concentration measurements. Atmos. Chem. Phys. 2014, 14, 1277–1297. [Google Scholar] [CrossRef]
- Gong, S. A parameterization of sea-salt aerosol source function for sub- and super-micron particles. Global Biogeochem. Cycles 2003, 17, 1097. [Google Scholar] [CrossRef]
- Matthews, E.; Bannan, T.J.; Khan, M.A.H.; Shallcross, D.E.; Stark, H.; Browne, E.C.; Archibald, A.T.; Mehra, A.; Bauguitte, S.J.-B.; Reed, C.; Thamban, N.M.; Wu, H.; Parker, B.; Lee, J.; Carpenter, L.J.; Yang, M.; Bell, T.G.; Allen, G.; Jayne, J.T.; Percival, C.J.; McFiggans, G.; Gallagher, M.; Coe, H. Airborne observations over the North Atlantic Ocean reveal the importance of gas-phase urea in the atmosphere. PNAS 2023, 120, e2218127120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Conway, W.; Burns, R.; McCann, N.; Maeder, M. Comprehensive study of the hydration and dehydration reactions of carbon dioxide in aqueous solution. J. Phys. Chem. A 2010, 114, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Sander, R. Compilation of Henry's law constants (version 5.0.0) for water as solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
| Category | Glyph | Relevance | Scaling 1 | Scaling 2 |
|---|---|---|---|---|
| 1 | 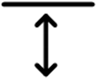 |
poorly soluble | water | difference |
| 2 | 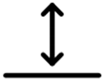 |
soluble | air | difference |
| 3 |  |
poorly soluble | complex | difference |
| 4 | 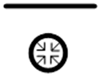 |
poorly soluble | air | absolute |
| 5 | 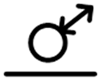 |
soluble | complex | difference |
| 6 | 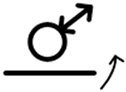 |
poorly soluble | complex | difference |
| 7 |  |
soluble | water | absolute |
| 8 | 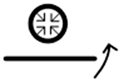 |
poorly soluble | water | absolute |
| pw (μatm) | pw/ Δp | Heff/H | ΔV (cm/h) | ΔV (x10-8 m/s) | HL (Wm-2) |
|---|---|---|---|---|---|
| 600 | 3 | 200 | 0.042 | 12.4 | 285 |
| 600 | 3 | 100 | 0.083 | 24.8 | 570 |
| 600 | 3 | 1 | 8.333 | 2480.2 | 57044 |
| 500 | 5 | 200 | 0.025 | 7.4 | 171 |
| 500 | 5 | 100 | 0.050 | 14.9 | 342 |
| 500 | 5 | 1 | 5.000 | 1488.1 | 34226 |
| 450 | 9 | 200 | 0.014 | 4.1 | 95 |
| 450 | 9 | 100 | 0.028 | 8.3 | 190 |
| 450 | 9 | 1 | 2.778 | 826.7 | 19015 |
| 350 | -7 | 200 | 0.018 | 5.3 | 122 |
| 350 | -7 | 100 | 0.036 | 10.6 | 244 |
| 350 | -7 | 1 | 3.571 | 1062.9 | 24447 |
| 300 | -3 | 200 | 0.042 | 12.4 | 285 |
| 300 | -3 | 100 | 0.083 | 24.8 | 570 |
| 300 | -3 | 1 | 8.333 | 2480.2 | 57044 |
| 200 | -1 | 200 | 0.125 | 37.2 | 856 |
| 200 | -1 | 100 | 0.250 | 74.4 | 1711 |
| 200 | -1 | 1 | 25.000 | 7440.5 | 171131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





