1. Introduction
Breast cancer (BC) is the most common malignancy among women globally, underscoring the importance of continuous advancements in diagnostic imaging to enhance early detection, precise characterization, and effective treatment planning [
1,
2,
3].
Among these innovations, contrast-enhanced mammography (CEM) has emerged as a promising modality, leveraging dual-energy technology to provide both anatomical and functional imaging through the use of contrast medium [
4,
5]. By capturing vascularization and neoangiogenesis characteristics of malignant lesions, CEM offers actionable insights that complement conventional mammography and ultrasound findings. CEM demonstrates notable advantages over magnetic resonance imaging (MRI), including shorter examination times, lower costs, and higher patient acceptability, particularly for individuals who are claustrophobic or have contraindications to MRI [
6]. Studies have demonstrated that CEM achieves comparable diagnostic performance to MRI, especially in preoperative staging, screening high-risk patients with dense breasts, and assessing responses to neoadjuvant therapy [
7,
8,
9,
10]. Moreover, despite its slightly lower sensitivity compared to MRI, CEM demonstrates higher specificity, supporting its use in clinical scenarios requiring precise lesion characterization [
11,
12].
The standardization of CEM reporting advanced significantly with the introduction of the Breast Imaging Reporting and Data System (BI-RADS) for CEM by the American College of Radiology in 2022. The introduction of standardized descriptors has facilitated consistent reporting of findings, enabling detailed evaluation of lesion morphology and contrast enhancement patterns [
13,
14]. Emerging research highlights the correlation between these imaging features and histopathological characteristics, offering potential prognostic insights [
15,
16,
17,
18].
While numerous studies have explored the association between CEM findings and BC characteristics, many have not comprehensively analyzed the full spectrum of imaging features [
19]. This study aims to bridge this gap by evaluating the relationship between CEM morphodynamic features and histopathological parameters, contributing to the growing body of evidence supporting CEM’s diagnostic and prognostic roles in BC management.
2. Materials and Methods
2.1. Study Design and Population
This prospective, monocentric study was approved by the Institutional Review Board (protocol identification number and approval date: 2771-15/12/2022), and written informed consent was obtained from all participants.
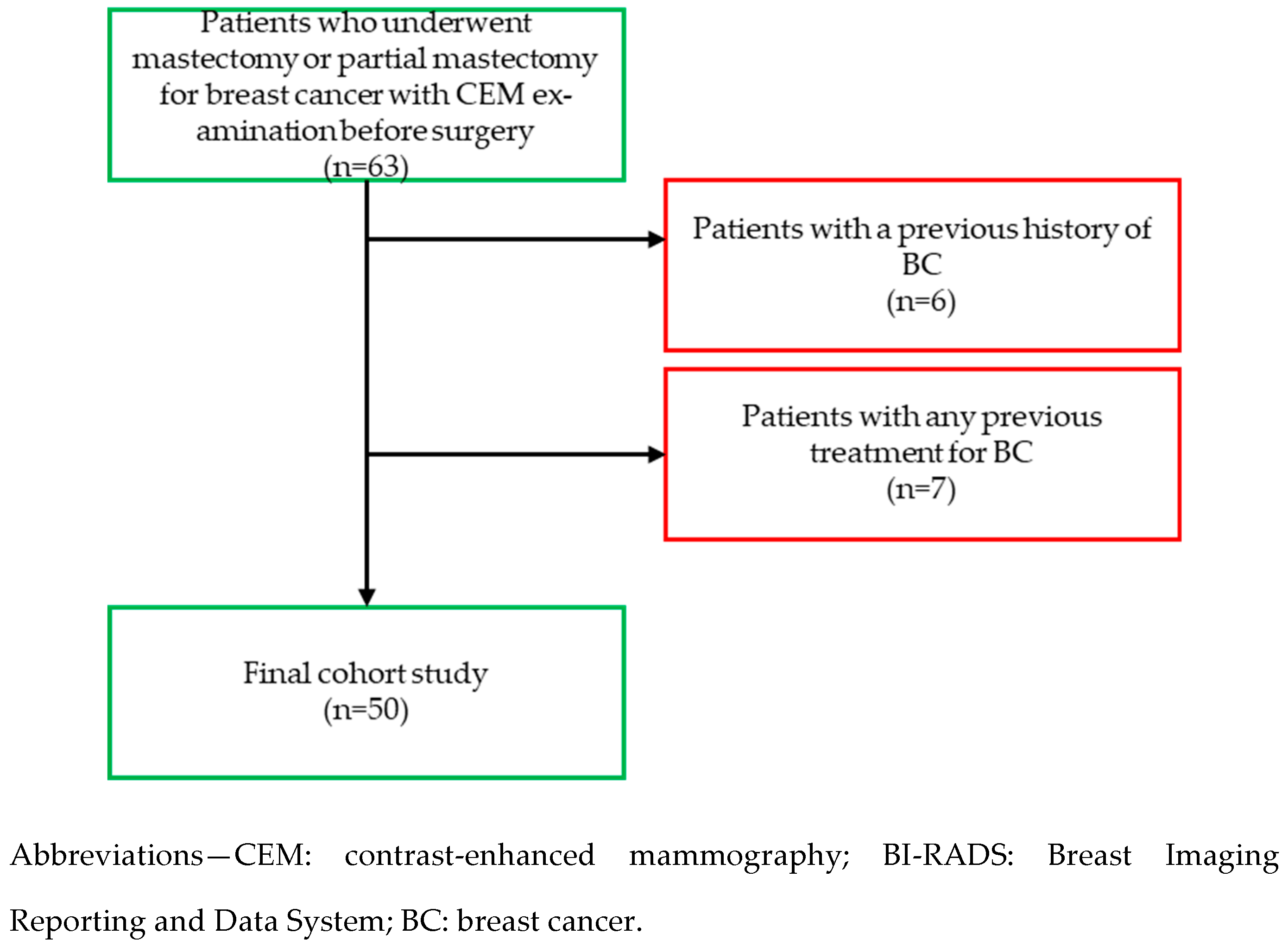
Between December 2022 and May 2024, we consecutively enrolled patients who underwent mastectomy or partial mastectomy for BC with CEM examination before surgery (n=63). The exclusion criteria were: patients with a previous history of BC (n=6); patients with any previous treatment for BC (n=7). For each patient, clinical, radiological, and histopathological data were collected. A total of 50 patients were included in this study (
Scheme 1).
2.2. Image Acquisition
All CEM examinations were performed using a dual-energy mammograph (Giotto Class IMS Series 30000, United States). Following intravenous administration of a non-ionic contrast agent (iopamidol, 1.2 mL/kg at a rate of 3 mL/sec) and saline solution (20 mL at a rate of 3 mL/sec), paired low-energy (LE) (23–32 kVp) and high-energy (45–49 kVp) images were obtained in craniocaudal and mediolateral oblique views. Recombined images were then generated from the LE and high-energy images to highlight areas with contrast enhancement. CEM images were acquired 2 minutes after contrast injection and completed within 10 minutes.
2.3. Reader Study
Two breast radiologists with 15 years of experience independently evaluated all CEM imaging features using the ACR BI-RADS lexicon for CEM (published in 2022). Both radiologists were blinded to clinical and histopathological information. In cases of disagreement, a consensus was reached. For LE images, the following CEM imaging characteristics were analyzed in the study: breast composition (A—almost entirely fatty, B—scattered areas of fibroglandular, C—heterogeneously dense, D—extremely dense); presence of microcalcifications; presence of architectural distortion. For recombined images, the following parameters were evaluated: size; location; shape (oval, round, irregular); margins (circumscribed, not circumscribed); internal enhancement characteristics (homogeneous, heterogeneous, rim enhancement); lesion conspicuity (low, moderate, high); associated features (nipple retraction, nipple invasion, skin retraction, skin thickening, skin invasion, axillary adenopathy). Moreover, background parenchymal enhancement (BPE) was evaluated. In cases with multicentric or multifocal lesions, the lesion with the largest dimension was selected for analysis.
2.4. Histopathological Evaluation
Pathological specimens were obtained through surgical excision and analyzed by a single pathologist with 15 years of experience in breast pathology. The following parameters were evaluated: tumor size; histotype (ductal or lobular); grade (G1, G2, or G3) determined using the Nottingham modification of the Bloom and Richardson method (Elston–Ellis criteria); presence of lymphovascular invasion; immunophenotype (IF) classification, based on the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Immunohistochemical (IHC) testing was used to determine receptor status. According to IHC, nuclear staining ≥10% was regarded as positive for ER and PR status. Expression of HER-2 protein was classified as 0, 1+, 2+, and 3+ by IHC. If a specimen was categorized as 2+, fluorescence in situ hybridization (FISH) was performed to test HER-2 gene. HER-2 positivity was defined as an IHC HER-2 score of 3+ or gene amplification by FISH. HER-2 negativity was defined as an IHC HER-2 score of 0 or 1+. Based on ER, PR, HER2, and Ki-67 expressions, BC were classified into four IFs: Luminal-A (ER-positive and/or PR-positive, HER2-negative and Ki-67 < 20%); Luminal-B (ER-positive and/or PR-positive, HER2-negative, and Ki-67 ≥ 20% or ER and/or PR-positive and HER2-positive, irrespective of Ki-67 expression); HER2-enriched (ER-negative, PR-negative, and HER2-positive); and triple-negative (ER-negative, PR- negative, and HER2-negative).
2.5. Statistical Analysis
All statistical analyses were performed by using SPSS 21.0 (IBM Corporation, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as frequencies and percentages. Variables found to be statistically significant in the univariate analysis were further examined using a multivariable regression model to assess the relationship between CEM findings and histopathological parameters. Due to the non-normal distribution of variables, Spearman rank correlation analysis was applied to identify correlations among statistically significant parameters. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Population
A total of 50 patients were included and 50 BCs were evaluated. The mean age of patients was 57.2 ± 13.7 years and 13 (26%) patients presented palpable nodules.
Table 1 summarizes the main demographic and clinical characteristics of the study population.
3.2. CEM Findings
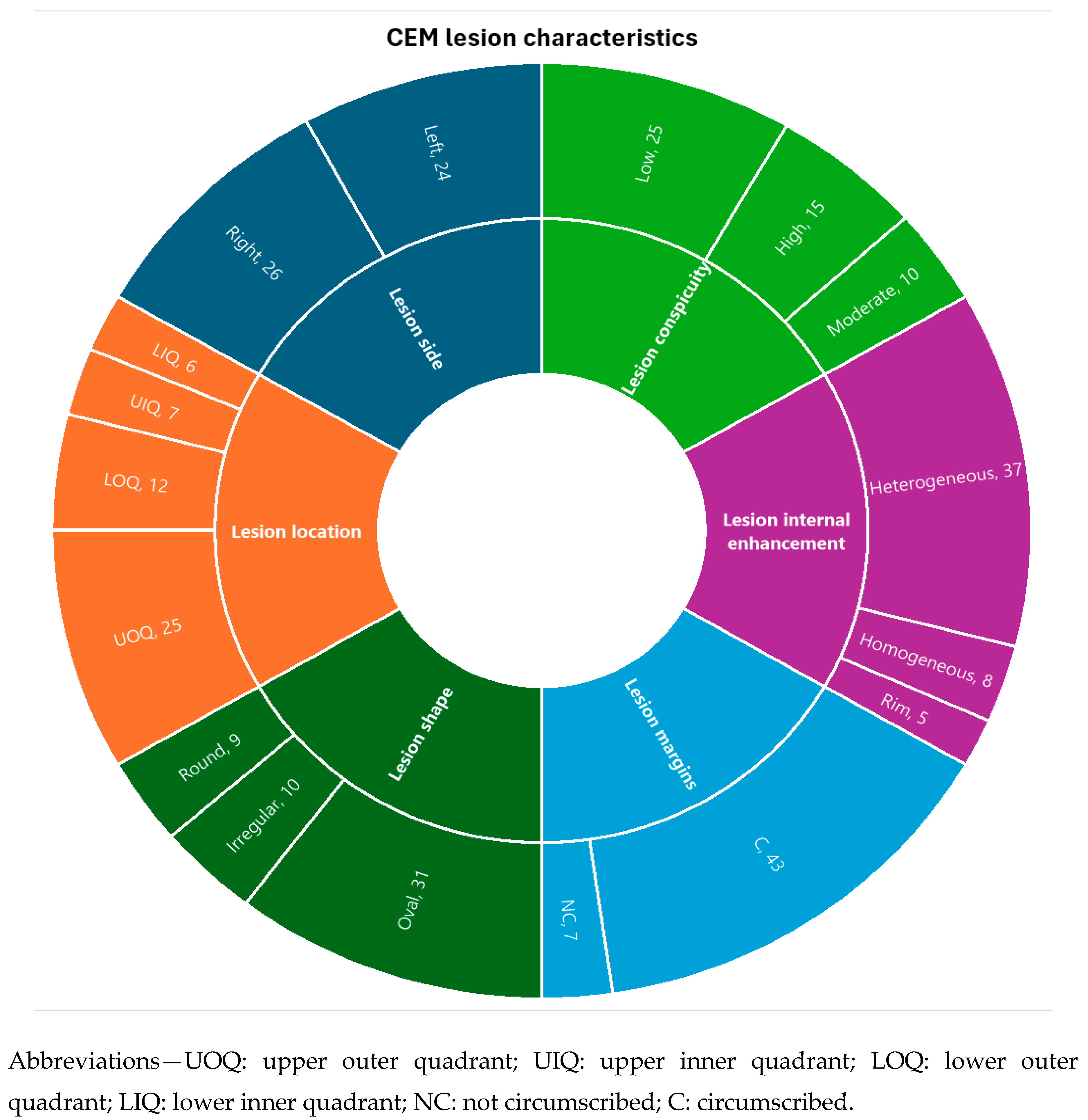
LE CEM images revealed that 60% of patients had a breast composition pattern classified as category B, while microcalcifications were observed in 11 lesions (22%). Architectural distortion was detected in 34 cases (68%). Recombined CEM images revealed an average larger cancer dimension of 23.4 ± 10.9 mm. Most lesions were located in the upper outer quadrant (50%) and demonstrated an oval shape (62%), not circumscribed margins (86%), and heterogeneous internal enhancement (74%). High conspicuity was observed in 30% of lesions. Finally, 52% of cases showed minimal BPE. Detailed findings from LE and recombined CEM images are presented in
Table 2 and
Table 3.
Scheme 2 illustrates CEM lesion characteristics.
3.2. Histopathological Findings
Histopathological analysis identified ductal carcinoma in 36 cases (72%), with grade 2 being the most frequent (50%). The predominant IF was Luminal-B (52%), and lymphovascular invasion was observed in 16 cases (32%). Pathological characteristics of the lesions are summarized in
Table 4.
3.2. Relationship between CEM findings and histopathological characteristics
No significant relationships were observed between breast composition, microcalcifications, and architectural distortion, and histopathological characteristics. However, the larger tumor dimension measured on CEM was strongly correlated with larger histopathological tumor size (ρ = 0.788, p < 0.001) and was associated with grade 3 tumors (p = 0.017). No significant associations were identified between BC side, location, shape, lesion internal enhancement, and other associated features and histopathological characteristics. Not circumscribed margins were significantly associated with the Luminal-B (p = 0.001). High lesion conspicuity was strongly associated with the Luminal-B (p = 0.001) and triple-negative IFs (p = 0.001); additionally, it was positively correlated with larger histopathological tumor size (ρ = 0.517, p < 0.001). Furthermore, BPE demonstrated a significant negative correlation with patient age (ρ = -0.286, p = 0.049).
Table 5 summarizes statistically significant relationships between CEM findings and histopathological/clinical characteristics.
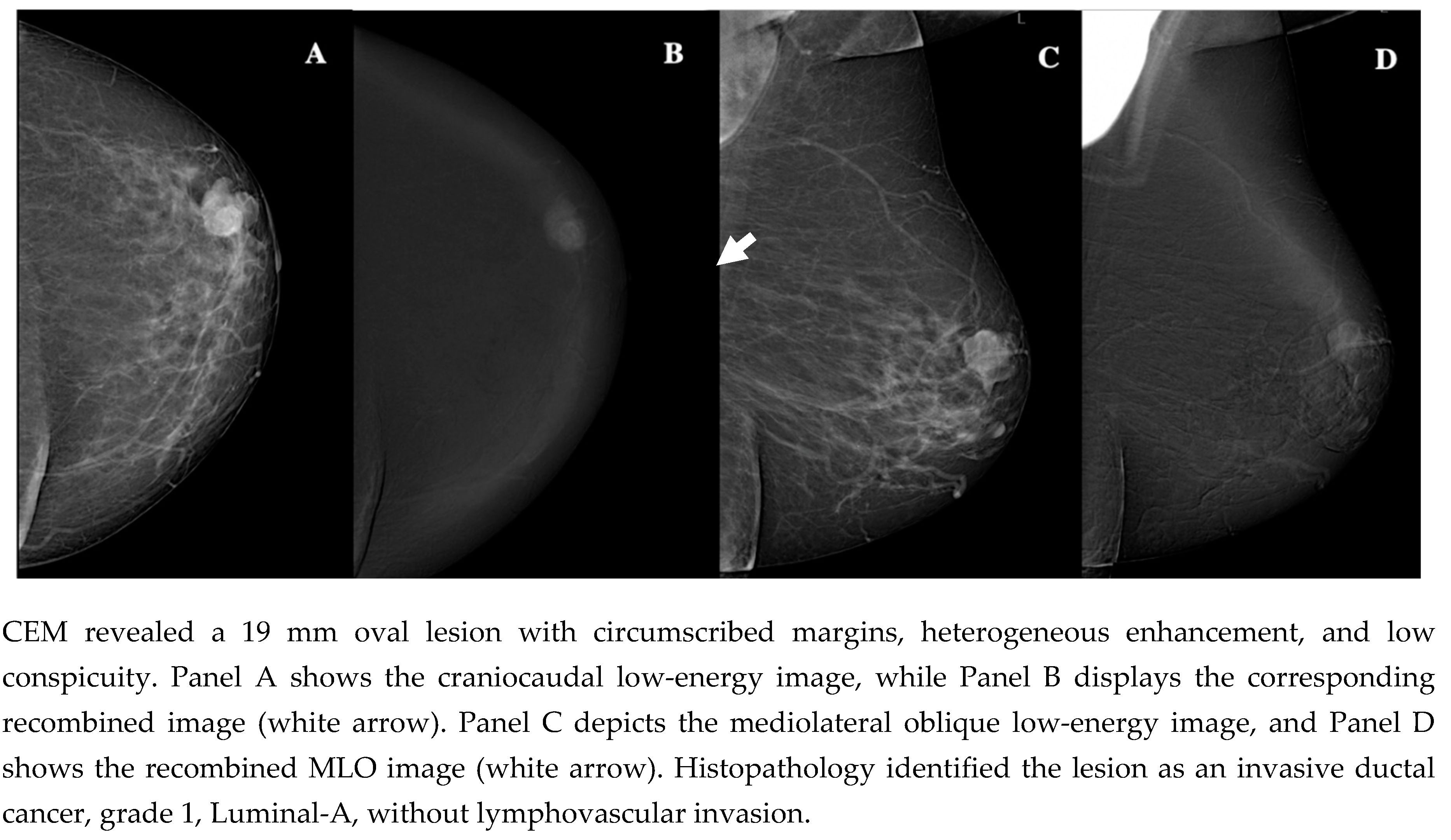
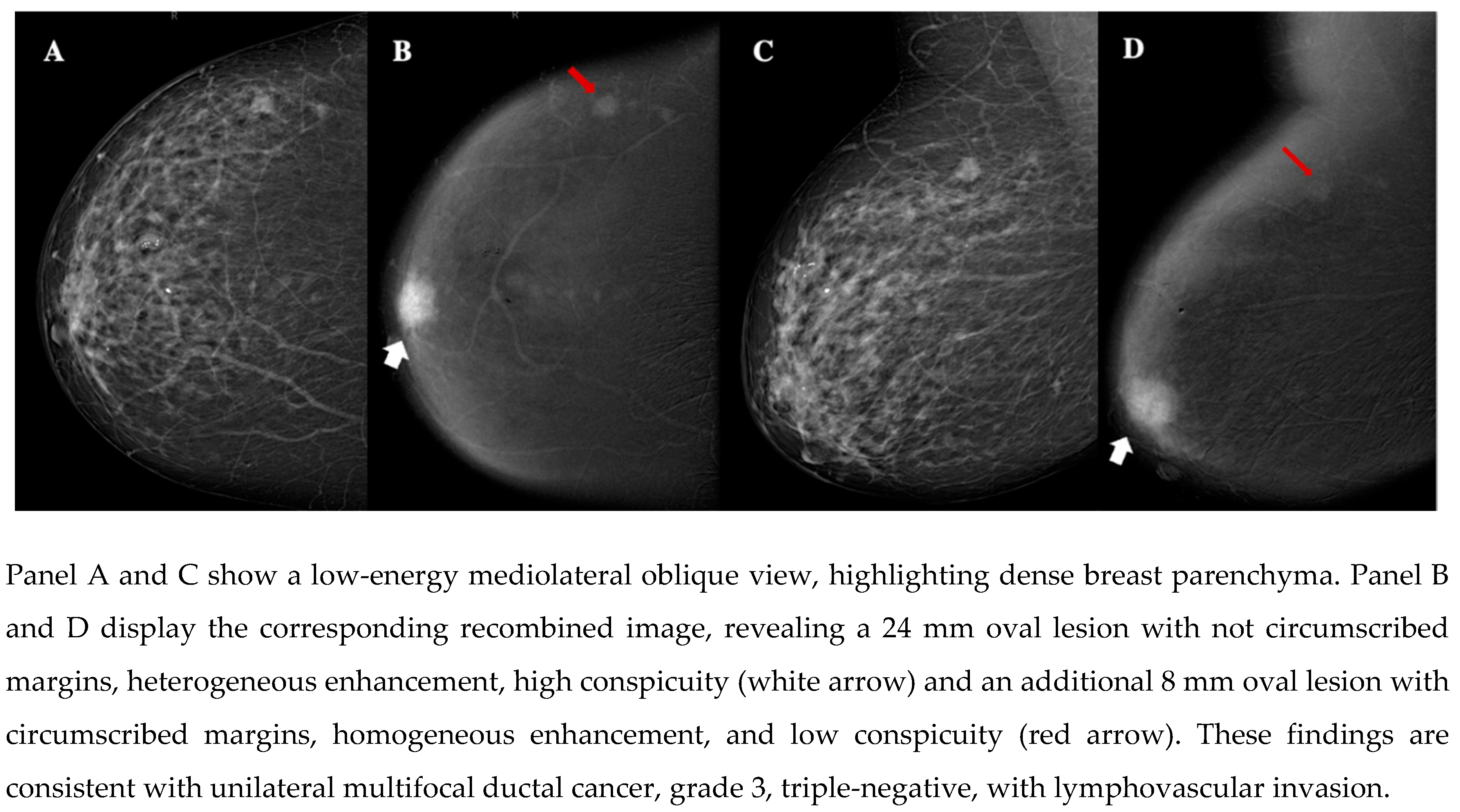
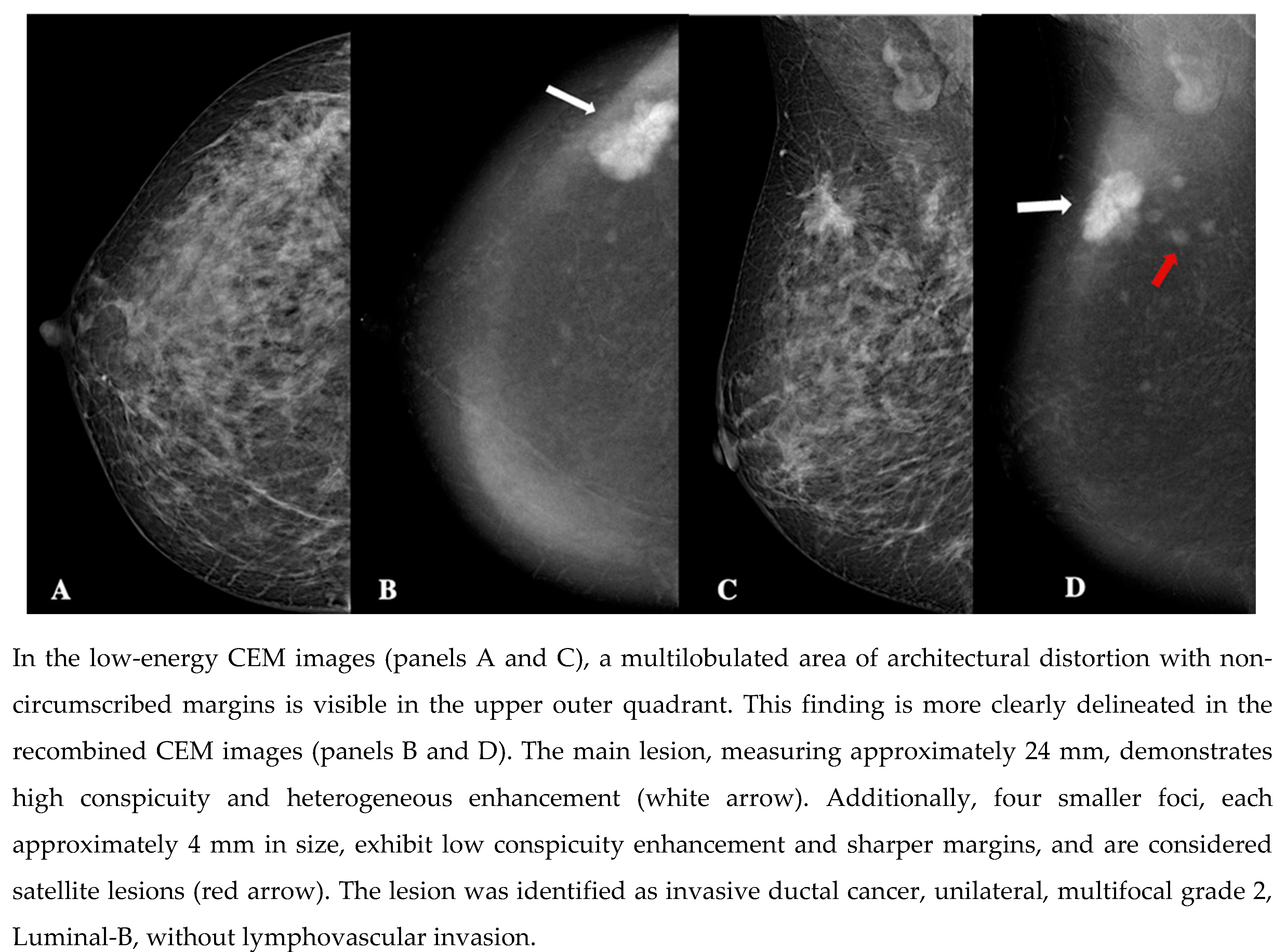
Figure 1,
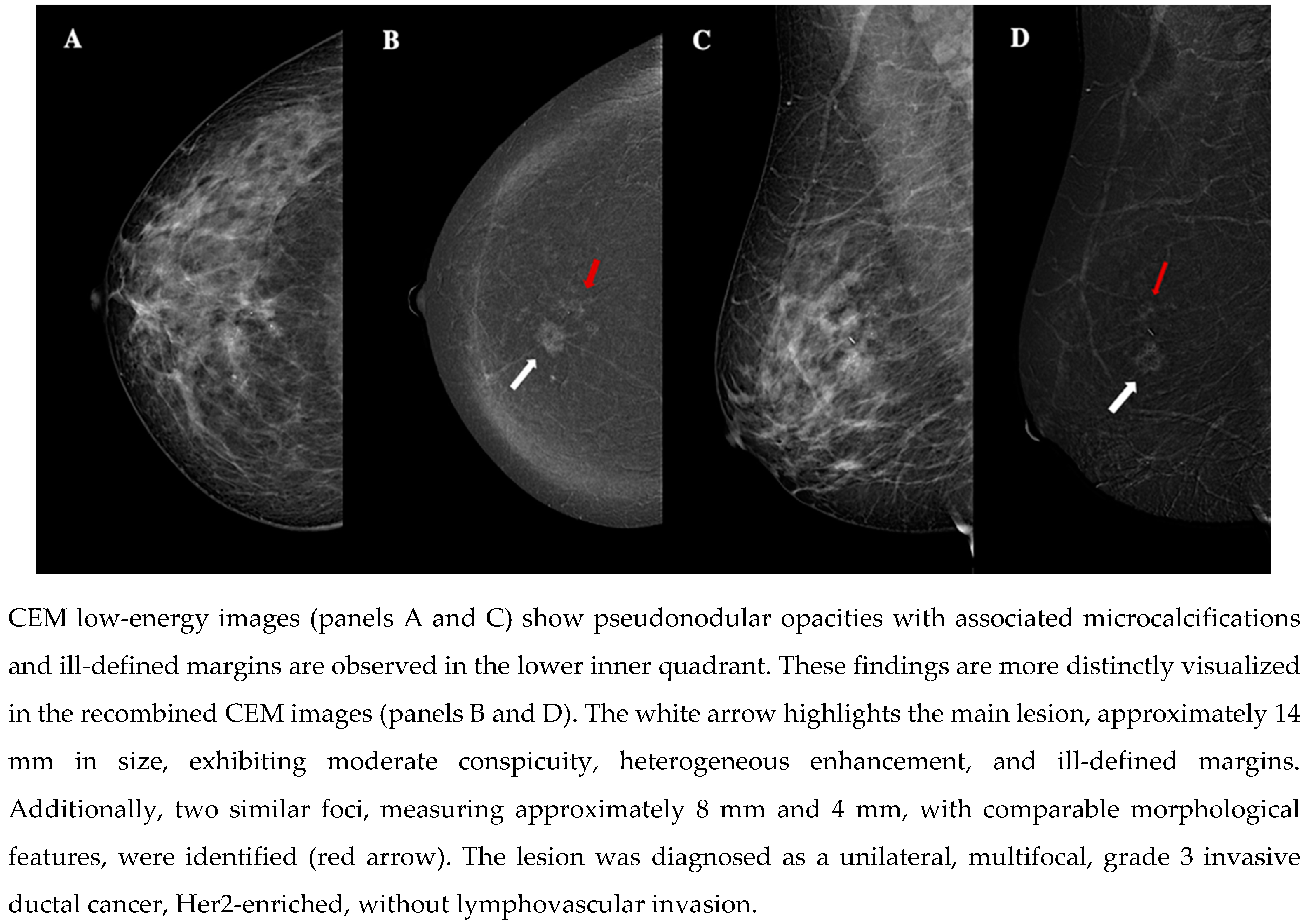
Figure 2,
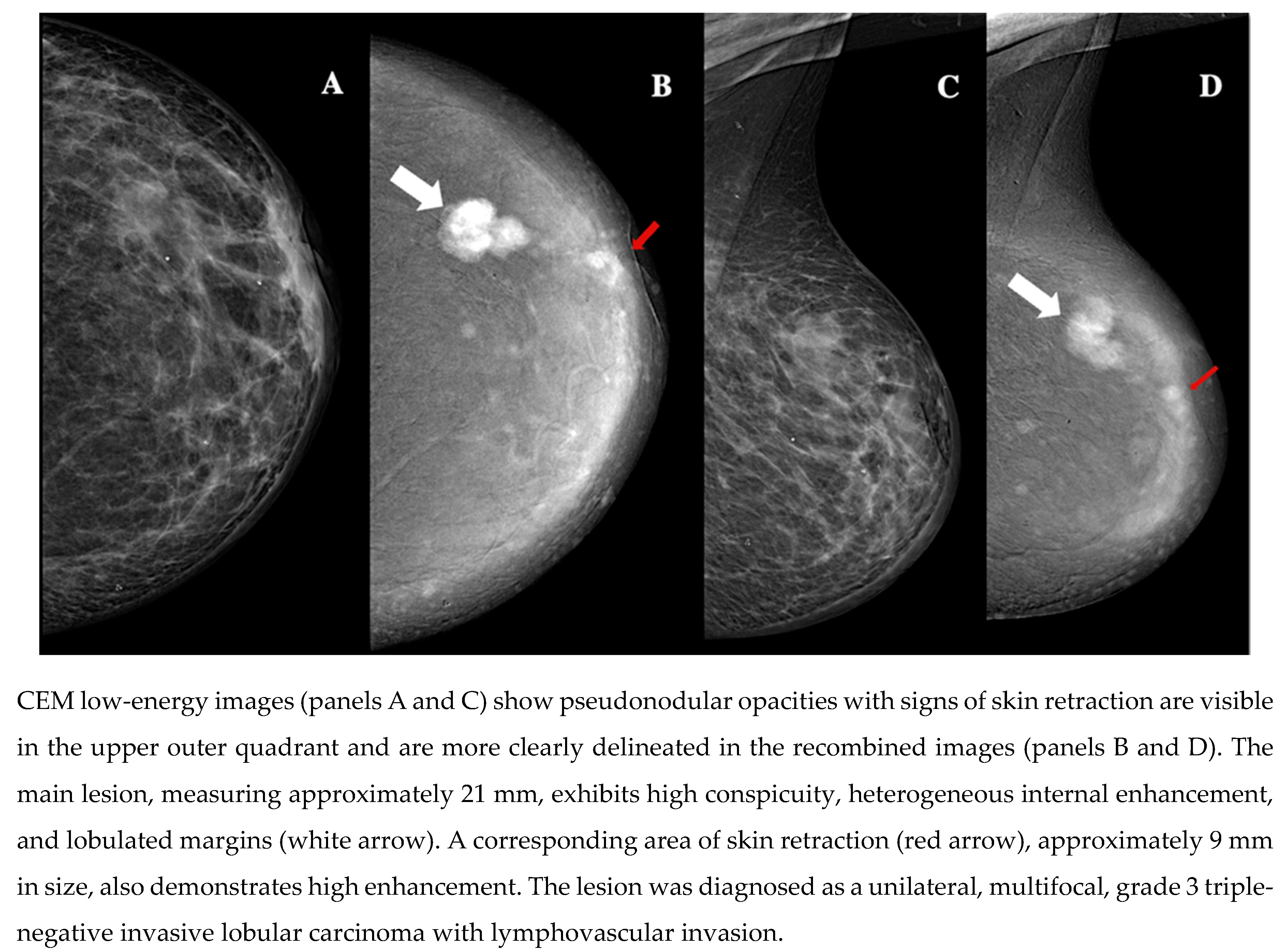
Figure 3,
Figure 4 and
Figure 5 show examples of patients included in our study.
4. Discussion
BC is the most prevalent malignancy among women worldwide, making it a critical focus for continuous innovation in diagnostic imaging. The advancements in imaging techniques have significantly impacted the early detection, characterization, and management of BC [
1,
20,
21]. Among these innovations, CEM has gained considerable attention due to its ability to provide both morphological and functional information using contrast agents. CEM offers high specificity, shorter exam times, and better patient acceptability compared to MRI, especially for those with contraindications [
5,
8,
9,
10]. While emerging research suggests correlations between CEM imaging features and histopathological characteristics, comprehensive evaluations of CEM’s capabilities remain limited [
19]. This study aims to analyze the morphodynamic features of CEM and their association with BC histopathology, advancing its diagnostic and prognostic utility.
CEM provided detailed imaging features that helped in identifying lesions with varying characteristics. The majority of lesions (74%) displayed heterogeneous internal enhancement, which is consistent with previous studies showing that this pattern is more commonly associated with malignancies [
22]. Additionally, most lesions (86%) had not circumscribed margins, which further supports the notion that irregular margins are indicative of invasive carcinoma. The conspicuity of lesions varied, with 50% showing low conspicuity, and 30% exhibiting high conspicuity. These findings suggest that while CEM can be helpful in visualizing malignancies, the conspicuity of the enhancement may limit its diagnostic power in some cases, similar to what has been reported in prior literature [
23].
One of the primary findings of this study is that CEM imaging provides detailed information that correlates with histopathological tumor size, a key factor in BC staging and treatment planning. The positive correlation between larger BC size on CEM images and histopathological evaluation (ρ = 0.788, p < 0.001) supports the use of CEM for accurate tumor size estimation. In this regard, the use of CEM for detecting and characterizing BC is well reported by existing literature. Some studies have demonstrated that CEM is an effective imaging technique, particularly for detecting BC in dense breast tissue, where conventional mammography may have reduced sensitivity. This highlights CEM’s reliability in accurately sizing lesions, regardless of breast density. Accurate preoperative tumor size estimation remains critical for surgical planning and treatment decisions [
24,
25]. Furthermore, research has shown that CEM is not inferior to MRI in preoperative evaluations, particularly in dense breasts [
10,
26,
27,
28]. However, it is important to note that false negatives in CEM are typically observed in smaller, monofocal lesions (less than 10 mm in size) or in cases of ductal carcinoma in situ, where the only indication might be suspicious microcalcifications [
29].
BPE was another feature examined in this study. We found a significant negative correlation between BPE and patient age (ρ = -0.286, p = 0.049), which is consistent with findings from previous studies suggesting that younger women tend to exhibit higher levels of BPE in CEM context [
15]. BPE is influenced by various factors, including hormonal status and can impact the sensitivity of mammography and other imaging modalities.
Moreover, high lesion conspicuity on CEM was also strongly associated with both Luminal-B and triple-negative IFs (p = 0.001). These IFs are known for their poor prognosis and more aggressive clinical behavior. This finding corroborates previous studies highlighting the role of contrast-enhanced imaging in identifying tumors with active blood vessel formation, a hallmark of malignancy [
23]. Moreover, the correlation between high lesion conspicuity and larger tumor size observed in our study further supports the use of CEM in assessing the extent of tumor spread, which is crucial for preoperative staging and treatment planning.
Furthermore, one of the key observations in our study was the correlation between not circumscribed margins on CEM and Luminal-B. This is in line with existing literature that suggests not circumscribed margins are indicative of more aggressive tumors and may be a manifestation of tumor cells invading surrounding tissues [
23]. The significant association of not circumscribed margins with Luminal-B subtype further underscores the value of CEM in characterizing biologically aggressive tumors. This is especially important for guiding clinical decision-making, as Luminal-B tumors are typically more aggressive than Luminal-A tumors and may require more intensive treatment regimens.
The results from this study emphasize the potential role of CEM as a complementary imaging tool in BC diagnosis and management. In addition to histopathological correlations, this study highlights the advantages of CEM in terms of diagnostic efficiency and patient acceptability. CEM offers a significant advantage over traditional mammography in patients with dense breasts, where conventional mammography may fail to identify lesions effectively. It also provides a viable alternative for patients who are unable to undergo MRI due to contraindications such as claustrophobia. The ability to capture both anatomical and functional imaging information within a single exam, with relatively short examination times, makes CEM an attractive option for clinical practice. Furthermore, the use of a standardized reporting system such as BI-RADS for CEM enhances its consistency and reproducibility, ensuring that radiologists can reliably communicate findings across clinical settings. Finally, emerging studies suggest artificial intelligence (AI) integration into CEM analysis. Advances in AI and machine learning could significantly enhance CEM’s diagnostic accuracy and prognostic value by analyzing complex patterns within imaging data that are not immediately apparent to human observers [
30,
31,
32,
33].
Despite the promising results, this study has some limitations. The sample size is relatively small, which may limit the generalizability of the findings. Additionally, the study was monocentric, and the findings might vary in a multi-center setting with a more diverse population. Moreover, this study did not assess interobserver variability among radiologists evaluating CEM images, which is an important factor in establishing the consistency and reliability of CEM-based diagnoses. Finally, the absence of smaller or non-enhancing lesions in our study sample may have introduced some selection bias. Further studies with larger sample sizes and comparisons with other imaging modalities are needed to fully establish the role of CEM in BC diagnosis.
5. Conclusions
In conclusion, CEM proves to be a versatile, patient-friendly imaging technique with significant diagnostic and prognostic capabilities. Its correlation with histopathological tumor characteristics, particularly the enhancement of biologically aggressive lesions, underscores its clinical value and suggest that CEM can provide important insights into tumor characteristics, potentially aiding in diagnosis, staging, and treatment planning. With continued technological advancements and larger-scale studies, CEM could become an essential component of BC imaging in clinical practice, complementing existing modalities and providing valuable insights into tumor biology.
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
Author Contributions
Conceptualization, C.V; methodology, C.V. and M.F; formal analysis, C.V. and M.F; investigation, C.V., E.M., B.F.S., S.B.G and N.C.; data curation, M.F.; writing—original draft preparation, C.V.; writing—review and editing, M.F., E.M., B.F.S.; S.B.G and N.C.; visualization, E.L. and G.A.; supervision, E.L. and G.A.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of University Hospital of Marche (protocol code 2771–15/12/2022 date of approval).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Dr.ssa Silvia Baldassarri—Dr.ssa Paola Ercolani—Dr. Federico Cerimele.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sibylle, L.; Philip, P.; Monica, M.; et al. Breast cancer. Lancet (London, England) 2021, 397, 1750–1769.
- Jong, R.A.; Yaffe, M.J.; Skarpathiotakis, M.; Bloomquist, A.; Lockwood, G.; Plewes, D.B.; et al. Contrast-enhanced digital mammography: Initial clinical experience. Radiology 2003, 228, 842–850. [CrossRef]
- Ventura, C.; Baldassarre, S.; Cerimele, F.; Pepi, L.; Marconi, E.; Ercolani, P. et al. 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiologia Medica 2022, 127, 1221–1227. [CrossRef]
- Lobbes, M.B.I.; Smidt, M.L.; Houwers, J.; Tjan-Heijnen, V.C.; Wildberger, J.E.; et al. Contrast-enhanced mammography: Techniques, current results, and potential indications. Clinical Radiology 2013, 68, 935–944. [CrossRef]
- Diekmann, F.; Freyer, M.; Diekmann, S.; Stengel, A.; Fischer, T.; Bick, U. Evaluation of contrast-enhanced digital mammography. European Journal of Radiology 2011, 78, 112–121. [CrossRef]
- Polat, D.S.; Evans, W.P.; Dogan, B.E.; Chen, H.L.; Burgess, M.; Paulsen, L.; et al. Contrast-enhanced digital mammography: Technique, clinical applications, and pitfalls. American Journal of Roentgenology 2020, 215, 1267–1278. [CrossRef]
- Covington, M.F.; Salmon, S.; Weaver, B.D.; Fajardo, L.L.; Rhodes, D.J. State-of-the-art for contrast-enhanced mammography. British Institute of Radiology 2024, 97, 695–704.
- Ainakulova, A.S.; Zholdybay, Z.Z.; Kaidarova, D.R.; Baimukhanova, A.B.; Nurtayeva, S.; Tulegenov, A.; et al. Contrast-enhanced spectral mammography without and with a delayed image for diagnosing malignancy among mass lesions in dense breast. Wspolczesna Onkologia 2021, 25, 17–22. [CrossRef]
- Sorin, V.; Yagil, Y.; Yosepovich, A.; Hershkovitz, D.; Ben-Baruch, G.; Khashper, A.; et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. American Journal of Roentgenology 2018, W267–W274.
- Bernardi, D.; Vatteroni, G.; Acquaviva, A.; Monti, S.; Bariani, M.; Petrillo, A. Contrast-enhanced mammography versus MRI in the evaluation of neoadjuvant therapy response in patients with breast cancer: A prospective study. American Journal of Roentgenology 2022, 219, 884–894. [CrossRef]
- Jochelson, M.S.; Lobbes, M.B.I.; Scaranelo, A.M.; Margolies, L.R.; Gilbert, F.J.; Lee, C.H. Contrast-enhanced mammography: State of the art. Radiology 2021, 299, 36–48.
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Baltzer, P.A.T.; Helbich, T.H. Contrast-enhanced mammography versus contrast-enhanced breast MRI: A systematic review and meta-analysis. Radiology 2022, 305, 94–103.
- Lee, C.H.; Phillips, J.; Sung, J.S.; Giess, C.S.; Jochelson, M.S.; Margolies, L.R. ACR BI-RADS® atlas-mammography contrast enhanced mammography (CEM). American College of Radiology 2022.
- Nicosia, L.; Battaglia, O.; Venturini, M.; Pellegrino, S.; Priola, S.; Bernardi, D. Contrast-enhanced mammography BI-RADS: A case-based approach to radiology reporting. Insights Imaging 2024, 15.
- Magni, V.; Cozzi, A.; Muscogiuri, G.; Pellegrino, S.; Nicosia, L.; De Marco, P. Background parenchymal enhancement on contrast-enhanced mammography: Associations with breast density and patient characteristics. Radiologia Medica 2024, 129, 1303–1312. [CrossRef]
- Petrillo, A.; Fusco, R.; Petrosino, T.; Bianchi, F.; Ferrara, D.; Barone, C. A multicentric study of radiomics and artificial intelligence analysis on contrast-enhanced mammography to identify different histotypes of breast cancer. Radiologia Medica 2024, 129, 864–878. [CrossRef]
- Bartolotta, T.V.; Militello, C.; Prinzi, F.; La Forgia, D.; Sarli, M.; Di Paolo, A.; et al. Artificial intelligence-based, semi-automated segmentation for the extraction of ultrasound-derived radiomics features in breast cancer: A prospective multicenter study. Radiologia Medica 2024, 129, 977–988. [CrossRef]
- Migliaro, G.; Bicchierai, G.; Valente, P.; Amato, F.; Vanzi, B.; Ventura, C. Contrast enhanced mammography (CEM) enhancing asymmetry: Single-center first case analysis. Diagnostics 2023, 13. [CrossRef]
- Li, N.; Gong, W.; Xie, Y.; Sheng, L. Correlation between the CEM imaging characteristics and different molecular subtypes of breast cancer. Radiology 2023.
- Fogante, M.; Tagliati, C.; De Lisa, M.; Berardi, R.; Giuseppetti, G.M.; Giovagnoni, A. Correlation between apparent diffusion coefficient of magnetic resonance imaging and tumor-infiltrating lymphocytes in breast cancer. Radiologia Medica 2019, 124, 581–587. [CrossRef]
- Prochowski Iamurri, A.; Ponziani, M.; Macchini, M.; Fogante, M.; Pistelli, M.; De Lisa, M.; et al. Evaluation of multifocality and multicentricity with breast magnetic resonance imaging in each breast cancer subtype. Clinical Breast Cancer 2018, 18, e231–e235. [CrossRef]
- Soylu Boy, F.N.; Goksu, K.; Tasdelen, I. Association between lesion enhancement and breast cancer in contrast-enhanced spectral mammography. Acta Radiologica 2023, 64, 74–79. [CrossRef]
- Marzogi, A.; Baltzer, P.A.T.; Kapetas, P.; Milos, R.I.; Bernathova, M.; Helbich, T.H.; et al. Is the level of contrast enhancement on contrast-enhanced mammography (CEM) associated with the presence and biological aggressiveness of breast cancer? Diagnostics 2023, 13, 754. [CrossRef]
- Travieso-Aja, M.M.; Naranjo-Santana, P.; Fernández-Ruiz, C.; Rodríguez-Abreu, D.; García-Hernández, N. Factors affecting the precision of lesion sizing with contrast-enhanced spectral mammography. Clinical Radiology 2018, 73, 296–303. [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Mantini, G.; Marini, P.; Pruneri, G. Contrast-enhanced spectral mammography and tumor size assessment: A valuable tool for appropriate surgical management of breast lesions. Radiologia Medica 2022, 127, 1228–1234. [CrossRef]
- Cozzi, A.; Schiaffino, S.; Sardanelli, F. The emerging role of contrast-enhanced mammography. Quantitative Imaging in Medicine and Surgery 2019, 9, 2012–2018.
- Bozzini, A.; Nicosia, L.; Pruneri, G.; Martella, E.; Testori, A.; Mastropasqua, M.G.; et al. Clinical performance of contrast-enhanced spectral mammography in pre-surgical evaluation of breast malignant lesions in dense breasts: A single-center study. Breast Cancer Research and Treatment 2020, 184, 723–731. [CrossRef]
- Wessam, R.; Gomaa, M.; Fouad, M.A.; Omar, W.; Mohamed, M. Added value of contrast-enhanced mammography in assessment of breast asymmetries. Clinical Radiology 2019.
- Magni, V.; Cozzi, A.; Muscogiuri, G.; Pellegrino, S.; Nicosia, L.; De Marco, P. Background parenchymal enhancement on contrast-enhanced mammography: Associations with breast density and patient characteristics. Radiologia Medica 2024, 129, 1303–1312. [CrossRef]
- Dominique, C.; Callonnec, F.; Berghian, A.; Foucher, G.; Mari, S.; Lemonnier, P.; et al. Deep learning analysis of contrast-enhanced spectral mammography to determine histoprognostic factors of malignant breast tumours. European Radiology 2022, 32, 4834–4844. [CrossRef]
- Petrillo, A.; Fusco, R.; Di Bernardo, E.; De Luca, L.; Di Giuda, D.; Petrosino, T.; et al. Prediction of breast cancer histological outcome by radiomics and artificial intelligence analysis in contrast-enhanced mammography. Cancers 2022, 14, 2132. [CrossRef]
- Piccolo, C.L.; Sarli, M.; Pileri, M.; Costa, F.; Chiarenza, M.; Tagliati, C. Radiomics for predicting prognostic factors in breast cancer: Insights from contrast-enhanced mammography (CEM). Journal of Clinical Medicine 2024, 13, 6486. [CrossRef]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Di Palma, D.; Di Giorgio, L.; Rizzi, F.; et al. Radiomic analysis in contrast-enhanced spectral mammography for predicting breast cancer histological outcome. Diagnostics 2020, 10, 0708. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).