A graphical abstract summarizing the review on innovative strategies to combat multidrug-resistant tuberculosis (MDR-TB). The figure highlights key approaches such as advanced drug delivery systems such as liposomes and/or transdermal patches, host-directed therapies such as cytokines, recombinant proteins, cellular and vitamin therapy, repurposed drugs and monoclonal antibodies. Inflammatory response modulation in alveolar macrophages infected with Mycobacterium tuberculosis is also depicted. Future directions include global collaboration, artificial intelligence and machine learning to address cost, accessibility and implementation barriers. This figure informs policymakers by emphasizing the need for investment in innovative therapeutic strategies, addressing practical challenges such as cost, implementation barriers, and accessibility, and outlining future directions involving artificial intelligence, machine learning and global collaboration. By presenting a holistic view of delivery strategies, obstacles, and opportunities, this figure facilitates and shapes policy development that prioritizes research funding, improves affordability and accessibility, shapes policies that prioritize research funding, improves affordability and accessibility, and fosters cross-border cooperation to tackle MDR-TB effectively. Created with BioRender.com.
1. Introduction
Tuberculosis (TB) continues to pose a formidable challenge to public health in the 21st century [
1]. Despite being a preventable and treatable disease, it has resulted in approximately 1.6 million fatalities worldwide in 2021, positioning it as the second leading cause of death from a singular infectious agent following COVID-19 [
2]. The rise of multidrug-resistant tuberculosis (MDR-TB) has compounded this burden, undermining decades of progress in the treatment and control of TB [
3]. MDR-TB is characterized by resistance to at least isoniazid and rifampin, the foremost first-line anti-TB medicines and accounts for approximately 450,000 new cases annually, with treatment success rates of approximately 60% [
4]. The emergence and persistence of MDR-TB are driven by a combination of factors, including incomplete or incorrect treatment regimens, genetic adaptability of
Mycobacterium tuberculosis and the lack of access to timely and accurate diagnostic tools [
4]. These challenges necessitate innovative strategies to improve the efficacy of existing treatments and the delivery of novel therapeutic agents. Recent advances in molecular biology, drug development and drug delivery systems offer a glimmer of hope in the fight against MDR-TB [
5,
6,
7,
8]. However, successfully translating these innovations into widespread clinical practice remains formidable.
This review provides an in-depth overview of the latest advances in managing MDR-TB, with a focus on cutting-edge drug delivery systems (DDS) and innovative drug therapies. By highlighting both the successes and challenges of these approaches, we seek to identify opportunities for future research and implementation that can bridge the gap between scientific discovery and clinical impact.
MDR-TB is part of a broader spectrum of drug-resistant TB, which includes extensively drug-resistant TB (XDR-TB) and, more recently, totally drug-resistant TB (TDR-TB) [
9]. XDR-TB is characterized by resistance to fluoroquinolones and a minimum of one second-line injectable drug and has been reported in over 120 countries [
10]. The management of drug-resistant TB incurs significant costs, estimated to be nearly 20 times greater than those associated with drug-susceptible TB. However, it is also associated with more severe side effects and prolonged treatment duration [
10]. One alarming statistic is the high mortality rate associated with untreated or poorly treated MDR-TB, which can reach up to 80% [
11]. Furthermore, MDR-TB often affects individuals in low- and middle-income nations, where healthcare infrastructure is ill-equipped to manage complex drug regimens [
12]. These realities underscore the urgent need for improved diagnostic, therapeutic and preventive measures.
In recent years, several novel pharmaceuticals have received approval for treating MDR-TB, including bedaquiline, delamanid and pretomanid. These drugs have shown promise in reducing treatment duration and improving clinical outcomes, mainly when used as part of an all-oral regimen [
13]. For instance, a recent study demonstrated that a combination of bedaquiline, pretomanid and linezolid (the BPaL regimen) achieved an 89% t success rate when treating individuals infected with XDR-TB [
13]. In addition, repurposing existing drugs, such as clofazimine and meropenem, has expanded treatment options for combatting MDR-TB. These strategies provide alternative options and help mitigate the risk of further development of resistance. However, challenges such as drug toxicity, high costs and limited availability persist, highlighting the need for further innovation [
14,
15].
Conventional TB treatment relies on systemic drug administration, which often leads to suboptimal drug concentrations at the site(s) of infection, thereby contributing to treatment failure and resistance [
16]. Innovative drug delivery systems, such as nanoparticles, liposomes and inhalation formulations, have emerged as potential game-changers. These technologies facilitate precise drug delivery, prolonged release and enhanced bioavailability, augmenting therapeutic efficacy and minimizing side effects. For example, inhaled formulations of anti-TB drugs have been shown to deliver drugs in high concentrations directly to the lungs, the primary site of infection. This approach reduces systemic toxicity and shortens treatment duration in preclinical models [
17]. Furthermore, delivery systems rooted in nanotechnology, including polymeric nanoparticles and lipid-based carriers, have shown significant promise in surmounting biological barriers and facilitating the delivery of drugs to the intracellular reservoirs of
Mycobacterium tuberculosis [
18,
19]. While the advances in treatment and drug delivery systems are promising, their impact on the global MDR-TB epidemic will depend on equitable access, robust healthcare infrastructure and effective policy implementation. Investment in research and development must complement strategies to ensure affordability and accessibility, particularly in high-burden settings.
Moreover, integrating novel diagnostics, such as rapid molecular tests and whole-genome sequencing, into TB programs can facilitate timely identification of drug resistance and guide the development of personalized treatment regimens [
20]. Such a comprehensive approach is essential to curbing the spread of MDR-TB and achieving the goals of the End MDR-TB Strategy by 2030 [
2]. This review analyses the distinctive features of MDR-TB and investigates the latest developments in treatment and drug delivery approaches. The discussion includes emerging drugs, innovative drug delivery mechanisms and immune modulation as central focus areas.
2. Peculiarities of TB and Mechanisms of MDR-TB
2.1. Tuberculosis Pathophysiology and Drug Resistance Mechanisms
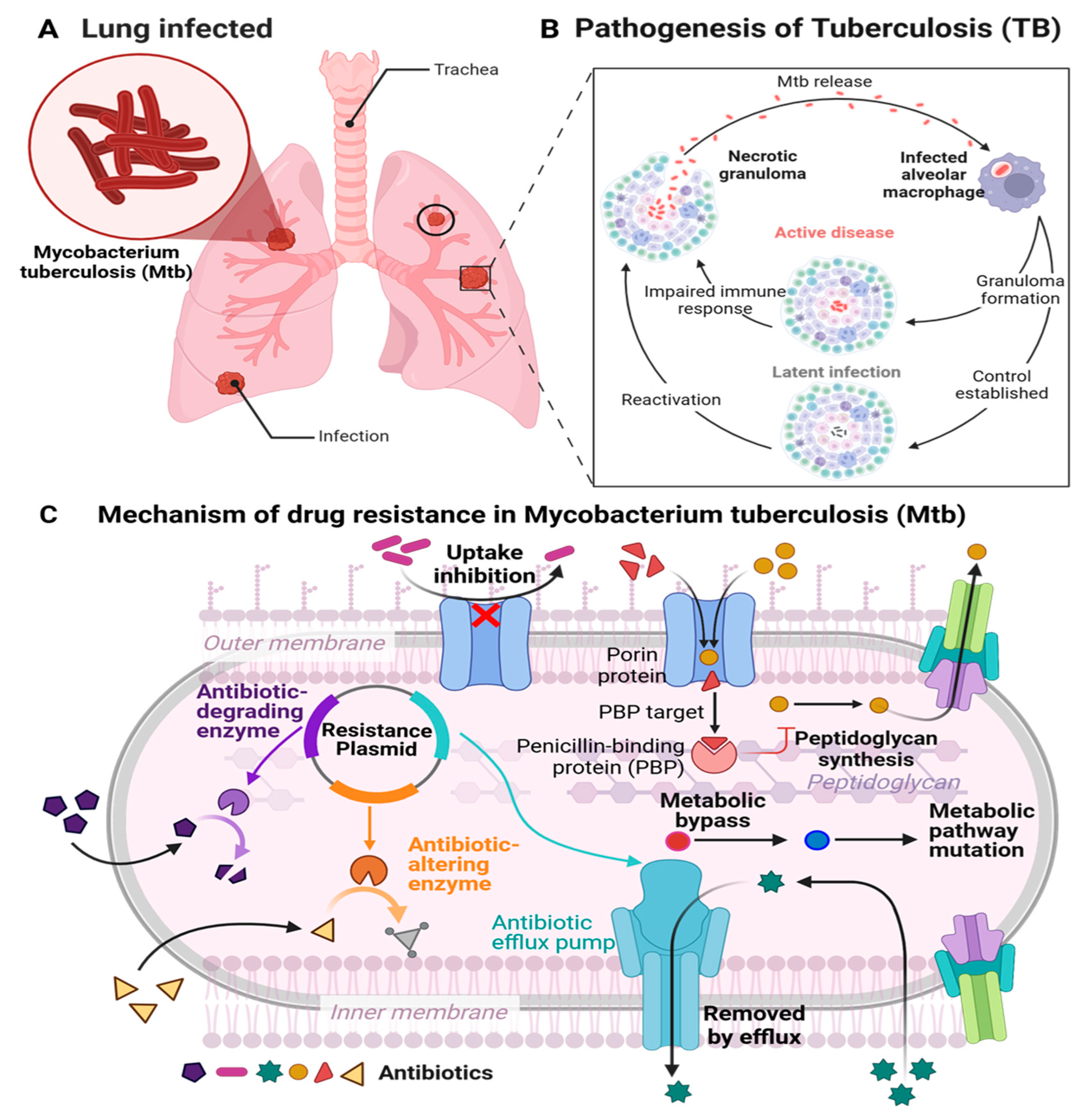
TB is a severe infectious disease that primarily impacts on the pulmonary system. Moreover, it can impact other body parts, including the kidneys, spine, bones, lymph nodes and brain and is attributed to
Mycobacterium tuberculosis (
Mtb) (
Figure 1A), a highly specialized human pathogen [
21,
22,
23].
2.1.1. Pathophysiology of TB
Mtb transmission occurs via airborne particles expelled by individuals who cough, sneeze or spit, thereby distributing the infectious organism. The bacteria can persist in the air for several hours, rapidly infecting others [
24,
25]. Following inhalation,
Mtb is primarily engulfed by resident alveolar macrophages in the lungs [
25]. Macrophages internalize pathogens via phagocytosis, which is facilitated by ligand-receptor interactions involving mannose receptors, scavenger receptors, complement receptors (CR1, CR3, CR4), Fc receptors, and surfactant protein receptors [
26,
27].
Mtb skillfully circumvents the bactericidal strategies of macrophages through mechanisms that include the prevention of bacterial vacuole acidification, inhibition of phagolysosome formation and the obstruction of apoptosis and autophagy in infected macrophages [
26,
28]. After internalization,
Mtb inhibits phagosome maturation, allowing it to merge with lysosome and persist within macrophages. Alternatively, it replicates intracellularly by preventing phagosome-lysosome fusion and transforming macrophages into a protective environment for the pathogen [
27,
29]. After infection of alveolar macrophages within the lower respiratory tract,
Mtb infiltrates the interstitium of the lung(s), thereby advancing the course of the infection process. The incursion of pathogens into the parenchyma triggers an immune response, resulting in the mobilization of T and B cells to the locus of infection. This recruitment precipitates a complex multicellular response by the host called granulomatous inflammation. Granulomas serve the purpose of efficiently confining the
Mtb pathogen at the site(s) of infection, thus inhibiting spreading in the host. Nonetheless, the progression of dysfunctional granulomas may lead to the persistence of pathogens, considerable tissue damage and insufficient responses to treatment.
Mtb utilizes granulomas (
Figure 1B) as focal points of infection, where phagocytic cells congregate, providing ample nutrients for replication [
26,
27]. Infected individuals who do not present with symptoms of the disease cannot transmit
Mtb; however, the bacteria persist within the body in an inactive or latent form, which if left untreated, can advance to become active TB (
Figure 1B) [
25].
2.1.2. Conventional Anti-Tubercular Pharmaceuticals
Tuberculosis is typically managed using antibiotics, including isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), ethambutol hydrochloride (EMB) and streptomycin (STREP) (
Table 1), and can be deadly, if left untreated. The existing treatment protocol for tuberculosis involves a daily dosing regimen for between four and six months. This treatment involves an intensive two-month phase, during which patients are administered four first-line antibiotics viz., EMB, INH, RIF and PZA. This is followed by a four-month continuous phase of treatment, where the regimen includes RIF and INH. When bacteria resist standard medications, it may become necessary to use second-line treatment such as injectable formulations of amikacin, capreomycin, and kanamycin, thereby leading to more intricate and extended therapeutic processes which frequently exhibit reduced efficacy, higher costs, and more pronounced adverse effects [
24,
30]. Fluoroquinolones, including moxifloxacin (MOX), gatifloxacin, levofloxacin and ofloxacin in combination with other oral agents such as para-aminosalicylic acid, prothionamide, terizidone, cycloserine and ethionamide, are used for the treatment of tuberculosis [
26,
31].
2.2. Mechanism of Action of Anti-Tubercular Agents
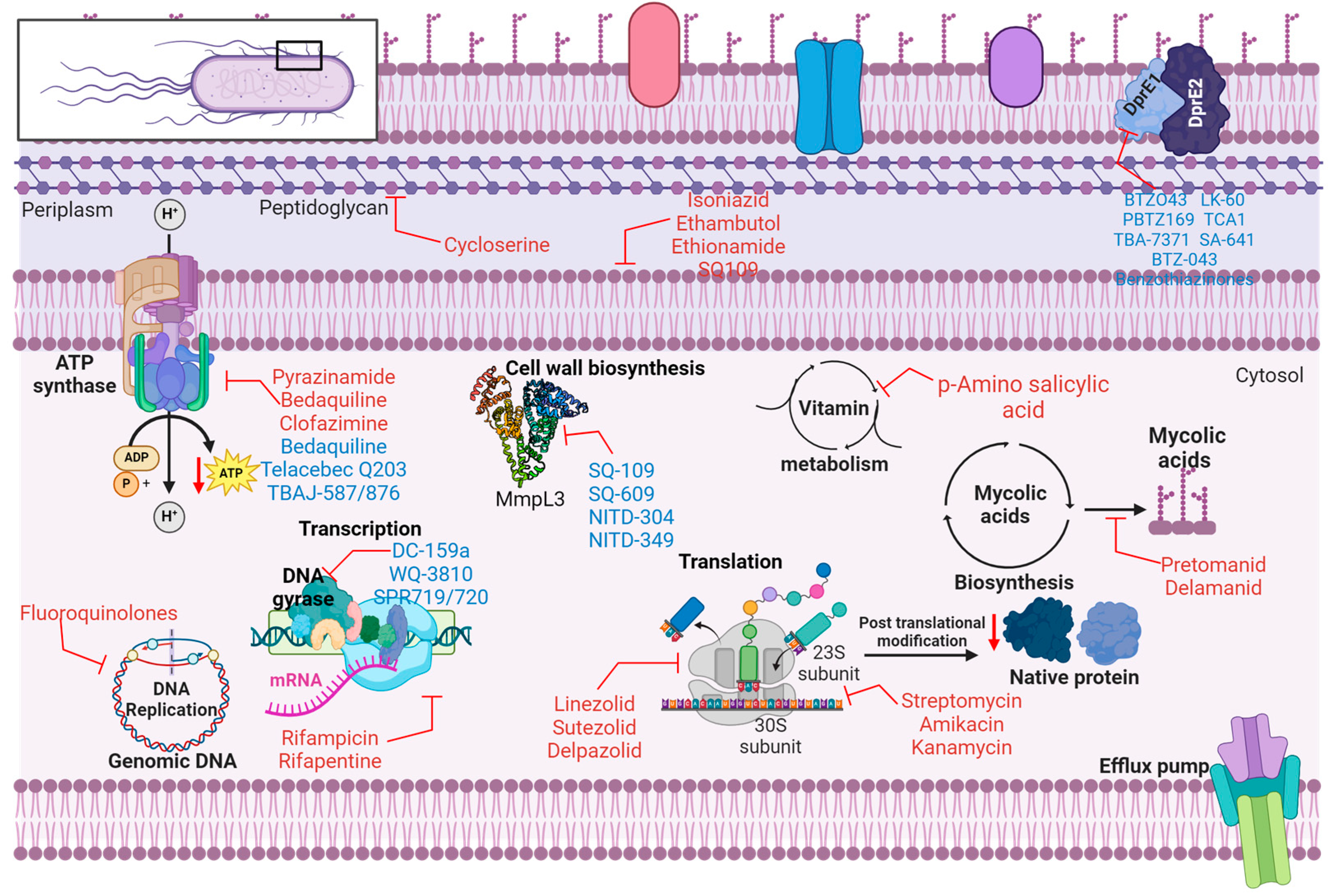
Isoniazid interacts with two specific proteins in
Mtb. The primary target is catalase-peroxidase, an enzyme that protects the organism against reactive oxygen species activity. The second target is enoyl-acyl-carrier-protein reductase, which is involved in mycolic acid production.
Mtb inhibits the biosynthesis of mycolic acid, impacting the cell wall (
Table 1 and Figure 2), and affects the metabolism of DNA, lipids, carbohydrates and NAD. Rifampin interacts with and obstructs DNA-dependent RNA polymerase, thereby impeding RNA synthesis. Ethambutol exhibits bacteriostatic properties by inhibiting arabinosyl transferase A, B and C, disrupting cell wall synthesis. Pyrazinamide inhibits
Mtb fatty acid synthetase, leading to plasma membrane disruption and intracellular acidification, impairing energy metabolism. Rifapentine is an antibiotic that similarly inhibits DNA-dependent RNA polymerase, similar to rifampicin. Streptomycin targets the S12 and 16S rRNA components of the 30S ribosomal subunit, inhibiting protein synthesis. Similarly, amikacin and kanamycin also target the 30S ribosomal subunit and inhibit protein synthesis. Para-amino salicylic acid inhibits folic acid and iron metabolism by targeting thymidylate synthase (
ThyA) and dihydropteroate synthase. Fluoroquinolones target DNA gyrase and DNA topoisomerase, leading to the inhibition of DNA supercoiling. Cycloserine inhibits D-alanine racemase and ligase, thereby disrupting peptidoglycan biosynthesis. Ethionamide inhibits Enoyl-[acyl-carrier protein] reductase (InhA), disrupting mycolic acid biosynthesis. Capreomycin targets Interbridge B2a and inhibits protein synthesis [
27,
31,
32]. Some investigational drugs for the treatment of TB are listed in
Table 2.
2.3. Mechanism of Drug Resistance (MDR-TB)
The increase in strains resistant to widely used anti-TB medications has created a quandary for researchers [
33]. Understanding the principal factors contributing to drug-resistant tuberculosis in clinical instances, such as genetic changes in drug targets or activation enzymes, the synthesis of drug-inactivating enzymes, compensatory evolution, and the activation of efflux pumps on the bacterial surface [
30,
51]. Moreover, spontaneous mutations in the
Mtb genome might modify enzymes/proteins that change the affinity of antibiotics for its target, rendering the bacteria resistant to the medicine contingent on the mechanism of action of the drug (
Figure 1C).
2.3.1. Genetic Mechanisms of Resistance
The primary mechanism behind the development of MDR-TB is the occurrence of specific genetic mutations in Mtb that confer first-line antibiotic resistance. Mycobacterium genomes that exhibit mutations and various structural alterations can resist the effects of commonly used drugs that inhibit growth.
Isoniazid Resistance
Isoniazid, classified as a pro-drug, undergoes activation through the catalase-peroxidase enzyme (
KatG), expressed by the
katG gene. Alterations in the
katG gene, responsible for encoding the activating enzyme catalase-peroxidase, are essential for understanding the core mechanism(s) of resistance. These mutations represent a significant area of study and understanding in microbiology and pharmacology. Furthermore, alterations in the
inhA gene, responsible for encoding the target enzyme for isoniazid, affect the target enzyme, enoyl-ACP reductase. Alterations in the
inhA gene represent the second most common mechanism by which resistance occurs. The expression of untargeted proteins, a significant factor in resistance to treatment, underscores the urgency of addressing this issue. The impact of resistance to treatment is a key area of concern and research. These alterations undermine the efficacy of drugs in the disruption of mycolic acid production, which is essential for mycobacterial cell wall integrity [
31,
33,
52].
Rifampicin Resistance
Rifampicin exhibits strong bactericidal properties and effectively sterilizes
Mtb. Rifampicin is recognized for its ability to disrupt RNA synthesis by binding to the ß subunit of RNA polymerase. Changes in the
rpoB gene, which encodes the beta-subunit of RNA polymerase, lead to modification of the β-subunit of RNA polymerase. The mutations hinder rifampicin binding to the enzyme, obstructing its inhibitory impact on bacterial transcription. Approximately 95% of rifampicin resistance is linked to mutations in an 81-base pair segment of
rpoB [
31,
52,
53]. The mutations that lead to resistance against these drugs frequently arise spontaneously, taking place during the replication of bacteria when exposed to suboptimal or incomplete treatment regimens. Mutations in the target genes of antibiotics are regarded as the primary mechanism of resistance in this bacterium [
4].
2.3.2. Non-Genetic Mechanisms
Efflux Pump Mechanisms
Drug resistance cannot solely be attributed to gene mutations in a subset of clinical
Mtb strains. Previous studies indicate that 30% of isoniazid-resistant and 5% of rifampicin-resistant
Mtb clinical strains exhibit this phenomenon, implying the existence of alternative mechanisms of drug resistance. Efflux has been identified as a potential mechanism underlying drug resistance in clinical strains that lack the previously described gene mutations [
54,
55,
56]. Bacterial efflux pumps, proteins that remove toxic substances from cells, play a crucial role in MDR-TB.
As integral membrane proteins, efflux pumps orchestrate a complex resistance mechanism that covers different classes of drug. The overexpression of efflux pumps such as Rv1258c (P55 efflux pump) enhances drug tolerance and potentially facilitates the acquisition of chromosomal mutations that confer increased levels of resistance. This intricate process leverages transmembrane electrochemical gradient of protons or sodium ions to expel drugs from the cell, effectively counteracting drug activity. PS5 actively extrudes and consequently confers resistance to multiple drugs, including rifampin. Efflux pumps, in combination with permeability barriers, reduce the movement of antimicrobials through the outer bacterial membrane, adding another layer of complexity to the mechanism of resistance [
57,
58]. While efflux pumps alone typically do not cause drug resistance, they contribute to other mechanisms of resistance in
Mtb and are crucial in causing elevated levels of resistance [
59,
60].
Phenotypic Adaptation
The remarkable success of
Mtb can be attributed to three key abilities viz, the reprogramming of macrophages post-primary infection/phagocytosis to evade destruction, the initiation of well-organized granuloma formation, which includes various immune cells to establish a controlled environment for the host-pathogen interaction and the ability to downregulate its central metabolism, halt replication and enter a dormant state, making it exceptionally resilient against the defenses of the host and pharmacological treatment interventions. The mechanism of phenotypic adaptation enables bacteria to alter their physiological state when faced with challenging environmental conditions, particularly when under antibiotic stress [
61]. Metabolic reconfiguration of
Mtb can significantly modify its metabolic state to endure demanding conditions. During exposure to drugs, the bacterium can enter a metabolically inactive state, which reduces susceptibility to the drug and leads to metabolic dormancy and establish safeguarding conditions that restrict drug infiltration via biofilm development or exploration of alternative metabolic pathways that circumvent drug-targeted processes by leveraging metabolic heterogeneity.
Mtb uses phenotypic adaptation as a vital survival strategy, enhancing its notable capacity to acquire and sustain multi-drug resistance [
61].
Oxidative Stress and Adaptation
Following
Mtb infraction the immune system of the host initiates its defense mechanisms against the pathogen, subsequently triggering the phagocyte process that leads to the production of reactive oxygen species (ROS) [
62]. The host cells elevate the production of ROS to eliminate mycobacterial infection. However, the potential harm of overproduction of ROS cannot be overstated. It can harm host cells by exacerbating inflammation and related tissue damage, underscoring the urgency of research. For example, when the generation of ROS such as hydrogen peroxide (H
2O
2) and superoxide anion exceeds the necessary levels for cellular metabolism in the lungs, it can result in excessive tissue exposure to a persistent redox imbalance [
63].
The role of oxidative stress induced by host macrophages is crucial in inhibiting the growth and development of
Mtb. Certain drug-resistant strains of
Mtb impede the redox defense of the host via different mechanisms. Mycolic acid, associated with the cell wall, is a physical barrier against host-related oxidative stress and can inhibit the oxidative stress response through its presence in the cell wall [
64]. A point alteration in the
ndh gene, responsible for encoding NADH II dehydrogenase in certain
Mtb strains, leads to increased levels of NADH/NAD+, resulting in co-resistance to ethionamide and isoniazid. These strains resist acidified nitrites and peroxides [
65]. However, certain strains of
Mtb possess a unique protein known as enhanced intracellular survival (Eis), which plays a fascinating role in identifying and countering ROS [
66]. Certain strains of
Mtb possess peroxiredoxin, including thioredoxin reductase (
TrxR) and thioredoxin, which can mitigate and modify oxidative stress via disulfide reductase activity [
67,
68].
DosS and
DosT act as redox sensors and activate the transcription factor
DosR, which assists in the anaerobic survival of
Mtb and contributes to the latent phase of infections [
69,
70]. In addition, to causing tissue damage, ROS activate antimycobacterial agents such as INH and pretomanid.
Mtb develops resistance to this drug through inactivation of host-mediated oxidative stress, highlighting the ability of
Mtb to manipulate the oxidative response of the host [
71]. Oxidative stress is a significant underlying factor, and further research is urgently required to improve the different regimens for treating tuberculosis.
3. Advances in MDR-TB Treatment Strategies
3.1. Emerging Drug Regimens
3.1.1. Bedaquiline-based Regimens
Bedaquiline (BDQ) or diarylquinoline TMC 207 represents the first adenosine triphosphate (ATP) synthase inhibitor targeting MRD-TB [
72]. This drug represents a significant advancement in treating MRD-TB infections with recent studies demonstrating remarkable efficacy against drug-resistant
Mtb strains [
73,
74,
75,
76]. Bedaquiline has a unique mechanism of action, which differs from existing anti-tuberculosis therapeutics as it explicitly targets mycobacterial ATP synthase (
Figure 2), a crucial membrane-bound enzyme found in dormant and actively replicating mycobacteria. By binding to a particular site on subunit c, it disrupts the rotational movement of this subunit during catalysis or at the interface between the oligomeric subunit c and subunit a, thereby hindering energy metabolism of the pathogen [
77,
78]. The substance has a remarkable capacity to eliminate mycobacteria across different microenvironments which is partly attributed to its affinity for TMC207 under low pH and low proton motive force values [
77].
It has been reported that BDQ can interact with the ε subunit [
78,
79] Bedaquiline has a significant impact on the immune system of the host and in a recent study it was reported that bedaquiline improves the innate immune resistance of host macrophages against bacterial infections. Treatment with BDQ initiated a range of antimicrobial defence mechanisms, such as the fusion of phagosomes and lysosomes and autophagy. The effects observed were linked to the activation of transcription factor EB, which plays a role in the transcription of lysosomal genes, leading to improved intracellular killing of different bacterial species inherently resistant to BDQ. Importantly, the role of BDQ as a host-directed therapy for different bacterial infections [
80] underscores its potential and importance in pharmacology and immunology and warrants additional exploration due to potentially significant and positive impacts when treating cancer patients with compromised immune systems [
72].
The effectiveness of BDQ has been shown to reduce the treatment period for MDR-TB patients and improve the success rate of therapy [
81,
82,
83,
84]. BDQ is a fundamental element of the six-month treatment protocol for MDR/rifampicin-resistant (RR)-TB/BPaLM/BPaL which includes bedaquiline, pretomanid, linezolid, with or without moxifloxacin and is the preferred regimen recommended by WHO for treating adolescents and adults > 14 years of age. BDQ is a key element of the nine-month all-oral regimen, recognized as the preferred treatment for eligible children and young adolescents < 14 years of age infected with MDR/RR-TB, as opposed to the longer (18-month) regimens [
83]. BDQ exhibits significant lipophilicity, characterized by an extended effective half-life, a complex distribution profile and a slow elimination profile characterized by a gradual release from peripheral tissues [
85]. BDQ is available as 400 mg tablets, administered daily for two weeks which is then reduced to 200 mg three times a week for 22 weeks. BDQ must be taken with food, as this enhances bioavailability two-fold BDQ undergoes metabolism via the cytochrome P450 isoenzyme 3A4 and is influenced by both inducers and inhibitors of this specific isoenzyme. Adverse drug reactions associated with BDQ typically affect the gastrointestinal, musculoskeletal, and central nervous systems and include nausea, vomiting, diarrhoea, abdominal discomfort, limb pain, joint pain, back pain, headache and dizziness [
86,
87].
3.2. Precision Medicine in MDR-TB
For more than five decades, managing patients infected with tuberculosis has followed a standardized approach which overlooks the differences in human vulnerability to infection, immune response, pharmacokinetics and the duration of treatment required to achieve a relapse-free cure. However, recent notable scientific discoveries and technological advancements offer a perspective for personalized rather than standardized management of patients with tuberculosis. This shift in approach can be used to optimize the selection of the most effective medications and host-directed therapies and tailor drug dosing and treatment durations, making it a topic of great interest for clinicians managing tuberculosis [
88]. Precision medicine for tuberculosis encompasses tailor-made treatment regimens, therapeutic drug monitoring, and biomarker-guided therapy.
3.2.1. Tailor-Made Treatment Regimens
Recent advancements in whole-genome sequencing are revolutionizing the application of genomics in the epidemiology and diagnosis of TB. The enhanced accuracy of innovative methods enables experts to pinpoint transmission with remarkable clarity and to develop customized approaches to decrease the occurrence of TB both locally and globally. Healthcare professionals, researchers and policymakers are crucial in integrating these advancements into clinical practice. Furthermore, in contrast to the restricted commercially available molecular tests, diagnosing drug resistance via the complete genome allows for examining all drug resistance targets by using the catalogue provided by the WHO. These advancements are progressively being integrated into clinical practice, ultimately facilitating the gradual control of TB and providing tailored care for every patient [
89].
3.2.2. Therapeutic Drug Monitoring (TDM)
Considering the complexities of managing MDR-TB, healthcare professionals can enhance the chances of achieving successful outcomes by implementing a TDM approach to therapy. TDM is a clinical approach that makes use of concentrations from the patient to modify therapy, enhancing the chances of achieving therapeutic drug levels while reducing the risk of toxicity [
82]. The use of TDM to strengthen the management of TB has received strong support from established guidelines, including the ATS/CDC/ERS/IDSA clinical practice guideline for treating drug-resistant TB [
90] and provides a solid foundation for implementation. Potential reasons for TDM may encompass compliance assessment, tailoring treatment, determining if a patient is receiving the correct doses , preventing toxicity related to drug concentration and addressing issues related to drug-drug interactions [
91]. It may be prudent to conduct TDM in all patients with MDR-TB instead of only using it when treating patients, those that exhibit a poor response to treatment [
90].
3.2.3. Biomarker-Guided Therapy
Without a timely indicator of treatment failure or relapses in patients infected with MDR-TB, biomarkers derived from host-miRNA and
Mtb-RNA, assessed in extracellular vesicles (EV) provide a viable alternative for monitoring MDR-TB infections. The analysis of the payload of EV to determine differentially expressed miRNA before and after treatment, in addition to monitoring
Mtb-derived RNA in serum EV from patients resistant to TB. A dual signal consisting of host-derived miR-let7e-5p and
Mtb-derived RNA could indicate treatment failure or relapse after the treatment period has ended [
92]. In numerous diseases, such as tuberculosis, biomarkers play a crucial role in diagnosing the condition, anticipating the emergence of an active phase, and assessing responses to treatment or vaccination [
93] thereby, keeping clinicians informed and aware of the progression of the disease.
3.3. Novel Drug Candidates
3.3.1. Delamanid
Delamanid (DLM)/OPC67683 is an anti-TB agent from the nitro-dihydro-imidazooxazole class of compounds which inhibit mycolic acid synthesis in the bacterial cell wall. It is a potent weapon in the fight against drug-resistant tuberculosis, demonstrating its effectiveness when used in combination with other antibiotics [
30,
32]. DLM is a prodrug that must be activated bioactivation before exerting antibacterial efficacy against both proliferating and dormant mycobacteria via the mycobacterial F420 coenzyme and the deazaflavin-dependent nitroreductase (Rv3547) enzyme systems. Following stimulation, the synthesis of methoxy mycolic and ketomycolic acids is inhibited via the radical intermediate generated between DLM and the desnitroimidazooxazole derivative, resulting in the depletion of mycobacterial cell wall components and, ultimately results in cell lysis [
40,
94].
The recommended oral dose of delamanid is 100 mg twice daily for people > 50 kg in weight and 50 mg twice daily for those weighing between 30 and 50 kg [
40]. Healthcare professionals must consider the weight of the patient when prescribing delamanid, as it can significantly affect the dose required and treatment outcome. DLM is poorly soluble in aqueous media, and absorption is enhanced two-fold when administered with meals. The absolute bioavailability is undetermined, but it ranges between 25% and approximately 47% [
40]. DLM is binds extensively to proteins with a capacity > 99% which translates into a volume of distribution of 2100 L and a half-life ranging between 30 and 38 hours. It has been suggested that albumin primarily facilitates the metabolism of DLM, supplemented by the involvement of P450 enzymes, particularly
CYP3A4 [
94]. Gastrointestinal adverse effects are associated with DLM. DLM may induce QTc prolongation, an adverse effect linked to various medicines used to treat MDR-TB, including BDQ and fluoroquinolones [
40].
3.3.2. Pretomanid
Pretomanid, or PA-824, is the third medication to receive authorization from the Food and Drug Administration (FDA) in 2019, following BDQ and DLM [
95]. It is an oral nitroimidazooxane that disrupts mycolic acid biosynthesis by obstructing hydroxy-mycolate oxidation to ketomycolate, thereby actively targeting replicating cells by disruption of cell wall synthesis. This agent demonstrates efficacy against non-replicating
Mtb in anaerobic environments, acting as a respiratory toxin and inhibiting protein synthesis, that is attributed to the generation of intracellular nitric oxide [
96,
97,
98]. However, the influence of nitric acid does not yield a notable bactericidal effect on bacteria that replicate aerobically [
98,
99]. Pretomanid is a prodrug that requires metabolic stimulation through a deazaflavin (cofactor F420)-dependent nitroreductase (
ddn) pathway [
99]. The potential of pretomanid for treating extensively resistant tuberculosis (MDR-TB and XDR-TB) in combination with BDQ and linezolid as part of the BPaL course of therapy [
95,
100,
101] brings hope and optimism to the field of infectious diseases.
The bioavailability of pretomanid at doses of 50−1500 mg in humans was favourable and is significantly enhanced following a high-calorific fat meal relative to fasting conditions [
102,
103]. Pretomanid has a half-life of 16 to 20 hours and can be administered once daily.
CYP3A4 constituted around 20% of the metabolism in vitro [
97] and everyday side events linked to pretomanid include peripheral neuropathy, acne, anemia, abdominal pain, nausea, vomiting, musculoskeletal pain and headache [
97].
3.4. Repurposed Drugs for MDR-TB
Patients encounter difficulties in adhering to prescribed regimens for treatment of multi-drug-resistant tuberculosis due to considerable toxicity, low efficacy and prolonged treatment durations leading to drug resistance (DR). The development of resistance to several first-line anti-TB medications requires the development of new TB therapies for treating drug-resistant individuals effectively. One key aspect of these new therapies is the need for a reduced treatment duration for drug-susceptible and resistant bacterial strains. However, establishing a new medicine regimen that integrates two or three innovative and effective pharmaceuticals is a lengthy process and may take between 20 and 30 years with substantial financial investment, as observed with the development of BDQ and DLM. These challenges make medication repurposing a necessity, and the repurposing of previously approved pharmaceuticals for other conditions holds significant promise for the treatment of anti-DR-TB. Consequently, drug repurposing/repositioning is a fascinating field that involves discovering novel therapeutic applications for an existing medication, focusing on its pharmacodynamics and interactions with other receptors. This approach could improve the treatment of various diseases for which the medication was not initially authorized. These repurposed pharmaceuticals target several routes, reducing the likelihood of treatment resistance. Examples of these compounds include sulfonamides, sulfanilamide, sulfadiazine, clofazimine, linezolid, amoxicillin/clavulanic acid, carbapenems, metformin, verapamil, fluoroquinolones, statins, and NSAID. Their mechanisms of action are associated with immunomodulatory effects on the host, facilitating both host-directed and pathogen-targeted therapy options [
104,
105].
3.4.1. Linezolid
Linezolid (LZD), an oxazolidinone derivative, is authorized for the treatment of severe skin and soft tissue diseases, bacteraemia and pneumonia caused by Gram-positive bacteria. LZD eradicates
Mtb by attaching to and obstructing tRNA in the peptidyltransferase centre of the 50S ribosomal subunit, which comprises 5S rRNA and 23S rRNA [
106,
107]. The progressive repurposing of LZD for the treatment of MDR and XDR-TB has been supported by encouraging clinical evidence [
107,
108]. The standard oral treatment dose is 1200 mg, administered once daily or 600 mg, administered twice daily. In the short term, headache, rash and gastrointestinal side effects such as diarrhoea and nausea are the most frequent adverse reactions [
40]. Severe adverse effects resulting in treatment interruption with LZD occur in approximately 3–4% of patients undergoing short-duration treatment. Myelosuppression is a significant side effect of LZD, impacting approximately 28–33% of patients undergoing prolonged treatment with the drug [
109]. Initial findings indicate that toxicity is dose-dependent and often manifests after a minimum of two weeks of treatment. Consequently, myelotoxicity is unlikely in patients receiving dosages of ≤ 600 mg daily, even with a duration of therapy exceeding 20 months. This underscores the safety when using lower doses and should give healthcare professionals confidence when considering their prescribing decisions [
110].
3.4.2. Clofazimine
Clofazimine (CFZ) is predominantly utilized for treating leprosy and is a lipophilic riminophenazine dye exhibiting both anti-mycobacterial and anti-inflammatory properties and was first identified as an anti-TB agent in the 1950s [
40,
111,
112,
113]. The precise mechanism of action of CFZ is still unknown, however it seems to exhibit various effects on
Mtb, such as disrupting redox cycling through the enzymatic reduction of CFZ by NDH-2, leading to the production of bactericidal ROS in addition to membrane destabilization and dysfunction by obstructing the electron transport chain in bacteria [
113,
114]. Evidence suggests s that CFZ and BDQ affect the electron transport chain of
Mtb [
114]. The typical oral dose of CFZ is 100 mg once daily, and the primary adverse effects of CFZ include skin pigmentation and gastrointestinal tract discomfort [
40].
3.4.3. Cycloserine
Cycloserine exhibits a unique mechanism of action and has been used to treat MDR-TB since the 1950s [
115]. It is a bacteriostatic agent indicated for incorporation and use in prolonged MDR-TB treatment regimens. Terizidone is a structural analogue of cycloserine that is also effective for the treatment of MDR-TB [
40]. Cycloserine is a cyclic counterpart of D-alanine and may inhibit alanine racemase and D-alanine ligase, thereby disrupting bacterial cell wall synthesis [
40,
116] without exhibiting cross-resistance with other anti-TB medications due to the distinct mechanism of action [
117]. Cycloserine, with its unique mechanism of action and promising potential, should intrigue healthcare professionals. In addition, its safety profile justifies its application in most instances, and it has been found to markedly enhance the likelihood of a positive result for patients with uncomplicated MDR-TB but not those who present with pre-XDR-TB or XDR-TB [
116]. The maximum daily dose is 1000 mg, and the principal side effects linked to cycloserine include psychiatric conditions and central nervous system toxicity [
40].
3.5. Host-Directed Therapies (HDT)
Adjunctive therapies, designed to 're-educate' the immune system, offer a viable and alternative strategy to customize the response of the host to TB infection [
118]. The proven effectiveness and attractiveness of HDT, given the significant influence of the immune response of the host on
Mtb infection outcomes [
119,
120] offer potential opportunities to combat drug resistance. By enhancing the efficacy of tuberculosis therapy and disrupting the mechanism(s) essential for the sustained persistence and replication while targeting routes affected by of
Mycobacterium tuberculosis without directly engaging with it offers a strategic alternative to therapy [
118,
121,
122]. As a result, HDT alleviates the burden of infection by functioning as an immunomodulator, thereby enabling the body to combat antibiotic-resistant pathogens while reducing the likelihood of developing resistance to susceptible new drugs, as
Mtb cannot acquire resistance to a drug that targets host cell functions [
104,
123].
Host-directed immunomodulation by reducing treatment duration and preventing the emergence of resistance whilst increasing the susceptibility of
Mtb to existing anti-tuberculosis medications and mitigating the response of the host inflammatory toxicity that compromises treatment efficacy will benefit patients [
105,
119] as the approach holds significant promise for the treatment of drug-resistant TB. Pharmaceutical compounds that target the host cell rather than the
Mtb bacillus directly are less susceptible to induced drug resistance, thereby reducing selection pressure on the bacterium [
122]. Furthermore, HDT offers a promising approach for the treatment of drug-resistant TB with minimal exposure to TB medications, thereby preventing or slowing the emergence and dissemination of superbugs. The combination of HDT with conventional therapy not only facilitates synergism but also allows for a dose reduction, which in turn, decreases toxicity whilst maintaining treatment efficacy [
104,
121,
122]. HDT may also mitigate the hazards related to drug-drug interactions in patients with co-morbidities, such as those treated with antiretrovirals in TB-HIV positive patients [
124]. Some promising HDT candidates include corticosteroids such as prednisolone and dexamethasone, ibuprofen, aspirin [
118,
125,
126], metformin [
121,
127], NSAID, vitamin A, zinc [
118,
128] and vitamin D
3 [
118,
121].
Repurposed medications used to treat tuberculosis function as host-directed therapy, conditioning the immune cells of the host to accommodate the presence of tuberculosis, enhancing their antibacterial efficacy, and significantly reducing the duration required to eradicate the illness while minimizing inflammation and tissue damage [
104]. This promising approach provides hope for the future of patient care. The primary mechanisms through which repurposed adjunctive compounds enhance tuberculosis treatment outcomes include modulation of inflammatory routes and pro-inflammatory mediators to attenuate inflammation and associated tissue pathology, thereby improving lung function and integrity, enhancement of the ability of the host immune response and reinforcement of immune and memory responses, augmentation of host bactericidal mechanisms, macrophage-mediated
Mycobacterium tuberculosis elimination in addition to a reduction of bacilli proliferation and disruption and penetration of any granuloma to expose
Mycobacterium tuberculosis to anti-tuberculosis therapy [
118].
Host-directed therapies (HDT), particularly those involving repurposed medications, are not just beneficial but essential for achieving the 2035 World Health Organization (WHO) End TB objectives [
118] which underscores the urgency and importance of our collective efforts in this field of research.
3.5.1. Metformin
Metformin is used for managing type 2 diabetes through mechanisms involving AMP-activated protein kinase (AMPK) dependence, independence and Sirtuin I inhibition. These are crucial for detecting cellular energy levels and may facilitate activation of autophagy to eliminate
Mtb, mechanisms which have, in recent studies, been supported [
71,
104,
105]. Metformin treatment augmented the protective immunological response and elevated ROS generation, inhibiting
Mtb growth. The medicine demonstrated efficacy in eradicating medication-resistant bacterial strains by facilitating effective phagosome-lysosome fusion, alleviating chronic lung inflammation, augmenting the immune response and boosting the efficacy of conventional TB medications [
129,
130]. Metformin augments the release of IFN-γ from CD4+ and CD8+ T cells, modulates inflammation, and activates intracellular antimicrobial defenses [
104,
130,
131]. Given the extensive utilization of metformin and current safety data, it is ideal as a primary choice for adjunct high-dose therapy when treating tuberculosis [
71].
3.5.2. Non-Steroidal Anti-Inflammatory Drugs (NSAID)
The
host-directed therapeutic actions of NSAID are primarily influenced by their distinct pharmacokinetic features rather than alternative pathways of activity which result in a variety of effects depending on tissue location and cell type. Ibuprofen has been extensively investigated for tuberculosis treatment and aspirin is a prevalent has garnered interest in tuberculosis research [
125]. The primary mechanism by which NSAID function in tuberculosis treatment is by alleviating inflammation resulting from the accumulation of monocytes, lymphocytes and neutrophils [
105]. NSAID demonstrate anti-inflammatory effects by inhibiting the cyclooxygenases COX-1 and COX-2 enzymes which regulate pro-inflammatory and immunosuppressive mediators, including prostaglandins and leukotrienes. Inhibiting cyclooxygenase enzymes halts chronic inflammatory responses in the host contributing to pathological lung lesions while enhancing bactericidal mechanisms and the immunological response to vaccines. The justification for using NSAID as HDT relates to the suppression of pro-inflammatory COX enzymes which mitigate excessive inflammation-related tissue damage and enhances host bactericidal function in persons with active tuberculosis [
105,
118].
3.5.3. Vitamin D3
Many patients with tuberculosis exhibit clinical deficiencies of different vitamins, including vitamin D
3 [
119]. Consequently, because of the association between vitamin D
3 deficiency and susceptibility to tuberculosis, vitamin D
3 has emerged as a significant focus of investigation for HDT-TB. Recent studies have highlighted the potential of vitamin D
3 to improve the production of reactive oxygen and nitrogen intermediates, promote autophagy and facilitate the production of antimicrobial peptides [
71,
121]. Vitamin D
3 exerts an immunomodulatory action on the innate immune system by upregulating its response and inflammatory response, respectively, through its active form 1,25-dihydroxy vitamin D
3 (1,25D) pathway, leading to a reduction in the proliferation of
Mtb in macrophages treated with 1,25D. Vitamin D significantly influences the innate immune system by promoting the expression of antimicrobial proteins and facilitating the formation of autophagosomes. Vitamin D also influences the adaptive immune system by promoting the formation of suppressive regulatory T cells and inhibition of the production of inflammatory Th17 cells. Consequently, the relationship between vitamin D and tuberculosis focuses mainly on enhancing bacterial removal via integrated innate and adaptive immune mechanisms and reduction of tissue damage [
121,
132]. In addition to enhancing innate immune function through various mechanisms, such as the induction of autophagy, vitamin D plays a crucial role in modulating inflammatory responses which is achieved by reducing the development of pro-inflammatory cytokines and chemokines, raising the levels of anti-inflammatory cytokines and influencing the T-cell response [
120].
3.6. Enhancing Autophagy in Therapy
3.6.1. Autophagy Inducers
The activation of autophagy in diseased cells by diverse autophagy-inducing compounds (AIC) has emerged as a promising alternative treatment strategy for tuberculosis [
121]. Autophagy is a catabolic mechanism that facilitates lysosomal breakdown of cellular constituents, including invading pathogens such as
Mtb to maintain cellular homeostasis [
133,
134]. Macrophages exhibit robust antimicrobial responses to
Mtb infection via autophagy. Nonetheless,
Mtb has developed advanced approaches to evade, disrupt, and manipulate the antimicrobial functions of macrophages by disrupting the production of protective Th1-type cytokine , vacuolar membrane trafficking or autophagy activation for extended survival rather than merely eliminating the host [
135]. Autophagy is vital in sustaining intracellular balance and essential to the immune response. Therefore, modulators of autophagy or activating this process through different drugs or agents is a promising opportunity in host-directed therapy against
Mtb infection, whether used alone or in combination with standard treatments, including for drug-resistant strains. The modulation of autophagy activation plays a crucial role in managing inflammation, enhancing the effectiveness of both innate and adaptive immune responses against
Mtb [
134,
136,
137]. Key factors that facilitate the activation of autophagy encompass vitamin D receptor signaling, the AMP-activated protein kinase pathway, sirtuin 1 activation and nuclear receptors [
134].
Innovative approaches in anti-TB treatment have been proposed by manipulating autophagy activation including the use of surface-functionalized or modified nanoparticles (NP) that encapsulate traditional anti-TB medications and other AIC designed for HDT [
121]. NP augment the efficacy of AIC, thereby enhancing stability, facilitating cell targeting and creating avenues for multimodal treatment [
137]. The established HDT drugs are crucial in pathological inflammation, phagolysosomal fusion, lysosomal functions and the antimicrobial response of host cells infected by
Mtb. While agents that activate autophagy could serve as potential therapeutic options for HDT-TB, numerous other biological pathways including autophagy, play a role in the ability of the host to defend itself against TB infection. For instance, vitamin D is often used for treating HDT-TB and was used before the advent of antibiotics. Beyond a capacity to activate autophagy, vitamin D exhibits a range of activity on diseased cells and tissues,
including a significant role in enhancing direct antimicrobial defense through cathelicidin and modulating inflammation [
134].
A limitation in the field pertains to clinical usage, specifically regarding the potential for targeted drug administration to disease sites and the possibility that autophagy-adjunctive therapies might shorten the period of antibiotic treatment. Ongoing and forthcoming investigations into autophagy-based HDT therapeutic candidates will enhance our understanding of the antibacterial role of autophagy. Nonetheless, this approach might also play a role in advancing therapeutic strategies for TB [
134].
4. Innovative Drug Delivery Systems
4.1. Nanoparticle-Based Drug Delivery
The primary challenges related to conventional tuberculosis medications include inadequate aqueous solubility, limited penetrability, systemic toxicity at therapeutic doses, off-target accumulation, bacterial mutation resulting in multidrug-resistant strains and diminished bactericidal efficacy against bacteria within macrophages or infected deep tissues. In this respect, nanomedicine have been recognized as providing unique benefits that may effectively tackle the previously reported challenges in respect of treating tuberculosis infection [
138]. Nanoparticles serve as drug nanocarriers or nanocontainers with the potential to augment therapeutic efficacy and enhance patient adherence to tuberculosis treatment, leading to more optimistic treatment outcomes. They offer advantages such as substantial and multifaceted drug encapsulation, improved immune response, significant passive permeability of the payload, sustained release, facilitation of autophagy-inducing activity, reduction of administered doses, precise delivery, decreased dosing frequency, minimal adverse effects, and the utilization of multiple synergistic mechanisms to enhance antimicrobial activity and counteract antibiotic resistance [
28,
121,
137,
139].
The mechanisms of antimicrobial activity when delivered from nanoparticles include alteration of essential proteins, inhibition of enzyme activity and protein synthesis, incorporation into DNA bases, generation of oxidative stress, disruption of cell signaling, inhibition of biofilm formation, penetration of cell membranes and inhibition of cell wall synthesis [
31]. The modulation of nanocarrier attributes, including surface composition, charge, shape, temperature, redox state, particle size, pH, hydrophobicity, hypoxia and Zeta potential, may influence drug uptake by alveolar macrophages (AM) [
26,
140]. An alternative method involves targeting ligands on the nanocarrier that engage with specific receptors on macrophages, which is referred to as active or ligand-mediated targeting. Nanoparticles (NP) can activate macrophages, driving them into a bactericidal state that effectively target and eliminate intracellular
Mtb. The surface of the nanoparticles can be modified with ligands to engage with particular macrophage surface receptors that play a role in macrophage activation [
141]. Approaches independent of specific ligands are termed enhanced permeability and retention effects or passive targeting. Carriers based on polymer and polysaccharides, liposomes, and metallic nanoparticles have gained interest in active and passive targeting of antimicrobial agents (AM) in MDR-TB medication administration. Their potential to improve drug solubility, stability, and bioavailability offers hope for the future of tuberculosis treatment furthermore, facilitated and regulated release and targeted medication administration to the infection site is possible [
139,
142].
4.1.1. Liposomal Systems
Liposomes are remarkable spherical vesicles comprised of lipids, for example, phospholipid and cholesterol, to enhance drug delivery and mitigation of drugs. They can encapsulate hydrophobic pharmaceuticals within the hydrophobic core of their bilayers and water-soluble substances in the hydrophilic centre region, thereby facilitating their transport across biological barriers. These are the well-studied systems for the controlled administration of medications to the lungs, as they can be formulated with phospholipids naturally present in the lungs as surfactants. The drug delivery mechanism requires either fusion with the cell membranes for drug release or the endocytosis process, which entails entry into mononuclear phagocytic macrophages. They can enhance the pharmacokinetics of medicines and mitigate toxicity by lowering systemic exposure to elevated drug concentrations [
28,
140].
Liposomes were successfully synthesized with dipalmitoylphosphatidylcholine and cholesterol as a carrier for Zn(II) phthalocyanine (ZnPc), a non-toxic photosensitizer for the inactivation of susceptible (ATCC 27294)
Mtb and MDR-TB (9037R) [
140]. The ZnPc-loaded liposomal formulation, when compared to the unmedicated or control liposomes, successfully inactivated the two pathogen strains used in this investigation, providing a significant breakthrough in the field. The duration of incubation and light exposure influenced the photoinactivation process. A significant reduction of three (3) log10 CFU/mL following two (2) hours incubation with 75 J/cm
2 or 150 J/cm
2 irradiation of the susceptible strain was observed. For MDR-TB, it was necessary to modify the incorporation time to four (4) hours and increase the light exposure to reduce three (3) log10 CFU/mL. Consequently, applying photodynamic antimicrobial chemotherapy with ZnPc-liposomes presents a potential alternative for treating MDR-TB, demonstrating an impressive reduction of 99.9% in mortality in vitro.
Niosomes are similar to liposomes but include a surfactant bilayer with external and internal hydrophilic termini bared to the aqueous phase, while the hydrophobic chains are oriented towards one another inside the surfactant bilayer [
143]. Ethionamide (ETH) is a second-line anti-TB agent, making it a preferable treatment option for MDR-TB. However, it is associated with transient, asymptomatic increases in serum aminotransferase levels and, in rare cases, can lead to severe acute liver injury [
144]. The negative impact of ethionamide prompted Sadhu et al. [
145] to develop niosomes to encapsulate the drug. This approach aims to enhance the therapeutic efficacy of the pharmaceutical by prolonging its presence in the bloodstream, using these vesicles as a reservoir for controlled drug release, addressing drug resistance challenges, shortening the duration of treatment and minimizing drug-drug interactions resulting in better patient adherence and treatment outcomes whilst minimizing drug-related toxicity. ETH-loaded niosomes prepared through thin film hydration exhibited sustained release of 94.89% of the payload over 24 hours, implying a potential reduction in the frequency of dosing [
145].
The encapsulation of ETH within niosomes prepared through thin-film hydration led to regulated drug release, enhanced efficacy and improved safety compared to the unencapsulated drug [
146]. The formulation effectively increased drug delivery to the lungs of mice over an extended duration, resulting in reduced bacterial counts in lung homogenates.
A long-acting dual drug-loaded self-assembling niosome technology incorporating lipophilic ETH and hydrophilic D-Cycloserine to effectively treat MDR-TB has been reported [
147]. A Box Behnken experimental design was used to develop and optimize the niosomes, and the formulation demonstrated commendable stability over a 6-month period. Haemodialysis studies revealed that administration of the dual drug-loaded niosomes via the intravenous route was safe, and the MIC for the niosome technology was the lowest MIC compared to free drug and single drug-loaded niosomes. The retarded rate of release of ETH and rapid initial release of D-Cycloserine played a significant role in the efficacy. The combined effect of the two drugs in the niosomes demonstrated a more effective treatment option for tuberculosis when compared to the pure drug combination [
147].
Notwithstanding the numerous advantages of liposomes, including their safety and biocompatibility profiles, their principal drawback as nanocarriers is their instability in plasma. Selective serum proteins such as opsonins adhere to the surfaces of liposomes on entry into the systemic circulation and alert the mononuclear phagocyte system (MPS) to their presence and resulting in removal from the bloodstream. Nonetheless opsonization and removal can be mitigated through functionalization with polyethene glycol (PEG) or other ligands, including antibodies and that enhance precision targeting to the infected location [
148].
4.1.2. Metallic Nanoparticles
Metal nanoparticles (MNP) have attracted considerable interest due to their medicinal applications, especially in antibacterial, drug delivery and theragnostic applications. These nanoparticles exhibit significant antibacterial efficacy and biocompatibility, rendering them promising agents in the fight against antimicrobial resistance. Different MNP, including iron oxide, zinc oxide, silver and gold, have demonstrated promise in boosting the efficacy of medicine against resistant microorganisms. MNP can disrupt bacterial cell membranes and produce ROS, including superoxide anions, hydrogen peroxide and hydroxyl radicals, which disrupt DNA replication and amino acid synthesis, thereby compromising microbial cell membranes. This disruption makes the development of bacterial resistance unlikely, providing reassurance about the effectiveness of MNP in combating antimicrobial resistance. The positively charged magnetic nanoparticles may engage with the negatively charged bacteria, resulting in lipid oxidation and cell death. Moreover, MNP can eliminate microbes through the release of ions [
31,
142,
149]. The mechanism by which MNP exerts their effects on bacteria is intricate and can simultaneously target multiple cellular structures, complicating the development of adaptive responses and consequently reducing the likelihood of developing bacterial resistance. MNP also serve as a unique delivery system for antibacterial drugs, protecting them against enzymatic and other degradation pathways. Their distinctive characteristics, including an ability to enhance delivery with greater specificity and diminished adverse effects, make them a fascinating area of research and development [
31,
142,
149].
The efficacy of rifampin (RIF) when treating MDR-TB has been enhanced [
150] using engineered polydopamine-coated silver nanoparticles (Ag-PDA NP) and revealed that minimum inhibitory concentration (MIC) tests performed with different ratios exhibited a synergistic interaction between Ag-PDA NP and RF, with the most effective antimycobacterial outcome against the multidrug-resistant strain of
Mtb occurring at a mass proportion of 2 Ag-PDA NP to 8 RF. The synthesized and characterized RF-loaded Ag-PDA nanoparticles show that this drug-loaded metallic nano-formulation is not just a potential solution but a promising one to limit the growth of multidrug-resistant strains of Mycobacterium and maintain the efficacy of RIF in clinical applications.
The antitubercular properties of silver nanoparticles when used in combination with antituberculosis medications has been reported and involved in vitro experiments in which 65 white mice were used with a model of resistant tuberculosis [
151] . The apparent antitubercular effects of the nanocomposite were demonstrated using silver nanoparticles and isoniazid.
In a 2019 study the antimycobacterial efficacy of combination treatment with transition metals and antibiotics against
Mtb strains resistant to first-line drugs was reported [
152]. The findings suggest that a combination of INH and AgNO
3 exhibited a synergistic effect, demonstrating bactericidal activity against a clinical strain of
Mtb resistant to isoniazid [
152].
Silver nanoparticles (AgNP) and zinc nanoparticles (ZnNP) exhibited effectiveness against
Mtb and a MDR strain, with a MIC of 1.25 mg/ml [
153]. The AgNP demonstrated superior antimicrobial efficacy when compared to ZnNP. The effectiveness of these nanoparticles in combating drug-resistant pathogens positions them as a promising option for therapeutic application.
A study showcasing the potent antimycobacterial properties of AgNP was reported in 2018 and focused on reference strains of
Mycobacterium bovis and
Mtb H37Rv, in addition to a MDR strain of
Mtb and clinical isolates of both
M. bovis and
Mtb to determine the MIC of AgNP using the microplate Alamar blue assay. This study brings hope and optimism as it reveals the potential of AgNP as a promising chemotherapeutic agent against Mycobacterium
spp [
154].
The exceptional potential of mixed metallic nanoparticles against MDR-TB has sparked interest in their application [
153]. The anti-tubercular efficacy of metallic nanoparticles, specifically magnesium oxide nanoparticles (MgONP) and zinc oxide nanoparticles (ZnONP), remain unexamined in respect of MDR-TB. A study was initiated to compare the effects of different doses of a blend of MgONP and ZnONP on two clinical isolates viz., MDR-
Mtb and reference strain [
155]. The research assessed the MIC and minimum bactericidal concentrations (MBC) of the ZnONP, MgONP and MgONP-ZnONP against H37Rv Mtb and MDR-Mtb. The ZnONP and MgONP-ZnONP exhibited bactericidal properties and exhibit synergistic benefits against MDR-TB [
155].
The antimycobacterial properties of Ag, ZnO and Ag-ZnO NP against MDR and XDR-
Mtb have been reported [
156]. The MIC results demonstrated the inhibitory effects of these nanoparticles on these two strains of
Mycobacterium tuberculosis. Nonetheless, MBC results demonstrated that Ag, ZnO, and Ag-ZnO NP whether used individually or in combination, were ineffective in eradicating MDR- or XDR-
Mtb. These nanoparticles are promising anti-mycobacterial nanodrugs due to their bacteriostatic activities against drug-resistant strains of
Mtb. However additional research is necessary to validate the bactericidal properties of these nanoparticles against TB.
Nanoparticles, with the bactericidal and immuno-potentiating attributes, are currently being explored as an antibiotic alternative for their potential to reduce antibiotic doses , minimize toxicity and reduce the likelihood of multi-drug resistance [
31]. This reduction in toxicity offers a reassuring prospect for the safety of future therapies however, further research on the synergistic effects of metallic nanoparticles with traditional anti-TB medications and novel anti-tubercular medicines is required. This research could significantly enhance the antibacterial efficacy of isoniazid and rifampicin against MDR-TB which has acquired resistance.
4.1.3. Polymeric Nanoparticles (PNP)
PNP nanosystems can enhance the efficacy of chemotherapeutic agents and mitigate the adverse reactions of anti-TB medications through encapsulation and conjugation of therapeutic agents in carrier technology. The nano-systems can be manufactured from many natural or synthetic precursors, including collagen, chitosan, gelatin, albumin, polyethylene glycol, polylactic acid, poly (lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL). PLGA copolymers are extensively used for the delivery of anti-TB medicines. Moreover, polymers used to manufacture PNP can be transformed into different forms such as micelles, vesicles, dendrimers or hybrid inorganic-polymer nano-carriers [
148,
157]. Polymeric nanoparticles have generated significant interest as promising antibacterial drug delivery systems as the exhibit numerous advantages, including effective cargo dissolution, entrapment, encapsulation, surface bonding and/or functionalization, antibiotic properties, capability to form antimicrobial groups for targeted destruction, biocompatibility, biodegradability, co-delivery of diverse drugs, accumulation on cell membranes, low toxicity, stability and potential synergistic therapeutic activity [
148,
157,
158,
159].
Due to the toxicity, cost and limited effectiveness of drugs used to treat MDR-TB, a nano-formulation was used clinically used to deliver moxifloxacin (MOX), econazole (ECZ) and ethionamide (ETH)loaded into PLGA nanoparticles and their therapeutic efficacy was assessed in an animal model infected with MDR-TB. The treatment of MDR-TB-diseased mice with weekly doses of a three-component nano-formulation viz., PLGA-NP-ECZ + PLGA-NP-MOX + PLGA-NP-ETH resulted in the elimination of bacilli from both the lungs and the spleen of the mice [
160]. This investigation represents the initial documentation regarding the possible effectiveness of a mixture of ECZ, MOX and ETH NP for treating MDR-TB, with promising potential for clinical application [
160].
The high intracellular drug concentration resulting when using PNP and their ready internalization into macrophages led to the development of alginate modified-PLGA nanoparticles loaded with the hydrophilic compounds amikacin and moxifloxacin and two water-oil-water (w/o/w) emulsion methods for the targeted therapy of MDR-TB [
161] . The antibacterial efficacy of the resultant PLGA NP in
Mtb-diseased macrophages was investigated and in the untreated group, the dual encapsulated NP exhibited a bacterial viability of 0.6%, whereas the nanoparticles containing amikacin and moxifloxacin exhibited a viability of 6.49% and 3.27%, respectively thereby indicating that the synergistic effect of amikacin and moxifloxacin in the PLGA NP resulted in greater inhibition of the viable bacteria. The alginate-encapsulated PLGA nanoparticles loaded with amikacin and moxifloxacin can be used to reduce the dosage of these medications, enhancing patient adherence to therapy and potentially mitigating undesirable side effects associated with higher doses however, additional in vivo investigations are necessary to validate this potential [
161].
Studies have demonstrated the safety and efficacy of sonodynamic antibacterial chemotherapy (SACT) as a potential solution to the rising threat of drug-resistant bacteria. By using sonosensitive-loaded nanoparticles with targeted therapeutic capabilities, SACT could effectively eradicate bacteria without the risk of precipitating drug resistance [
162,
163]. In response to the challenge of MDR-TB, this concept was used to develop levofloxacin-loaded PLGA-PEG NP conjugated with the BM2 aptamer on their surface using1-ethyl-3-(3-dimethylamino propyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) as cross-linking agents to prepare BM2-LVFX-NP [
164]. The nanoparticles were designed to investigate antimycobacterial efficacy and fundamental mechanism of activity of the levofloxacin-loaded nano-sonosensitizer with specific therapeutic action against Bacillus Calmette-Guérin bacteria (BCG) used as a model for
Mtb. The study revealed a significant production of ROS during treatment with BM2-LVFX-NP using ultrasonic activation. Both in vitro and in vivo studies demonstrated the robust targeting specificity of BM2-LVFX-NP for BCG and the BM2-LVFX-NP-mediated SACT exhibited potent antibacterial efficacy against BCG and suppressed the proliferation of subcutaneous abscesses without any discernible adverse reactions. Consequently, an ultrasonic -activated therapeutic nano-platform incorporating an aptamer-specific moiety demonstrates significant capability as a practical approach for additive targeted treatment against
Mtb infections, ensuring biosafety [
164].
The polysaccharide 1,3-β-glucan is recognized for activating macrophages, producing pro-inflammatory signals, including ROS and nitrogen species (ROS). β-glucans interact with Dectin-1 located on the surfaces of macrophages, activating multiple downstream signaling pathways that enhance pro-inflammatory gene expression and the production of intracellular ROS/RNS. Pro-inflammatory cytokines generated via Dectin-1 activation encompass IL-12 and TNF-α [
165], which are vital for managing
Mtb infections [
166]. Curdlan functionalized PLGA NP designed for HDT exhibited enhanced and expedited release of the pro-inflammatory cytokine TNF-α in macrophages, reducing intracellular
Mtb amount inside these cells [
141]. This investigation further demonstrated that curdlan exhibits a strong binding affinity for the Dectin-1 receptor, facilitating targeted delivery of anti-TB drugs and AIC to macrophages. This mechanism enhances pharmaceutical activity in specific cells simultaneously reducing immunotoxicity. The findings indicate that the nanocarrier exhibited bio-safety properties, highlighting the need for further investigation into its potential as a host-directed therapeutic approach for intracellularly activity against viable
Mtb [
141] and MDR-TB.
Macrophages have mannose receptors on their surfaces that recognize and bind to non-reducing terminals of mannose moieties, thereby promoting the cellular uptake of nanoparticles. Consequently, using mannosylated nano-formulations may be a promising approach for targeting alveolar macrophages for the delivery of active therapeutics to treat MDR-TB. Incorporating mannose into a drug delivery vehicle which exhibits enhanced uptake of carriers by macrophages could result in improved efficacy with a reduction in side effects. This phenomenon may be linked to the preferential absorption of mannose by alveolar macrophages facilitating the targeted accumulation of drug-loaded nanoparticles at the desired sites of action [
167,
168].
Mannose receptor-targeted bioadhesive chitosan NP encapsulating clofazimine to treat drug-resistant tuberculosis has been reported [
169]. In-vitro drug release at pH 7.4 was gradual and prolonged and uptake tests in C2C12 cell lines demonstrated that the mannosylated nanoparticles were more efficiently absorbed than non-targeted and conventional medicines. The luciferase reporter phage (LRP) was tested against the H37Rv strain and revealed that clofazimine NP exhibited 49.5 times greater inhibition and anti-mycobacterial efficacy compared to pure clofazimine alone, reassuring the potential of this combination in tuberculosis treatment. This remarkable activity may be ascribed to improved drug distribution due to the favorable bioadhesive characteristics of the chitosan-based nanoparticles. Following regulatory validation, these nanoparticles may be clinically relevant to target macrophages infected with mycobacterium and the treatment of drug-resistant tuberculosis.
Innovative combinations of drugs and advanced targeted drug delivery technologies may enhance the management of anti-TB drug resistance, significantly. Research indicates that fluoxetine, a serotonin reuptake inhibitor, may be beneficial in treating infectious diseases caused by mycobacteria[
170,
171]. A nano-system containing isoniazid and fluoxetine-conjugated multi-walled carbon nanotube nanofluid was developed to enhance drug delivery efficiency and address resistance in vitro [
172]. It was established that fluoxetine exhibited a synergistic effect when used in combination with isoniazid, suggesting that their combined application in treatment regimens may significantly impact the management of infections caused by all clinical strains of
Mtb. The expression of isoniazid resistance genes, such as
inhA and
katG, along with the secretion of cytokines TNFα and IL6, is effectively regulated by this drug delivery system [
172]. These findings corroborate those reported in earlier studies indicating that serotonin receptor agonists or antagonists can stimulate the autophagy pathway and promote elimination of TB mycobacteria. In conjunction with host-targeted molecules, this nano-drug delivery approach can advance the production of a new generation of antibiotics characterized by high therapeutic efficacy, reduced side effects and an ability to address drug resistance challenges however, further comprehensive studies on this nano-formulation pertinent to signaling pathways is required [
172].
Notwithstanding recent progress in the development of PLGA NP DDS for the treatment of TB, numerous challenges persist, requiring urgent resolution. The lack of specific regulatory criteria for the characterization, research design and statistical analysis for these studies are a major obstacle to the clinical translation of nano-formulation use. Enhancing collaborative practices is crucial for translating nanotechnology from successful proof of concept experimentally to clinical use and supplementary in vivo data are necessary. Some studies suggest that the PLGA NP medication delivery technology exhibits efficacy in preclinical models of infectious TB. However, this is insufficient as upcoming clinical trials will depend on available preclinical evidence. Investigations into PLGA nano DDS for TB therapy are still in their infancy and additional funding is required to develop the capability to produce commercially viable micro/nano-formulations [
173].
4.2. Gene Therapy and RNA-Based Therapy in Treatment of MDR-TB
TB, particularly the MDR-TB forms, pose a significant global health challenge requiring therapeutic strategies, including the use of gene therapy and non-coding RNA such as small interfering RNA (siRNA), microRNA (mRNA), Long non-coding RNA (lncRNA) and RNA interference (RNAi) which have emerged as promising avenues to combat the challenge. These technologies facilitate targeting the Mtb pathogen and host cellular mechanisms to enhance therapeutic efficacy.
4.2.1. Gene Therapy
Gene therapy involves the introduction, removal or alteration of genetic material within the cells of patients to treat diseases. In MDR-TB, gene therapy strategies aim to enhance the immune response of the host or target
Mtb directly. The CRISPR-Cas9 system enables precise editing of genetic material and has been applied to study the pathogenesis and drug resistance mechanisms of
Mtb. Researchers can identify novel drug targets and understand bacterial survival strategies by knocking out or modifying specific genes in the
Mtb. CRISPR-based diagnostics have been developed to detect drug-resistant TB strains rapidly thereby, facilitating timely and appropriate treatment interventions. While direct therapeutic applications of CRISPR in TB treatment are still in the early stages of development and use, the technology holds promise for developing targeted antimicrobial agents and enhancing our understanding of the biology of
Mtb species [
174]. Examples of the proposed application of CRISPR System in MDR-TB treatment include reprogramming the endogenous CRISPR System of
Mtb. Researchers have harnessed the endogenous type III-A CRISPR/Cas10 system of
Mtb for efficient gene editing and RNA interference by transforming a mini-CRISPR array plasmid and avoiding the introduction of exogenous proteins whilst minimizing proteotoxicity. This system has been applied to single- and multiple-gene RNAi and genome-wide RNAi screening to identify
Mtb genes regulating in vitro and intracellular growth [
175]. A CRISPR-guided mutagenic DNA polymerase system has been developed in fast-growing
Mycobacterium smegmatis and slow-growing
Mtb. This system combines a Cas9 nickase with an error-prone DNA polymerase to introduce random substitution mutations within target gene(s). It has been used to detect novel resistant mutant organisms, potentially aiding in identifying drug-resistant mutations of
Mtb [
176]. The finding low abundance sequences by hybridization (FLASH-TB) diagnostic tool and ) techniques have been applied to detect antibiotic-resistant mutations in
Mtb. This method uses CRISPR to amplify candidate genes linked to resistance against first- and second-line drugs, facilitating swift and precise identification of drug-resistant TB strains [
177].
Furthermore, researchers have explored gene therapy to boost the immune defense of the host organisms against Mtb. For example, enhancing the expression of cytokines such as interferon-gamma (IFN-γ) has been investigated to improve macrophage activation and mycobacterial clearance and gene editing tools like CRISPR-Cas9 have been proposed to disrupt essential genes in Mtb, inhibiting survival and replication. While direct application in clinical settings is still under investigation, this approach holds potential for future therapeutic developments.
A study by Rahman et al. reprogrammed the endogenous type III-A CRISPR system of
Mtb was reprogrammed to create a versatile tool for effective gene editing and RNA interference, demonstrating its potential for robust gene knock-in/knockout processes and genome-wide RNAi screening [
175]. In addition, CRISPR-guided mutagenesis and gene editing have been proposed as strategies to combat drug-resistant strains of
Mtb, offering new avenues for tuberculosis research and drug development [
178]. CRISPR screening has been used for genetic interaction mapping of
Mtb, providing insight into gene function and identification of potential therapeutic targets [
179]. Other gene therapy strategies have been investigated for treating
Mtb and MDR-TB. One notable example involved the use of adenoviral vectors to deliver therapeutic genes that enhance the immune response of the host against
Mtb. An adenoviral vector encoding for osteopontin (AdOPN) was administered to
Mtb-infected mice and led to heightened immune responses and improved control of bacterial load in the lungs, suggesting that AdOPN may be a potential adjunct to traditional chemotherapy [
180]. Another approach focused on host-directed therapies (HDT) that modulate the immune system of the host to combat
Mtb infection. Gene therapy techniques have been used to alter the expression of specific host genes involved in immune regulation, thereby enhancing the ability of the body to control or eliminate the pathogen. The modulation of gene expression of genes encoding for cytokines or other immune mediators through gene therapy has shown promise in preclinical models [
181] and highlight the potential of gene therapy approaches beyond CRISPR-Cas9 for the treatment of
Mtb and MDR-TB, offering alternative strategies to enhance host immunity and improve disease outcomes.
4.2.2. RNA-Based Therapy for Treatment of MDR-TB
Research into RNA-based therapies for TB, including MDR-TB, has focused on using RNA interference (RNAi) mechanisms with small interfering RNA (siRNA) and microRNA (miRNA) molecules to modulate the immune response(s) in the host and target
Mtb. For example, the inhibition of miR-27a was reported to enhance the ability of the host to control
Mtb infections in in mice and the miR-27a antagomir reduced bacterial loads and diminished lung pathology, indicating that inhibition of miR-27a could be a promising therapeutic strategy for treating TB [
182]. The mi RNA miR-let-7e-5p has been identified as a potential biomarker for monitoring MDR-TB treatment and variations in levels of expression align with the different phases of treatment, supporting its use for evaluating therapeutic efficacy [
92]. Another miRNA, miR-155, is upregulated during
Mtb infection, enhancing the antimicrobial activity of macrophages through an increased generation of pro-inflammatory cytokines. Modulating miRNA compounds such as miR-155 may strengthen the defenses of hosts against MDR-TB [
183]. Recent studies have emphasized the significance of long non-coding RNA (lncRNA) in combination with miRNA in TB pathogenesis and host-pathogen interactions. The lncRNA MEG3, for instance, has been shown to regulate genes involved in immune responses to
Mtb, presenting a novel target for improving host immunity against MDR-TB [
184]. Similarly, differentially expressed lncRNA in peripheral blood mononuclear cells (PBMC) of MDR-TB patients have been proposed as potential biomarkers and therapeutic targets [
185]. Specific lncRNA in PBMC have been identified in patients with active TB, further underscoring their potential utility as biomarkers for the diagnosis and monitoring of treatment in patients with TB [
186]. These findings collectively suggest that targeting non-coding RNA, including miRNA and lncRNA, may be significant for advancing RNA-based therapies in treating MDR-TB.
Despite their therapeutic potential, both RNA-based and gene therapy approaches for treating MDR-TB pose significant challenges that limit their clinical application. A major hurdle is the delivery of the therapeutic agents to infected cells while avoiding off-target effects, which may lead to unintended gene silencing, toxicity or genetic modification. Drug delivery systems must shield RNA molecules from degradation by nucleases and ensure precise targeting. However, these systems often raise safety concerns, such as immune responses triggered by viral vectors used in gene therapy. Furthermore, both ncRNA and gene therapy are complex due to interactions in the biological system. A single molecule may regulate multiple genes for ncRNA, making it difficult to predict and control exact therapeutic outcomes. Gene therapy carries the risk of insertional mutagenesis when therapeutic genes integrate into the host genome. The cost and complexity of producing these therapies with high purity and functionality further limit their scalability and accessibility, particularly in TB-endemic regions. The absence of thorough research on long-term safety and efficacy for both approaches is a significant barrier to clinical adoption. Addressing these challenges requires innovative stabilization, delivery and specificity technologies in addition to rigorous preclinical and clinical investigation. Furthermore, vector design and regulatory framework advancements are critical to making these cutting-edge therapies viable options for combating MDR-TB.
4.3. Pulmonary Delivery Systems
Transforming current anti-TB medications into inhalable nanoparticulate systems presents a potential approach to address the difficulties linked with oral treatment, as these systems may improve drug retention in pulmonary tissues and maintain therapeutic concentrations in the diseased lung(s) [
187]. Since
Mtb targets the lungs, administering drugs via the pulmonary route is a useful approach to promote efficient alveolar transcytosis. This approach circumvents hepatic first-pass metabolism and, due to the extensive vascular network, enhances the delivery of therapeutic agents, potentially increasing drug concentrations at the site of infection site which may improve their effectiveness [
188]. Interestingly, administering lower doses of TB medications via inhalation can still yield effective treatment outcomes, thereby minimizing the risk of toxicity while improving localized drug concentration [
189]. Aerosolized formulations and inhalation devices, including nebulizers, dry powder inhalers (DPI), soft mist inhalers (SMI) and metered-dose inhalers (MDI), represent advancements in pulmonary DDS designed to deliver anti-TB medications directly to the lungs. This approach increases local drug concentrations at the site of action while minimizing systemic side effects. Nevertheless, dry powder inhalers (DPI) have significant benefits owing to their stability compared to liquid or suspension-based formulations, in addition to their capacity to administer a high dose of drugs directly to the lungs [
188,
190,
191].
Concerns regarding the administration of anti-TB drugs via the oral route in tablets or capsules and parenteral route include potential drug-drug interactions, a slower onset of action and the possibility of degradation in the gastrointestinal environment. In addition, subtherapeutic concentrations of drug in the lungs due to hepatic first-pass metabolism may result in the development of resistant
Mtb strains and require elevated doses to achieve high concentrations at the site of action and insufficient drug may penetrate necrotic lesions. Other considerations include excessive peripheral neuropathy, serious side effects, and low tolerability of intravenous formulations. However, the rationale for inhalation delivery includes low systemic exposure of the drugs, high concentration of drugs at the site of action, which can potentially reduce the frequency of dosing requirements for successful therapy, avoid potential pharmacokinetic drug-drug interactions, food-drug interactions, high penetration of the drugs in necrotic lesions of the lungs, early sterilization of the sputum or early bactericidal activity
all of which may increase patient adherence [
188,
191].
Numerous antimicrobial drugs have received approval or are in pre-clinical investigation or clinical trials for evaluation of inhalable delivery systems to manage respiratory infections [
191]. Inhalable formulations for treating drug-resistant TB represent the least explored area of drug delivery for clinical validation. Only one dry powder inhalation formulation containing capreomycin has achieved a milestone, phase I, for treating drug-resistant tuberculosis. If demonstrated effectively, dry powder capreomycin inhalers could substantially enhance the treatment of MDR-TB treatment by broadening its application in different clinical settings including community-based, resource-limited and supervised self-administration settings. The application of inhalable delivery technologies in pediatric drug-resistant TB is also appealing. However, additional research is required formulate and evaluate dry powder versions of tuberculosis medications and cannot be overstated [
191,
192].
Linezolid has demonstrated potential as a good option for inclusion in the treatment regimen for MDR-TB however, it exhibits concentration-dependent pharmacokinetics, efficacy in killing
Mycobacterium tuberculosis, acquired resistance and associated toxicity. Concentration-dependent efficacy and toxicity could be effectively regulated by administering a high dose of linezolid to the pulmonary tissues in which
Mtb is located. Developing orally inhaled dry powder formulations as adjunctive therapy may offer an effective solution for tuberculosis patients and reduce treatment duration [
191,
193].
Rudolph and colleagues successfully developed amorphous drug NP for inhalation therapy to target MDR-TB [
194]. The NP contained bedaquiline (BDQ) and 1,3-benzothiazin-4-one 043 (BTZ) and were designed to overcome the lipophilicity of these drugs, which poses a challenge when used in aqueous environments. The lipophilic TB antibiotics were included in an elevated-dose in the amorphous nanoparticles using a solvent-antisolvent technique for site-specific delivery in granulomas present in
Mtb-infected lungs. Importantly, minimal BDQ/BTZ nanoparticles were detected in the spleen or liver, indicating that drug targeting was focused. Animal studies using the pulmonary delivery route demonstrated the enhanced efficacy of the nano-formulations compared to using nonformulated drugs. This successful technology yielded promising results suggesting that the pulmonary delivery of BDQ/BTZ in NP could significantly improve the treatment of tuberculosis in the future[
194].
BDQ-encapsulated fucosylated and nonfucosylated liposomes (BDQ-Lipofuc/BDQ-Lipo) were developed for intranasal delivery[
195]. The pharmacodynamic and pharmacokinetic studies conducted in mice revealed that the intranasal delivery of liposomal BDQ formulations at a dose of 5 mg/kg BDQ and administered on alternate days for 14 days resulted in a substantial decrease in the lung burden of
Mtb and an elevated concentration of BDQ in the lung when compared to BDQ delivered via the oral or intravenous routes of administration. These findings suggest that a liposomal BDQ inhalation suspension could significantly improve antitubercular efficacy, suggesting that further exploration and development of this promising new treatment for tuberculosis is needed.
The poor aqueous solubility and absorption of delamanid pose considerable challenges to achieving efficient oral administration [
196]. To address this therapeutic hurdle, a self-microemulsifying DDS designed for administration via a pressurized metered dose inhaler (pMDI) to treat MDR-pulmonary tuberculosis was developed [
196]. The performance of the self-micro emulsifying drug delivery system (SMEDDS)-pMDI was evaluated using a next-generation impactor, and the ability to transport and deliver the medication to the innermost regions of the pulmonary system was demonstrated. Cellular internalization tests revealed effective delivery of the formulations into macrophage cells, suggesting the capability of achieving intracellular antimycobacterial activity. These results underscore the promise of using delamanid-SMEDDS-pMDI in enhancing the efficacy of delamanid for treating MRD-TB and offer a bright outlook for the future of tuberculosis treatment.
All trans-retinoic acid (ATRA) is an active and potent metabolite of vitamin A that has exhibited significant in vitro and in vivo efficacy against TB [
128]. In response to the increasing global challenge of treating MDR-TB, Bahlool et al. [
197] successfully developed a targeted treatment approach utilizing ATRA-loaded nanoparticles (NP) designed for nebulization that is a potential new approach for effective treatment of TB. Targeted inhaled HDT may provide a novel adjunctive treatment option for tuberculosis, with the potential to significantly improve existing dosage regimens, thereby improving the outcomes of MDR-TB cases, which may lead to improved patient outcomes and a reduction in the incidence of MDR-TB, offering a ray of hope in the fight against TB.
4.3.1. Dry Powder Inhalers (DPI)
DPI offers ease of use and is ideal for high-dose formulations to administer medications as a dry powder inhaled directly into the lungs for local and systemic activity. The propellant-free design enhances patient adherence and allows for high drug loading and improved stability, providing a reliable solution for patients. The formulations can be engineered to incorporate either single drugs or combination therapies for the treatment of MDR-TB. They offer significant practical advantages in Sub-Saharan environments, primarily due to their enhanced portability, stability, and lack of reliance on refrigeration. Nonetheless, the efficacy of DPI is contingent on several factors, including the design of the inhaler, the powder formulation and the airflow during inhalation. The performance of DPI has seen significant enhancement over the past ten years and is attributed to the incorporation of engineered nanoparticles and advancements in formulation technologies [
19,
188].
Inhalation formulation strategies encompass different approaches, including the use of liposomes, proliposomes, solid lipid nanoparticles, microemulsions, and metallic nanoparticles made of gold, silver and zinc in addition to microparticles, polymeric nanoparticles and dendrimers [
188,
191]. Liposomes have been the subject of extensive research and have been thoroughly investigated for pulmonary application. Numerous studies have highlighted their effectiveness in treating pulmonary conditions, providing a strong foundation to evaluate their potential for lung delivery with distinct benefits due to inclusion of phospholipids which are similar to naturally occurring pulmonary surfactant [
198].
The potential of pulmonary administration of low drug doses, alongside reduced oral doses of the same substances, to significantly improve patient outcomes has been reported [
199]. By formulating dry powder inhalation (DPI) preparations containing sutezolid (SUT), a second-generation pretomanid analogue TBA-354 (TBA), or a fluorinated derivative of TBA-354 (32,625) within a matrix of the biodegradable polymer poly(L-lactide), a promising new approach was evaluated and revealed that oral doses of 100 mg/kg/day and DPI doses of 0.25–0.5 mg/kg/day of SUT, TBA-354, or 32,625 over 28 days, were inadequately efficient in diminishing the lung and spleen burden of
Mtb in infected mice. It was concluded that the incorporation of inhaled second-line medications as adjunct therapy could allow for a reduced oral dose for MDR-TB, offering hope for improved patient outcomes and adherence.
Despite the significant clinical effectiveness of linezolid (LNZ) against drug-resistant TB, safety and tolerability issues prompted Makled et al. [
200] to formulate inhalable LNZ nano-embedded microparticles in a dry powder form. LNZ was integrated into non-structured lipid carriers (NLC) and the capability of LNZ-NLC to traverse mucosal barriers and infiltrate alveolar macrophages (AM, MH-S cells) was evaluated. The dried powder inhalation of LNZ-NLC in microparticles demonstrated sustained release of LNZ, enhanced mucus penetrability and promises safety at therapeutic doses, in vitro and in vivo targeting of macrophages and better deposition in the alveolar regions of the lung. The favorable results, including the potential for less frequent administration, depend on lower doses and enhanced safety, which mitigates life-threatening systemic side effects.
Pretomanid (PA-824) exhibits significant effectiveness against both active and dormant types of
Mtb and is recognized for additive benefits when combined with PZA and MOX [
201]. An inhalable combination powder formulation for the treatment of latent and MDR-TB was developed [
201] with a high-dose fixed-dose combination (FDC) DPI formulations with elevated aerosolization efficiency, incorporating PZA and PA-824, or PZA and MOX, for targeted delivery to the alveolar regions of the lung where latent and resistant
Mtb persists. Pretomanid and MOX exhibited better stability in the combination powders than PZA. Additional research is required to enhance the stability of PZA in FDC powders intended for inhalation.
Administering inhalable formulations of repurposed drugs or HDT, with or without autophagy modulation, as an adjunct in the treatment of drug-resistant TB may alleviate the challenges associated with oral delivery by delivering drugs directly to the vicinity of Mtb within the interior granuloma of the infected lung regions.
4.4. Combination Delivery Systems
The treatment of MDR-TB necessitates the administration of different drugs concomitantly to address the resistance effectively. In 2022, the WHO recommended a six-month course of therapy consisting of BDQ, PA-824, 600 mg of LZD and MOX (BPaL) as the preferred treatment option over a nine-month or eighteen-month regimen for patients with MDR/rifampicin resistance-TB. Nanotechnology offers the capability to integrate these medicines into a single dose thereby minimizing the frequency of administration needed. Systems for drug delivery, which are engineered to deliver different anti-TB agents simultaneously, can enhance the effectiveness of combination therapy while minimizing the likelihood of resistance emerging. These systems may be nanoparticles such as liposomes or other drug nanocarriers [
139,
202].
The combined administration of antimicrobial agents that engage with different targets offers significant potential in reducing the likelihood of the development of resistance. This approach not only restores the sensitivity of multidrug-resistant bacteria to previously ineffective antibiotics but also reduces harmful side effect and allows for comparable antimicrobial effectiveness with reduced drug doses [
203]. Integrating antibiotics with compounds that enhance or revive antibiotic efficacy against multidrug-resistant bacteria could revolutionize the treatment of MDR-TB using traditional anti-tubercular medications like RIF and INH.
4.4.1. Fixed-Dose Combination (FDC) Formulations
FDC formulations integrate multiple drugs into a single dosage form, streamlining the treatment process and enhancing patient adherence [
204,
205].
Typically, FDC have one or more of the following objectives viz., (i) reducing the rate of acquired resistance though use of drug combinations that exhibit minimal cross-resistance, thereby making the emergence of resistance dependent on the occurrence of multiple mutations in rapid succession which is unlikely,(ii) decreasing doses of drugs that have non-overlapping toxicity and comparable therapeutic profiles to improve efficacy while minimizing side effects, (iii) increasing cell sensitivity to drug effects through the administration of a different drug for chemosensitization or radiation for radiosensitization, by modifying the stages or growth characteristics of the cell cycle or cytokinetic optimization and (iv) achieving greater potency by leveraging additivity or synergistic effects, in the biochemical activity of two or three drugs [
205]. The formulation of FDC of drugs can be integrated into nanoparticles or microparticles to achieve sustained, prolonged and/or extended drug release [
204].
The future of successful MDR therapy lies in using FDC medications and further investigations should be undertaken to discover and develop such FDC [
205]. However, the challenge of funding meticulously designed clinical trials and advancing the development of viable formulations, particularly for off-patent pharmaceuticals, remains a significant policy issue [
205].
5. Challenges in the Implementation of Novel Delivery Strategies
Nano-therapeutics and nano-pharmaceuticals offer promising opportunities for earlier and more accurate diagnoses, better targeting of therapies, fewer adverse events and improved therapeutic monitoring. These benefits may significantly improve the quality of life, promote healthier ageing and maximize healthcare cost-effectiveness. However, the domain of nano-medicine is still in its infancy, with most research confined to laboratories and limited success in clinical trials or medical applications having been demonstrated [
206]. Despite the potential of these drug delivery systems, several significant challenges must be addressed.
5.1. Manufacture and Scale-Up
The intricate formulation and preparation of nano-therapeutics poses challenges for large-scale manufacturing, often leading to increased costs. There are very few, if any lipid-based nanomedicines for antitubercular drug delivery due to scalability issues, high patient costs and the need for specialized manufacturing equipment [
207].
One of the primary obstacles in scaling up nanomedicine manufacture is maintaining consistent physicochemical properties of the technology including but not limited to size and drug loading across batches. While pilot-scale processes can attain reproducibility using well-characterized nanoparticles, industrial-scale production often faces challenges in controlling the polydispersity of nanomaterials. Differences in the physical and chemical properties between batches of nanomaterials can complicate pharmaceutical development [
208].
Nanomedicines are complex three-dimensional products that require sophisticated chemistry, manufacturing and process control. The lack of extensive experience in nanoscale, multi-component systems within traditional pharmaceutical manufacturing environments further exacerbates these challenges. In addition the high cost of raw materials and multistep production adds to the complexity and contributes to elevated costs further deterring attempts at large-scale production of nanocarriers [
206].
5.2. Safety and Biocompatibility
To achieve clinical success, drug carriers must be non-toxic and biocompatible over extended periods of time. However, the toxicity of some nanomaterials poses a significant challenge. Experimental studies have demonstrated that nanoparticles such as silver, gold, silica, and titanium may adversely affect drug coupling and delivery [
209].
Although pre-clinical studies provide insight into the pharmacokinetics of nanotechnology-based drug delivery systems (NDDS), they fail to fully capture interactions in the human body and other biological systems. Clinical trials remain essential to determine the safety and efficacy of drugs as findings from animal models and cell lines often differ significantly from human responses [
210,
211]. For instance, only inhaled capreomycin has, to date, exhibited tolerability in Phase I clinical trials [
192].
5.3. Regulatory Approval
Advancements in nanotechnology and nanomedicine have the potential to revolutionize diagnostics and treatment of different diseases. However, the clinical translation process is complex, resource-intensive and time-consuming. Success requires collaboration with many stakeholders, resource mobilization and rigorous study designs to meet regulatory standards. Preclinical and clinical testing must establish safety and efficacy comprehensively, before approval can be granted [
212,
213].
5.4. Patient Adherence
Patient acceptability of nano-systems is critical to their successful implementation and use [
212]. However, maintaining adherence to lengthy and complex treatment regimens is challenging. Addressing this issue requires comprehensive solutions, including minimization of adverse effects and enhancing patient involvement in development activities. A multi-level approach that includes patients, healthcare professionals and healthcare systems is necessary to optimize adherence [
214].
5.5. Cost-Effectiveness
Nanotechnology-based solutions face significant budgetary constraints. Developing new pharmaceuticals for global health is costly, and reformulating existing drugs in nanocarriers may be a cost-effective alternative with a better chance of demonstrating efficacy and safety [
212]. However, some nano-systems, such as liposomal amphotericin B, are prohibitively expensive for use in low-resource settings [
215] and the cost of excipients, specific synthetic procedures and storage conditions pose practical challenges, especially in the developing world [
202,
212].
5.6. Stability Issues
Nanoparticles and systems are prone to instability that manifests as agglomeration, disintegration or premature drug release. Storage factors, including temperature, humidity and exposure to light may further compromise stability [
208]. While novel drug delivery systems have the capability to transform the treatment of MDR-TB by improving drug bioavailability, targeting to sites of infection and reducing side effects, overcoming technical, safety, stability, regulatory, and logistical barriers is crucial. Rapid nanotechnological advancements, as seen during the SARS-CoV-2 pandemic, highlight the potential for translating laboratory innovations into practical healthcare solutions. Addressing these challenges through focused research and development may enable similar successes in advancing the treatment of patients with MDR-TB [
212].
6. Future Directions
The battle against TB, particularly MDR-TB, requires innovative approaches to overcome existing challenges. Three critical areas for advancing immunomodulatory nanoparticle-based treatments have been identified and include addressing MDR infections, co-morbidities such as HIV/TB and HDT. Combining nanotechnology solutions with immunomodulatory agents or autophagy-inducing compounds (AICs) within an HDT frameworks, may improve treatment outcomes. Such approaches may reduce drug resistance, dosing frequency and costs while significantly enhancing the immune response of the host to TB infections, leading to better patient outcomes [
121].
One promising strategy involves combining bedaquiline (BDQ) or other novel drug candidates with autophagy-inducing agents, such as vitamin D3 together with functionalized nanoparticles targeting novel mycobacterial pathways. Administration of these treatment options via the pulmonary route could further enhance the management of drug-resistant TB. Furthermore, combining these advanced therapies with existing anti-TB drugs may mitigate the emergence of drug resistance.
Emerging technologies, including artificial intelligence (AI) and machine learning (ML), also offer considerable promise in developing approaches to TB management. AI algorithms can track drug resistance, enhance diagnostic precision and predict adverse reactions, treatment duration and outcomes. The analysis of large datasets, including genetic and treatment history may facilitate the development of personalized treatment plans using AI thereby optimizing therapeutic regimens [
216,
217]. In addition, AI-powered tools, mobile applications and reminder systems would allow predict and improvement of patient adherence patterns. By identifying individuals at risk of non-adherence, healthcare providers can proactively intervene to enhance compliance [
214,
218].
Telemedicine and digital adherence technologies are increasingly integrated with diagnostic tools to improve timely diagnoses, follow-up and real-time therapeutic monitoring, particularly in remote areas. These tools allow automated reminders to be sent and better communication between patients and healthcare providers, improving treatment outcomes [
219,
220]. However, successfully implementing AI and ML in TB management necessitates addressing several challenges, including data availability and quality, training healthcare workers, integrating AI with existing healthcare systems and ensuring algorithm interpretability and reproducibility. Furthermore, ethical considerations, adequate funding, community awareness and supportive policies must be prioritized to harness the potential of AI fully [
216,
219,
220,
221]. Incorporating AI technologies into routine clinical practice could revolutionize MDR-TB management. By enabling personalized treatment approaches, strengthening global TB control efforts and facilitating adherence support, AI represents a transformative tool for improving patient outcomes and advancing efforts to eradicate TB.
7. Conclusions
Addressing multidrug-resistant tuberculosis (MDR-TB) demands a comprehensive approach that integrates innovative drugs, advanced drug delivery systems, and precision medicine development. Key research efforts should focus on developing and identifying functionalized nanocarriers to deliver fixed-dose combinations of existing anti-TB drugs, new therapeutic compounds and host-directed therapies (HDT) to enhance autophagy in the macrophages of the host. Although these strategies are vital, they are unlikely to be the sole solution to the challenges posed by MDR-TB. A future paradigm for TB treatment must incorporate a personalized approach that considers the genetics of different Mycobacterium tuberculosis strains and the host in combination with the drug susceptibility profiles for those strains. Furthermore, treatment regimens should address co-infections and comorbidities, tailor therapy for different forms of tuberculosis, including drug-susceptible TB, MDR-TB, latent TB, pre-extensively drug-resistant TB (Pre-XDR-TB), XDR-TB, and TDR-TB. Overcoming current obstacles, such as balancing treatment efficacy with minimizing adverse effects and addressing cost barriers, is essential to improving global TB care and outcomes. As MDR-TB spreads rapidly, there is an urgent need for cost-effective, efficient treatment strategies and technologies. The ongoing advancements in MDR-TB research highlight the critical role scientists play in developing accessible, personalized therapeutic strategies to combat this global health challenge.
Author Contributions
Conceptualization, O.A.O.; methodology, O.A.O. and A.O.F.; software, O.A.O. and A.O.F.; validation, O.A.O. and A.O.F.; formal analysis, O.A.O. and A.O.F.; investigation, O.A.O. and A.O.F.; resources, O.A.O. and A.O.F.; data curation, O.A.O. and A.O.F.; writing—original draft preparation, O.A.O. and A.O.F.; writing—review and editing, O.A.O., A.O.F., R.B.W. and S.K.; visualization, A.O.F.; supervision, R.B.W. and S.K.; project administration, O.A.O.; funding acquisition, R.B.W. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors R.B.W. and S.M.K. acknowledge financial support from Rhodes University, and the author O.A.O. acknowledges funding from Rhodes University for a Postdoctoral Fellowship. Rhodes University funded the APC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions featured in this study are incorporated in the article. Additional inquiries may be sent to the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raviglione, M.; Sulis, G. Tuberculosis 2015: Burden, Challenges and Strategy for Control and Elimination. Infect. Dis. Rep. 2016, 8, 6570. [Google Scholar] [CrossRef] [PubMed]
- G: Global Tuberculosis Report 2021; World Health Organization, 2021.
- Song, H.-W.; Tian, J.-H.; Song, H.-P.; Guo, S.-J.; Lin, Y.-H.; Pan, J.-S. Tracking Multidrug Resistant Tuberculosis: A 30-Year Analysis of Global, Regional, and National Trends. Front. Public Health 2024, 12. [Google Scholar] [CrossRef]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial Resistance in Mycobacterium Tuberculosis: Mechanistic and Evolutionary Perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kakkar, V. Recent Technological Advancements in Tuberculosis Diagnostics – A Review. Biosens. Bioelectron. 2018, 115, 14–29. [Google Scholar] [CrossRef]
- Nurkanto, A.; Masrukhin; Tampubolon, J. C.E.; Ewaldo, M.F.; Putri, A.L.; Ratnakomala, S.; Setiawan, R.; Fathoni, A.; Palupi, K.D.; Rahmawati, Y.; et al. Exploring Indonesian Actinomycete Extracts for Anti-Tubercular Compounds: Integrating Inhibition Assessment, Genomic Analysis, and Prediction of Its Target by Molecular Docking. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Parida, K.K.; Lahiri, M.; Ghosh, M.; Dalal, A.; Kalia, N.P. P-Glycoprotein Inhibitors as an Adjunct Therapy for TB. Drug Discov. Today 2024, 29, 104108. [Google Scholar] [CrossRef]
- van Staden, D. Development of a Topical Self-Emulsifying Drug Delivery System for Optimised Delivery, North West University: South Africa, 2020.
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Acc. Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef]
- Dheda, K.; Gumbo, T.; Maartens, G.; Dooley, K.E.; McNerney, R.; Murray, M.; Furin, J.; Nardell, E.A.; London, L.; Lessem, E.; et al. The Epidemiology, Pathogenesis, Transmission, Diagnosis, and Management of Multidrug-Resistant, Extensively Drug-Resistant, and Incurable Tuberculosis. Lancet Respir. Med. 2017, 5, 291–360. [Google Scholar] [CrossRef]
- Shah, N.S.; Auld, S.C.; Brust, J.C.M.; Mathema, B.; Ismail, N.; Moodley, P.; Mlisana, K.; Allana, S.; Campbell, A.; Mthiyane, T.; et al. Transmission of Extensively Drug-Resistant Tuberculosis in South Africa. N. Engl. J. Med. 2017, 376, 243–253. [Google Scholar] [CrossRef]
- Chandila, S.; Kaushik, H. Current trends and challenges in tuberculosis: a systemic review. 2023, 12. 12.
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Dartois, V.A.; Rubin, E.J. Anti-Tuberculosis Treatment Strategies and Drug Development: Challenges and Priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The Challenge of New Drug Discovery for Tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Deb, P.K.; Venugopala, K.N.; Al-Shar’i, N.A.; Singh, V.; Deka, S.; Srivastava, A.; Tiwari, V.; Mailavaram, R.P. Tuberculosis: An Update on Pathophysiology, Molecular Mechanisms of Drug Resistance, Newer Anti-TB Drugs, Treatment Regimens and Host- Directed Therapies. Curr. Top. Med. Chem. 2021, 21, 547–570. [Google Scholar] [CrossRef]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory Risks from Household Air Pollution in Low and Middle Income Countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-Based Drug Delivery Systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef]
- Kia, P.; Ruman, U.; Pratiwi, A.R.; Hussein, M.Z. Innovative Therapeutic Approaches Based on Nanotechnology for the Treatment and Management of Tuberculosis. Int. J. Nanomed. 2023, Volume 18, 1159–1191. [Google Scholar] [CrossRef]
- Lawn, S.D.; Mwaba, P.; Bates, M.; Piatek, A.; Alexander, H.; Marais, B.J.; Cuevas, L.E.; McHugh, T.D.; Zijenah, L.; Kapata, N.; et al. Advances in Tuberculosis Diagnostics: The Xpert MTB/RIF Assay and Future Prospects for a Point-of-Care Test. Lancet Infect. Dis. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Sunny, P.S. The Lessons Learned from An Active Tuberculosis Genotyping Cluster Investigation in Allegheny County. Doctoral dissertation, University of Pittsburgh: United States, 2024.
- Shirley Feldmann-Jensen, D.; Terrence O’Sullivan, P. Informing Adaptation with Lessons Learned from Key 21st Century Infectious Disease Outbreaks. 2024.
- Sparrow, A.; Smith-Torino, M.; Shamamba, S.; Chirakarhula, B.; Lwaboshi, M.; Benn, C.; Chumakov, K. A Risk Management Approach to Global Pandemics of Infectious Disease and Anti-Microbial Resistance. Trop. Med. Infect. Dis. 2024, 9, 280. [Google Scholar] [CrossRef]
- WHO, 2024a Tuberculosis (TB) Available online:. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 30 January 2025).
- CDC, 2024a Clinical Overview of Latent Tuberculosis Infection Available online:. Available online: https://www.cdc.gov/tb/hcp/clinical-overview/latent-tuberculosis-infection.html (accessed on 30 January 2025).
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 2100193. [Google Scholar] [CrossRef]
- Maison, D.P. Tuberculosis Pathophysiology and Anti-VEGF Intervention. J. Clin. Tuberc. Other Mycobact. Dis. 2022, 27, 100300. [Google Scholar] [CrossRef]
- Ibrahim Bekraki, A. Liposomes-and Niosomes-Based Drug Delivery Systems for Tuberculosis Treatment. In Nanotechnology Based Approaches for Tuberculosis Treatment; Elsevier, 2020; pp. 107–122 ISBN 978-0-12-819811-7.
- De Chastellier, C. The Many Niches and Strategies Used by Pathogenic Mycobacteria for Survival within Host Macrophages. Immunobiology 2009, 214, 526–542. [Google Scholar] [CrossRef]
- Jang, J.G.; Chung, J.H. Diagnosis and Treatment of Multidrug-Resistant Tuberculosis. Yeungnam Univ. J. Med. 2020, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, A.K.; Singh, S. Molecular Mechanisms of Drug Resistance in Mycobacterium Tuberculosis: Role of Nanoparticles Against Multi-Drug-Resistant Tuberculosis (MDR-TB). In NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; ISBN 978-981-329-898-9. [Google Scholar]
- Mamatha Bhanu, L.S. Anti-Tuberculosis Drugs and Mechanisms of Action: Review. Int. J. Infect. Dis. Res. 2023, 4, 1–7. [Google Scholar]
- Khawbung, J.L.; Nath, D.; Chakraborty, S. Drug Resistant Tuberculosis: A Review. Comparative Immunology, Microbiol. Infect. Dis. 2021, 74, 101574. [Google Scholar] [CrossRef] [PubMed]
- Batt, J.; Khan, K. Responsible Use of Rifampin for the Treatment of Latent Tuberculosis Infection. Can. Med. Assoc. J. 2019, 191, E678–E679. [Google Scholar] [CrossRef]
- Santucci, P.; Greenwood, D.J.; Fearns, A.; Chen, K.; Jiang, H.; Gutierrez, M.G. Intracellular Localisation of Mycobacterium Tuberculosis Affects Efficacy of the Antibiotic Pyrazinamide. Nat. Commun. 2021, 12, 3816. [Google Scholar] [CrossRef]
- Lee, N.; Patel, P.; Nguyen, H. Ethambutol. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2024. [Google Scholar]
- Campbell, R.E.; Chen, C.H.; Edelstein, C.L. Overview of Antibiotic-Induced Nephrotoxicity. Kidney Int. Rep. 2023, 8, 2211–2225. [Google Scholar] [CrossRef]
- Sabur, N.F.; Brar, M.S.; Wu, L.; Brode, S.K. Low-Dose Amikacin in the Treatment of Multidrug-Resistant Tuberculosis (MDR-TB). BMC Infect. Dis. 2021, 21, 254. [Google Scholar] [CrossRef]
- Shibeshi, W.; Sheth, A.N.; Admasu, A.; Berha, A.B.; Negash, Z.; Yimer, G. Nephrotoxicity and Ototoxic Symptoms of Injectable Second-Line Anti-Tubercular Drugs among Patients Treated for MDR-TB in Ethiopia: A Retrospective Cohort Study. BMC Pharmacol. Toxicol. 2019, 20, 31. [Google Scholar] [CrossRef]
- Espinosa-Pereiro, J.; Sánchez-Montalvá, A.; Aznar, M.L.; Espiau, M. MDR Tuberculosis Treatment. Medicina 2022, 58, 188. [Google Scholar] [CrossRef]
- Täubel, J.; Prasad, K.; Rosano, G.; Ferber, G.; Wibberley, H.; Cole, S.T.; Van Langenhoven, L.; Fernandes, S.; Djumanov, D.; Sugiyama, A. Effects of the Fluoroquinolones Moxifloxacin and Levofloxacin on the QT Subintervals: Sex Differences in Ventricular Repolarization. J. Clin. Pharma 2020, 60, 400–408. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Van Deun, A.; Rieder, H.L. Gatifloxacin for Short, Effective Treatment of Multidrug-Resistant Tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.J.; Nhung, N.V.; Cam Binh, N.; Hoa, N.B.; Garden, F.L.; Benedetti, A.; Ngoc Yen, P.; Cuong, N.K.; MacLean, E.L.; Yapa, H.M.; et al. Levofloxacin for the Prevention of Multidrug-Resistant Tuberculosis in Vietnam. N. Engl. J. Med. 2024, 391, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Angula, K.T.; Legoabe, L.J.; Beteck, R.M. Chemical Classes Presenting Novel Antituberculosis Agents Currently in Different Phases of Drug Development: A 2010–2020 Review. Pharmaceuticals 2021, 14, 461. [Google Scholar] [CrossRef]
- Imran, Mohd. ; Abida; Alotaibi, N.M.; Thabet, H.K.; Alruwaili, J.A.; Asdaq, S.M.B.; Eltaib, L.; Alshehri, A.; Alsaiari, A.A.; Almehmadi, M.; et al. QcrB Inhibition as a Potential Approach for the Treatment of Tuberculosis: A Review of Recent Developments, Patents, and Future Directions. J. Infect. Public Health 2023, 16, 928–937. [Google Scholar] [CrossRef]
- Wallis, R.S.; Dawson, R.; Friedrich, S.O.; Venter, A.; Paige, D.; Zhu, T.; Silvia, A.; Gobey, J.; Ellery, C.; Zhang, Y.; et al. Mycobactericidal Activity of Sutezolid (PNU-100480) in Sputum (EBA) and Blood (WBA) of Patients with Pulmonary Tuberculosis. PLoS ONE 2014, 9, e94462. [Google Scholar] [CrossRef]
- Gao, C.; Peng, C.; Shi, Y.; You, X.; Ran, K.; Xiong, L.; Ye, T.; Zhang, L.; Wang, N.; Zhu, Y.; et al. Benzothiazinethione Is a Potent Preclinical Candidate for the Treatment of Drug-Resistant Tuberculosis. Sci. Rep. 2016, 6, 29717. [Google Scholar] [CrossRef]
- Lu, X.; Williams, Z.; Hards, K.; Tang, J.; Cheung, C.-Y.; Aung, H.L.; Wang, B.; Liu, Z.; Hu, X.; Lenaerts, A.; et al. Pyrazolo[1,5- a ]Pyridine Inhibitor of the Respiratory Cytochrome Bcc Complex for the Treatment of Drug-Resistant Tuberculosis. ACS Infect. Dis. 2019, 5, 239–249. [Google Scholar] [CrossRef]
- Hoelscher, M.; Barros-Aguirre, D.; Dara, M.; Heinrich, N.; Sun, E.; Lange, C.; Tiberi, S.; Wells, C. Candidate Anti-Tuberculosis Medicines and Regimens under Clinical Evaluation. Clin. Microbiol. Infect. 2024, 30, 1131–1138. [Google Scholar] [CrossRef]
- Heinrich, N.; De Jager, V.; Dreisbach, J.; Gross-Demel, P.; Schultz, S.; Gerbach, S.; Kloss, F.; Dawson, R.; Narunsky, K.; Leonie, M.; et al. BTZ-043 Shows Good Safety and Strong Bactericidal Activity in a Combined Phase1b/2a Study in Tuberculosis Patients 2023.
- Miotto, P.; Zhang, Y.; Cirillo, D.M.; Yam, W.C. Drug Resistance Mechanisms and Drug Susceptibility Testing for Tuberculosis. Respirology 2018, 23, 1098–1113. [Google Scholar] [CrossRef]
- Zhang, Y.; Yew, W.-W. Mechanisms of Drug Resistance in <I>Mycobacterium Tuberculosis</I>: Update 2015. Int. J. Tuberc. Lung Dis. 2015, 19, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Musser, J.M. Molecular Genetic Basis of Antimicrobial Agent Resistance inMycobacterium Tuberculosis: 1998 Update. Tuber. Lung Dis. 1998, 79, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hasan, Z.; McNerney, R.; Mallard, K.; Hill-Cawthorne, G.; Coll, F.; Nair, M.; Pain, A.; Clark, T.G.; Hasan, R. Whole Genome Sequencing Based Characterization of Extensively Drug-Resistant Mycobacterium Tuberculosis Isolates from Pakistan. PLoS ONE 2015, 10, e0117771. [Google Scholar] [CrossRef]
- Kanji, A.; Hasan, R.; Ali, A.; Zaver, A.; Zhang, Y.; Imtiaz, K.; Shi, W.; Clark, T.G.; McNerney, R.; Phelan, J.; et al. Single Nucleotide Polymorphisms in Efflux Pumps Genes in Extensively Drug Resistant Mycobacterium Tuberculosis Isolates from Pakistan. Tuberculosis 2017, 107, 20–30. [Google Scholar] [CrossRef]
- Louw, G.E.; Warren, R.M.; Van Helden, P.D.; Victor, T.C. Rv2629 191A/C Nucleotide Change Is Not Associated with Rifampicin Resistance in Mycobacterium Tuberculosis. Clin. Chem. Lab. Med. 2009, 47. [Google Scholar] [CrossRef]
- Ghajavand, H.; Kargarpour Kamakoli, M.; Khanipour, S.; Pourazar Dizaji, S.; Masoumi, M.; Rahimi Jamnani, F.; Fateh, A.; Yaseri, M.; Siadat, S.D.; Vaziri, F. Scrutinizing the Drug Resistance Mechanism of Multi- and Extensively-Drug Resistant Mycobacterium Tuberculosis: Mutations versus Efflux Pumps. Antimicrob. Resist. Infect. Control 2019, 8, 70. [Google Scholar] [CrossRef]
- Ramón-García, S.; Martín, C.; Thompson, C.J.; Aínsa, J.A. Role of the Mycobacterium Tuberculosis P55 Efflux Pump in Intrinsic Drug Resistance, Oxidative Stress Responses, and Growth. Antimicrob. Agents Chemother. 2009, 53, 3675–3682. [Google Scholar] [CrossRef]
- Kanji, A.; Hasan, R.; Hasan, Z. Efflux Pump as Alternate Mechanism for Drug Resistance in Mycobacterium Tuberculosis. Indian J. Tuberc. 2019, 66, 20–25. [Google Scholar] [CrossRef]
- Machado, D.; Couto, I.; Perdigão, J.; Rodrigues, L.; Portugal, I.; Baptista, P.; Veigas, B.; Amaral, L.; Viveiros, M. Contribution of Efflux to the Emergence of Isoniazid and Multidrug Resistance in Mycobacterium Tuberculosis. PLoS ONE 2012, 7, e34538. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H.E. Mycobacterium Tuberculosis : Success through Dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef]
- Dua, K.; Rapalli, V.K.; Shukla, S.D.; Singhvi, G.; Shastri, M.D.; Chellappan, D.K.; Satija, S.; Mehta, M.; Gulati, M.; Pinto, T.D.J.A.; et al. Multi-Drug Resistant Mycobacterium Tuberculosis & Oxidative Stress Complexity: Emerging Need for Novel Drug Delivery Approaches. Biomed. Pharmacother. 2018, 107, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Sukumar, S.; Coscolla, M.; Shui, G.; Li, B.; Guan, X.L.; Bendt, A.K.; Young, D.; Gagneux, S.; Wenk, M.R. Lipidomics and Genomics of Mycobacterium Tuberculosis Reveal Lineage-specific Trends in Mycolic Acid Biosynthesis. MicrobiologyOpen 2014, 3, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Weisbrod, T.R.; Chen, B.; Kremer, L.; Hazbón, M.H.; Wang, F.; Alland, D.; Sacchettini, J.C.; Jacobs, W.R. Altered NADH/NAD+ Ratio Mediates Coresistance to Isoniazid and Ethionamide in Mycobacteria. Antimicrob. Agents Chemother. 2005, 49, 708–720. [Google Scholar] [CrossRef]
- Awuh, J.A.; Flo, T.H. Molecular Basis of Mycobacterial Survival in Macrophages. Cell. Mol. Life Sci. 2017, 74, 1625–1648. [Google Scholar] [CrossRef]
- Jaeger, T. Peroxiredoxin Systems in Mycobacteria. In Peroxiredoxin Systems; Flohé, L., Harris, J.R., Eds.; Subcellular Biochemistry; Springer Netherlands: Dordrecht, 2007; ISBN 978-1-4020-6050-2. [Google Scholar]
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radical Bio. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Sivaramakrishnan, S.; Ortiz De Montellano, P. The DosS-DosT/DosR Mycobacterial Sensor System. Biosensors 2013, 3, 259–282. [Google Scholar] [CrossRef]
- Wadhwa, R.; Sehgal, N.; G, N.; Aggarwal, T.; Satija, S.; Mehta, M.; Gupta, G.; Chellappan, D.K.; Tambuwala, M.M.; Oliver, B.; et al. Oxidative Stress and Immunological Complexities in Multidrug-Resistant Tuberculosis. In Role of Oxidative Stress in Pathophysiology of Diseases; Maurya, P.K., Dua, K., Eds.; Springer Singapore: Singapore, 2020; ISBN 978-981-15-1567-5. [Google Scholar]
- Tiwari, D.; Martineau, A.R. Inflammation-Mediated Tissue Damage in Pulmonary Tuberculosis and Host-Directed Therapeutic Strategies. Seminars in Immunology 2023, 65, 101672. [Google Scholar] [CrossRef]
- Mackieh, R.; Al-Bakkar, N.; Kfoury, M.; Roufayel, R.; Sabatier, J.-M.; Fajloun, Z. Inhibitors of ATP Synthase as New Antibacterial Candidates. Antibiotics 2023, 12, 650. [Google Scholar] [CrossRef]
- Hatami1, H.; Sotgiu2, G.; Bostanghadiri3, N.; Shafiee Dolat Abadi4, S.; Mesgarpour5, B.; Goudarzi4, H.; Battista Migliori6, G.; Javad Nasiri4, M. Bedaquiline-Containing Regimens and Multidrug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis. J. Bras. Pneumol. 2022, e20210384. [Google Scholar] [CrossRef]
- Ur Rehman, O.; Fatima, E.; Ali, A.; Akram, U.; Nashwan, A.; Yunus, F. Efficacy and Safety of Bedaquiline Containing Regimens in Patients of Drug-Resistant Tuberculosis: An Updated Systematic Review and Meta-Analysis. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 34, 100405. [Google Scholar] [CrossRef] [PubMed]
- Farid Thariqulhaq, M.; Yunis Miko Wahyono, T. The effectiveness and safety of bedaquiline-containing regimens in the treatment of patients with multi-drug resistant tuberculosis (MDR-TB): A Systematic Literature Review. Jurnal EduHealth 2023, 14, 1382–1392. [Google Scholar] [CrossRef]
- Trevisi, L.; Hernán, M.A.; Mitnick, C.D.; Khan, U.; Seung, K.J.; Rich, M.L.; Bastard, M.; Huerga, H.; Melikyan, N.; Atwood, S.A.; et al. Effectiveness of Bedaquiline Use beyond Six Months in Patients with Multidrug-Resistant Tuberculosis. Am. J. Respir. Crit. Care Med. 2023, 207, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, A.C.; Podasca, I.; Koul, A.; Andries, K.; Guillemont, J.; Lill, H.; Bald, D. Probing the Interaction of the Diarylquinoline TMC207 with Its Target Mycobacterial ATP Synthase. PLoS ONE 2011, 6, e23575. [Google Scholar] [CrossRef]
- Guo, H.; Courbon, G.M.; Bueler, S.A.; Mai, J.; Liu, J.; Rubinstein, J.L. Structure of Mycobacterial ATP Synthase Bound to the Tuberculosis Drug Bedaquiline. Nature 2021, 589, 143–147. [Google Scholar] [CrossRef]
- Krah, A.; Grüber, G.; Bond, P.J. Binding Properties of the Anti-TB Drugs Bedaquiline and TBAJ-876 to a Mycobacterial F-ATP Synthase. Curr. Res. Struct. Biol. 2022, 4, 278–284. [Google Scholar] [CrossRef]
- Giraud-Gatineau, A.; Coya, J.M.; Maure, A.; Biton, A.; Thomson, M.; Bernard, E.M.; Marrec, J.; Gutierrez, M.G.; Larrouy-Maumus, G.; Brosch, R.; et al. The Antibiotic Bedaquiline Activates Host Macrophage Innate Immune Resistance to Bacterial Infection. eLife 2020, 9, e55692. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Guglielmetti, L.; Hewison, C.; Bakare, N.; Bastard, M.; Caumes, E.; Fréchet-Jachym, M.; Robert, J.; Veziris, N.; Khachatryan, N.; et al. Outcomes of Bedaquiline Treatment in Patients with Multidrug-Resistant Tuberculosis. Emerg. Infect. Dis. 2019, 25, 936–943. [Google Scholar] [CrossRef]
- Maranchick, N.F.; Peloquin, C.A. Role of Therapeutic Drug Monitoring in the Treatment of Multi-Drug Resistant Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 36, 100444. [Google Scholar] [CrossRef]
- WHO, 2023b WHO Publishes Information Notes on the Use of Bedaquiline and Delamanid in Children and Adolescents with Drug-Resistant Tuberculosis Available online:. Available online: https://www.who.int/news/item/28-06-2023-who-publishes-information-notes-on-the-use-of-bedaquiline-and-delamanid-in-children-and-adolescents-with-drug-resistant-tuberculosis (accessed on 30 January 2025).
- Vanino, E.; Granozzi, B.; Akkerman, O.W.; Munoz-Torrico, M.; Palmieri, F.; Seaworth, B.; Tiberi, S.; Tadolini, M. Update of Drug-Resistant Tuberculosis Treatment Guidelines: A Turning Point. Int. J. Infect. Dis. 2023, 130, S12–S15. [Google Scholar] [CrossRef]
- Van Heeswijk, R.P.G.; Dannemann, B.; Hoetelmans, R.M.W. Bedaquiline: A Review of Human Pharmacokinetics and Drug-Drug Interactions. J. Antimicrob. Chemother. 2014, 69, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Matteelli, A.; Carvalho, A.C.; Dooley, K.E.; Kritski, A. Tmc207: The First Compound of A New Class of Potent Anti-Tuberculosis Drugs. Future Microbiol. 2010, 5, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Worley, M.V.; Estrada, S.J. Bedaquiline: A Novel Antitubercular Agent for the Treatment of Multidrug-Resistant Tuberculosis. Pharmacotherapy 2014, 34, 1187–1197. [Google Scholar] [CrossRef]
- Lange, C.; Aarnoutse, R.; Chesov, D.; Van Crevel, R.; Gillespie, S.H.; Grobbel, H.-P.; Kalsdorf, B.; Kontsevaya, I.; Van Laarhoven, A.; Nishiguchi, T.; et al. Perspective for Precision Medicine for Tuberculosis. Front. Immunol. 2020, 11, 566608. [Google Scholar] [CrossRef]
- Comas, I.; López, M.G.; Chiner-Oms, Á.; Farhat, M.R.; Ngabonziza, J.C.S.; Campos, J.; Moreno-Molina, M. Genomic Approaches for Tuberculosis Management and Control. The Challenge of Tuberculosis in the 21st Century:; ERS Monograph Series; 3rd ed. European Respiratory Society: Sheffield, 2023; ISBN 978-1-84984-169-6. [Google Scholar]
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Cegielski, J.P.; Chen, L.; Daley, C.L.; et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2019, 200, e93–e142. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, M.-H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1. [Google Scholar] [CrossRef]
- Carranza, C.; Herrera, M.T.; Guzmán-Beltrán, S.; Salgado-Cantú, M.G.; Salido-Guadarrama, I.; Santiago, E.; Chávez-Galán, L.; Gutiérrez-González, L.H.; González, Y. A Dual Marker for Monitoring MDR-TB Treatment: Host-Derived miRNAs and M. Tuberculosis-Derived RNA Sequences in Serum. Front. Immunol. 2021, 12, 760468. [Google Scholar] [CrossRef]
- Goletti, D.; Lee, M.; Wang, J.; Walter, N.; Ottenhoff, T.H.M. Update on Tuberculosis Biomarkers: From Correlates of Risk, to Correlates of Active Disease and of Cure from Disease. Respirology 2018, 23, 455–466. [Google Scholar] [CrossRef]
- Lynch, J.; Szumowski, J. Profile of Delamanid for the Treatment of Multidrug-Resistant Tuberculosis. DDDT 2015, 677. [Google Scholar] [CrossRef]
- Nguyen, T.V.A.; Nguyen, Q.H.; Nguyen, T.N.T.; Anthony, R.M.; Vu, D.H.; Alffenaar, J.-W.C. Pretomanid Resistance: An Update on Emergence, Mechanisms and Relevance for Clinical Practice. Int. J. Antimicrob. Agents 2023, 62, 106953. [Google Scholar] [CrossRef]
- Lee, S.F.K.; Laughon, B.E.; McHugh, T.D.; Lipman, M. New Drugs to Treat Difficult Tuberculous and Nontuberculous Mycobacterial Pulmonary Disease. Curr. Opin. Pulm. Med. 2019, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Pretomanid: First Approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Stover, C.K.; Warrener, P.; VanDevanter, D.R.; Sherman, D.R.; Arain, T.M.; Langhorne, M.H.; Anderson, S.W.; Towell, J.A.; Yuan, Y.; McMurray, D.N.; et al. A Small-Molecule Nitroimidazopyran Drug Candidate for the Treatment of Tuberculosis. Nature 2000, 405, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Haver, H.L.; Chua, A.; Ghode, P.; Lakshminarayana, S.B.; Singhal, A.; Mathema, B.; Wintjens, R.; Bifani, P. Mutations in Genes for the F420 Biosynthetic Pathway and a Nitroreductase Enzyme Are the Primary Resistance Determinants in Spontaneous In Vitro -Selected PA-824-Resistant Mutants of Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 5316–5323. [Google Scholar] [CrossRef]
- Mudde, S.E.; Upton, A.M.; Lenaerts, A.; Bax, H.I.; De Steenwinkel, J.E.M. Delamanid or Pretomanid? A Solomonic Judgement! J. Antimicrob. Chemother. 2022, 77, 880–902. [Google Scholar] [CrossRef]
- Peloquin, C.A.; Davies, G.R. The Treatment of Tuberculosis. Clin. Pharma. Ther. 2021, 110, 1455–1466. [Google Scholar] [CrossRef]
- Ginsberg, A.M.; Laurenzi, M.W.; Rouse, D.J.; Whitney, K.D.; Spigelman, M.K. Safety, Tolerability, and Pharmacokinetics of PA-824 in Healthy Subjects. Antimicrob. Agents Chemother. 2009, 53, 3720–3725. [Google Scholar] [CrossRef]
- Winter, H.; Ginsberg, A.; Egizi, E.; Erondu, N.; Whitney, K.; Pauli, E.; Everitt, D. Effect of a High-Calorie, High-Fat Meal on the Bioavailability and Pharmacokinetics of PA-824 in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2013, 57, 5516–5520. [Google Scholar] [CrossRef]
- Ayodele, S.; Kumar, P.; Van Eyk, A.; Choonara, Y.E. Advances in Immunomodulatory Strategies for Host-Directed Therapies in Combating Tuberculosis. Biomed. Pharmacother. 2023, 162, 114588. [Google Scholar] [CrossRef]
- Fatima, S.; Bhaskar, A.; Dwivedi, V.P. Repurposing Immunomodulatory Drugs to Combat Tuberculosis. Front. Immunol. 2021, 12, 645485. [Google Scholar] [CrossRef]
- Kadura, S.; King, N.; Nakhoul, M.; Zhu, H.; Theron, G.; Köser, C.U.; Farhat, M. Systematic Review of Mutations Associated with Resistance to the New and Repurposed Mycobacterium Tuberculosis Drugs Bedaquiline, Clofazimine, Linezolid, Delamanid and Pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, J.; Carroll, M.W.; Choi, H.; Min, S.; Song, T.; Via, L.E.; Goldfeder, L.C.; Kang, E.; Jin, B.; et al. Linezolid for Treatment of Chronic Extensively Drug-Resistant Tuberculosis. N. Engl. J. Med. 2012, 367, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Cocker, D.; Ryan, H.; Sloan, D.J. Linezolid for Drug-Resistant Pulmonary Tuberculosis. Cochrane Database of Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Ofori-Asenso, R. Efficacy and Safety Profile of Linezolid in the Treatment of Multidrug-Resistant (MDR) and Extensively Drug-Resistant (XDR) Tuberculosis: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 41. [Google Scholar] [CrossRef]
- Ramírez-Lapausa, M.; Pascual Pareja, J.F.; Carrillo Gómez, R.; Martínez-Prieto, M.; González-Ruano Pérez, P.; Noguerado Asensio, A. Retrospective Study of Tolerability and Efficacy of Linezolid in Patients with Multidrug-Resistant Tuberculosis (1998–2014). Enferm. Infecc. Microbiol. Clín. 2016, 34, 85–90. [Google Scholar] [CrossRef]
- Lange, C.; Chesov, D.; Heyckendorf, J. Clofazimine for the Treatment of Multidrug-Resistant Tuberculosis. Clinical Microbiology and Infection 2019, 25, 128–130. [Google Scholar] [CrossRef]
- Browne, S.G.; Hogerzeil, L.M. “B 663” in the Treatment of Leprosy. Preliminary Report of a Pilot Trial. Lepr. Rev. 1962, 33, 6–10. [Google Scholar]
- Naranjo, M.F.; Kumar, A.; Ratrey, P.; Hudson, S.P. Pre-Formulation of an Additive Combination of Two Antimicrobial Agents, Clofazimine and Nisin A, to Boost Antimicrobial Activity. J. Mater. Chem. B 2024, 12, 1558–1568. [Google Scholar] [CrossRef]
- Lamprecht, D.A.; Finin, P.M.; Rahman, Md.A.; Cumming, B.M.; Russell, S.L.; Jonnala, S.R.; Adamson, J.H.; Steyn, A.J.C. Turning the Respiratory Flexibility of Mycobacterium Tuberculosis against Itself. Nat. Commun. 2016, 7, 12393. [Google Scholar] [CrossRef]
- Epstein, I.G.; Nair, K.G.S.; Boyd, L.J. Cycloserine, a New Antibiotic, in the Treatment of Human Pulmonary Tuberculosis: A Preliminary Report. Antibiotic Med. 1955, 1, 80–93. [Google Scholar]
- Li, Y.; Wang, F.; Wu, L.; Zhu, M.; He, G.; Chen, X.; Sun, F.; Liu, Q.; Wang, X.; Zhang, W. Cycloserine for Treatment of Multidrug-Resistant Tuberculosis: A Retrospective Cohort Study in China. IDR 2019, Volume 12, 721–731. [Google Scholar] [CrossRef]
- Wang, J.; Pang, Y.; Jing, W.; Chen, W.; Guo, R.; Han, X.; Wu, L.; Yang, G.; Yang, K.; Chen, C.; et al. Efficacy and Safety of Cycloserine-Containing Regimens in the Treatment of Multidrug-Resistant Tuberculosis: A Nationwide Retrospective Cohort Study in China. Infect. Drug Resist. 2019, 12, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic Host-Directed Strategies to Improve Outcome in Tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Gangwar, A.; Sharma, R. Harnessing Host-Pathogen Interactions for Innovative Drug Discovery and Host-Directed Therapeutics to Tackle Tuberculosis. Microbiol. Res. 2023, 275, 127466. [Google Scholar] [CrossRef] [PubMed]
- Torfs, E.; Piller, T.; Cos, P.; Cappoen, D. Opportunities for Overcoming Mycobacterium Tuberculosis Drug Resistance: Emerging Mycobacterial Targets and Host-Directed Therapy. Int. J. Mol. Sci. 2019, 20, 2868. [Google Scholar] [CrossRef]
- Khoza, L.J.; Kumar, P.; Dube, A.; Demana, P.H.; Choonara, Y.E. Insights into Innovative Therapeutics for Drug-Resistant Tuberculosis: Host-Directed Therapy and Autophagy Inducing Modified Nanoparticles. Int. J. Pharm. 2022, 622, 121893. [Google Scholar] [CrossRef]
- Kilinç, G.; Saris, A.; Ottenhoff, T.H.M.; Haks, M.C. Host-directed Therapy to Combat Mycobacterial Infections*. Immunol. Rev. 2021, 301, 62–83. [Google Scholar] [CrossRef]
- Singh, P.; Subbian, S. Harnessing the mTOR Pathway for Tuberculosis Treatment. Front. Microbiol. 2018, 9, 70. [Google Scholar] [CrossRef]
- Kolloli, A.; Subbian, S. Host-Directed Therapeutic Strategies for Tuberculosis. Front. Med. 2017, 4, 171. [Google Scholar] [CrossRef]
- Lee, C.; Bhakta, S. The Prospect of Repurposing Immunomodulatory Drugs for Adjunctive Chemotherapy against Tuberculosis: A Critical Review. Antibiotics 2021, 10, 91. [Google Scholar] [CrossRef]
- Rao, M.; Ippolito, G.; Mfinanga, S.; Ntoumi, F.; Yeboah-Manu, D.; Vilaplana, C.; Zumla, A.; Maeurer, M. Improving Treatment Outcomes for MDR-TB — Novel Host-Directed Therapies and Personalised Medicine of the Future. Int. J. Infect. Dis. 2019, 80, S62–S67. [Google Scholar] [CrossRef]
- Naicker, N.; Sigal, A.; Naidoo, K. Metformin as Host-Directed Therapy for TB Treatment: Scoping Review. Front. Microbiol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Bahlool, A.Z.; Grant, C.; Cryan, S.-A.; Keane, J.; O’Sullivan, M.P. All Trans Retinoic Acid as a Host-Directed Immunotherapy for Tuberculosis. Curr. Res. Immunol. 3, 54–72. [CrossRef]
- Lee, Y.-J.; Han, S.K.; Park, J.H.; Lee, J.K.; Kim, D.K.; Chung, H.S.; Heo, E.Y. The Effect of Metformin on Culture Conversion in Tuberculosis Patients with Diabetes Mellitus. Korean J. Intern. Med. 2018, 33, 933–940. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.-S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as Adjunct Antituberculosis Therapy. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef]
- Yew, W.W.; Chang, K.C.; Chan, D.P.; Zhang, Y. Metformin as a Host-Directed Therapeutic in Tuberculosis: Is There a Promise? Tuberculosis 2019, 115, 76–80. [Google Scholar] [CrossRef]
- Chun, R.F.; Adams, J.S.; Hewison, M. Immunomodulation by Vitamin D: Implications for TB. Expert Rev. Clin. Pharmacol. 2011, 4, 583–591. [Google Scholar] [CrossRef]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in Infection, Inflammation and Immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Chung, C.; Jo, E.-K. Autophagy: A New Strategy for Host-Directed Therapy of Tuberculosis. Virulence 2019, 10, 448–459. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New Insights into the Evasion of Host Innate Immunity by Mycobacterium Tuberculosis. Cell Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Kimmey, J.M.; Stallings, C.L. Bacterial Pathogens versus Autophagy: Implications for Therapeutic Interventions. Trends Mol. Med. 2016, 22, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Maphasa, R.E.; Meyer, M.; Dube, A. The Macrophage Response to Mycobacterium Tuberculosis and Opportunities for Autophagy Inducing Nanomedicines for Tuberculosis Therapy. Front. Cell. Infect. Microbiol. 2021, 10, 618414. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Kumar, M.; Jha, A.; Bharti, K.; Das, M.; Mishra, B. Nanocarriers for Tuberculosis Therapy: Design of Safe and Effective Drug Delivery Strategies to Overcome the Therapeutic Challenges. J. Drug Deliv. Sci. Technol. 2022, 67, 102850. [Google Scholar] [CrossRef]
- Nair, A.; Greeny, A.; Nandan, A.; Sah, R.K.; Jose, A.; Dyawanapelly, S.; Junnuthula, V.; K. V., A.; Sadanandan, P. Advanced Drug Delivery and Therapeutic Strategies for Tuberculosis Treatment. J. Nanobiotechnol. 2023, 21, 414. [Google Scholar] [CrossRef]
- Miretti, M.; Juri, L.; Cosiansi, M.C.; Tempesti, T.C.; Baumgartner, M.T. Antimicrobial Effects of ZnPc Delivered into Liposomes on Multidrug Resistant (MDR)- Mycobacterium Tuberculosis. ChemistrySelect 2019, 4, 9726–9730. [Google Scholar] [CrossRef]
- D’Souza, S.; Du Plessis, S.; Egieyeh, S.; Bekale, R.; Maphasa, R.; Irabin, A.; Sampson, S.; Dube, A. Physicochemical and Biological Evaluation of Curdlan-Poly(Lactic-Co-Glycolic Acid) Nanoparticles as a Host-Directed Therapy Against Mycobacterium Tuberculosis. J. Pharm. Sci. 2022, 111, 469–478. [Google Scholar] [CrossRef]
- Akinnawo, C.A.; Dube, A. Clinically Relevant Metallic Nanoparticles in Tuberculosis Diagnosis and Therapy. Adv. Ther. 2024, 2400189. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Das, S.S.; Anjireddy, K.; Karpagam, S.; Shakeel, F. Nanomedicines as Drug Delivery Carriers of Anti-Tubercular Drugs: From Pathogenesis to Infection Control. Curr. Drug Deliv. 2019, 16, 400–429. [Google Scholar] [CrossRef]
- Caminero, J.A.; Lasserra, P.; Piubello, A.; Singla, R. Adverse Anti-Tuberculosis Drug Events and Their Management.; Eur. Respir. Monogr. 2018; Vol. 82; ISBN 978-1-84984-100-9.
- Sadhu, P.K.; Saisivam, S.; Debnath, S.K. Design and characterization of niosomes of ethionamide for multi drug resistance tuberculosis.
- El-Ridy, M.S.; Yehia, S.A.; Kassem, M.A.-E.-M.; Mostafa, D.M.; Nasr, E.A.; Asfour, M.H. Niosomal Encapsulation of Ethambutol Hydrochloride for Increasing Its Efficacy and Safety. Drug Deliv. 2015, 22, 21–36. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D.; Barot, T. Formulation and Optimization of Long Acting Dual Niosomes Using Box-Behnken Experimental Design Method for Combinative Delivery of Ethionamide and D-Cycloserine in Tuberculosis Treatment. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 565, 131–142. [Google Scholar] [CrossRef]
- Kumar, M.; Virmani, T.; Kumar, G.; Deshmukh, R.; Sharma, A.; Duarte, S.; Brandão, P.; Fonte, P. Nanocarriers in Tuberculosis Treatment: Challenges and Delivery Strategies. Pharmaceuticals 2023, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Gong, Y.; Yan, X.; Wang, L.; Zheng, W.; Ai, H.; Zhao, Y. Recent Advances in Nanoantibiotics against Multidrug-Resistant Bacteria. Nanoscale Adv. 5, 6278–6317. [CrossRef] [PubMed]
- Yu, D.; Xu, J.; Li, R.; Zhao, J.; Li, F.; Zhai, Y.; Xue, J.; Song, H.; Yang, F.; Xu, P.; et al. Synergetic Effect of Rifampin Loaded Mussel-Inspired Silver Nanoparticles for Enhanced Antibacterial Activity Against Multidrug-Resistant Strain of Mycobacterium Tuberculosis. ChemistrySelect 2021, 6, 10682–10687. [Google Scholar] [CrossRef]
- Kreytsberg, G.N.; Gracheva, I.E.; Kibrik, B.S.; Golikov, I.V. Antituberculous Effect of Silver Nanoparticles. J. Phys.: Conf. Ser. 2011, 291, 012030. [Google Scholar] [CrossRef]
- Montelongo-Peralta, L.Z.; León-Buitimea, A.; Palma-Nicolás, J.P.; Gonzalez-Christen, J.; Morones-Ramírez, J.R. Antibacterial Activity of Combinatorial Treatments Composed of Transition-Metal/Antibiotics against Mycobacterium Tuberculosis. Sci. Rep. 2019, 9, 5471. [Google Scholar] [CrossRef]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of Biosynthesized Silver and Zinc Nanoparticles Against Multi-Drug Resistant Pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef]
- Selim, A.; Elhaig, M.M.; Taha, S.A.; Nasr, E.A. Antibacterial Activity of Silver Nanoparticles against Field and Reference Strains of Mycobacterium Tuberculosis, Mycobacterium Bovis and Multiple-Drug-Resistant Tuberculosis Strains: -EN- -FR- Activité Antibactérienne Des Nanoparticules d’argent Contre Des Souches de Terrain et de Référence de Mycobacterium Tuberculosis et Mycobacterium Bovis et Des Souches Multirésistantes Aux Médicaments Contre La Tuberculose -ES- Actividad Antibacteriana de Las Nanopartículas de Plata Contra Cepas Salvajes y de Referencia de Mycobacterium Tuberculosis y Mycobacterium Bovis y Cepas de Tuberculosis Multirresistente. Rev. Sci. Tech. OIE 2018, 37, 823–830. [Google Scholar] [CrossRef]
- Yaghubi Kalurazi, T.; Jafari, A. Evaluation of Magnesium Oxide and Zinc Oxide Nanoparticles against Multi-Drug-Resistance Mycobacterium Tuberculosis. Indian J. Tuberc. 2021, 68, 195–200. [Google Scholar] [CrossRef]
- Heidary, M.; Zaker Bostanabad, S.; Amini, S.M.; Jafari, A.; Ghalami Nobar, M.; Ghodousi, A.; Kamalzadeh, M.; Darban-Sarokhalil, D. The Anti-Mycobacterial Activity Of Ag, ZnO, And Ag- ZnO Nanoparticles Against MDR- And XDR-Mycobacterium Tuberculosis. IDR 2019, Volume 12, 3425–3435. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-Date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-Art Polymeric Nanoparticles as Promising Therapeutic Tools against Human Bacterial Infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, N.; K. Khuller, G.; Prabhakar, T.; Pal, N.; Gupta, P.; Gupta, U. Nanoformulations of Moxifloxacin, Econozole and Ethionamide as Novel Treatment Regimens Against MDR TB - An Experimental Study. Curr, Nanosci. 2016, 12, 110–117. [Google Scholar]
- Abdelghany, S.; Parumasivam, T.; Pang, A.; Roediger, B.; Tang, P.; Jahn, K.; Britton, W.J.; Chan, H.-K. Alginate Modified-PLGA Nanoparticles Entrapping Amikacin and Moxifloxacin as a Novel Host-Directed Therapy for Multidrug-Resistant Tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 52, 642–651. [Google Scholar] [CrossRef]
- Pang, X.; Xiao, Q.; Cheng, Y.; Ren, E.; Lian, L.; Zhang, Y.; Gao, H.; Wang, X.; Leung, W.; Chen, X.; et al. Bacteria-Responsive Nanoliposomes as Smart Sonotheranostics for Multidrug Resistant Bacterial Infections. ACS Nano, 0933. [Google Scholar] [CrossRef]
- Sun, D.; Pang, X.; Cheng, Y.; Ming, J.; Xiang, S.; Zhang, C.; Lv, P.; Chu, C.; Chen, X.; Liu, G.; et al. Ultrasound-Switchable Nanozyme Augments Sonodynamic Therapy against Multidrug-Resistant Bacterial Infection. ACS Nano 2020, 14, 2063–2076. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Hou, Y.; Xie, S.; Xu, J.; Yang, M.; Li, D.; Du, Y. Levofloxacin-Loaded Nanosonosensitizer as a Highly Efficient Therapy for Bacillus Calmette-Guérin Infections Based on Bacteria-Specific Labeling and Sonotheranostic Strategy. Int. J. Nanomed. 2021, Volume 16, 6553–6573. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Β-glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Quesniaux, V.F.J.; Jacobs, M.; Allie, N.; Grivennikov, S.; Nedospasov, S.A.; Garcia, I.; Olleros, M.L.; Shebzukhov, Y.; Kuprash, D.; Vasseur, V.; et al. TNF in Host Resistance to Tuberculosis Infection. In Current Directions in Autoimmunity; Kollias, G., Sfikakis, P.P., Eds.; KARGER: Basel, 2010; ISBN 978-3-8055-9383-0. [Google Scholar]
- Kumar, P.V.; Asthana, A.; Dutta, T.; Jain, N.K. Intracellular Macrophage Uptake of Rifampicin Loaded Mannosylated Dendrimers. J. Drug Target. 2006, 14, 546–556. [Google Scholar] [CrossRef]
- Praphakar, R.A.; Shakila, H.; Azger Dusthackeer, V.N.; Munusamy, M.A.; Kumar, S.; Rajan, M. A Mannose-Conjugated Multi-Layered Polymeric Nanocarrier System for Controlled and Targeted Release on Alveolar Macrophages. Polym. Chem. 2018, 9, 656–667. [Google Scholar] [CrossRef]
- Pawde, D.M.; Viswanadh, M.K.; Mehata, A.K.; Sonkar, R.; Narendra; Poddar, S. ; Burande, A.S.; Jha, A.; Vajanthri, K.Y.; Mahto, S.K.; et al. Mannose Receptor Targeted Bioadhesive Chitosan Nanoparticles of Clofazimine for Effective Therapy of Tuberculosis. Saudi Pharm. J. 2020, 28, 1616–1625. [Google Scholar] [CrossRef]
- Karine De Sousa, A.; Rocha, J.E.; Gonçalves De Souza, T.; Sampaio De Freitas, T.; Ribeiro-Filho, J.; Melo Coutinho, H.D. New Roles of Fluoxetine in Pharmacology: Antibacterial Effect and Modulation of Antibiotic Activity. Microb. Pathog. 2018, 123, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcı̀a-Rodrı̀guez, J.A. Antimicrobial Activity of Psychotropic Drugs. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Delorme, V.; Kasaeian, A.; Amiri, V.; Masoumi, M.; Sadeghinia, M.; Ebrahimzadeh, N.; Maleki, M.; Pourazar, S. An Effective Nano Drug Delivery and Combination Therapy for the Treatment of Tuberculosis. Sci. Rep. 2022, 12, 9591. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Shen, S.; Liu, H. Recent Advances in PLGA Micro/Nanoparticle Delivery Systems as Novel Therapeutic Approach for Drug-Resistant Tuberculosis. Front. Bioeng. Biotechnol. 2022, 10, 941077. [Google Scholar] [CrossRef]
- Shetty, A.; Kwas, H.; Rajhi, H.; Rangareddy, H.; Fryer, J. Revolutionizing Tuberculosis Management With Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas Technology: A Comprehensive Literature Review. Cureus 2024. [Google Scholar] [CrossRef]
- Rahman, K.; Jamal, M.; Chen, X.; Zhou, W.; Yang, B.; Zou, Y.; Xu, W.; Lei, Y.; Wu, C.; Cao, X.; et al. Reprogramming Mycobacterium Tuberculosis CRISPR System for Gene Editing and Genome-Wide RNA Interference Screening. Genom. Proteom. Bioinf. 2022, 20, 1180–1196. [Google Scholar] [CrossRef]
- Feng, S.; Liang, L.; Shen, C.; Lin, D.; Lyu, L.; Liang, W.; Zhong, L.L.; Cook, G.M.; Doi, Y.; Chen, C. A CRISPR-Guided Mutagenic DNA Polymerase Strategy for the Detection of Antibiotic-Resistant Mutations in M. Available online: https://www.cell.com/molecular-therapy-family/nucleic-acids/fulltext/S2162-2531(22)00173-1 (accessed on day month year).
- Tram, T.T.B.; Ha, V.T.N.; Trieu, L.P.T.; Ashton, P.M.; Crawford, E.D.; Thu, D.D.A.; Quang, N.L.; Thwaites, G.E.; Walker, T.M.; Anscombe, C.; et al. FLASH-TB: An Application of Next-Generation CRISPR to Detect Drug Resistant Tuberculosis from Direct Sputum. J. Clin. Microbiol. 2023, 61, e01634–22. [Google Scholar] [CrossRef]
- Hussen, B.M.; Najmadden, Z.B.; Abdullah, S.R.; Rasul, M.F.; Mustafa, S.A.; Ghafouri-Fard, S.; Taheri, M. CRISPR/Cas9 Gene Editing: A Novel Strategy for Fighting Drug Resistance in Respiratory Disorders. Cell Commun. Signal. 2024, 22, 329. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Zheng, D.; Li, S.-S.; Ding, X.-Y.; Wang, C.-L.; Guo, X.-P.; Zhan, L.; Jin, Q.; Yang, J.; Sun, Y.-C. Application of Combined CRISPR Screening for Genetic and Chemical-Genetic Interaction Profiling in Mycobacterium Tuberculosis. Sci. Adv. 2022, 8, eadd5907. [Google Scholar] [CrossRef]
- Hernández-Bazán, S.; Mata-Espinosa, D.; Lozano-Ordaz, V.; Ramos-Espinosa, O.; Barrios-Payán, J.; López-Casillas, F.; Hernández Pando, R. Immune Regulatory Effect of Osteopontin Gene Therapy in a Murine Model of Multidrug Resistant Pulmonary Tuberculosis. Hum. Gene Ther. 2022, 33, 1037–1051. [Google Scholar] [CrossRef]
- Ahmed, S.; Raqib, R.; Guðmundsson, G.H.; Bergman, P.; Agerberth, B.; Rekha, R.S. Host-Directed Therapy as a Novel Treatment Strategy to Overcome Tuberculosis: Targeting Immune Modulation. Antibiotics 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, J.; Wang, P.; Li, H.; Zhou, Y.; Liu, H.; Liu, Z.; Zheng, R.; Wang, L.; Yang, H.; et al. MicroRNA-27a Controls the Intracellular Survival of Mycobacterium Tuberculosis by Regulating Calcium-Associated Autophagy. Nat. Commun. 2018, 9, 4295. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Halder, P.; Sahu, S.K.; Kumar, M.; Kumari, M.; Jana, K.; Ghosh, Z.; Sharma, P.; Kundu, M.; Basu, J. Identification of a Novel Role of ESAT-6-Dependent miR-155 Induction during Infection of Macrophages with Mycobacterium Tuberculosis: M. Tuberculosis Induces miR-155 in Macrophages. Cell Microbiol. 2012, 14, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lou, H.; Cao, J.; Wang, P.; Sha, W.; Sun, Q. RETRACTED: LncRNA MEG3 Control Mycobacterium Tuberculosis Infection via Controlled MiR-145-5p Expression and Modulation of Macrophages Proliferation. Microb. Pathog. 2020, 149, 104550. [Google Scholar] [CrossRef]
- Yan, H.; Xu, R.; Zhang, X.; Wang, Q.; Pang, J.; Zhang, X.; Chang, X.; Zhang, Y. Identifying Differentially Expressed Long Non-Coding RNAs in PBMCs in Response to the Infection of Multidrug-Resistant Tuberculosis. Infect. Drug Resist. 2018, 11, 945–959. [Google Scholar] [CrossRef]
- Li, G.; Feng, Z.; Song, H.; Wang, Y.; Zhu, L.; Li, Y. Long Non-Coding RNA Expression in PBMCs of Patients with Active Pulmonary Tuberculosis. Front. Microbiol. 2023, 14, 1257267. [Google Scholar] [CrossRef]
- Ramachandran, S.; Prakash, P.; Mohtar, N.; Kumar, K.S.; Parumasivam, T. Review of Inhalable Nanoparticles for the Pulmonary Delivery of Anti-Tuberculosis Drugs. Pharm. Dev. Technol. 2023, 28, 978–991. [Google Scholar] [CrossRef]
- Mehta, P.; Bothiraja, C.; Kadam, S.; Pawar, A. Potential of Dry Powder Inhalers for Tuberculosis Therapy: Facts, Fidelity and Future. Artif. Cells Nanomed. Biotechnol. 2018, 46, S791–S806. [Google Scholar] [CrossRef]
- Braunstein, M.; Hickey, A.J.; Ekins, S. Why Wait? The Case for Treating Tuberculosis with Inhaled Drugs. Pharm. Res. 2019, 36, 166. [Google Scholar] [CrossRef]
- Nainwal, N.; Sharma, Y.; Jakhmola, V. Dry Powder Inhalers of Antitubercular Drugs. Tuberculosis 2022, 135, 102228. [Google Scholar] [CrossRef]
- Ranjan, R.; Devireddy, V.S.R. Prospects of Inhalable Formulations of Conventionally Administered Repurposed Drugs for Adjunctive Treatment of Drug-Resistant Tuberculosis: Supporting Evidence from Clinical Trials and Cohort Studies. J. Aerosol Med. Pulm. Drug Deliv. 2024. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Kabadi, M.; Gerety, B.; Hickey, A.J.; Fourie, P.B.; Nardell, E. Phase I, Single-Dose, Dose-Escalating Study of Inhaled Dry Powder Capreomycin: A New Approach to Therapy of Drug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- McGee, B.; Dietze, R.; Hadad, D.J.; Molino, L.P.D.; Maciel, E.L.N.; Boom, W.H.; Palaci, M.; Johnson, J.L.; Peloquin, C.A. Population Pharmacokinetics of Linezolid in Adults with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2009, 53, 3981–3984. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.; Redinger, N.; Schwarz, K.; Li, F.; Hädrich, G.; Cohrs, M.; Dailey, L.A.; Schaible, U.E.; Feldmann, C. Amorphous Drug Nanoparticles for Inhalation Therapy of Multidrug-Resistant Tuberculosis. ACS Nano 2023, 17, 9478–9486. [Google Scholar] [CrossRef] [PubMed]
- Marwitz, F.; Hädrich, G.; Redinger, N.; Besecke, K.F.W.; Li, F.; Aboutara, N.; Thomsen, S.; Cohrs, M.; Neumann, P.R.; Lucas, H.; et al. Intranasal Administration of Bedaquiline-Loaded Fucosylated Liposomes Provides Anti-Tubercular Activity While Reducing the Potential for Systemic Side Effects. ACS Infect. Dis. 2024, 10, 3222–3232. [Google Scholar] [CrossRef]
- Paliwal, H.; Nakpheng, T.; Kumar Paul, P.; Prem Ananth, K.; Srichana, T. Development of a Self-Microemulsifying Drug Delivery System to Deliver Delamanid via a Pressurized Metered Dose Inhaler for Treatment of Multi-Drug Resistant Pulmonary Tuberculosis. Int. J. Pharm. 2024, 655, 124031. [Google Scholar] [CrossRef]
- Bahlool, A.Z.; Fattah, S.; O’Sullivan, A.; Cavanagh, B.; MacLoughlin, R.; Keane, J.; O’Sullivan, M.P.; Cryan, S.-A. Development of Inhalable ATRA-Loaded PLGA Nanoparticles as Host-Directed Immunotherapy against Tuberculosis. Pharmaceutics 2022, 14, 1745. [Google Scholar] [CrossRef]
- Mehta, P.P.; Ghoshal, D.; Pawar, A.P.; Kadam, S.S.; Dhapte-Pawar, V.S. Recent Advances in Inhalable Liposomes for Treatment of Pulmonary Diseases: Concept to Clinical Stance. J. Drug Deliv. Sci. Technol. 2020, 56, 101509. [Google Scholar] [CrossRef]
- Verma, S.; Dal, N.-J.K.; Srivastava, A.; Bharti, R.; Siva Reddy, D.V.; Sofi, H.S.; Roy, T.; Verma, K.; Raman, S.K.; Azmi, L.; et al. Inhaled Adjunct Therapy with Second-Line Drug Candidates for Dose Reduction in Chemotherapeutic Regimens for Multi-Drug-Resistant Tuberculosis. AAPS PharmSciTech 2023, 24, 130. [Google Scholar] [CrossRef]
- Makled, S.; Boraie, N.; Nafee, N. Nanoparticle-Mediated Macrophage Targeting—a New Inhalation Therapy Tackling Tuberculosis. Drug Deliv. Transl. Res. 2021, 11, 1037–1055. [Google Scholar] [CrossRef]
- Eedara, B.B.; Fan, C.; Sinha, S.; Khadka, P.; Das, S.C. Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization. Pharmaceutics 2023, 15, 2354. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Carlson, E.E. Tiny Things with Enormous Impact: Nanotechnology in the Fight Against Infectious Disease. ACS Infect. Dis. 2018, 4, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Sheard, D.E.; O’Brien-Simpson, N.M.; Wade, J.D.; Separovic, F. Combating Bacterial Resistance by Combination of Antibiotics with Antimicrobial Peptides. Pure Appl. Chem. 2019, 91, 199–209. [Google Scholar] [CrossRef]
- Alyami, M.H.; Dahmash, E.Z.; Ali, D.K.; Alyami, H.S.; AbdulKarim, H.; Alsudir, S.A. Novel Fluticasone Propionate and Salmeterol Fixed-Dose Combination Nano-Encapsulated Particles Using Polyamide Based on L-Lysine. Pharmaceuticals 2022, 15, 321. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashmi, A.A. Chapter 1 - Combination Therapy: Current Status and Future Perspectives. In Combination Therapy Against Multidrug Resistance; Wani, M.Y., Ahmad, A., Eds.; Academic Press, 2020; pp. 1–38 ISBN 978-0-12-820576-1.
- Wu, L.-P.; Wang, D.; Li, Z. Grand Challenges in Nanomedicine. Mater. Sci. Eng. C 2020, 106, 110302. [Google Scholar] [CrossRef]
- Buya, A.B.; Witika, B.A.; Bapolisi, A.M.; Mwila, C.; Mukubwa, G.K.; Memvanga, P.B.; Makoni, P.A.; Nkanga, C.I. Application of Lipid-Based Nanocarriers for Antitubercular Drug Delivery: A Review. Pharmaceutics 2021, 13, 2041. [Google Scholar] [CrossRef]
- Madkhali, O.A. Drug Delivery of Gelatin Nanoparticles as a Biodegradable Polymer for the Treatment of Infectious Diseases: Perspectives and Challenges. Polymers 2023, 15, 4327. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Chan, Y.; Ng, S.W.; Mehta, M.; Anand, K.; Kumar Singh, S.; Gupta, G.; Chellappan, D.K.; Dua, K. Advanced Drug Delivery Systems Can Assist in Managing Influenza Virus Infection: A Hypothesis. Med. Hypotheses 2020, 144, 110298. [Google Scholar] [CrossRef]
- Verma, N.; Arora, V.; Awasthi, R.; Chan, Y.; Jha, N.K.; Thapa, K.; Jawaid, T.; Kamal, M.; Gupta, G.; Liu, G.; et al. Recent Developments, Challenges and Future Prospects in Advanced Drug Delivery Systems in the Management of Tuberculosis. J. Drug Deliv. Sci. Technol. 2022, 75, 103690. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology Approaches for Global Infectious Diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Satalkar, P.; Elger, B.S.; Hunziker, P.; Shaw, D. Challenges of Clinical Translation in Nanomedicine: A Qualitative Study. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 893–900. [Google Scholar] [CrossRef]
- Babel, A.; Taneja, R.; Mondello Malvestiti, F.; Monaco, A.; Donde, S. Artificial Intelligence Solutions to Increase Medication Adherence in Patients With Non-Communicable Diseases. Front. Digit. Health 2021, 3, 669869. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Liposomal Amphotericin B and Leishmaniasis: Dose and Response. J. Global Infect. Dis. 2010, 2, 159. [Google Scholar] [CrossRef]
- K, S.P.; Parivakkam Mani, A.; S, G.; Yadav, S. Advancements in Artificial Intelligence for the Diagnosis of Multidrug Resistance and Extensively Drug-Resistant Tuberculosis: A Comprehensive Review. Cureus 2024. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, F.; Li, L.; Pang, Y. Clinical Utilization of Artificial Intelligence in Predicting Therapeutic Efficacy in Pulmonary Tuberculosis. J. Infect. Public Health 2024, 17, 632–641. [Google Scholar] [CrossRef]
- Singh, M.; Pujar, G.V.; Kumar, S.A.; Bhagyalalitha, M.; Akshatha, H.S.; Abuhaija, B.; Alsoud, A.R.; Abualigah, L.; Beeraka, N.M.; Gandomi, A.H. Evolution of Machine Learning in Tuberculosis Diagnosis: A Review of Deep Learning-Based Medical Applications. Electronics 2022, 11, 2634. [Google Scholar] [CrossRef]
- Olawade, D.B.; Eberhardt, J.; David-Olawade, A.C.; Balogun, M.A.; Bolarinwa, O.A.; Esan, D.T. Transforming Multidrug-Resistant Tuberculosis Care: The Potentials of Telemedicine in Resource-Limited Settings. Health Sci. Rev. 2024, 12, 100185. [Google Scholar] [CrossRef]
- Yadav, S.; Jeyaraman, N.; Jeyaraman, M.; Rawal, G. Artificial Intelligence in Tuberculosis Diagnosis: Revolutionizing Detection and Treatment. Indian J. Immunol. Respir. Med. 2024, 9, 85–87. [Google Scholar] [CrossRef]
- Patel, M.N.; Patel, A.J.; Nandpal, M.N.; Raval, M.A.; Patel, R.J.; Patel, A.A.; Paudel, K.R.; Hansbro, P.M.; Singh, S.K.; Gupta, G.; et al. Advancing against Drug-Resistant Tuberculosis: An Extensive Review, Novel Strategies and Patent Landscape. Naunyn-Schmiedeberg’s Arch Pharmacol. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).