Submitted:
02 March 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
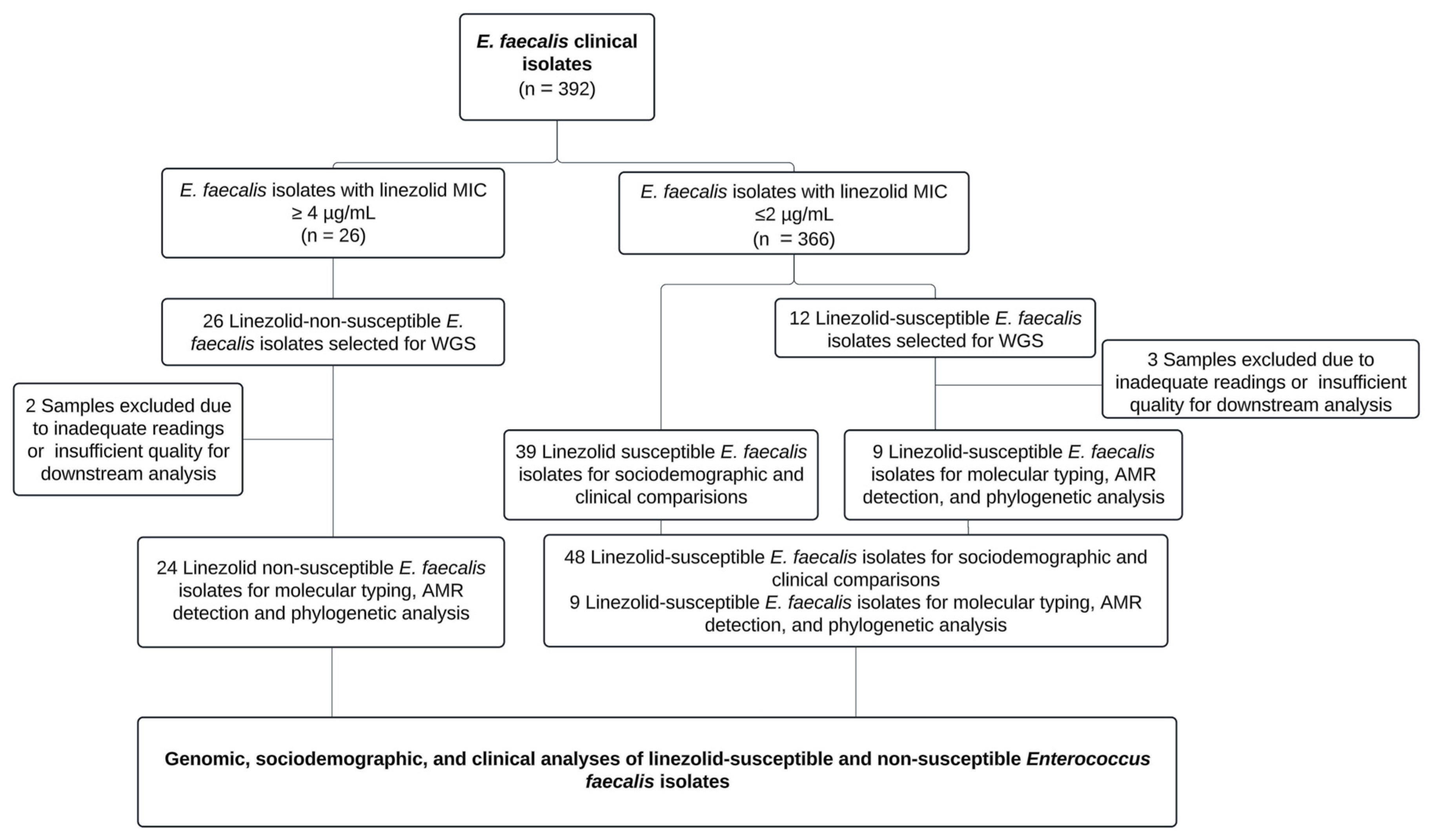
2.1. Bacterial Strain Collection, Clinical Data Gathering, and Antimicrobial Susceptibility Testing
2.2. DNA Extraction, Library Construction, and Whole-Genome Sequencing
2.3. Bioinformatic Analyses
2.4. Statistical Analysis
3. Results
| Variable | Total (n = 24) |
Women (%) (n = 7) |
Men (%) (n = 17) |
p value |
|---|---|---|---|---|
| Age – median (IQR) | 48 (31-57) | 56 (53.5-62.25) | 43 (27-55) | 0.092 |
| Previous antibiotic use in 90 days – n (%) | 11 (45.8) | 3 (27.3) | 8 (72.7) | 1 |
| Previous Antibiotic in 14 days – n (%) | 9 (37.5) | 3 (33.3) | 6 (66.7) | 0.643 |
| Previous hospitalization in 90 days – n (%) | 13 (54.2) | 4 (30.8) | 9 (69.2) | 0.66 |
| Comorbidities – n (%) | 16 (66.7) | 6 (37.5) | 10 (62.5) | 0.1243 |
| Charlson comorbidity index – median (IQR) | 1 (0-3) | 3 (1.5-3.75) | 0 (0-2) | 0.059 |
| Diabetes Mellitus – n (%) | 6 (25.0) | 3 (50) | 3 (50) | 0.2786 |
| Hypertension – n (%) | 5 (20.8) | 3 (60) | 2 (40) | 0.0886 |
| Cardiovascular disease – n (%) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Obesity – n (%) | 1 (4.2) | 1 (100) | 0 (0) | 0.260 |
| Immunosuppression – n (%) | 3 (12.5) | 1 (33.3) | 2 (66.7) | 1 |
| Chronic kidney disease – n (%) | 2 (8.3) | 0 (0) | 2 (100) | 1 |
| Surgical Intervention – n (%) | 16 (66.7) | 2 (12.5) | 14 (87.5) | 0.045 |
| Central line insertion – n (%) | 9 (37.5) | 3 (33.3) | 6 (66.7) | 0.643 |
| Urinary catheter – n (%) | 16 (66.7) | 5 (31.2) | 11 (68.8) | 0.621 |
| Need for vasopressors – n (%) | 7 (29.2) | 3 (42.9) | 4 (57.1) | 0.318 |
| ICU admission – n (%) | 3 (12.5) | 2 (66.7) | 1 (33.3) | 0.155 |
| Hospital stay – median (IQR) | 14 (11.25-20.25) | 14 (10.25-14.75) | 15 (12-23) | 0.343 |
| In-hospital mortality – n (%) | 4 (16.7) | 3 (75) | 1 (25) | 0.040 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| AMR | Antimicrobial Resistance |
| AMP | Ampicillin |
| ARGs | Antimicrobial Resistance Genes |
| AST | Antimicrobial Susceptibility Testing |
| ATP | Adenosine Triphosphate |
| CARD | Comprehensive Antibiotic Resistance Database |
| CAR | Cardiology |
| cgMLST | Core Genome Multilocus Sequence Typing |
| CIP | Ciprofloxacin |
| CLSI | Clinical and Laboratory Standards Institute |
| CSF | Cerebrospinal Fluid |
| DAP | Daptomycin |
| DNA | Deoxyribonucleic Acid |
| FASTQ | Format for Sequence Data |
| GAS | Gastroenterology |
| GEN | Gentamicin |
| HEM | Hematology |
| HIV | Human Immunodeficiency Virus |
| ICU | Intensive Care Unit |
| INF | Infectious Diseases |
| INT | Internal Medicine |
| LEV | Levofloxacin |
| LNZ | Linezolid |
| LNSEf | Linezolid-Non-Susceptible Enterococcus faecalis |
| LRE | Linezolid-Resistant Enterococcus |
| LSEf | Linezolid-Susceptible Enterococcus faecalis |
| MDR-TB | Multidrug-Resistant Tuberculosis |
| MIC | Minimum Inhibitory Concentration |
| ML | Maximum Likelihood |
| MLST | Multilocus Sequence Typing |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NEP | Nephrology |
| NEU | Neurosurgery |
| NIT | Nitrofurantoin |
| NR | Non-Redundant Database |
| PCR | Polymerase Chain Reaction |
| PED | Pediatrics |
| PEN | Benzylpenicillin |
| PLA | Plastic Surgery |
| QC | Quality Control |
| rRNA | Ribosomal Ribonucleic Acid |
| RGI | Resistance Gene Identifier |
| SNP | Single Nucleotide Polymorphism |
| ST | Sequence Type |
| STR | Streptomycin |
| SUR | General Surgery |
| TCV | Thoracic and Cardiovascular Surgery |
| TET | Tetracycline |
| TRA | Traumatology |
| TRAU | Transplant Unit |
| URO | Urology |
| VA | Vancomycin |
| WHO | World Health Organization |
| WGS | Whole-Genome Sequencing |
| SUR | General Surgery |
| TCV | Thoracic and Cardiovascular Surgery |
| TET | Tetracycline |
| TRA | Traumatology |
| TRAU | Transplant Unit |
| URO | Urology |
| VA | Vancomycin |
| VFDB | Virulence Factor Database |
| WHO | World Health Organization |
| WGS | Whole-Genome Sequencing |
References
- Zahedi Bialvaei, A.; Rahbar, M.; Yousefi, M.; Asgharzadeh, M.; Samadi Kafil, H. Linezolid: A Promising Option in the Treatment of Gram-Positives. J Antimicrob Chemother 2017, 72, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the First Oxazolidinone Antibacterial Agent. Ann N Y Acad Sci 2011, 1222, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. DDDT 2018, 12, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal Structure of the Oxazolidinone Antibiotic Linezolid Bound to the 50S Ribosomal Subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef]
- Makarov, G.I.; Makarova, T.M. A Noncanonical Binding Site of Linezolid Revealed via Molecular Dynamics Simulations. J Comput Aided Mol Des 2020, 34, 281–291. [Google Scholar] [CrossRef]
- Alloush, H.; Salisbury, V.; Lewis, R.; Macgowan, A. Pharmacodynamics of Linezolid in a Clinical Isolate of Streptococcus Pneumoniae Genetically Modified to Express Lux Genes. The Journal of antimicrobial chemotherapy 2003, 52 3, 511–513. [Google Scholar] [CrossRef]
- Brauers, J.; Kresken, M.; Menke, A.; Orland, A.; Weiher, H.; Morrissey, I. Bactericidal Activity of Daptomycin, Vancomycin, Teicoplanin and Linezolid against STaphylococcus Aureus, Enterococcus Faecalis and Enterococcus Faecium Using Human Peak Free Serum Drug Concentrations. International Journal of Antimicrobial Agents 2007, 29, 322–325. [Google Scholar] [CrossRef]
- Nahid, P.; Mase, S.R.; Migliori, G.B.; Sotgiu, G.; Bothamley, G.H.; Brozek, J.L.; Cattamanchi, A.; Cegielski, J.P.; Chen, L.; Daley, C.L.; et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am J Respir Crit Care Med 2019, 200, e93–e142. [Google Scholar] [CrossRef]
- Welshman, I.R.; Sisson, T.A.; Jungbluth, G.L.; Stalker, D.J.; Hopkins, N.K. Linezolid Absolute Bioavailability and the Effect of Food on Oral Bioavailability. Biopharmaceutics & Drug Disposition 2001, 22, 91–97. [Google Scholar] [CrossRef]
- Gargis, A.S.; Spicer, L.M.; Kent, A.G.; Zhu, W.; Campbell, D.; McAllister, G.; Ewing, T.O.; Albrecht, V.; Stevens, V.A.; Sheth, M.; et al. Sentinel Surveillance Reveals Emerging Daptomycin-Resistant ST736 Enterococcus Faecium and Multiple Mechanisms of Linezolid Resistance in Enterococci in the United States. Frontiers in Microbiology 2022, 12. [Google Scholar] [CrossRef]
- Seyedolmohadesin, M.; Kouhzad, M.; Götz, F.; Ashkani, M.; Aminzadeh, S.; Bostanghadiri, N. Emergence of Lineage ST150 and Linezolid Resistance in Enterococcus Faecalis: A Molecular Epidemiology Study of UTIs in Tehran, Iran. Front Microbiol 2024, 15, 1464691. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Huh, H.J.; Song, D.J.; Shim, H.J.; Park, K.S.; Kang, C.-I.; Ki, C.-S.; Lee, N.Y. Resistance Mechanisms of Linezolid-Nonsusceptible Enterococci in Korea: Low Rate of 23S rRNA Mutations in Enterococcus Faecium. J Med Microbiol 2017, 66, 1730–1735. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, F.; Bai, B.; Lin, Z.; Xu, G.; Chen, Z.; Sun, X.; Zheng, J.; Deng, Q.; Yu, Z. Linezolid Resistance in Enterococcus Faecalis Associated With Urinary Tract Infections of Patients in a Tertiary Hospitals in China: Resistance Mechanisms, Virulence, and Risk Factors. Frontiers in Public Health 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pan, H.; Lou, Y.; Wu, Z.; Zhang, J.; Huang, Y.; Yu, W.; Qiu, Y. Epidemiological Characteristics and Genetic Structure of Linezolid-Resistant Enterococcus Faecalis. IDR 2018, Volume 11, 2397–2409. [Google Scholar] [CrossRef]
- Hua, R.; Xia, Y.; Wu, W.; Yang, M.; Yan, J. Molecular Epidemiology and Mechanisms of 43 Low-Level Linezolid-Resistant Enterococcus Faecalis Strains in Chongqing, China. Ann Lab Med 2019, 39, 36–42. [Google Scholar] [CrossRef]
- Shen, W.; Hu, Y.; Liu, D.; Wang, Y.; Schwarz, S.; Zhang, R.; Cai, J. Prevalence and Genetic Characterization of Linezolid Resistance Gene Reservoirs in Hospital Sewage from Zhejiang Province, China. Sci Total Environ 2024, 955, 177162. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, S.; Han, L.; Wu, Q. Antimicrobial Resistance, Virulence Gene Profiles, and Molecular Epidemiology of Enterococcal Isolates from Patients with Urinary Tract Infections in Shanghai, China. Microbiol Spectr 2025, 13, e0121724. [Google Scholar] [CrossRef]
- Brenciani, A.; Cinthi, M.; Coccitto, S.N.; Massacci, F.R.; Albini, E.; Cucco, L.; Paniccià, M.; Freitas, A.R.; Schwarz, S.; Giovanetti, E.; et al. Global Spread of the Linezolid-Resistant Enterococcus Faecalis ST476 Clonal Lineage Carrying optrA. J Antimicrob Chemother 2024, 79, 846–850. [Google Scholar] [CrossRef]
- Shan, X.; Li, C.; Zhang, L.; Zou, C.; Yu, R.; Schwarz, S.; Shang, Y.; Li, D.; Brenciani, A.; Du, X.-D. poxtA Amplification and Mutations in 23S rRNA Confer Enhanced Linezolid Resistance in Enterococcus Faecalis. J Antimicrob Chemother 2024, 79, 3199–3203. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, N.; Chen, M.; Liu, Y.; Wang, S.; Mao, J.; Li, J.; Huang, X. Synergistic Combination of Linezolid and Fosfomycin Closing Each Other’s Mutant Selection Window to Prevent Enterococcal Resistance. Front Microbiol 2020, 11, 605962. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Bernal, J.F.; Zankari, E.; Léon, M.; Hendriksen, R.S.; Perez-Gutierrez, E.; Aarestrup, F.M.; Donado-Godoy, P. Detection of Linezolid Resistance Due to the optrA Gene in Enterococcus Faecalis from Poultry Meat from the American Continent (Colombia). Journal of Antimicrobial Chemotherapy 2017, 72, 678–683. [Google Scholar] [CrossRef]
- Long, K.S.; Vester, B. Resistance to Linezolid Caused by Modifications at Its Binding Site on the Ribosome. Antimicrob Agents Chemother 2012, 56, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Munck, C.; Andersen, T.M.B.; Schaub, M.A.; Hobbie, S.N.; Böttger, E.C.; Vester, B. Mutations in 23S rRNA at the Peptidyl Transferase Center and Their Relationship to Linezolid Binding and Cross-Resistance. Antimicrob Agents Chemother 2010, 54, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.; Sawant, A.R.; Prashanth, K.; Sistla, S. Multiple Mechanisms of Linezolid Resistance in Staphylococcus Haemolyticus Detected by Whole-Genome Sequencing. J Med Microbiol 2023, 72. [Google Scholar] [CrossRef]
- Locke, J.B.; Morales, G.; Hilgers, M.; G C, K.; Rahawi, S.; José Picazo, J.; Shaw, K.J.; Stein, J.L. Elevated Linezolid Resistance in Clinical Cfr-Positive Staphylococcus Aureus Isolates Is Associated with Co-Occurring Mutations in Ribosomal Protein L3. Antimicrob Agents Chemother 2010, 54, 5352–5355. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A Novel Gene, optrA, That Confers Transferable Resistance to Oxazolidinones and Phenicols and Its Presence in Enterococcus Faecalis and Enterococcus Faecium of Human and Animal Origin. J Antimicrob Chemother 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Deng, Z.; Shen, Y.; Wei, W.; Xiang, Q.; Liu, Z.; Hanf, K.; Huang, S.; Lv, Z.; Cao, T.; et al. High Prevalence and Plasmidome Diversity of optrA-Positive Enterococci in a Shenzhen Community, China. Front Microbiol 2024, 15, 1505107. [Google Scholar] [CrossRef]
- Cai, J.; Schwarz, S.; Chi, D.; Wang, Z.; Zhang, R.; Wang, Y. Faecal Carriage of optrA-Positive Enterococci in Asymptomatic Healthy Humans in Hangzhou, China. Clin Microbiol Infect 2019, 25, 630.e1–630.e6. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, K.; Liu, Z.; Li, Y.; Xiao, X.; Du, X.-D.; Li, R.; Wang, Z. Genomic Insights into Linezolid-Resistant Enterococci Revealed Its Evolutionary Diversity and poxtA Copy Number Heterogeneity. Int J Antimicrob Agents 2023, 62, 106929. [Google Scholar] [CrossRef]
- Sun, W.; Liu, H.; Liu, J.; Jiang, Q.; Pan, Y.; Yang, Y.; Zhu, X.; Ge, J. Detection of optrA and poxtA Genes in Linezolid-Resistant Enterococcus Isolates from Fur Animals in China. Lett Appl Microbiol 2022, 75, 1590–1595. [Google Scholar] [CrossRef]
- Hou, J.; Xu, Q.; Zhou, L.; Chai, J.; Lin, L.; Ma, C.; Zhu, Y.; Zhang, W. Identification of an Enterococcus Faecium Strain Isolated from Raw Bovine Milk Co-Harbouring the Oxazolidinone Resistance Genes optrA and poxtA in China. Vet Microbiol 2024, 293, 110103. [Google Scholar] [CrossRef] [PubMed]
- LaMarre, J.; Mendes, R.E.; Szal, T.; Schwarz, S.; Jones, R.N.; Mankin, A.S. The Genetic Environment of the Cfr Gene and the Presence of Other Mechanisms Account for the Very High Linezolid Resistance of Staphylococcus Epidermidis Isolate 426-3147L. Antimicrob Agents Chemother 2013, 57, 1173–1179. [Google Scholar] [CrossRef]
- Shore, A.C.; Lazaris, A.; Kinnevey, P.M.; Brennan, O.M.; Brennan, G.I.; O’Connell, B.; Feßler, A.T.; Schwarz, S.; Coleman, D.C. First Report of Cfr-Carrying Plasmids in the Pandemic Sequence Type 22 Methicillin-Resistant Staphylococcus Aureus Staphylococcal Cassette Chromosome Mec Type IV Clone. Antimicrob Agents Chemother 2016, 60, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Zhang, W.; Du, X.-D.; Krüger, H.; Feßler, A.T.; Ma, S.; Zhu, Y.; Wu, C.; Shen, J.; Wang, Y. Mobile Oxazolidinone Resistance Genes in Gram-Positive and Gram-Negative Bacteria. Clin Microbiol Rev 2021, 34, e0018820. [Google Scholar] [CrossRef]
- M100Ed35 | Performance Standards for Antimicrobial Susceptibility Testing, 35th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 28 January 2025).
- Kent, A.G.; Spicer, L.M.; Campbell, D.; Breaker, E.; McAllister, G.A.; Ewing, T.O.; Longo, C.; Balbuena, R.; Burroughs, M.; Burgin, A.; et al. Sentinel Surveillance Reveals Phylogenetic Diversity and Detection of Linear Plasmids Harboring vanA and optrA among Enterococci Collected in the United States. Antimicrob Agents Chemother 2024, 68, e0059124. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Chen, J.; Zhang, G.; Li, J.; Zhang, J.; Wang, T.; Kang, W.; Gao, H.; Zhang, Z.; et al. Multicentre Evaluation of in Vitro Activity of Contezolid against Drug-Resistant Staphylococcus and Enterococcus. J Antimicrob Chemother 2024, 79, 3132–3141. [Google Scholar] [CrossRef]
- Dadashi, M.; Sharifian, P.; Bostanshirin, N.; Hajikhani, B.; Bostanghadiri, N.; Khosravi-Dehaghi, N.; Belkum, A. van; Darban-Sarokhalil, D. The Global Prevalence of Daptomycin, Tigecycline, and Linezolid-Resistant Enterococcus Faecalis and Enterococcus Faecium Strains From Human Clinical Samples: A Systematic Review and Meta-Analysis. Frontiers in Medicine 2021, 8. [Google Scholar] [CrossRef]
- Quiles-Melero, I.; Gómez-Gil, R.; Romero-Gómez, M.P.; Sánchez-Díaz, A.M.; de Pablos, M.; García-Rodriguez, J.; Gutiérrez, A.; Mingorance, J. Mechanisms of Linezolid Resistance among Staphylococci in a Tertiary Hospital. J Clin Microbiol 2013, 51, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Mendes, R.E.; Streit, J.M.; Hogan, P.A.; Flamm, R.K. Five-Year Summary of In Vitro Activity and Resistance Mechanisms of Linezolid against Clinically Important Gram-Positive Cocci in the United States from the LEADER Surveillance Program (2011 to 2015). Antimicrob Agents Chemother 2017, 61, e00609-17. [Google Scholar] [CrossRef]
- Besier, S.; Ludwig, A.; Zander, J.; Brade, V.; Wichelhaus, T.A. Linezolid Resistance in Staphylococcus Aureus: Gene Dosage Effect, Stability, Fitness Costs, and Cross-Resistances. Antimicrob Agents Chemother 2008, 52, 1570–1572. [Google Scholar] [CrossRef]
- Alonso, M.; Marín, M.; Iglesias, C.; Cercenado, E.; Bouza, E.; García de Viedma, D. Rapid Identification of Linezolid Resistance in Enterococcus Spp. Based on High-Resolution Melting Analysis. J Microbiol Methods 2014, 98, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Gawryszewska, I.; Żabicka, D.; Hryniewicz, W.; Sadowy, E. Linezolid-Resistant Enterococci in Polish Hospitals: Species, Clonality and Determinants of Linezolid Resistance. Eur J Clin Microbiol Infect Dis 2017, 36, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, G.; Li, J.; Chen, L.; Liu, H.; Bi, W.; Lu, H.; Zhou, T. A High Incidence and Coexistence of Multiresistance Genes Cfr and optrA among Linezolid-Resistant Enterococci Isolated from a Teaching Hospital in Wenzhou, China. Eur J Clin Microbiol Infect Dis 2018, 37, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lucas, C.; Fernández, J.; Vázquez, X.; de Toro, M.; Ladero, V.; Fuster, C.; Rodicio, R.; Rodicio, M.R. Detection of the optrA Gene Among Polyclonal Linezolid-Susceptible Isolates of Enterococcus Faecalis Recovered from Community Patients. Microbial Drug Resistance 2022, 28, 773–779. [Google Scholar] [CrossRef]
- Egan, S.A.; Shore, A.C.; O’Connell, B.; Brennan, G.I.; Coleman, D.C. Linezolid Resistance in Enterococcus Faecium and Enterococcus Faecalis from Hospitalized Patients in Ireland: High Prevalence of the MDR Genes optrA and poxtA in Isolates with Diverse Genetic Backgrounds. Journal of Antimicrobial Chemotherapy 2020, 75, 1704–1711. [Google Scholar] [CrossRef]
- He, T.; Shen, Y.; Schwarz, S.; Cai, J.; Lv, Y.; Li, J.; Feßler, A.T.; Zhang, R.; Wu, C.; Shen, J.; et al. Genetic Environment of the Transferable Oxazolidinone/Phenicol Resistance Gene optrA in Enterococcus Faecalis Isolates of Human and Animal Origin. J Antimicrob Chemother 2016, 71, 1466–1473. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Schwarz, S.; Cai, J.; Fan, R.; Li, J.; Feßler, A.T.; Zhang, R.; Wu, C.; Shen, J. Co-Location of the Oxazolidinone Resistance Genes optrA and Cfr on a Multiresistance Plasmid from Staphylococcus Sciuri. J Antimicrob Chemother 2016, 71, 1474–1478. [Google Scholar] [CrossRef]
- Yoon, S.; Son, S.H.; Kim, Y.B.; Seo, K.W.; Lee, Y.J. Molecular Characteristics of optrA-Carrying Enterococcus Faecalis from Chicken Meat in South Korea. Poult Sci 2020, 99, 6990–6996. [Google Scholar] [CrossRef]
| Variable | Total (n = 72) |
LSEf (n = 48) |
LNSEf (n = 24) |
p value |
|---|---|---|---|---|
| Age – median (IQR) | 48 (31.25-57) | 48 (31.25-57.25) | 48 (31-57) | 0.9096 |
| Male sex – n (%) | 51 (70.8) | 34 (66.7) | 17 (70.8) | 0.928 |
| Previous antibiotic use in 90 days – n (%) | 34 (47.2) | 23 (47.9) | 11 (45.8) | 1 |
| Previous Antibiotic in 14 days – n (%) | 29 (40.3) | 20 (41.7) | 9 (37.5) | 0.932 |
| Previous hospitalization in 90 days – n (%) | 28 (38.9) | 15 (31.2) | 13 (54.2) | 0.104 |
| Comorbidities – n (%) | 40 (55.6) | 23 (47.9) | 17 (70.8) | 0.111 |
| Charlson comorbidity index – median (IQR) | 1 (0–3) | 1.5 (0–3) | 1 (0–3) | 0.905 |
| Diabetes Mellitus – n (%) | 15 (20.8) | 9 (19.6) | 6 (25) | 0.826 |
| Hypertension – n (%) | 15 (20.8) | 10 (20.8) | 5 (20.8) | 1 |
| Cardiovascular disease – n (%) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Obesity – n (%) | 2 (2.8) | 1 (2.1) | 1 (4.2) | 1 |
| Immunosuppression – n (%) | 3 (4.2) | 0 (0) | 3 (12.5) | 0.033 |
| Chronic kidney disease – n (%) | 10 (13.9) | 8 (16.7) | 2 (8.3) | 0.478 |
| Surgical Intervention – n (%) | 27 (37.5) | 11 (22.9) | 16 (66.7) | <0.001 |
| Central line insertion – n (%) | 25 (34.8) | 15 (31.2) | 10 (41.7) | 0.540 |
| Urinary catheter – n (%) | 40 (55.6) | 23 (47.9) | 17 (70.8) | 0.111 |
| Need for vasopressors – n (%) | 14 (19.4) | 7 (14.6) | 7 (29.2) | 0.246 |
| ICU admission – n (%) | 11 (15.3) | 8 (16.7) | 3 (13) | 1 |
| Hospital stay – median (IQR) | 12 (4–17.25) | 9.5 (3.75–15) | 14 (11.25–20.25) | 0.02 |
| In-hospital mortality – n (%) | 12 (16.7) | 8 (16.7) | 4 (16.7) | 1 |
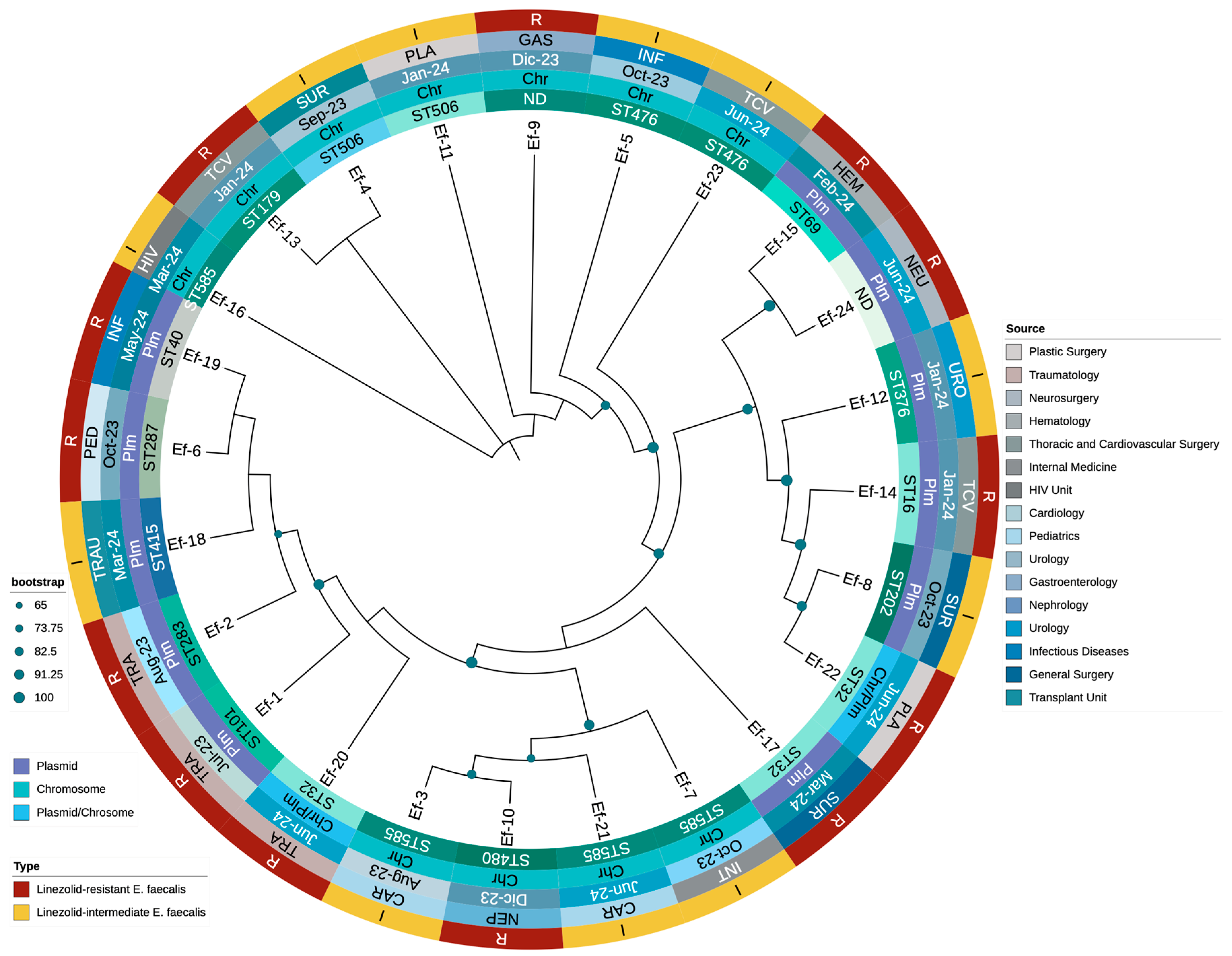
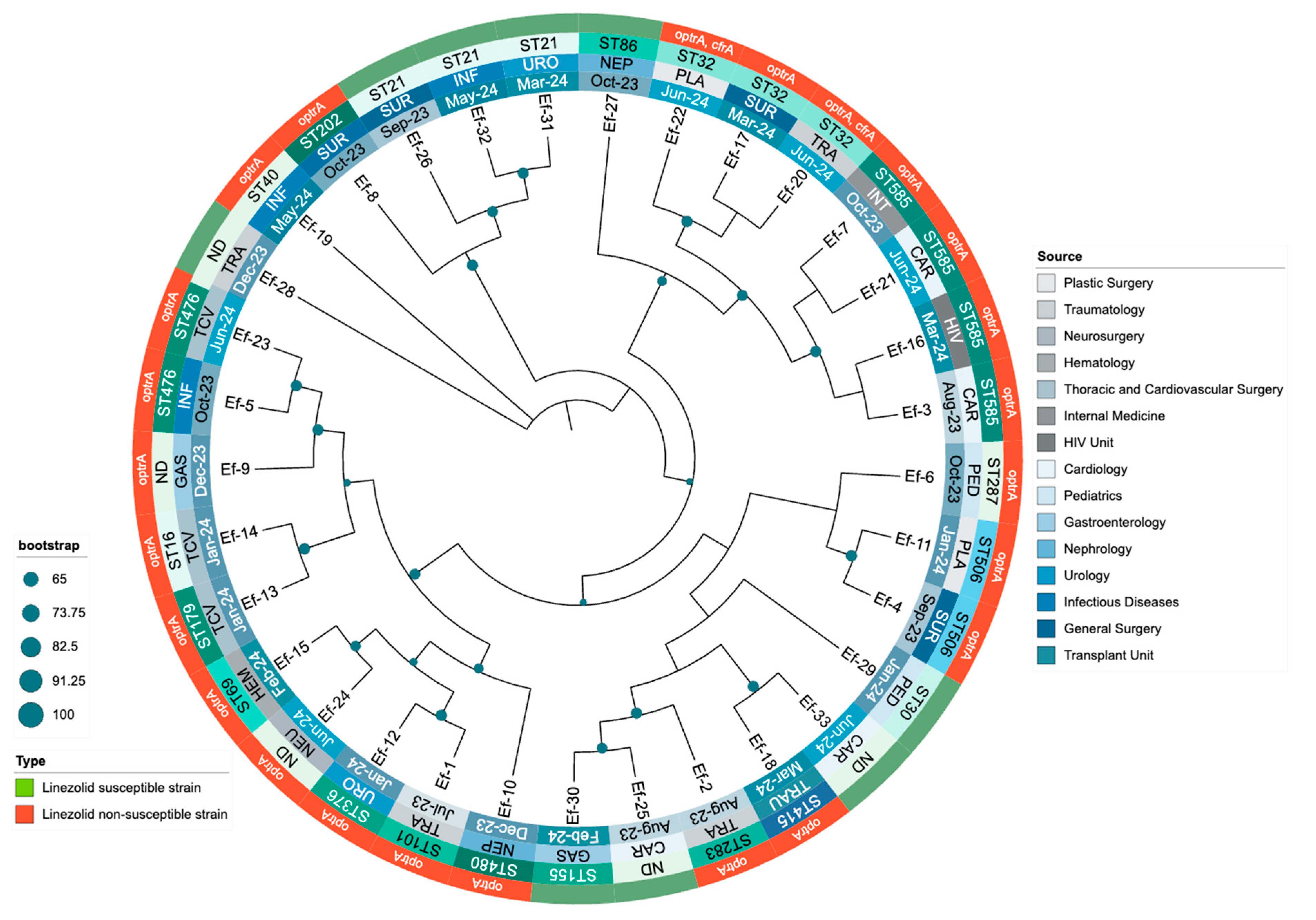
| Label | Date | Source | AMP | CIP | DAP | NIT | HLG | HLS | LEV | LNZ | PEN | TET | VA | MLST | Genes associated with linezolid resistance | Locations of linezolid resistance Genes | Other AMR genes | Other identified plasmids |
| Ef-1 | Jul-23 | Traumatology | <=2 | 1 | 4 | <=16 | SYN-S | SYN-S | 1 | >=8 | 2 | >=16 | 1 | ST101 | optrA (T10G, T35A, C54T, A91G, A107G, T626G, C949T, A1966G) | Plasmid | erm(B), fexA, lsa(A), NarA, NarB, tet(L) | repUS43 |
| Ef-2 | Aug-23 | Traumatology | <=2 | 1 | 1 | <=16 | SYN-S | SYN-R | 1 | >=8 | 2 | >=16 | <=0.5 | ST283 | optrA (T626G, A1541C) | Plasmid | ant(6)-Ia, aph(2'')-Ic, aph(3')-III, dfrG, erm(A), erm(B), fexA, lsa(A), tet(L), tet(M) | rep2, rep6, rep9b |
| Ef-3 | Aug-23 | Cardiology | <=2 | >=8 | 2 | <=16 | SYN-R | SYN-R | >=8 | 4 | 2 | >=16 | 1 | ST585 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(6)-Ia, ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E),, parC (S80I), str, tet(L) | rep7a, repUS43 |
| Ef-4 | Sep-23 | General Surgery | <=2 | 1 | 4 | <=16 | SYN-S | SYN-S | 1 | 4 | 1 | >=16 | 1 | ST506 | optrA (G1879A, C1933T) | Chromosome | ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lsa(A), tet(L) | - |
| Ef-5 | Oct-23 | Infectious Diseases | <=2 | >=8 | 2 | <=16 | SYN-R | SYN-S | >=8 | 4 | 2 | <=1 | 1 | ST476 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, gyrA (S83I), lsa(A), parC (S80I | - |
| Ef-6 | Oct-23 | Pediatrics | <=2 | 4 | 2 | <=16 | SYN-R | SYN-R | 4 | >=8 | 2 | >=16 | 1 | ST287 | optrA (G1879A, C1933T) | Plasmid | aac(6')-aph(2''), ant(6)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lsa(A), NarA, NarB, tet(L) | rep1, rep9b |
| Ef-7 | Oct-23 | Internal Medicine | <=2 | >=8 | 1 | <=16 | SYN-R | SYN-S | >=8 | 4 | 2 | >=16 | 1 | ST585 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, dfrG, erm(A), erm(B), fexA, lsa(A), parC (S80I), tet(L) | repUS43 |
| Ef-8 | Oct-23 | General Surgery | <=2 | 4 | 2 | <=16 | SYN-S | SYN-S | 4 | 4 | 2 | >=16 | 1 | ST202 | optrA (T10G, T35A, C54T, A91G, A107G, T626G, C949T, A1966G) | Plasmid | ant(9)-Ia, dfrG, erm(B), erm(B), fexA, fosB, lsa(A), NarA, NarB, tet(L) | - |
| Ef-9 | Dec-23 | Gastroenterology | <=2 | >=8 | 2 | <=16 | SYN-R | SYN-S | >=8 | >=8 | 1 | >=16 | 1 | ND | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, dfrG, erm(A), erm(B), fexA, fosB, gyrA (S83I), lsa(A), NarA, NarB, parC (S80I), tet(L) | - |
| Ef-10 | Dec-23 | Nephrology | <=2 | >=8 | 2 | <=16 | SYN-S | SYN-S | >=8 | >=8 | 2 | >=16 | 1 | ST480 | optrA ((G1879A, C1933T) | Chromosome | ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lsa(A), NarA, NarB, tet(L) | repUS43 |
| Ef-11 | Jan-24 | Plastic Surgery | <=2 | 1 | 2 | <=16 | SYN-R | SYN-R | 1 | 4 | 1 | >=16 | <=0.5 | ST506 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, dfrG, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E),tet(L) | repUS43 |
| Ef-12 | Jan-24 | Urology | <=2 | 4 | 2 | <=16 | SYN-R | SYN-R | 2 | 4 | 8 | >=16 | 1 | ST376 | optrA (T411G, T626G, G866A) | Plasmid | aac(6')-aph(2''), ant(6)-Ia, ant(6)-Ia, aph(3')-III, cat, dfrG, erm(A), fexA, lnu(B), lsa(A), lsa(E), tet(L) | repUS43 |
| Ef-13 | Jan-24 | Thoracic and Cardiovascular Surgery | <=2 | <=0.5 | 4 | <=16 | SYN-R | SYN-R | 1 | >=8 | 2 | >=16 | 1 | ST179 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E), tet(M) | repUS43 |
| Ef-14 | Jan-24 | Thoracic and Cardiovascular Surgery | <=2 | <=0.5 | 2 | <=16 | SYN-R | SYN-R | 1 | >=8 | 2 | >=16 | 1 | ST16 | optrA (T626G, A1541C) | Plasmid | aac(6')-aph(2''), aph(3')-III, dfrG, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E), tet(M)c | rep9b, repUS43 |
| Ef-15 | Feb-24 | Hematology | <=2 | >=8 | 2 | <=16 | SYN-S | SYN-S | >=8 | >=8 | 2 | >=16 | <=0.5 | ST69 | optrA (T411G, T626G, G866A) | Plasmid | cat, dfrG, erm(B), fexA, lsa(A), parC | rep22, repUS43 |
| Ef-16 | Mar-24 | HIV Unit | <=2 | >=8 | 1 | <=16 | SYN-R | SYN-R | >=8 | 4 | 2 | >=16 | <=0.5 | ST585 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, dfrG, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E), parC (S80I), str, tet(L) | rep7a, repUS43 |
| Ef-17 | Mar-24 | General Surgery | <=2 | >=8 | 4 | <=16 | SYN-S | SYN-S | >=8 | >=8 | 2 | >=16 | <=0.5 | ST32 | optrA (T411G, T626G, G866A) | Plasmid | ant(6)-Ia, aph(3')-III, erm(A), erm(B), fexA, gyrA (S83I), lsa(A), parC (S80I), tet(L) | repUS43 |
| Ef-18 | Mar-24 | Transplant Unit | <=2 | 4 | 2 | <=16 | SYN-S | SYN-S | 4 | 4 | 2 | >=16 | 2 | ST415 | optrA (T411G, T626G, G866A) | Plasmid | cat, dfrG, erm(A), erm(B), fexA, fosB3, lsa(A), NarA, NarB, tet(L) | rep1, rep9c |
| Ef-19 | May-24 | Infectious Diseases | <=2 | <=0.5 | 2 | <=16 | SYN-R | SYN-R | 0.5 | >=8 | 2 | >=16 | 1 | ST40 | optrA (T411G, T626G, G866A) | Plasmid | aac(6')-aph(2''), ant(6)-Ia, ant(6)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lnu(B), lsa(A), lsa(E), tet(L) | rep18b, rep9b, repUS43 |
| Ef-20 | Jun-24 | Traumatology | <=2 | 4 | 2 | <=16 | SYN-R | SYN-R | >=8 | >=8 | 2 | >=16 | 1 | ST32 | optrA (T10G, T35A, C54T, A91G, A107G, A134T, T626G, C949T, G1278A, A1331G, A1541C, C1933T ), cfrA | optrA Chromosome, cfrA plasmid | aadD, ant(9)-Ia, aph(2'')-Ic, aph(3')-III, bleO, cat, dfrG, erm(A), erm(B), fexA, fosB3, lnu(A), lsa(A), tet(L) | repUS43 |
| Ef-21 | Jun-24 | Cardiology | <=2 | >=8 | 1 | <=16 | SYN-R | SYN-S | >=8 | 4 | 2 | >=16 | 1 | ST585 | optrA (G1879A, C1933T) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, cat, dfrG, erm(A), fexA, lnu(B), lsa(A), lsa(E), parC (S80I), str, tet(L) | rep7a, repUS43 |
| Ef-22 | Jun-24 | Plastic Surgery | <=2 | 4 | 2 | <=16 | SYN-R | SYN-R | >=8 | >=8 | 2 | >=16 | 1 | ST32 | optrA (T10G, T35A, C54T, A91G, A107G, A134T, T626G, C949T, G1278A, A1331G, A1541C, C1933T), cfrA | optrA Chromosome, cfrA plasmid | aadD, ant(9)-Ia, aph(2'')-Ic, aph(3')-III, bleO, cat, dfrG, erm(A), erm(B), fexA, fosB3, lnu(A), lsa(A), tet(L) | repUS43 |
| Ef-23 | Jun-24 | Thoracic and Cardiovascular Surgery | <=2 | >=8 | 2 | <=16 | SYN-R | SYN-S | >=8 | 4 | 2 | <=1 | 1 | ST476 | optrA (T411G, T626G, G866A) | Chromosome | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, gyrA (S83I), lnu(B), lsa(A), lsa(E), parC (S80I) | rep9b, rep9c |
| Ef-24 | Jun-24 | Neurosurgery | <=2 | <=0.5 | 2 | <=16 | SYN-R | SYN-R | 0.5 | >=8 | 2 | >=16 | 1 | ND | optrA (T411G, T626G, G866A) | Plasmid | aac(6')-aph(2''), ant(6)-Ia, ant(9)-Ia, aph(3')-III, dfrG, erm(A), erm(B), fexA, lsa(A), parC (S80I), tet(L) | repUS43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





