Submitted:
27 February 2025
Posted:
28 February 2025
You are already at the latest version
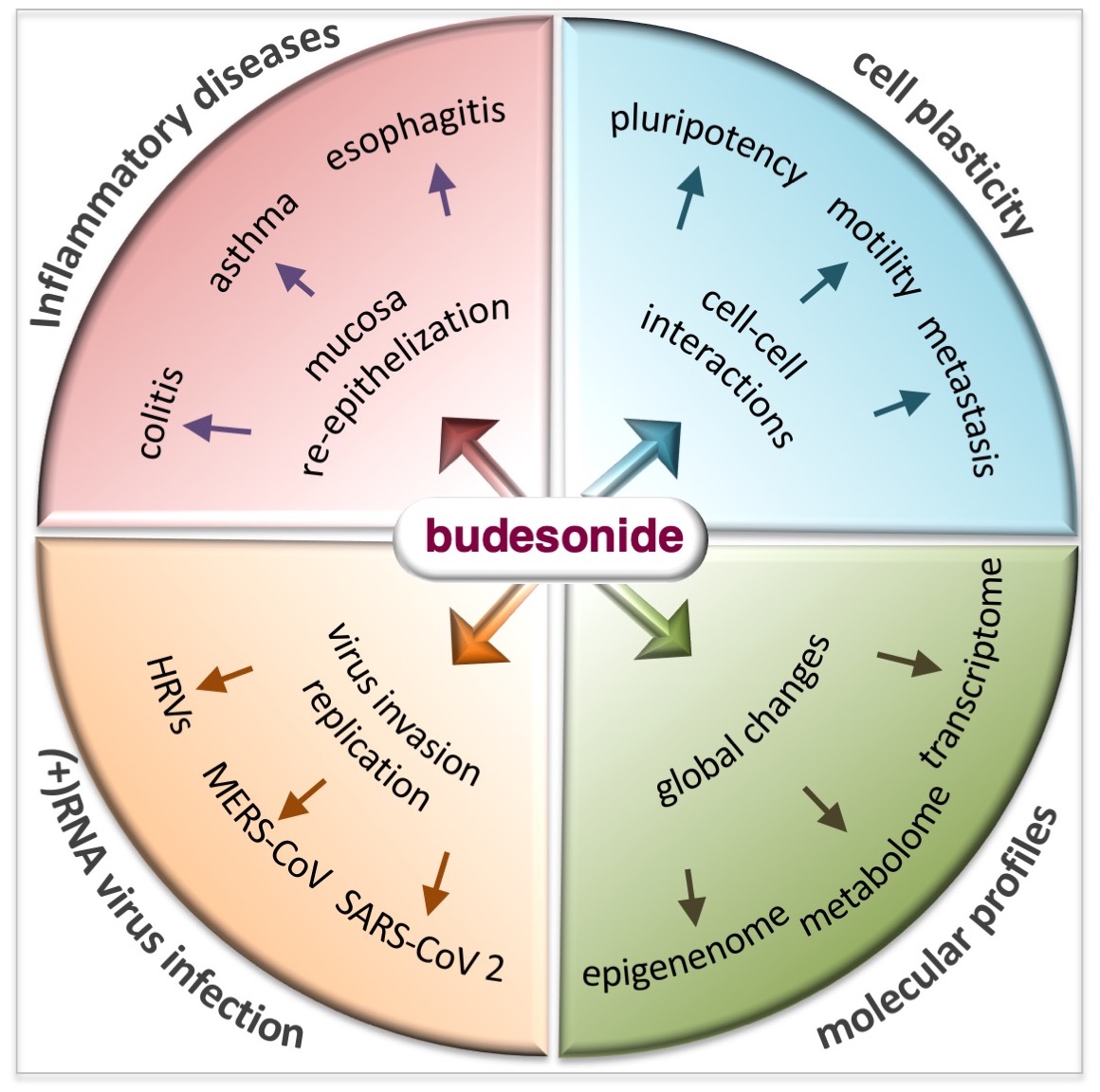
Abstract
Keywords:
1. Introduction
2. Physicochemical Properties of Budesonide
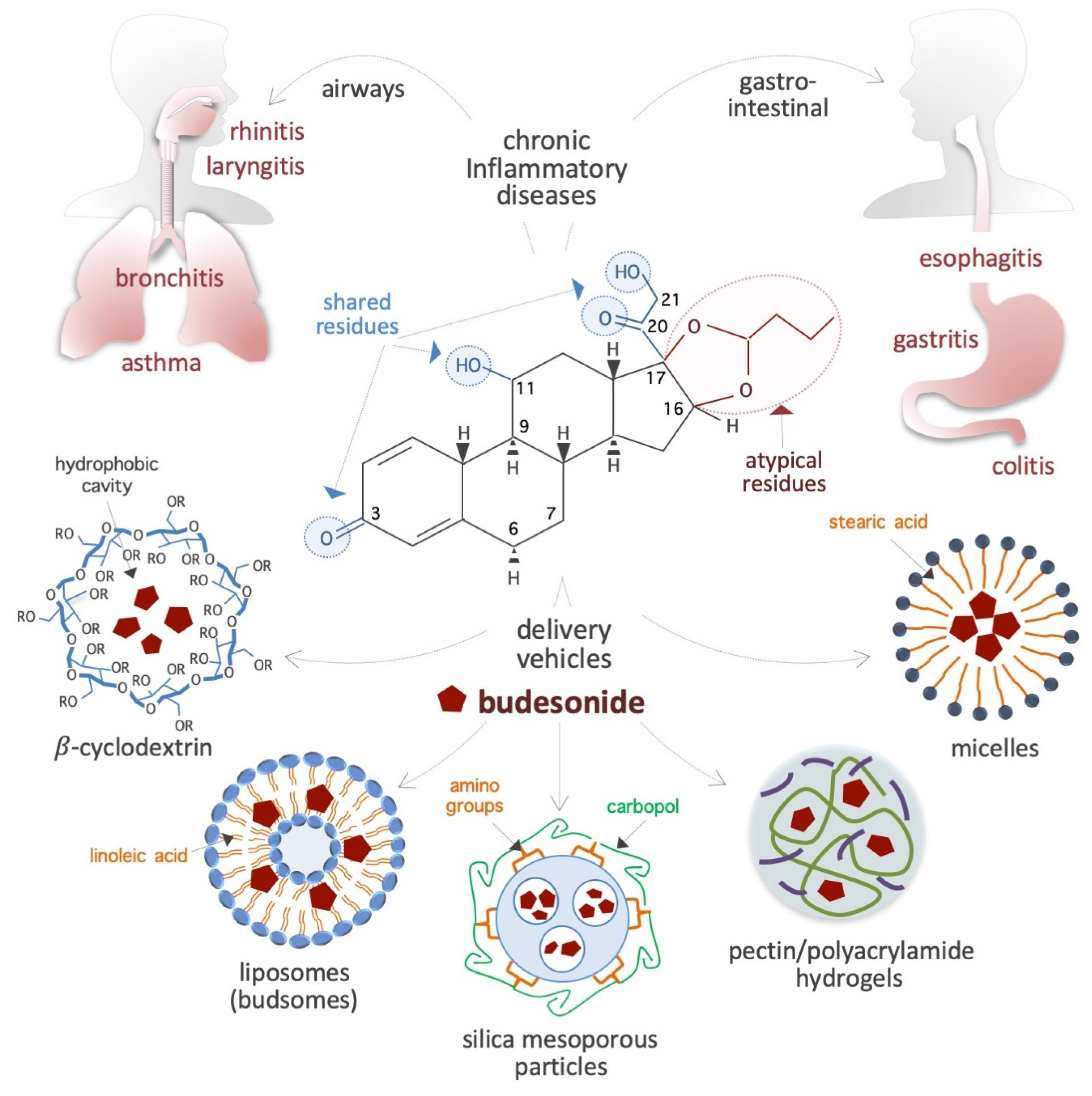
2.1. Chemical Structure and Properties
2.2. Bioavailability and Carriers for Delivery
2.3. Pharmacokinetic Parameters
2.4. Molecular Targets and Mechanisms of Action
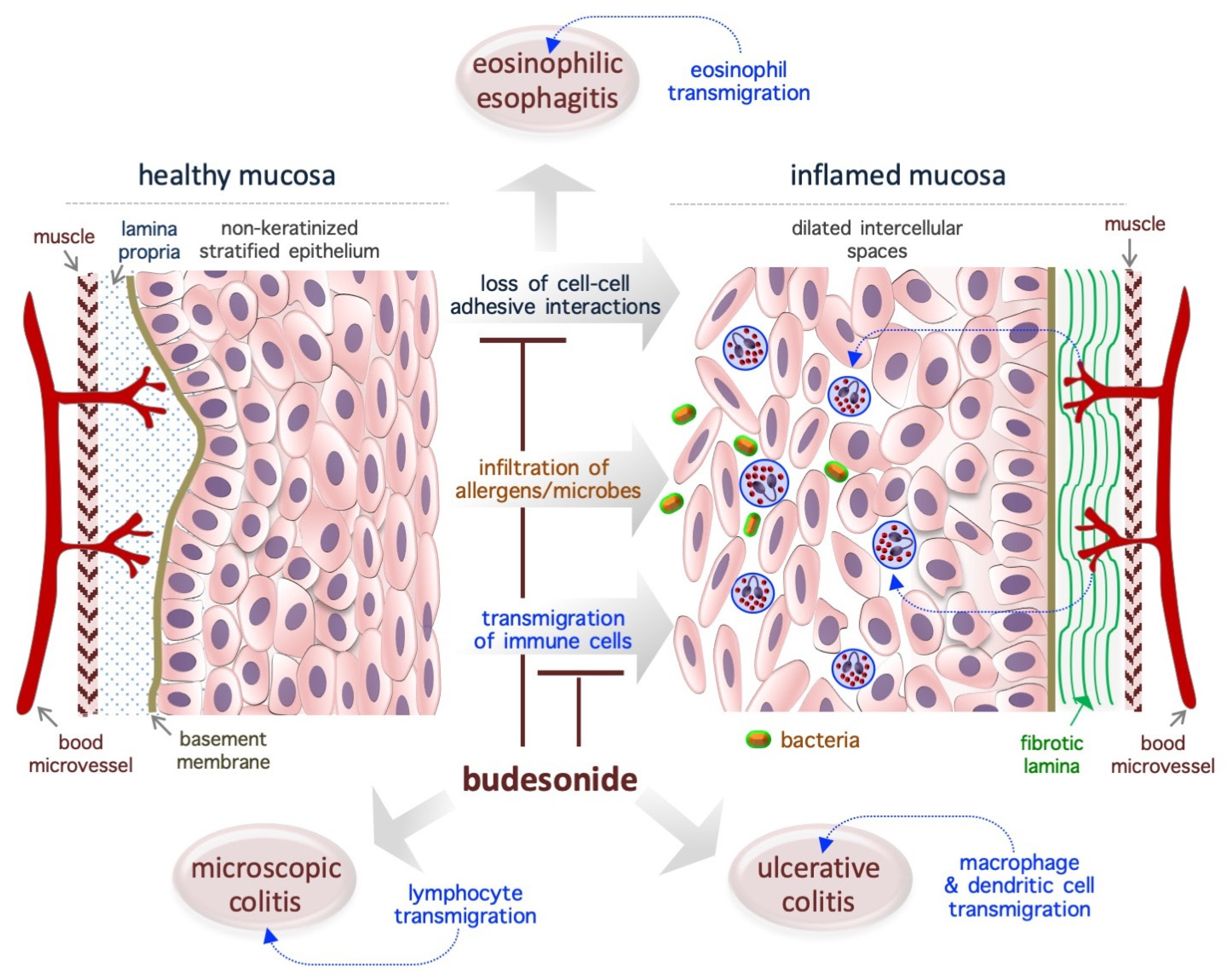
3. Budesonide-Mediated Mucosal Re-Epithelialization
3.1. Eosinophilic Esophagitis
3.2. Ulcerative Colitis
3.3. Microscopic Colitis
3.4. Asthma
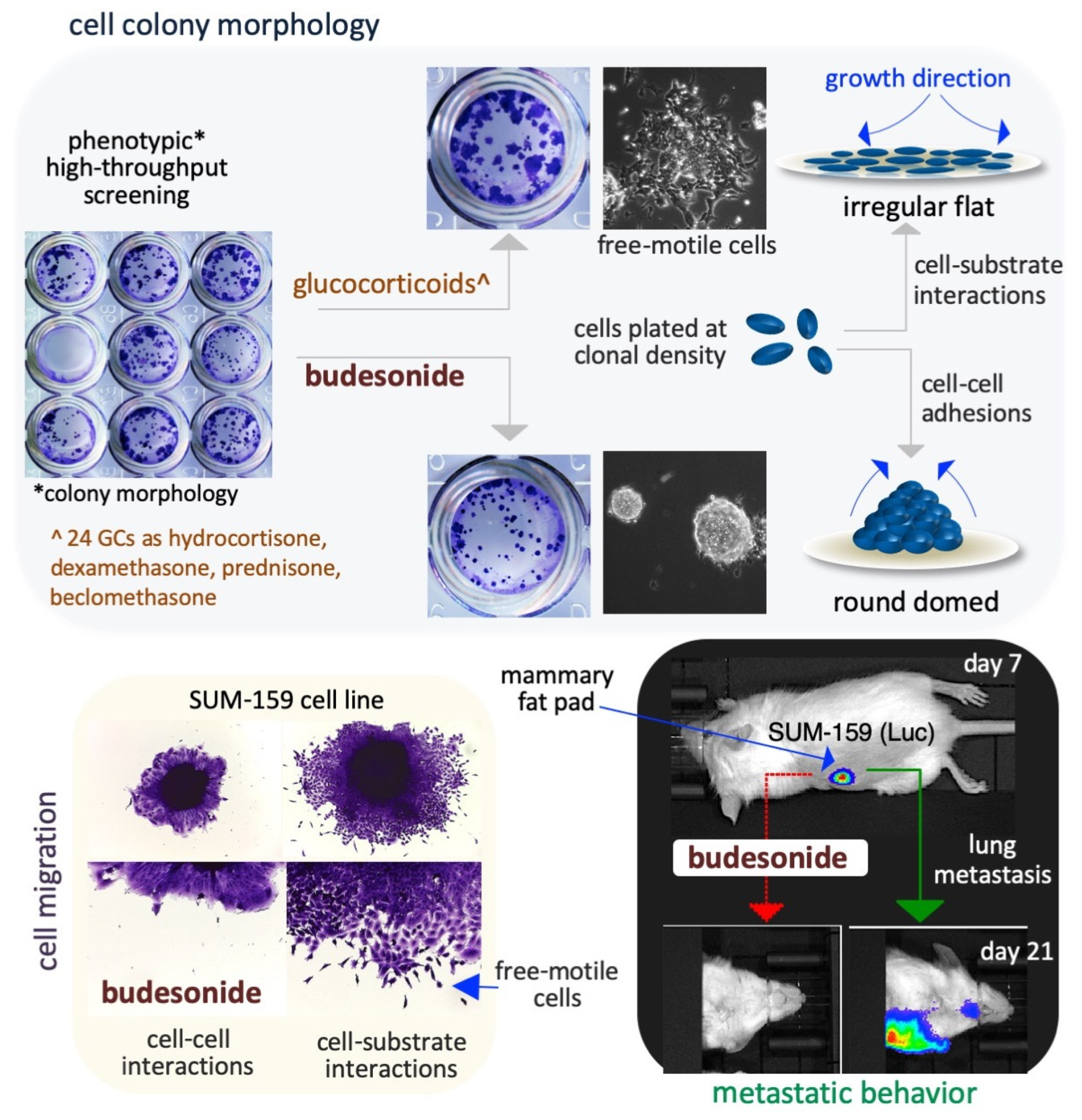
4. Budesonide-Mediated Inhibition of Plasticity in Stem and Cancer Cells
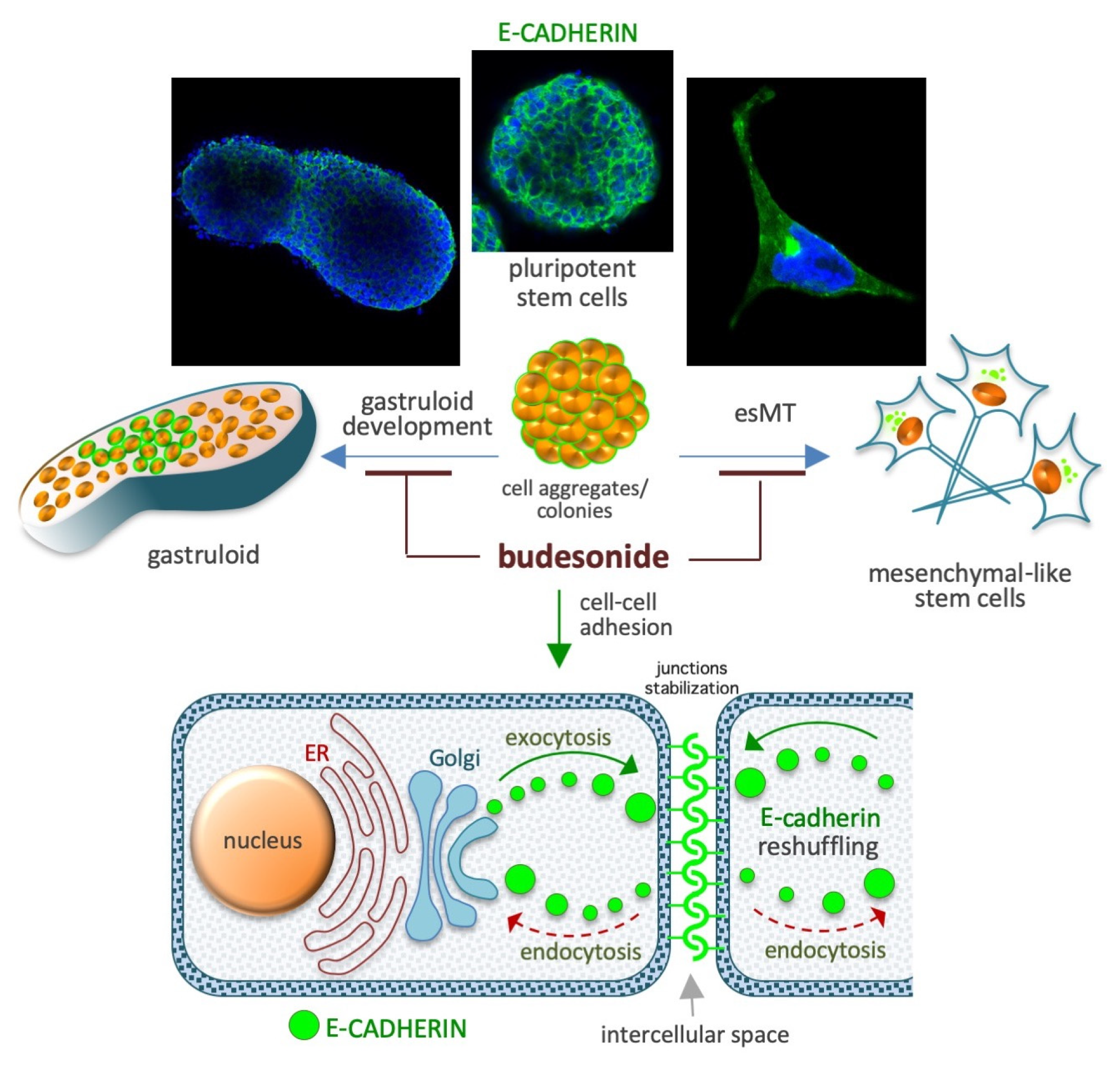
4.1. Embryonic stem-to-Mesenchymal Transition
4.2. Lung Cancer Cells
4.3. Breast Cancer Cells
4.4. Pancreatic Cancer Cells
5. Budesonide-Mediated Stabilization of Cell–Cell Interactions
5.1. Preservation of the Naïve Ground State in Stem Cells
5.2. Naïve-to-Primed Transition in Pluripotent Stem Cells
5.3. Gastruloid Development
5.4. Gastroenteric Mucosa
5.5. Airway Mucosa
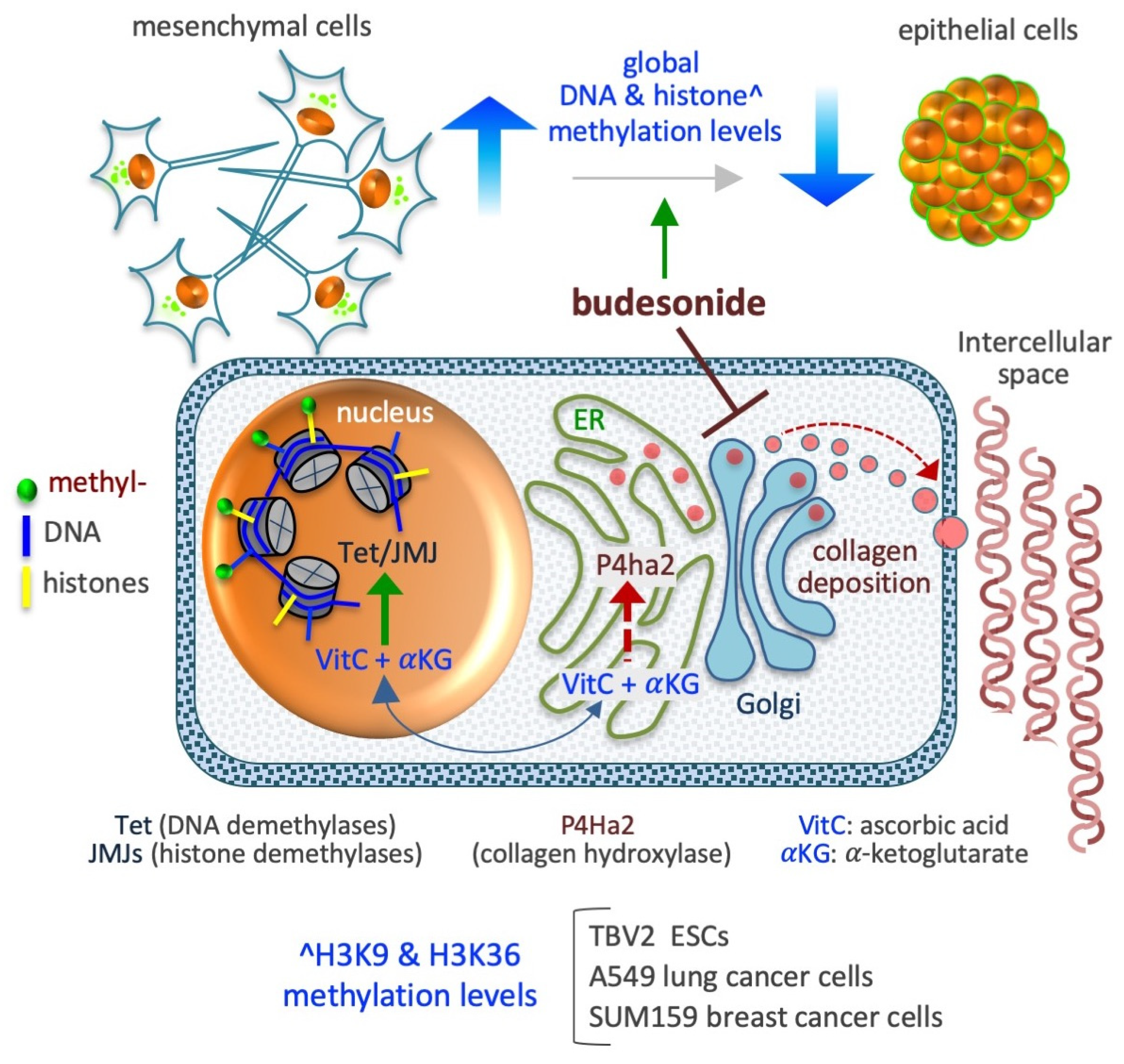
6. Budesonide-Mediated Inhibition of Collagen Deposition and Modulation of the Epigenetic Landscape
6.1. DNA Methylation
6.2. Histone Methylation
6.3. Histone Deacetylation
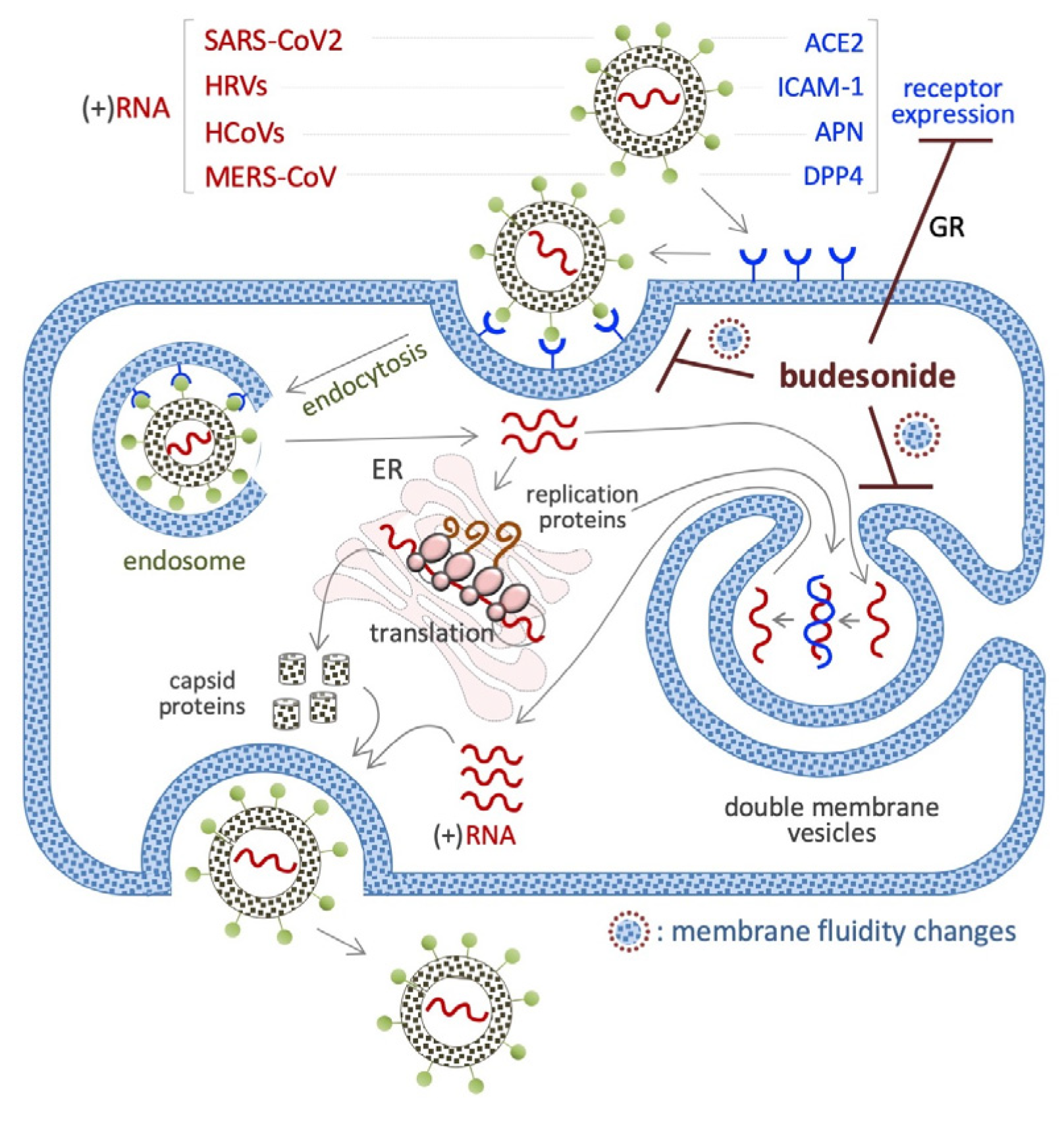
7. Budesonide-Mediated Inhibition of Replication of SARS-CoV-2 and Single-Stranded (+)RNA Virus
7.1. SARS-CoV2 (+)RNA Virus
7.2. HRVs (+)RNA Virus
7.3. HCoV (+)RNA Virus
7.4. MERS-CoV (+)RNA Virus
7.5. Viral Mimetic dsRNA
8. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| GCs | Glucocorticoids |
| BUD | budesonide |
| SARS-CoV-2 | severe acute respiratory syndrome corona virus 2 |
| IDs | chronic inflammatory diseases |
| P450/CYP3A | cytochrome P4503A |
| GR | glucocorticoid receptor |
| N3CR1 | nuclear receptor subfamily 3 group C member 1 |
| TGFβ1 | transforming growth factor-β1 |
| TNF | tumor necrosis factor |
| ASM | airway smooth muscle |
| GPCR | G protein-coupled receptor Gα |
| cAMP | cyclic adenosine monophosphate |
| EoE | eosinophilic esophagitis |
| TGM1_3 | transglutaminase enzymes 1 and 3 |
| UC | ulcerative colitis |
| MPO | myeloperoxidase |
| COX-2 | cyclooxygenase-2 |
| MC | microscopic colitis |
| EMT | epithelial-to-mesenchymal transition |
| ESCs | embryonic stem cells |
| esMT | embryonic-stem-to-mesenchymal transition |
| HTS | high-throughput screening |
| P4HA | prolyl-collagen hydroxylation |
| VitC | vitamin C |
| PDAC | pancreatic adenocarcinoma |
| CDKN1C | cyclin dependent kinase inhibitor 1C |
| CDH1 | Cadherin 1 |
| LIF | leukemia inhibitory factor |
| WNT | Wingless and Int-1 |
| FGF | fibroblast growth factor |
| EGF | extracellular signal-regulated kinase |
| ATF4 | activating transcription factor 4 |
| ECM1 | extracellular matrix protein 1 |
| LMNB1 | extracellular matrix glycoprotein laminin B1 |
| 5hMC | 5-hydroxymethylcytosine |
| IGF-2 | insulin-like growth factor 2 |
| c-MYC | cellular myelocytomatosis |
| Nrf2 | nuclear factor erythroid 2-related factor |
| HDAC | histone deacetylase |
| αKG | alpha-ketoglutarate |
| TET | ten-eleven translocation |
| JMJ | Jumonji |
| ER | endoplasmic reticulum |
| MERS | middle east respiratory syndrome |
| ACE2 | angiotensin-converting enzyme-2 |
| IFN-β | type I interferon-beta |
| HRVs | human rhinoviruses |
| ICAM-1 | intercellular adhesion molecule-1 |
| IL-1β | inflammatory cytokine pro-interleukin-1β |
| NF-kB | nuclear factor kappa-B |
| IL-1β | Interleukin-1 β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| HCoV | Human Coronavirus |
References
- Chrousos, G. P., The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med, 1995. 332(20): p. 1351-62. [CrossRef]
- Benedek, T. G., History of the development of corticosteroid therapy. Clin Exp Rheumatol, 2011. 29(5 Suppl 68): p. S-5-12.
- Ellul-Micallef, R.; Hansson, E.Johansson, S. A., Budesonide: a new corticosteroid in bronchial asthma. Eur J Respir Dis, 1980. 61(3): p. 167-73.
- Ellul-Micallef, R.Johansson, S. A., Acute dose-response studies in bronchial asthma with a new corticosteroid, budesonide. Br J Clin Pharmacol, 1983. 15(4): p. 419-22. [CrossRef]
- Johansson, S. A.; Andersson, K. E.; Brattsand, R.; Gruvstad, E.Hedner, P., Topical and systemic glucocorticoid potencies of budesonide and beclomethasone dipropionate in man. Eur J Clin Pharmacol, 1982. 22(6): p. 523-9. [CrossRef]
- Nilsson, K.; Andersson, M.Beck, O., Phospholipid removal combined with a semi-automated 96-well SPE application for determination of budesonide in human plasma with LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci, 2014. 970: p. 31-5. [CrossRef]
- Streel, B.; Cahay, B.Klinkenberg, R., Using total error concept for the validation of a liquid chromatography-tandem mass spectrometry method for the determination of budesonide epimers in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci, 2009. 877(23): p. 2290-300. [CrossRef]
- Berg, S.; Melamies, M.; Rajamaki, M.; Vainio, O.Peltonen, K., Liquid chromatography tandem mass spectrometry determination of total budesonide levels in dog plasma after inhalation exposure. Anal Bioanal Chem, 2012. 402(3): p. 1209-15. [CrossRef]
- Borges, N. C.; Astigarraga, R. B.; Sverdloff, C. E.; Borges, B. C.; Paiva, T. R.; Galvinas, P. R.Moreno, R. A., Budesonide quantification by HPLC coupled to atmospheric pressure photoionization (APPI) tandem mass spectrometry. Application to a comparative systemic bioavailability of two budesonide formulations in healthy volunteers. J Chromatogr B Analyt Technol Biomed Life Sci, 2011. 879(3-4): p. 236-42. [CrossRef]
- Li, X.; Tong, H.; Xu, B.; Deng, Y.; Li, Y.; Huang, J.; Mao, Y.; Liu, M.; Zhang, P.Guo, S., A sensitive and high-throughput LC-ESI-MS/MS method to detect budesonide in human plasma: application to an evaluation of pharmacokinetics of budesonide intranasal formulations with and without charcoal-block in healthy volunteers. Drug Dev Ind Pharm, 2021. 47(2): p. 329-336. [CrossRef]
- Ponzetto, F.; Parasiliti-Caprino, M.; Settanni, F.; Nonnato, A.; Mengozzi, G.; Ghigo, E.Giordano, R., Simultaneous Measurement of Cortisol, Cortisone, Dexamethasone and Additional Exogenous Corticosteroids by Rapid and Sensitive LC-MS/MS Analysis. Molecules, 2022. 28(1). [CrossRef]
- Rower, J. E.; Anderson, D. J.; Sherwin, C. M.; Reilly, C. A.; Ballard, P. L.; Mcevoy, C. T.Wilkins, D. G., Development and validation of an assay for quantifying budesonide in dried blood spots collected from extremely low gestational age neonates. J Pharm Biomed Anal, 2019. 167: p. 7-14. [CrossRef]
- Jonsson, G.; Astrom, A.Andersson, P., Budesonide is metabolized by cytochrome P450 3A (CYP3A) enzymes in human liver. Drug Metab Dispos, 1995. 23(1): p. 137-42.
- Silverman, J.Otley, A., Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol, 2011. 7(4): p. 419-28. [CrossRef]
- Dilger, K.; Schwab, M.Fromm, M. F., Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm Bowel Dis, 2004. 10(5): p. 578-83. [CrossRef]
- Szefler, S. J., Pharmacodynamics and pharmacokinetics of budesonide: a new nebulized corticosteroid. J Allergy Clin Immunol, 1999. 104(4 Pt 2): p. 175-83. [CrossRef]
- Claytor, J.; Kumar, P.; Ananthakrishnan, A. N.; Colombel, J. F.; Agrawal, M.Ungaro, R. C., Mild Crohn’s Disease: Definition and Management. Curr Gastroenterol Rep, 2023. 25(3): p. 45-51. [CrossRef]
- Lichtenstein, G. R.; Loftus, E. V.; Isaacs, K. L.; Regueiro, M. D.; Gerson, L. B.Sands, B. E., ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol, 2018. 113(4): p. 481-517. [CrossRef]
- Leigh, R.; Mostafa, M. M.; King, E. M.; Rider, C. F.; Shah, S.; Dumonceaux, C.; Traves, S. L.; Mcwhae, A.; Kolisnik, T.; Kooi, C.; Slater, D. M.; Kelly, M. M.; Bieda, M.; Miller-Larsson, A.Newton, R., An inhaled dose of budesonide induces genes involved in transcription and signaling in the human airways: enhancement of anti- and proinflammatory effector genes. Pharmacol Res Perspect, 2016. 4(4): p. e00243. [CrossRef]
- Toropainen, T.; Velaga, S.; Heikkila, T.; Matilainen, L.; Jarho, P.; Carlfors, J.; Lehto, V. P.; Jarvinen, T.Jarvinen, K., Preparation of budesonide/gamma-cyclodextrin complexes in supercritical fluids with a novel SEDS method. J Pharm Sci, 2006. 95(10): p. 2235-45. [CrossRef]
- Dos Santos, A. G.; Bayiha, J. C.; Dufour, G.; Cataldo, D.; Evrard, B.; Silva, L. C.; Deleu, M.Mingeot-Leclercq, M. P., Changes in membrane biophysical properties induced by the Budesonide/Hydroxypropyl-beta-cyclodextrin complex. Biochim Biophys Acta Biomembr, 2017. 1859(10): p. 1930-1940. [CrossRef]
- Bayiha, J. C.; Evrard, B.; Cataldo, D.; De Tullio, P.Mingeot-Leclercq, M. P., The Budesonide-Hydroxypropyl-beta-Cyclodextrin Complex Attenuates ROS Generation, IL-8 Release and Cell Death Induced by Oxidant and Inflammatory Stress. Study on A549 and A-THP-1 Cells. Molecules, 2020. 25(21). [CrossRef]
- Dufour, G.; Bigazzi, W.; Wong, N.; Boschini, F.; De Tullio, P.; Piel, G.; Cataldo, D.Evrard, B., Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int J Pharm, 2015. 495(2): p. 869-78. [CrossRef]
- Xian, S.; Zhu, J.; Wang, Y.; Song, H.Wang, H., Oral liposomal delivery of an activatable budesonide prodrug reduces colitis in experimental mice. Drug Deliv, 2023. 30(1): p. 2183821. [CrossRef]
- Mishra, R. K.; Ahmad, A.; Kanika; Kumar, A.; Vyawahare, A.; Sakla, R.; Nadeem, A.; Siddiqui, N.; Raza, S. S.Khan, R., Caffeic Acid-Conjugated Budesonide-Loaded Nanomicelle Attenuates Inflammation in Experimental Colitis. Mol Pharm, 2023. 20(1): p. 172-182. [CrossRef]
- Pandey, M.; Choudhury, H.; Sk, D. O. Segar Singh; Chetty Annan, N.; Bhattamisra, S. K.; Gorain, B.Mohd Amin, M. C. I., Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules, 2021. 26(9). [CrossRef]
- Vafaei, S. Y.; Abdolghaffari, A. H.; Mahjub, R.; Eslami, S. M.; Esmaeili, M.; Abdollahi, M.; Atyabi, F.Dinarvand, R., Budesonide-Loaded Hyaluronic Acid Nanoparticles for Targeted Delivery to the Inflamed Intestinal Mucosa in a Rodent Model of Colitis. Biomed Res Int, 2022. 2022: p. 7776092. [CrossRef]
- Ali, H.; Weigmann, B.; Neurath, M. F.; Collnot, E. M.; Windbergs, M.Lehr, C. M., Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release, 2014. 183: p. 167-77. [CrossRef]
- Kompella, U. B.; Bandi, N.Ayalasomayajula, S. P., Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci, 2003. 44(3): p. 1192-201. [CrossRef]
- Yathavan, B.; Ellis, A.; Jedrzkiewicz, J.; Subrahmanyam, N.; Khurana, N.; Pulsipher, A.; Alt, J. A.Ghandehari, H., Systemic administration of budesonide in pegylated liposomes for improved efficacy in chronic rhinosinusitis. J Control Release, 2023. 360: p. 274-284. [CrossRef]
- Ferri, D.; Costero, A. M.; Gavina, P.; Parra, M.; Merino, V.; Teruel, A. H.; Sancenon, F.Martinez-Manez, R., Efficacy of budesonide-loaded mesoporous silica microparticles capped with a bulky azo derivative in rats with TNBS-induced colitis. Int J Pharm, 2019. 561: p. 93-101. [CrossRef]
- Sguizzato, M.; Ferrara, F.; Baraldo, N.; Bondi, A.; Guarino, A.; Drechsler, M.; Valacchi, G.Cortesi, R., Bilosomes and Biloparticles for the Delivery of Lipophilic Drugs: A Preliminary Study. Antioxidants (Basel), 2023. 12(12). [CrossRef]
- Peng, G.; Cai, J.; Wang, Z.; Zhang, W.; Xu, J.; Zhang, D.Gong, D., Facile fabrication of diatomite biosilica-based nasal drug delivery vehicle for enhanced treatment of allergic rhinitis. Colloids Surf B Biointerfaces, 2024. 234: p. 113715. [CrossRef]
- Pinheiro Do Nascimento, L.; Tsapis, N.; Reynaud, F.; Desmaele, D.; Moine, L.; Vergnaud, J.; Abreu, S.; Chaminade, P.Fattal, E., Mannosylation of budesonide palmitate nanoprodrugs for improved macrophage targeting. Eur J Pharm Biopharm, 2022. 170: p. 112-120. [CrossRef]
- Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Tibi, I. P.; Stefanova, D.; Tzankova, V.; Petrov, P. D.Yoncheva, K., Formulation of Budesonide-Loaded Polymeric Nanoparticles into Hydrogels for Local Therapy of Atopic Dermatitis. Gels, 2024. 10(1). [CrossRef]
- Padula, C.; Machado, I. P.; Vigato, A. A.De Araujo, D. R., New Strategies for Improving Budesonide Skin Retention. Pharmaceutics, 2021. 14(1). [CrossRef]
- Barratt, J.; Lafayette, R. A.; Rovin, B. H.Fellstrom, B., Budesonide delayed-release capsules to reduce proteinuria in adults with primary immunoglobulin A nephropathy. Expert Rev Clin Immunol, 2023. 19(7): p. 699-710. [CrossRef]
- Del Vecchio, L.; Allinovi, M.; Comolli, S.; Peiti, S.; Rimoldi, C.Locatelli, F., Drugs in Development to Treat IgA Nephropathy. Drugs, 2024. [CrossRef]
- Awad, A.; Hollis, E.; Goyanes, A.; Orlu, M.; Gaisford, S.Basit, A. W., 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis. Int J Pharm X, 2023. 5: p. 100165. [CrossRef]
- Seoane-Viano, I.; Ong, J. J.; Luzardo-Alvarez, A.; Gonzalez-Barcia, M.; Basit, A. W.; Otero-Espinar, F. J.Goyanes, A., 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J Pharm Sci, 2021. 16(1): p. 110-119. [CrossRef]
- Chen, Q.; Hu, C.; Yu, H.; Shen, K.; Assam, P. N.; Gillen, M.; Liu, Y.Dorinsky, P., Pharmacokinetics and Tolerability of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate and Glycopyrronium/Formoterol Fumarate Dihydrate Metered Dose Inhalers in Healthy Chinese Adults: A Randomized, Double-blind, Parallel-group Study. Clin Ther, 2019. 41(5): p. 897-909 e1. [CrossRef]
- Gupta, S. K.; Hill, M.; Vitanza, J. M.; Farber, R. H.; Desai, N. K.; Williams, J.Song, I. H., Pharmacokinetics of Budesonide Oral Suspension in Children and Adolescents With Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr, 2022. 75(2): p. 186-191. [CrossRef]
- Dilger, K.; Alberer, M.; Busch, A.; Enninger, A.; Behrens, R.; Koletzko, S.; Stern, M.; Beckmann, C.Gleiter, C. H., Pharmacokinetics and pharmacodynamic action of budesonide in children with Crohn’s disease. Aliment Pharmacol Ther, 2006. 23(3): p. 387-96. [CrossRef]
- O’donnell, S.O’morain, C. A., Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis, 2010. 1(4): p. 177-86. [CrossRef]
- Moore, C. D.; Roberts, J. K.; Orton, C. R.; Murai, T.; Fidler, T. P.; Reilly, C. A.; Ward, R. M.Yost, G. S., Metabolic pathways of inhaled glucocorticoids by the CYP3A enzymes. Drug Metab Dispos, 2013. 41(2): p. 379-89. [CrossRef]
- Donnelly, R.Seale, J. P., Clinical pharmacokinetics of inhaled budesonide. Clin Pharmacokinet, 2001. 40(6): p. 427-40. [CrossRef]
- Van Den Bosch, J. M.; Westermann, C. J.; Aumann, J.; Edsbacker, S.; Tonnesson, M.Selroos, O., Relationship between lung tissue and blood plasma concentrations of inhaled budesonide. Biopharm Drug Dispos, 1993. 14(5): p. 455-9. [CrossRef]
- Corvino, A.; Granato, E.; Scognamiglio, A.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Cirino, G.; Cerqua, I.; Pavese, R.; Petti, A.; Pavese, F.; Petti, F.; Roviezzo, F.; Severino, B.Caliendo, G., A New Process for the Synthesis of Budesonide 21-Phosphate and Evaluation in a Murine Model of Inflammation. Molecules, 2024. 29(18). [CrossRef]
- Barrette, A. M.; Roberts, J. K.; Chapin, C.; Egan, E. A.; Segal, M. R.; Oses-Prieto, J. A.; Chand, S.; Burlingame, A. L.Ballard, P. L., Antiinflammatory Effects of Budesonide in Human Fetal Lung. Am J Respir Cell Mol Biol, 2016. 55(5): p. 623-632. [CrossRef]
- Nunez, F. J.; Johnstone, T. B.; Corpuz, M. L.; Kazarian, A. G.; Mohajer, N. N.; Tliba, O.; Panettieri, R. A., Jr.; Koziol-White, C.; Roosan, M. R.Ostrom, R. S., Glucocorticoids rapidly activate cAMP production via G(alphas) to initiate non-genomic signaling that contributes to one-third of their canonical genomic effects. FASEB J, 2020. 34(2): p. 2882-2895. [CrossRef]
- Sun, H. W.; Miao, C. Y.; Liu, L.; Zhou, J.; Su, D. F.; Wang, Y. X.Jiang, C. L., Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids, 2006. 71(2): p. 154-9. [CrossRef]
- Triggiani, M.; Granata, F.; Petraroli, A.; Loffredo, S.; Frattini, A.; Staiano, R. I.; Monaco, G.Marone, G., Inhibition of secretory phospholipase A2-induced cytokine production in human lung macrophages by budesonide. Int Arch Allergy Immunol, 2009. 150(2): p. 144-55. [CrossRef]
- Wang, Y.; Davidow, L.; Arvanites, A. C.; Blanchard, J.; Lam, K.; Xu, K.; Oza, V.; Yoo, J. W.; Ng, J. M.; Curran, T.; Rubin, L. L.Mcmahon, A. P., Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Biol, 2012. 19(8): p. 972-82. [CrossRef]
- Rana, R.; Carroll, C. E.; Lee, H. J.; Bao, J.; Marada, S.; Grace, C. R.; Guibao, C. D.; Ogden, S. K.Zheng, J. J., Structural insights into the role of the Smoothened cysteine-rich domain in Hedgehog signalling. Nat Commun, 2013. 4: p. 2965. [CrossRef]
- Recchia, A. D.; Dominicis, A.; D’amore, V. M.; Fabiano, T.; Al Jaf, A. I. A.; Peria, S.; Basoli, F.; Rainer, A.; Marinelli, L.; Di Leva, F. S.Ragnini-Wilson, A., Pharmacological targeting of smoothened receptor cysteine-rich domain by Budesonide promotes in vitro myelination. Front Mol Neurosci, 2024. 17: p. 1473960. [CrossRef]
- Orta, M. L.; Dominguez, I.; Pastor, N.; Cortes, F.Mateos, S., The role of the DNA hypermethylating agent Budesonide in the decatenating activity of DNA topoisomerase II. Mutat Res, 2010. 694(1-2): p. 45-52. [CrossRef]
- Laserna-Mendieta, E. J.; Navarro, P.; Casabona-Frances, S.; Savarino, E. V.; Amorena, E.; Perez-Martinez, I.; Guagnozzi, D.; Blas-Jhon, L.; Betore, E.; Guardiola-Arevalo, A.; Pellegatta, G.; Krarup, A. L.; Perello, A.; Barrio, J.; Gutierrez-Junquera, C.; Teruel Sanchez-Vegazo, C.; Fernandez-Fernandez, S.; Naves, J. E.; Oliva, S.; Rodriguez-Oballe, J. A.; Carrion, S.; Espina, S.; Llorente Barrio, M.; Masiques-Mas, M. L.; Dainese, R.; Feo-Ortega, S.; Martin-Dominguez, V.; Fernandez-Pacheco, J.; Perez-Fernandez, M. T.; Ghisa, M.; Maniero, D.; Nantes-Castillejo, O.; Nicolay-Maneru, J.; Suarez, A.; Maray, I.; Llerena-Castro, R.; Ortega-Larrode, A.; Alcedo, J.; Granja Navacerrada, A.; Racca, F.; Santander, C.; Arias, A.; Lucendo, A. J.; EureosEo, E. Connect Research Group, Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real-world efficacy from the EoE CONNECT registry. United European Gastroenterol J, 2024. [CrossRef]
- Hirano, I.; Dellon, E. S.; Gupta, S. K.; Katzka, D. A.; Collins, M. H.; Wojtowicz, A. M.; Terreri, B.; Zhang, W.; Boules, M.; Bhatia, S.Desai, N. K., Safety of an investigational formulation of budesonide (budesonide oral suspension) for eosinophilic oesophagitis: an integrated safety analysis of six phase 1-3 clinical trials. Aliment Pharmacol Ther, 2023. 57(10): p. 1117-1130. [CrossRef]
- Gupta, S. K.; Vitanza, J. M.Collins, M. H., Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol, 2015. 13(1): p. 66-76 e3. [CrossRef]
- Straumann, A.; Lucendo, A. J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C. S.; Ciriza De Los Rios, C.; Schmoecker, C.; Madisch, A.; Hruz, P.; Hayat, J.; Von Arnim, U.; Bredenoord, A. J.; Schubert, S.; Mueller, R.; Greinwald, R.; Schoepfer, A.; Attwood, S.International, E. O. S. Study Group, Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients With Eosinophilic Esophagitis. Gastroenterology, 2020. 159(5): p. 1672-1685 e5. [CrossRef]
- Lucendo, A. J.; Molina-Infante, J.; Arias, A.; Von Arnim, U.; Bredenoord, A. J.; Bussmann, C.; Amil Dias, J.; Bove, M.; Gonzalez-Cervera, J.; Larsson, H.; Miehlke, S.; Papadopoulou, A.; Rodriguez-Sanchez, J.; Ravelli, A.; Ronkainen, J.; Santander, C.; Schoepfer, A. M.; Storr, M. A.; Terreehorst, I.; Straumann, A.Attwood, S. E., Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J, 2017. 5(3): p. 335-358. [CrossRef]
- Lucendo, A. J.; Miehlke, S.; Schlag, C.; Vieth, M.; Von Arnim, U.; Molina-Infante, J.; Hartmann, D.; Bredenoord, A. J.; Ciriza De Los Rios, C.; Schubert, S.; Bruckner, S.; Madisch, A.; Hayat, J.; Tack, J.; Attwood, S.; Mueller, R.; Greinwald, R.; Schoepfer, A.; Straumann, A.International, E. O. S. Study Group, Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology, 2019. 157(1): p. 74-86 e15. [CrossRef]
- O’shea, K. M.; Aceves, S. S.; Dellon, E. S.; Gupta, S. K.; Spergel, J. M.; Furuta, G. T.Rothenberg, M. E., Pathophysiology of Eosinophilic Esophagitis. Gastroenterology, 2018. 154(2): p. 333-345. [CrossRef]
- Dellon, E. S.Hirano, I., Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology, 2018. 154(2): p. 319-332 e3. [CrossRef]
- Racca, F.; Pellegatta, G.; Cataldo, G.; Vespa, E.; Carlani, E.; Pelaia, C.; Paoletti, G.; Messina, M. R.; Nappi, E.; Canonica, G. W.; Repici, A.Heffler, E., Type 2 Inflammation in Eosinophilic Esophagitis: From Pathophysiology to Therapeutic Targets. Front Physiol, 2021. 12: p. 815842. [CrossRef]
- Adel-Patient, K.; Campeotto, F.; Grauso, M.; Guillon, B.; Moroldo, M.; Venot, E.; Dietrich, C.; Machavoine, F.; Castelli, F. A.; Fenaille, F.; Molina, T. J.; Barbet, P.; Delacourt, C.; Leite-De-Moraes, M.Lezmi, G., Assessment of local and systemic signature of eosinophilic esophagitis (EoE) in children through multi-omics approaches. Front Immunol, 2023. 14: p. 1108895. [CrossRef]
- Shoda, T.; Wen, T.; Aceves, S. S.; Abonia, J. P.; Atkins, D.; Bonis, P. A.; Caldwell, J. M.; Capocelli, K. E.; Carpenter, C. L.; Collins, M. H.; Dellon, E. S.; Eby, M. D.; Gonsalves, N.; Gupta, S. K.; Falk, G. W.; Hirano, I.; Menard-Katcher, P.; Kuhl, J. T.; Krischer, J. P.; Leung, J.; Mukkada, V. A.; Spergel, J. M.; Trimarchi, M. P.; Yang, G. Y.; Zimmermann, N.; Furuta, G. T.; Rothenberg, M. E.Consortium of Eosinophilic Gastrointestinal Disease, Researchers, Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross-sectional study. Lancet Gastroenterol Hepatol, 2018. 3(7): p. 477-488. [CrossRef]
- Sherrill, J. D.; Kc, K.; Wu, D.; Djukic, Z.; Caldwell, J. M.; Stucke, E. M.; Kemme, K. A.; Costello, M. S.; Mingler, M. K.; Blanchard, C.; Collins, M. H.; Abonia, J. P.; Putnam, P. E.; Dellon, E. S.; Orlando, R. C.; Hogan, S. P.Rothenberg, M. E., Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol, 2014. 7(3): p. 718-29. [CrossRef]
- Rochman, M.; Travers, J.; Miracle, C. E.; Bedard, M. C.; Wen, T.; Azouz, N. P.; Caldwell, J. M.; Kc, K.; Sherrill, J. D.; Davis, B. P.; Rymer, J. K.; Kaufman, K. M.; Aronow, B. J.Rothenberg, M. E., Profound loss of esophageal tissue differentiation in patients with eosinophilic esophagitis. J Allergy Clin Immunol, 2017. 140(3): p. 738-749 e3. [CrossRef]
- Blanchard, C.; Wang, N.; Stringer, K. F.; Mishra, A.; Fulkerson, P. C.; Abonia, J. P.; Jameson, S. C.; Kirby, C.; Konikoff, M. R.; Collins, M. H.; Cohen, M. B.; Akers, R.; Hogan, S. P.; Assa’ad, A. H.; Putnam, P. E.; Aronow, B. J.Rothenberg, M. E., Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest, 2006. 116(2): p. 536-47. [CrossRef]
- Sherrill, J. D.; Kiran, K. C.; Blanchard, C.; Stucke, E. M.; Kemme, K. A.; Collins, M. H.; Abonia, J. P.; Putnam, P. E.; Mukkada, V. A.; Kaul, A.; Kocoshis, S. A.; Kushner, J. P.; Plassard, A. J.; Karns, R. A.; Dexheimer, P. J.; Aronow, B. J.Rothenberg, M. E., Analysis and expansion of the eosinophilic esophagitis transcriptome by RNA sequencing. Genes Immun, 2014. 15(6): p. 361-9. [CrossRef]
- Blanchard, C.; Mingler, M. K.; Vicario, M.; Abonia, J. P.; Wu, Y. Y.; Lu, T. X.; Collins, M. H.; Putnam, P. E.; Wells, S. I.Rothenberg, M. E., IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol, 2007. 120(6): p. 1292-300. [CrossRef]
- Ma, S.; Xian, M.; Wang, Y.; Wang, C.Zhang, L., Budesonide repairs decreased barrier integrity of eosinophilic nasal polyp epithelial cells caused by PM(2.5). Clin Transl Allergy, 2021. 11(5): p. e12019. [CrossRef]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D. C.Sandborn, W. J., Ulcerative colitis. Lancet, 2012. 380(9853): p. 1606-19. [CrossRef]
- Barnes, E. L.; Loftus, E. V., Jr.Kappelman, M. D., Effects of Race and Ethnicity on Diagnosis and Management of Inflammatory Bowel Diseases. Gastroenterology, 2021. 160(3): p. 677-689. [CrossRef]
- Sherlock, M. E.; Macdonald, J. K.; Griffiths, A. M.; Steinhart, A. H.Seow, C. H., Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev, 2015. 2015(10): p. CD007698. [CrossRef]
- Xue, N. N.; He, M.; Li, Y.; Wu, J. Z.; Du, W. W.; Wu, X. M.; Yang, Z. Z.; Zhang, C. G.; Li, Q. Y.Xiao, H., Periplaneta americana extract promotes intestinal mucosa repair of ulcerative colitis in rat. Acta Cir Bras, 2020. 35(10): p. e202001002. [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A. P.; Ng, N.; Umapathy, C.; Ziade, N.Hashash, J. G., A comprehensive review and update on ulcerative colitis(). Dis Mon, 2019. 65(12): p. 100851. [CrossRef]
- Naganuma, M.; Aoyama, N.; Tada, T.; Kobayashi, K.; Hirai, F.; Watanabe, K.; Watanabe, M.Hibi, T., Complete mucosal healing of distal lesions induced by twice-daily budesonide 2-mg foam promoted clinical remission of mild-to-moderate ulcerative colitis with distal active inflammation: double-blind, randomized study. J Gastroenterol, 2018. 53(4): p. 494-506. [CrossRef]
- Burke, K. E.; D’amato, M.; Ng, S. C.; Pardi, D. S.; Ludvigsson, J. F.Khalili, H., Microscopic colitis. Nat Rev Dis Primers, 2021. 7(1): p. 39. [CrossRef]
- Nielsen, O. H.; Fernandez-Banares, F.; Sato, T.Pardi, D. S., Microscopic colitis: Etiopathology, diagnosis, and rational management. Elife, 2022. 11. [CrossRef]
- Pervez, A.; Siddique, K.Khan, M. A. S., A Literature Review of Microscopic Colitis. Cureus, 2024. 16(1): p. e52862. [CrossRef]
- Nielsen, O. H.Pardi, D. S., Diagnosis and Pharmacological Management of Microscopic Colitis in Geriatric Care. Drugs Aging, 2024. 41(2): p. 113-123. [CrossRef]
- Langner, C.; Aust, D.; Ensari, A.; Villanacci, V.; Becheanu, G.; Miehlke, S.; Geboes, K.; Munch, A.; Working Group of Digestive Diseases of the European Society Of, PathologyThe European Microscopic Colitis, Group, Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology, 2015. 66(5): p. 613-26. [CrossRef]
- Pardi, D. S., Diagnosis and Management of Microscopic Colitis. Am J Gastroenterol, 2017. 112(1): p. 78-85. [CrossRef]
- Sehgal, K.; Tome, J.; Kamboj, A. K.; Dierkhising, R. A.; Pardi, D. S.Khanna, S., The natural history of histological changes in microscopic colitis. Therap Adv Gastroenterol, 2023. 16: p. 17562848231168237. [CrossRef]
- Miehlke, S.; Guagnozzi, D.; Zabana, Y.; Tontini, G. E.; Kanstrup Fiehn, A. M.; Wildt, S.; Bohr, J.; Bonderup, O.; Bouma, G.; D’amato, M.; Heiberg Engel, P. J.; Fernandez-Banares, F.; Macaigne, G.; Hjortswang, H.; Hultgren-Hornquist, E.; Koulaouzidis, A.; Kupcinskas, J.; Landolfi, S.; Latella, G.; Lucendo, A.; Lyutakov, I.; Madisch, A.; Magro, F.; Marlicz, W.; Mihaly, E.; Munck, L. K.; Ostvik, A. E.; Patai, A. V.; Penchev, P.; Skonieczna-Zydecka, K.; Verhaegh, B.Munch, A., European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United European Gastroenterol J, 2021. 9(1): p. 13-37. [CrossRef]
- Tome, J.; Sehgal, K.; Kamboj, A. K.; Comstock, B.; Harmsen, W. S.; Khanna, S.Pardi, D. S., Budesonide Maintenance in Microscopic Colitis: Clinical Outcomes and Safety Profile From a Population-Based Study. Am J Gastroenterol, 2022. 117(8): p. 1311-1315. [CrossRef]
- Escudero-Hernandez, C.; Van Beelen Granlund, A.; Bruland, T.; Sandvik, A. K.; Koch, S.; Ostvik, A. E.Munch, A., Transcriptomic Profiling of Collagenous Colitis Identifies Hallmarks of Nondestructive Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol, 2021. 12(2): p. 665-687. [CrossRef]
- Royce, S. G.; Cheng, V.; Samuel, C. S.Tang, M. L., The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol, 2012. 351(2): p. 167-75. [CrossRef]
- Mottais, A.; Riberi, L.; Falco, A.; Soccal, S.; Gohy, S.De Rose, V., Epithelial-Mesenchymal Transition Mechanisms in Chronic Airway Diseases: A Common Process to Target? Int J Mol Sci, 2023. 24(15). [CrossRef]
- Tsartsali, L.; Hislop, A. A.; Mckay, K.; James, A. L.; Elliot, J.; Zhu, J.; Rosenthal, M.; Payne, D. N.; Jeffery, P. K.; Bush, A.Saglani, S., Development of the bronchial epithelial reticular basement membrane: relationship to epithelial height and age. Thorax, 2011. 66(4): p. 280-5. [CrossRef]
- Roche, W. R.; Beasley, R.; Williams, J. H.Holgate, S. T., Subepithelial fibrosis in the bronchi of asthmatics. Lancet, 1989. 1(8637): p. 520-4. [CrossRef]
- D’aniello, C.; Cermola, F.; Palamidessi, A.; Wanderlingh, L. G.; Gagliardi, M.; Migliaccio, A.; Varrone, F.; Casalino, L.; Matarazzo, M. R.; De Cesare, D.; Scita, G.; Patriarca, E. J.Minchiotti, G., Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res, 2019. 79(13): p. 3235-3250. [CrossRef]
- Comes, S.; Gagliardi, M.; Laprano, N.; Fico, A.; Cimmino, A.; Palamidessi, A.; De Cesare, D.; De Falco, S.; Angelini, C.; Scita, G.; Patriarca, E. J.; Matarazzo, M. R.Minchiotti, G., L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Reports, 2013. 1(4): p. 307-21. [CrossRef]
- Minchiotti, G.; D’aniello, C.; Fico, A.; De Cesare, D.Patriarca, E. J., Capturing Transitional Pluripotency through Proline Metabolism. Cells, 2022. 11(14). [CrossRef]
- Casalino, L.; Comes, S.; Lambazzi, G.; De Stefano, B.; Filosa, S.; De Falco, S.; De Cesare, D.; Minchiotti, G.Patriarca, E. J., Control of embryonic stem cell metastability by L-proline catabolism. J Mol Cell Biol, 2011. 3(2): p. 108-22. [CrossRef]
- Ibello, E.; Saracino, F.; Delle Cave, D.; Buonaiuto, S.; Amoroso, F.; Andolfi, G.; Corona, M.; Guardiola, O.; Colonna, V.; Sainz, B., Jr.; Altucci, L.; De Cesare, D.; Cobellis, G.; Lonardo, E.; Patriarca, E. J.; D’aniello, C.Minchiotti, G., Three-dimensional environment sensitizes pancreatic cancer cells to the anti-proliferative effect of budesonide by reprogramming energy metabolism. J Exp Clin Cancer Res, 2024. 43(1): p. 165. [CrossRef]
- Gomez-Rubio, P.; Zock, J. P.; Rava, M.; Marquez, M.; Sharp, L.; Hidalgo, M.; Carrato, A.; Ilzarbe, L.; Michalski, C.; Molero, X.; Farre, A.; Perea, J.; Greenhalf, W.; O’rorke, M.; Tardon, A.; Gress, T.; Barbera, V.; Crnogorac-Jurcevic, T.; Dominguez-Munoz, E.; Munoz-Bellvis, L.; Alvarez-Urturi, C.; Balcells, J.; Barneo, L.; Costello, E.; Guillen-Ponce, C.; Kleeff, J.; Kong, B.; Lawlor, R.; Lohr, M.; Mora, J.; Murray, L.; O’driscoll, D.; Pelaez, P.; Poves, I.; Scarpa, A.; Real, F. X.; Malats, N.Pangen, E. U. Study Investigators, Reduced risk of pancreatic cancer associated with asthma and nasal allergies. Gut, 2017. 66(2): p. 314-322. [CrossRef]
- Willey, R. F.; Godden, D. J.; Carmichael, J.; Preston, P.; Frame, M. H.Crompton, G. K., Twice daily inhalation of a new corticosteroid, budesonide, in the treatment of chronic asthma. Eur J Respir Dis Suppl, 1982. 122: p. 138-42.
- Del Valle, I.; Rudloff, S.; Carles, A.; Li, Y.; Liszewska, E.; Vogt, R.Kemler, R., E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development, 2013. 140(8): p. 1684-92. [CrossRef]
- Soncin, F.; Mohamet, L.; Eckardt, D.; Ritson, S.; Eastham, A. M.; Bobola, N.; Russell, A.; Davies, S.; Kemler, R.; Merry, C. L.Ward, C. M., Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells, 2009. 27(9): p. 2069-80. [CrossRef]
- Wong, S. H. M.; Fang, C. M.; Chuah, L. H.; Leong, C. O.Ngai, S. C., E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol, 2018. 121: p. 11-22. [CrossRef]
- Sharaireh, A. M.; Fitzpatrick, L. M.; Ward, C. M.; Mckay, T. R.Unwin, R. D., Epithelial cadherin regulates transition between the naive and primed pluripotent states in mouse embryonic stem cells. Stem Cells, 2020. 38(10): p. 1292-1306. [CrossRef]
- Chen, C.; Zhang, X.; Wang, Y.; Chen, X.; Chen, W.; Dan, S.; She, S.; Hu, W.; Dai, J.; Hu, J.; Cao, Q.; Liu, Q.; Huang, Y.; Qin, B.; Kang, B.Wang, Y. J., Translational and post-translational control of human naive versus primed pluripotency. iScience, 2022. 25(1): p. 103645. [CrossRef]
- Soncin, F.; Mohamet, L.; Ritson, S.; Hawkins, K.; Bobola, N.; Zeef, L.; Merry, C. L.Ward, C. M., E-cadherin acts as a regulator of transcripts associated with a wide range of cellular processes in mouse embryonic stem cells. PLoS One, 2011. 6(7): p. e21463. [CrossRef]
- Ying, Q. L.; Wray, J.; Nichols, J.; Batlle-Morera, L.; Doble, B.; Woodgett, J.; Cohen, P.Smith, A., The ground state of embryonic stem cell self-renewal. Nature, 2008. 453(7194): p. 519-23. [CrossRef]
- Pieters, T.Van Roy, F., Role of cell-cell adhesion complexes in embryonic stem cell biology. J Cell Sci, 2014. 127(Pt 12): p. 2603-13. [CrossRef]
- Cermola, F.; Amoroso, F.; Saracino, F.; Ibello, E.; De Cesare, D.; Fico, A.; Cobellis, G.; Scalera, E.; Casiraghi, C.; D’aniello, C.; Patriarca, E. J.Minchiotti, G., Stabilization of cell-cell adhesions prevents symmetry breaking and locks in pluripotency in 3D gastruloids. Stem Cell Reports, 2022. 17(11): p. 2548-2564. [CrossRef]
- Amoroso, F.; Ibello, E.; Saracino, F.; Cermola, F.; Ponticelli, G.; Scalera, E.; Ricci, F.; Villetti, G.; Cobellis, G.; Minchiotti, G.; Patriarca, E. J.; De Cesare, D.D’aniello, C., Budesonide Analogues Preserve Stem Cell Pluripotency and Delay 3D Gastruloid Development. Pharmaceutics, 2023. 15(7). [CrossRef]
- D’aniello, C.; Habibi, E.; Cermola, F.; Paris, D.; Russo, F.; Fiorenzano, A.; Di Napoli, G.; Melck, D. J.; Cobellis, G.; Angelini, C.; Fico, A.; Blelloch, R.; Motta, A.; Stunnenberg, H. G.; De Cesare, D.; Patriarca, E. J.Minchiotti, G., Vitamin C and l-Proline Antagonistic Effects Capture Alternative States in the Pluripotency Continuum. Stem Cell Reports, 2017. 8(1): p. 1-10. [CrossRef]
- D’aniello, C.; Fico, A.; Casalino, L.; Guardiola, O.; Di Napoli, G.; Cermola, F.; De Cesare, D.; Tate, R.; Cobellis, G.; Patriarca, E. J.Minchiotti, G., A novel autoregulatory loop between the Gcn2-Atf4 pathway and (L)-Proline [corrected] metabolism controls stem cell identity. Cell Death Differ, 2015. 22(7): p. 1094-105. [CrossRef]
- Glover, H. J.; Holliday, H.; Shparberg, R. A.; Winkler, D.; Day, M.Morris, M. B., Signalling pathway crosstalk stimulated by L-proline drives mouse embryonic stem cells to primitive-ectoderm-like cells. Development, 2023. 150(20). [CrossRef]
- Van Den Brink, S. C.Van Oudenaarden, A., 3D gastruloids: a novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol, 2021. 31(9): p. 747-759. [CrossRef]
- Sullivan, A. E.Santos, S. D., The ever-growing world of gastruloids: autogenous models of mammalian embryogenesis. Curr Opin Genet Dev, 2023. 82: p. 102102. [CrossRef]
- Cermola, F.; D’aniello, C.; Tate, R.; De Cesare, D.; Martinez-Arias, A.; Minchiotti, G.Patriarca, E. J., Gastruloid Development Competence Discriminates Different States of Pluripotency. Stem Cell Reports, 2021. 16(2): p. 354-369. [CrossRef]
- Hashmi, A.; Tlili, S.; Perrin, P.; Lowndes, M.; Peradziryi, H.; Brickman, J. M.; Martinez Arias, A.Lenne, P. F., Cell-state transitions and collective cell movement generate an endoderm-like region in gastruloids. Elife, 2022. 11. [CrossRef]
- Riethmacher, D.; Brinkmann, V.Birchmeier, C., A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A, 1995. 92(3): p. 855-9. [CrossRef]
- Bandyopadhyay, C.; Schecterson, L.Gumbiner, B. M., E-cadherin activating antibodies limit barrier dysfunction and inflammation in mouse inflammatory bowel disease. Tissue Barriers, 2021. 9(4): p. 1940741. [CrossRef]
- Thompson, A. I.Lees, C. W., Genetics of ulcerative colitis. Inflamm Bowel Dis, 2011. 17(3): p. 831-48. [CrossRef]
- Muise, A. M.; Walters, T. D.; Glowacka, W. K.; Griffiths, A. M.; Ngan, B. Y.; Lan, H.; Xu, W.; Silverberg, M. S.Rotin, D., Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn’s disease. Gut, 2009. 58(8): p. 1121-7. [CrossRef]
- Petrova, Y. I.; Spano, M. M.Gumbiner, B. M., Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol Biol Cell, 2012. 23(11): p. 2092-108. [CrossRef]
- Seoudi, S. S.; Allam, E. A.; El-Kamel, A. H.; Elkafrawy, H.El-Moslemany, R. M., Targeted delivery of budesonide in acetic acid induced colitis: impact on miR-21 and E-cadherin expression. Drug Deliv Transl Res, 2023. 13(11): p. 2930-2947. [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S. E.Reddel, H. K., Asthma. Lancet, 2018. 391(10122): p. 783-800. [CrossRef]
- De Boer, W. I.; Sharma, H. S.; Baelemans, S. M.; Hoogsteden, H. C.; Lambrecht, B. N.Braunstahl, G. J., Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol, 2008. 86(3): p. 105-12. [CrossRef]
- Heijink, I. H.; Brandenburg, S. M.; Noordhoek, J. A.; Postma, D. S.; Slebos, D. J.Van Oosterhout, A. J., Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur Respir J, 2010. 35(4): p. 894-903. [CrossRef]
- Xiao, C.; Puddicombe, S. M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H. M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; Cremin, C.; Sones, J.; Djukanovic, R.; Howarth, P. H.; Collins, J. E.; Holgate, S. T.; Monk, P.Davies, D. E., Defective epithelial barrier function in asthma. J Allergy Clin Immunol, 2011. 128(3): p. 549-56 e1-12. [CrossRef]
- Veronesi, G.; Guerrieri-Gonzaga, A.; Infante, M.Bonanni, B., Chemoprevention studies within lung cancer screening programmes. Ecancermedicalscience, 2015. 9: p. 597. [CrossRef]
- Veronesi, G.; Szabo, E.; Decensi, A.; Guerrieri-Gonzaga, A.; Bellomi, M.; Radice, D.; Ferretti, S.; Pelosi, G.; Lazzeroni, M.; Serrano, D.; Lippman, S. M.; Spaggiari, L.; Nardi-Pantoli, A.; Harari, S.; Varricchio, C.Bonanni, B., Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev Res (Phila), 2011. 4(1): p. 34-42. [CrossRef]
- Veronesi, G.; Lazzeroni, M.; Szabo, E.; Brown, P. H.; Decensi, A.; Guerrieri-Gonzaga, A.; Bellomi, M.; Radice, D.; Grimaldi, M. C.; Spaggiari, L.Bonanni, B., Long-term effects of inhaled budesonide on screening-detected lung nodules. Ann Oncol, 2015. 26(5): p. 1025-1030. [CrossRef]
- Wattenberg, L. W.; Wiedmann, T. S.; Estensen, R. D.; Zimmerman, C. L.; Steele, V. E.Kelloff, G. J., Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res, 1997. 57(24): p. 5489-92.
- Wattenberg, L. W.; Wiedmann, T. S.; Estensen, R. D.; Zimmerman, C. L.; Galbraith, A. R.; Steele, V. E.Kelloff, G. J., Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis, 2000. 21(2): p. 179-82. [CrossRef]
- Pereira, M. A.; Li, Y.; Gunning, W. T.; Kramer, P. M.; Al-Yaqoub, F.; Lubet, R. A.; Steele, V. E.; Szabo, E.Tao, L., Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis, 2002. 23(7): p. 1185-92. [CrossRef]
- Pereira, M. A.; Tao, L. H.; Wang, W.; Gunning, W. T.Lubet, R., Chemoprevention: mouse colon and lung tumor bioassay and modulation of DNA methylation as a biomarker. Exp Lung Res, 2005. 31(2): p. 145-63. [CrossRef]
- Alyaqoub, F. S.; Tao, L.; Kramer, P. M.; Steele, V. E.; Lubet, R. A.; Gunning, W. T.Pereira, M. A., Prevention of mouse lung tumors and modulation of DNA methylation by combined treatment with budesonide and R115777 (ZarnestraMT). Carcinogenesis, 2006. 27(12): p. 2442-7. [CrossRef]
- Galbraith, A. R.; Seabloom, D. E.; Wuertz, B. R.; Antonides, J. D.; Steele, V. E.; Wattenberg, L. W.Ondrey, F. G., Chemoprevention of Lung Carcinogenesis by Dietary Nicotinamide and Inhaled Budesonide. Cancer Prev Res (Phila), 2019. 12(2): p. 69-78. [CrossRef]
- Pereira, M. A.; Tao, L.; Liu, Y.; Li, L.; Steele, V. E.Lubet, R. A., Modulation by budesonide of DNA methylation and mRNA expression in mouse lung tumors. Int J Cancer, 2007. 120(5): p. 1150-3. [CrossRef]
- Tao, L.; Li, Y.; Wang, W.; Kramer, P. M.; Gunning, W. T.; Lubet, R. A.; Steele, V. E.Pereira, M. A., Effect of budesonide on the methylation and mRNA expression of the insulin-like growth factor 2 and c-myc genes in mouse lung tumors. Mol Carcinog, 2002. 35(2): p. 93-102. [CrossRef]
- Ciechomska, M.; Roszkowski, L.Maslinski, W., DNA Methylation as a Future Therapeutic and Diagnostic Target in Rheumatoid Arthritis. Cells, 2019. 8(9). [CrossRef]
- D’aniello, C.; Cermola, F.; Patriarca, E. J.Minchiotti, G., Vitamin C in Stem Cell Biology: Impact on Extracellular Matrix Homeostasis and Epigenetics. Stem Cells Int, 2017. 2017: p. 8936156. [CrossRef]
- D’aniello, C.; Cermola, F.; Patriarca, E. J.Minchiotti, G., Metabolic-Epigenetic Axis in Pluripotent State Transitions. Epigenomes, 2019. 3(3). [CrossRef]
- Barnes, P. J., Inhaled Corticosteroids. Pharmaceuticals (Basel), 2010. 3(3): p. 514-540. [CrossRef]
- Adenuga, D.; Caito, S.; Yao, H.; Sundar, I. K.; Hwang, J. W.; Chung, S.Rahman, I., Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem Biophys Res Commun, 2010. 403(3-4): p. 452-6. [CrossRef]
- Heinen, N.; Meister, T. L.; Klohn, M.; Steinmann, E.; Todt, D.Pfaender, S., Antiviral Effect of Budesonide against SARS-CoV-2. Viruses, 2021. 13(7). [CrossRef]
- Kim, S. R.; Song, J. H.; Ahn, J. H.; Lee, G. S.; Ahn, H.; Yoon, S. I.; Kang, S. G.; Kim, P. H.; Jeon, S. M.; Choi, E. J.; Shin, S.; Cha, Y.; Cho, S.; Kim, D. E.; Chang, S. Y.Ko, H. J., Antiviral and anti-inflammatory activity of budesonide against human rhinovirus infection mediated via autophagy activation. Antiviral Res, 2018. 151: p. 87-96. [CrossRef]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.Kawase, T., Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig, 2020. 58(3): p. 155-168. [CrossRef]
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.Fukushi, S., The Inhaled Steroid Ciclesonide Blocks SARS-CoV-2 RNA Replication by Targeting the Viral Replication-Transcription Complex in Cultured Cells. J Virol, 2020. 95(1). [CrossRef]
- Ramakrishnan, S.; Nicolau, D. V., Jr.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; Bright, S.; Butler, C.; Cane, J. L.; Halner, A.; Matthews, P. C.; Donnelly, L. E.; Simpson, J. L.; Baker, J. R.; Fadai, N. T.; Peterson, S.; Bengtsson, T.; Barnes, P. J.; Russell, R. E. K.Bafadhel, M., Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med, 2021. 9(7): p. 763-772. [CrossRef]
- Finney, L. J.; Glanville, N.; Farne, H.; Aniscenko, J.; Fenwick, P.; Kemp, S. V.; Trujillo-Torralbo, M. B.; Loo, S. L.; Calderazzo, M. A.; Wedzicha, J. A.; Mallia, P.; Bartlett, N. W.; Johnston, S. L.Singanayagam, A., Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol, 2021. 147(2): p. 510-519 e5. [CrossRef]
- Patriarca, E. J.; Cermola, F.; D’aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.Minchiotti, G., The Multifaceted Roles of Proline in Cell Behavior. Front Cell Dev Biol, 2021. 9: p. 728576. [CrossRef]
- Yamaya, M.; Nishimura, H.; Nadine, L.; Kubo, H.Nagatomi, R., Formoterol and budesonide inhibit rhinovirus infection and cytokine production in primary cultures of human tracheal epithelial cells. Respir Investig, 2014. 52(4): p. 251-60. [CrossRef]
- Fehr, A. R.Perlman, S., Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol, 2015. 1282: p. 1-23. [CrossRef]
- Heijink, I. H.; Jonker, M. R.; De Vries, M.; Van Oosterhout, A. J.; Telenga, E.; Ten Hacken, N. H.; Postma, D. S.Van Den Berge, M., Budesonide and fluticasone propionate differentially affect the airway epithelial barrier. Respir Res, 2016. 17: p. 2. [CrossRef]
- Rimmer, C.; Hetelekides, S.; Eliseeva, S. I.; Georas, S. N.Veazey, J. M., Budesonide promotes airway epithelial barrier integrity following double-stranded RNA challenge. PLoS One, 2021. 16(12): p. e0260706. [CrossRef]
- Tunek, A.; Sjodin, K.Hallstrom, G., Reversible formation of fatty acid esters of budesonide, an antiasthma glucocorticoid, in human lung and liver microsomes. Drug Metab Dispos, 1997. 25(11): p. 1311-7.
- Miller-Larsson, A.; Mattsson, H.; Hjertberg, E.; Dahlback, M.; Tunek, A.Brattsand, R., Reversible fatty acid conjugation of budesonide. Novel mechanism for prolonged retention of topically applied steroid in airway tissue. Drug Metab Dispos, 1998. 26(7): p. 623-30.
- Van Den Brink, K. I.; Boorsma, M.; Staal-Van Den Brekel, A. J.; Edsbacker, S.; Wouters, E. F.Thorsson, L., Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol, 2008. 66(1): p. 27-35. [CrossRef]
- Brattsand, R.Miller-Larsson, A., The role of intracellular esterification in budesonide once-daily dosing and airway selectivity. Clin Ther, 2003. 25 Suppl C: p. C28-41. [CrossRef]
- Nave, R.; Fisher, R.Mccracken, N., In vitro metabolism of beclomethasone dipropionate, budesonide, ciclesonide, and fluticasone propionate in human lung precision-cut tissue slices. Respir Res, 2007. 8(1): p. 65. [CrossRef]
- Redmer, T.; Diecke, S.; Grigoryan, T.; Quiroga-Negreira, A.; Birchmeier, W.Besser, D., E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep, 2011. 12(7): p. 720-6. [CrossRef]
- Cheung, K. J.; Gabrielson, E.; Werb, Z.Ewald, A. J., Collective invasion in breast cancer requires a conserved basal epithelial program. Cell, 2013. 155(7): p. 1639-51. [CrossRef]
- Cai, D.; Chen, S. C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J. K.; Danuser, G.Montell, D. J., Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell, 2014. 157(5): p. 1146-59. [CrossRef]
- Cabrera, A. J. H.; Gumbiner, B. M.Kwon, Y. V., Remodeling of E-cadherin subcellular localization during cell dissemination. Mol Biol Cell, 2023. 34(5): p. ar46. [CrossRef]
- Na, T. Y.; Schecterson, L.; Mendonsa, A. M.Gumbiner, B. M., The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A, 2020. 117(11): p. 5931-5937. [CrossRef]
- Troyanovsky, S. M., Adherens junction: the ensemble of specialized cadherin clusters. Trends Cell Biol, 2023. 33(5): p. 374-387. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




