Submitted:
27 February 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Pesticides are essential in agriculture for protecting crops and boosting productivity, but their widespread use may pose significant health risks. Farmworkers face direct exposure through skin contact and inhalation, which may lead to hormonal imbalances, neurological disorders, and elevated cancer risks. Moreover, pesticide residues in food and water may affect surrounding communities. One of the lesser investigated issues is immunotoxicity, mostly because chronic effects of compound exposure are very complex to study. As a case study, this work utilized pharmacophore modeling and virtual screening to identify pesticides that may inhibit Janus kinases (JAK1, JAK2, JAK3) and tyrosine kinase 2 (TYK2), which are pivotal in immune response regulation and associated with cancer development and increased infection susceptibility. We identified 64 potential pesticide candidates, 22 of which have previously been detected in the human body, as confirmed by the Human Metabolome Database. These results underscore the critical need for further research into potential immunotoxic and chronic impacts of the respective pesticides on human health.
Keywords:
1. Introduction
2. Results
2.1. Datasets
2.2. Pharmacophore Modeling
2.3. Theoretical Evaluation of the Generated Pharmacophore Models
2.4. Identified Pesticides During Virtual Screening Campaign
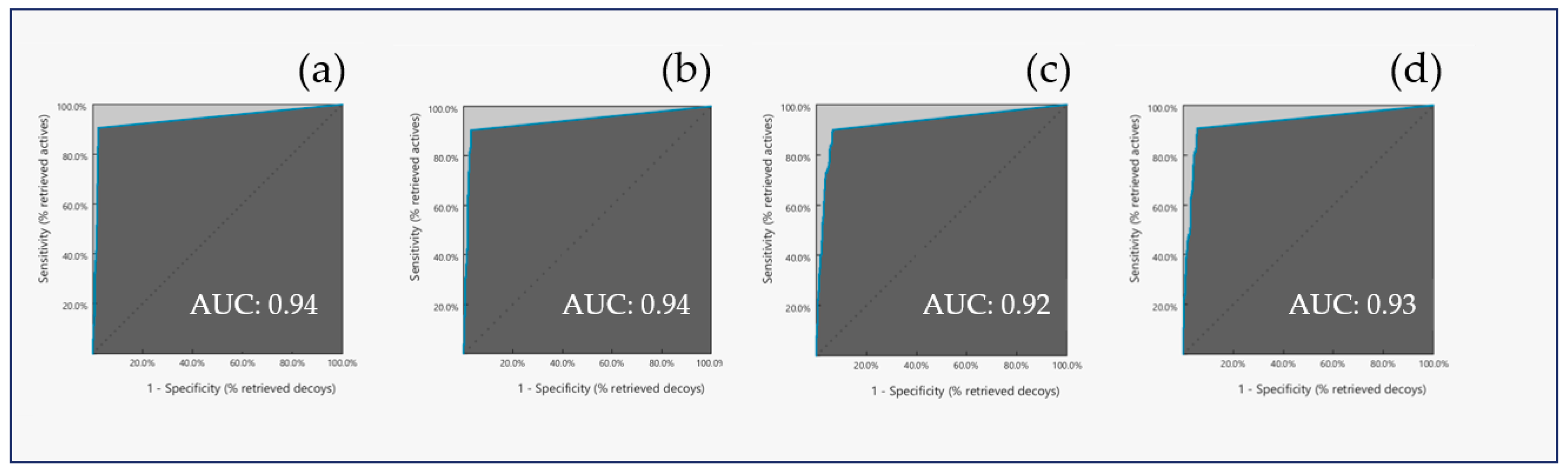
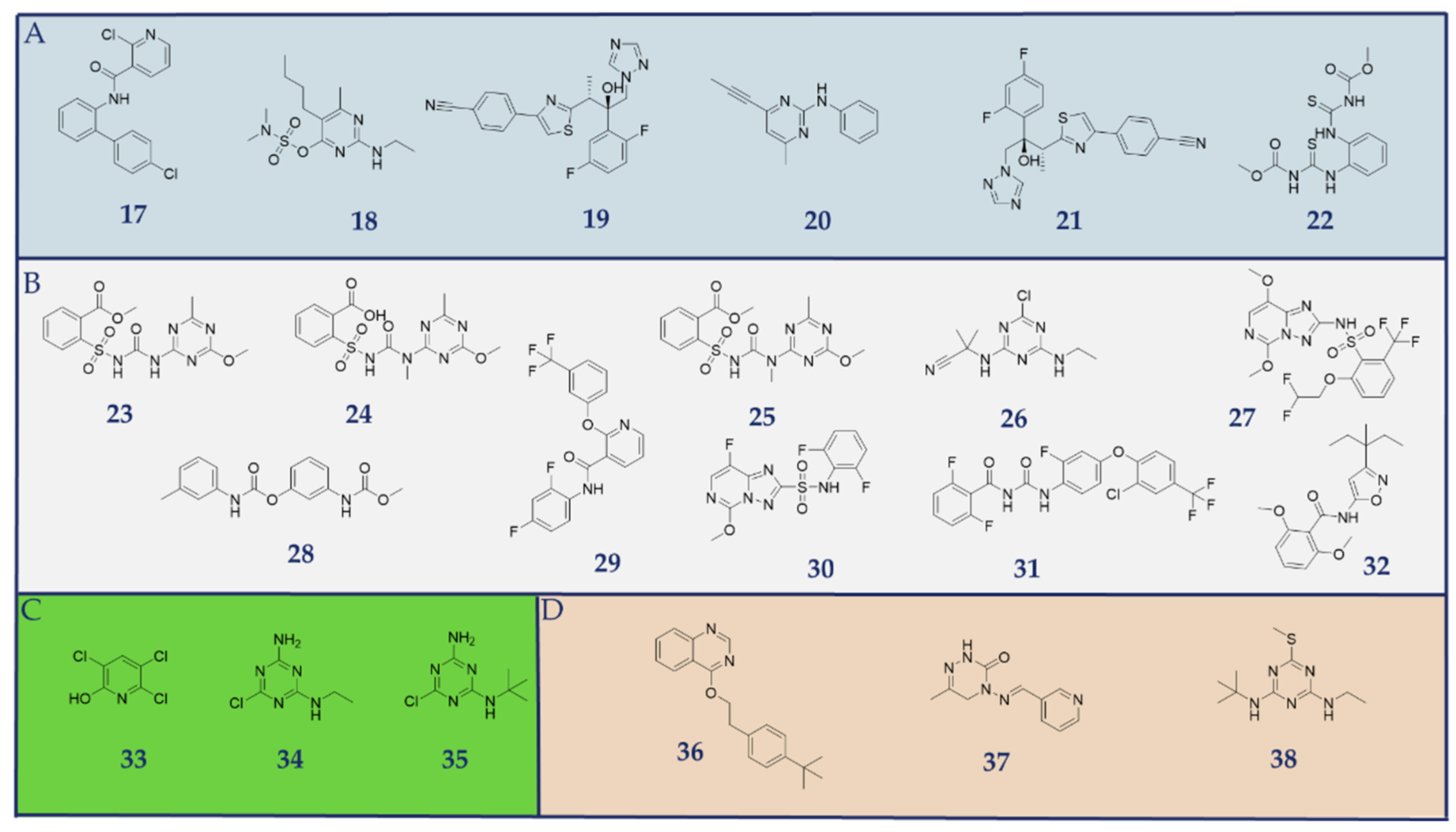
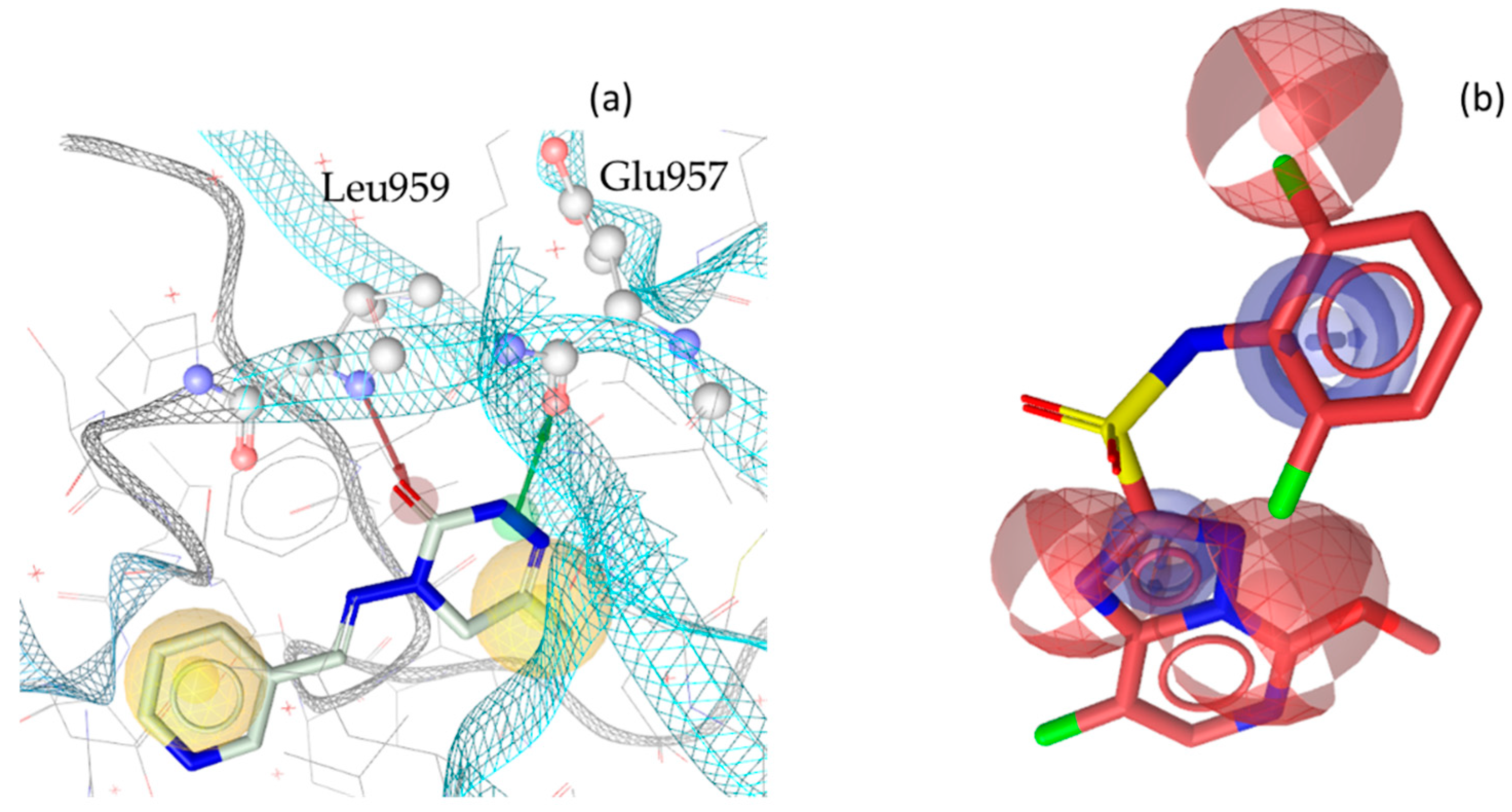
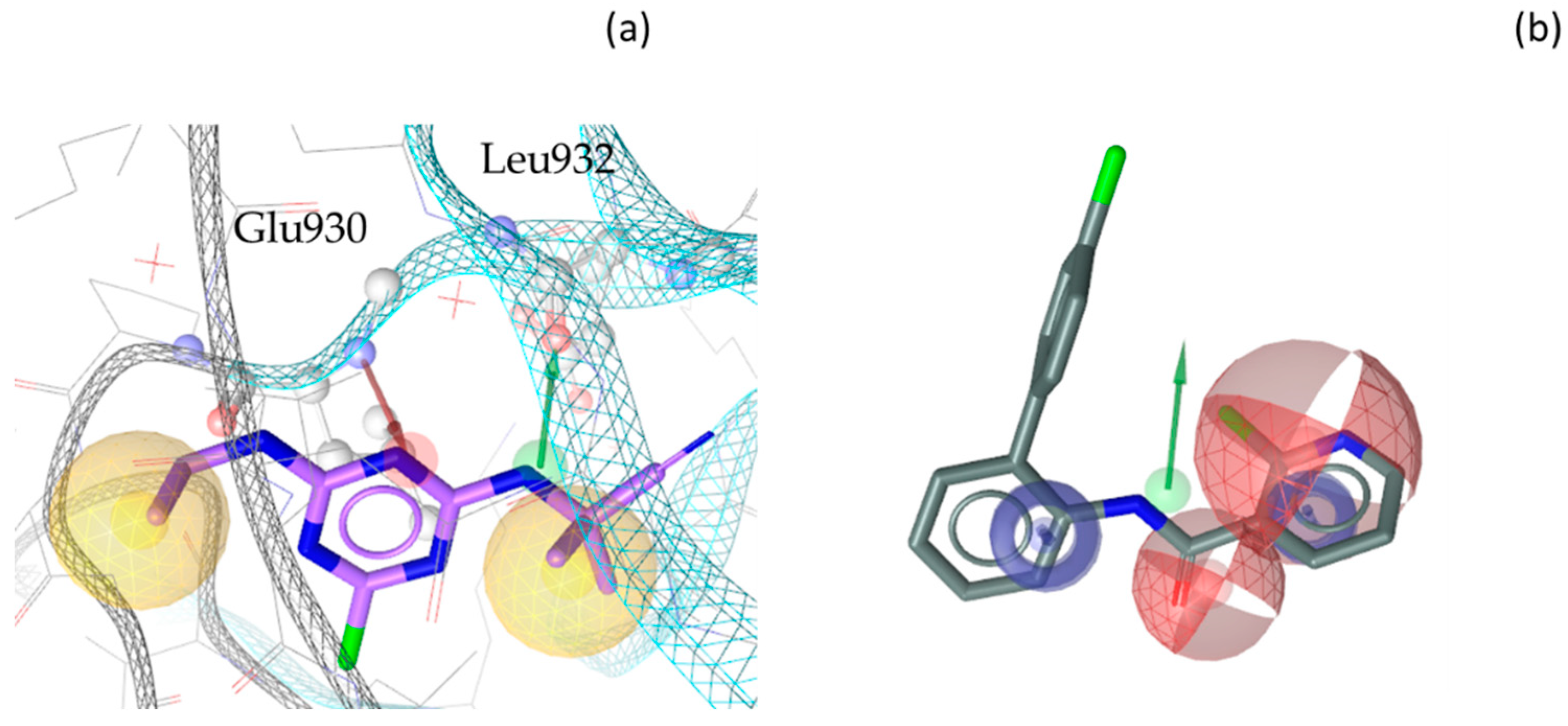
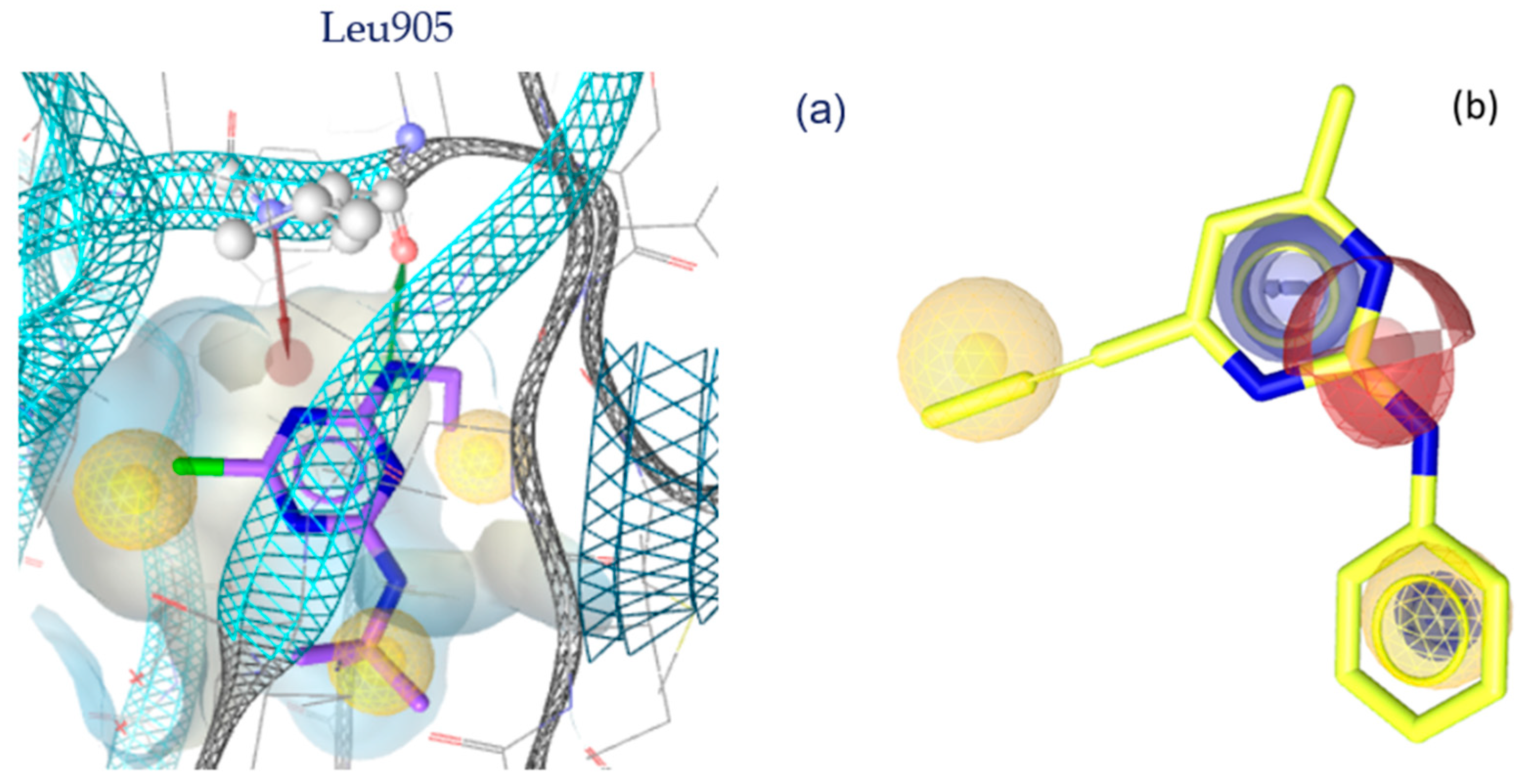
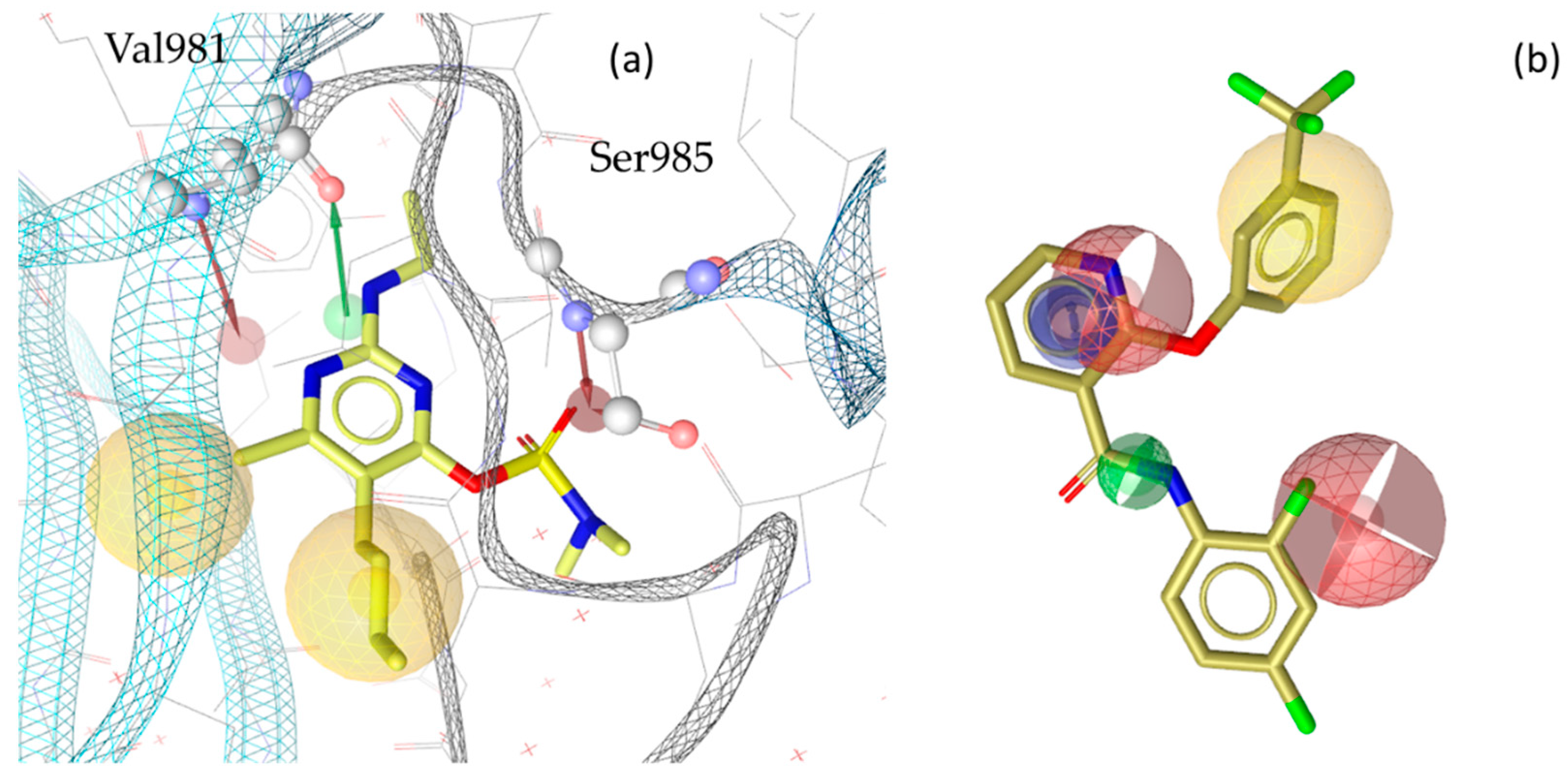
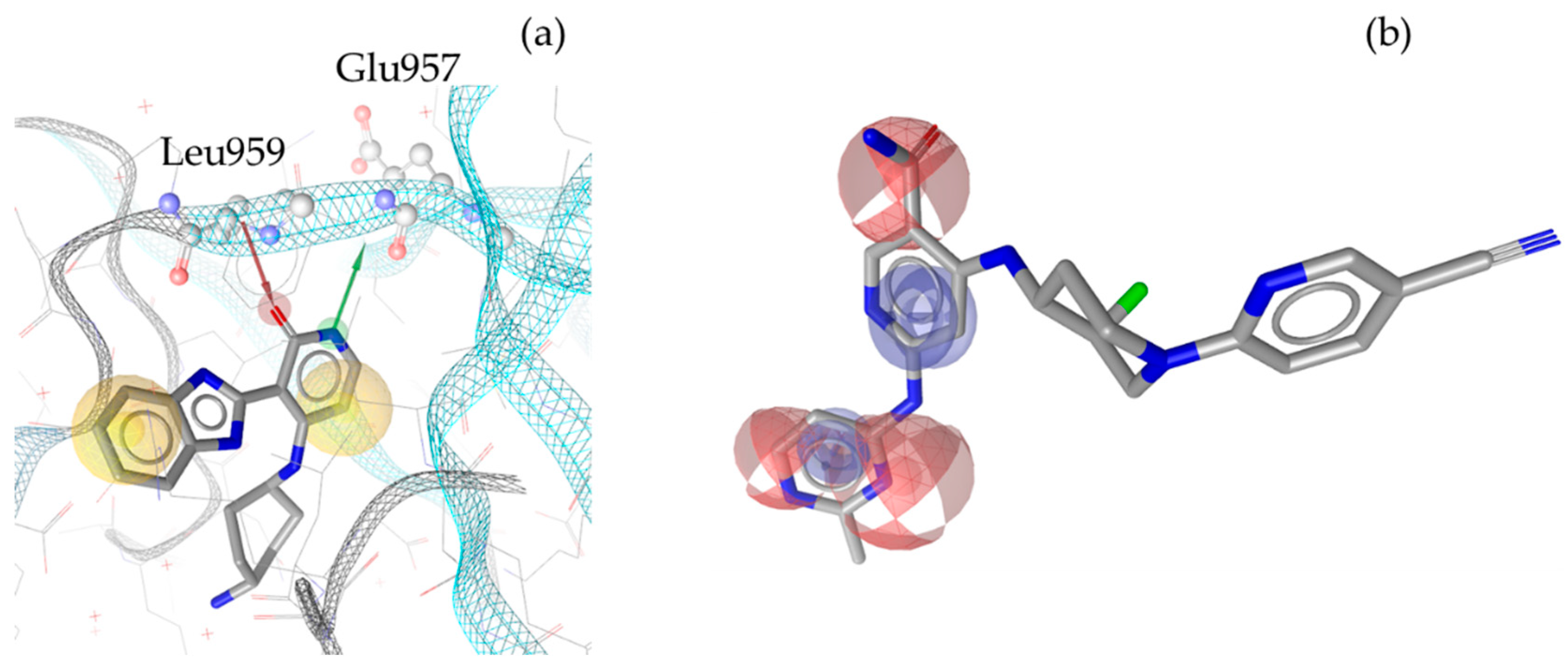
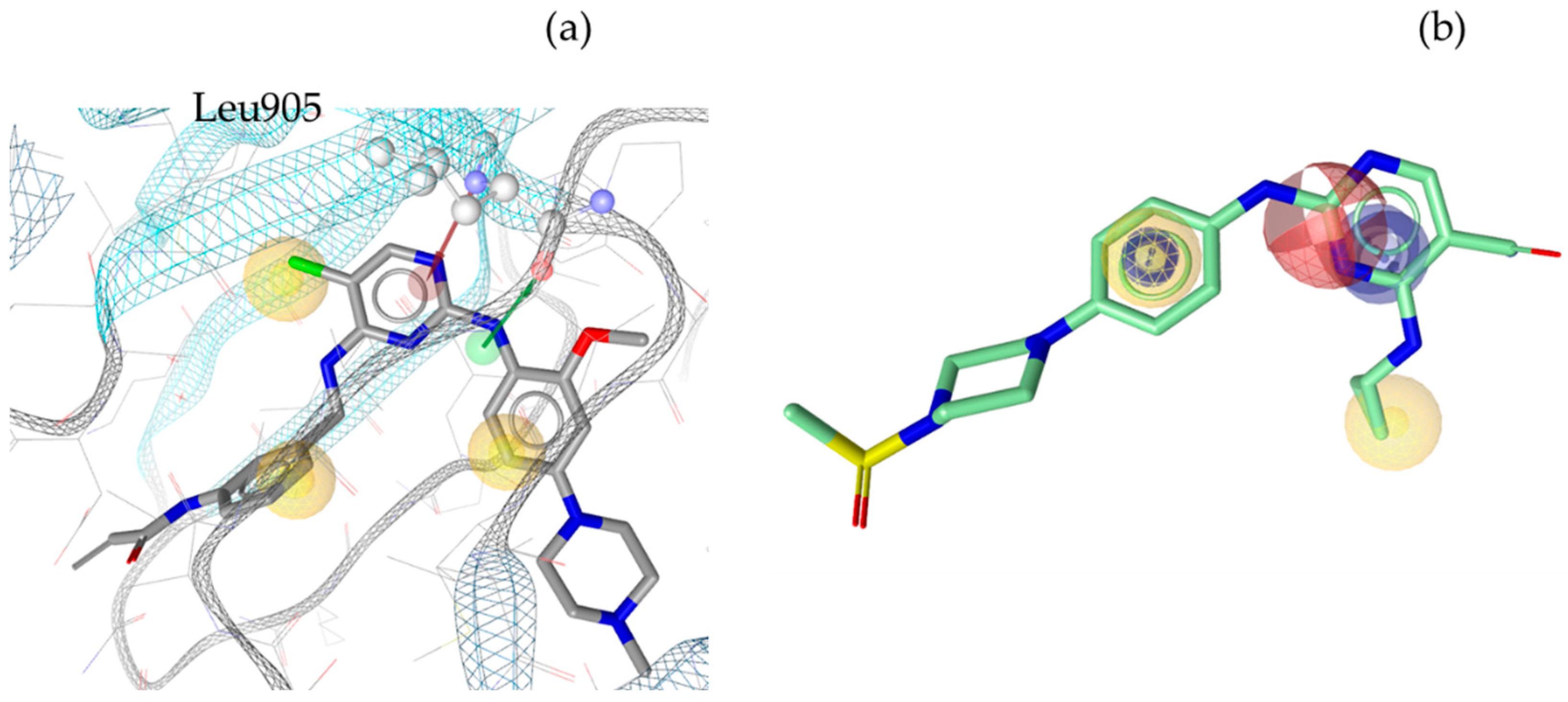
3. Discussion
4. Materials and Methods
4.1. Generation of Databases
4.2. Definition of a Pharmacophore
4.3. Pharmacophore Model Generation
4.4. Theoretical Validation
- a)
- Sensitivity = number of ACs identified by the model / number of ACs in the dataset
- b)
- Specificity = number of ACs not identified by the model / number of IAs in the dataset
- c)
- Accuracy = (number of TP / number of TN) / number of all the compounds in the database
- d)
- YoA = number of TP / number of total hits
- e)
- EF = YoA / (number of ACs in the database / number of all compounds in the database)
4.5. Virtual Screening
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- E. Macfarlane, R. Carey, T. Keegel, S. El-Zaemay, and L. Fritschi, "Dermal exposure associated with occupational end use of pesticides and the role of protective measures," (in eng), Saf Health Work, vol. 4, no. 3, pp. 136-41, Sep 2013. [CrossRef]
- X. Zhang et al., "Pesticide poisoning and neurobehavioral function among farm workers in Jiangsu, People's Republic of China," (in eng), Cortex, vol. 74, pp. 396-404, Jan 2016. [CrossRef]
- L. Wang, Z. Liu, J. Zhang, Y. Wu, and H. Sun, "Chlorpyrifos exposure in farmers and urban adults: Metabolic characteristic, exposure estimation, and potential effect of oxidative damage," (in eng), Environ Res, vol. 149, pp. 164-170, Aug 2016. [CrossRef]
- C. A. Damalas and S. D. Koutroubas, "Farmers' Exposure to Pesticides: Toxicity Types and Ways of Prevention," (in eng), Toxics, vol. 4, no. 1, Jan 08 2016. [CrossRef]
- K. H. Kim, E. Kabir, and S. A. Jahan, "Exposure to pesticides and the associated human health effects," (in eng), Sci Total Environ, vol. 575, pp. 525-535, Jan 01 2017. [CrossRef]
- M. Syafrudin et al., "Pesticides in Drinking Water-A Review," (in eng), Int J Environ Res Public Health, vol. 18, no. 2, Jan 08 2021. [CrossRef]
- B. H. Jensen et al., "Cumulative dietary risk assessment of pesticides in food for the Danish population for the period 2012-2017," (in eng), Food Chem Toxicol, vol. 168, p. 113359, Oct 2022. [CrossRef]
- A. Fucic et al., "Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides," (in eng), Int J Environ Res Public Health, vol. 18, no. 12, Jun 18 2021. [CrossRef]
- J. R. Richardson, V. Fitsanakis, R. H. S. Westerink, and A. G. Kanthasamy, "Neurotoxicity of pesticides," (in eng), Acta Neuropathol, vol. 138, no. 3, pp. 343-362, Sep 2019. [CrossRef]
- M. C. Alavanja, M. K. Ross, and M. R. Bonner, "Increased cancer burden among pesticide applicators and others due to pesticide exposure," (in eng), CA Cancer J Clin, vol. 63, no. 2, pp. 120-42, 2013. [CrossRef]
- M. P. Purdue, J. A. Hoppin, A. Blair, M. Dosemeci, and M. C. Alavanja, "Occupational exposure to organochlorine insecticides and cancer incidence in the Agricultural Health Study," (in eng), Int J Cancer, vol. 120, no. 3, pp. 642-9, Feb 01 2007. [CrossRef]
- R. Mahajan et al., "Fonofos exposure and cancer incidence in the agricultural health study," (in eng), Environ Health Perspect, vol. 114, no. 12, pp. 1838-42, Dec 2006. [CrossRef]
- A. Mokarizadeh, M. R. Faryabi, M. A. Rezvanfar, and M. Abdollahi, "A comprehensive review of pesticides and the immune dysregulation: mechanisms, evidence and consequences," (in eng), Toxicol Mech Methods, vol. 25, no. 4, pp. 258-78, 2015. [CrossRef]
- C. Costa, V. Rapisarda, S. Catania, C. Di Nola, C. Ledda, and C. Fenga, "Cytokine patterns in greenhouse workers occupationally exposed to α-cypermethrin: an observational study," (in eng), Environ Toxicol Pharmacol, vol. 36, no. 3, pp. 796-800, Nov 2013. [CrossRef]
- T. Takeuchi, "Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases-RA as a role model," (in eng), Inflamm Regen, vol. 42, no. 1, p. 35, Dec 01 2022. [CrossRef]
- M. Gadina et al., "Janus kinases to jakinibs: from basic insights to clinical practice," (in eng), Rheumatology (Oxford), vol. 58, no. Suppl 1, pp. i4-i16, Feb 01 2019. [CrossRef]
- A. A. Zarrin, K. Bao, P. Lupardus, and D. Vucic, "Kinase inhibition in autoimmunity and inflammation," (in eng), Nat Rev Drug Discov, vol. 20, no. 1, pp. 39-63, Jan 2021. [CrossRef]
- C. Xue et al., "Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer," (in eng), Signal Transduct Target Ther, vol. 8, no. 1, p. 204, May 19 2023. [CrossRef]
- J. D. Clark, M. E. Flanagan, and J. B. Telliez, "Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases," (in eng), J Med Chem, vol. 57, no. 12, pp. 5023-38, Jun 26 2014. [CrossRef]
- L. Hoisnard et al., "Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database," (in eng), Sci Rep, vol. 12, no. 1, p. 7140, May 03 2022. [CrossRef]
- C. G. Wermuth, C. R. Ganellin, P. Lindberg, and L. A. Mitscher, "Glossary of terms used in medicinal chemistry (IUPAC Recommendations 1998)," vol. 70, no. 5, pp. 1129-1143, 1998. [CrossRef]
- D. Schuster and G. Wolber, "Identification of bioactive natural products by pharmacophore-based virtual screening," (in eng), Curr Pharm Des, vol. 16, no. 15, pp. 1666-81, May 2010. [CrossRef]
- V. Simov et al., "Structure-based design and development of (benz)imidazole pyridones as JAK1-selective kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 7, pp. 1803-8, Apr 01 2016. [CrossRef]
- Y. Nakajima et al., "Design, synthesis, and evaluation of 4,6-diaminonicotinamide derivatives as novel and potent immunomodulators targeting JAK3," (in eng), Bioorg Med Chem, vol. 24, no. 19, pp. 4711-4722, Oct 01 2016. [CrossRef]
- S. H. Spergel et al., "Discovery of a JAK1/3 Inhibitor and Use of a Prodrug To Demonstrate Efficacy in a Model of Rheumatoid Arthritis," (in eng), ACS Med Chem Lett, vol. 10, no. 3, pp. 306-311, Mar 14 2019. [CrossRef]
- R. Raghuvanshi and S. B. Bharate, "Recent Developments in the Use of Kinase Inhibitors for Management of Viral Infections," (in eng), J Med Chem, vol. 65, no. 2, pp. 893-921, Jan 27 2022. [CrossRef]
- R. R. Davis et al., "Structural Insights into JAK2 Inhibition by Ruxolitinib, Fedratinib, and Derivatives Thereof," (in eng), J Med Chem, vol. 64, no. 4, pp. 2228-2241, Feb 25 2021. [CrossRef]
- C. M. A. U. D. B. D. U. D. B. J. U. G. D. E. U. M. E. F. (US), "US-8633173-B2," United States, 2009/06/05.
- C. J. N. Mathison et al., "Efficacy and Tolerability of Pyrazolo[1,5-," (in eng), ACS Med Chem Lett, vol. 11, no. 4, pp. 558-565, Apr 09 2020. [CrossRef]
- Y. Yin, C. J. Chen, R. N. Yu, L. Shu, T. T. Zhang, and D. Y. Zhang, "Discovery of novel selective Janus kinase 2 (JAK2) inhibitors bearing a 1H-pyrazolo[3,4-d]pyrimidin-4-amino scaffold," (in eng), Bioorg Med Chem, vol. 27, no. 8, pp. 1562-1576, Apr 15 2019. [CrossRef]
- D. L. Sloman et al., "Optimization of microtubule affinity regulating kinase (MARK) inhibitors with improved physical properties," (in eng), Bioorg Med Chem Lett, vol. 26, no. 17, pp. 4362-6, Sep 01 2016. [CrossRef]
- A. Fensome et al., "Design and optimization of a series of 4-(3-azabicyclo[3.1.0]hexan-3-yl)pyrimidin-2-amines: Dual inhibitors of TYK2 and JAK1," (in eng), Bioorg Med Chem, vol. 28, no. 10, p. 115481, May 15 2020. [CrossRef]
- L. Tan et al., "Development of Selective Covalent Janus Kinase 3 Inhibitors," (in eng), J Med Chem, vol. 58, no. 16, pp. 6589-606, Aug 27 2015. [CrossRef]
- R. S. Bhide et al., "Discovery and structure-based design of 4,6-diaminonicotinamides as potent and selective IRAK4 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 27, no. 21, pp. 4908-4913, Nov 01 2017. [CrossRef]
- B. S. M. (US) and P. A. (US), "National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9868729-B2, Inhibitors of protein kinases. Retrieved December 16, 2024 from https://pubchem.ncbi.nlm.nih.gov/patent/US-9868729-B2 ." United States,.
- H. R. Lawrence et al., "Development of novel ACK1/TNK2 inhibitors using a fragment-based approach," (in eng), J Med Chem, vol. 58, no. 6, pp. 2746-63, Mar 26 2015. [CrossRef]
- C. J. Menet et al., "Triazolopyridines as selective JAK1 inhibitors: from hit identification to GLPG0634," (in eng), J Med Chem, vol. 57, no. 22, pp. 9323-42, Nov 26 2014. [CrossRef]
- X. Ma et al., "Discovery and optimization of 2-aminopyridine derivatives as novel and selective JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 30, no. 8, p. 127048, Apr 15 2020. [CrossRef]
- J. Krier et al., "Discovering pesticides and their TPs in Luxembourg waters using open cheminformatics approaches," (in eng), Environ Int, vol. 158, p. 106885, Jan 2022. [CrossRef]
- D. S. Wishart et al., "HMDB 5.0: the Human Metabolome Database for 2022," (in eng), Nucleic Acids Res, vol. 50, no. D1, pp. D622-D631, Jan 07 2022. [CrossRef]
- P. Xin et al., "The role of JAK/STAT signaling pathway and its inhibitors in diseases," (in eng), Int Immunopharmacol, vol. 80, p. 106210, Mar 2020. [CrossRef]
- S. Cohen et al., "Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis," (in eng), Arthritis Rheumatol, vol. 66, no. 11, pp. 2924-37, Nov 2014. [CrossRef]
- M. D. Russell et al., "JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications," (in eng), Ann Rheum Dis, vol. 82, no. 8, pp. 1059-1067, Aug 2023. [CrossRef]
- J. Zhang et al., "Risk of venous thromboembolism with janus kinase inhibitors in inflammatory immune diseases: a systematic review and meta-analysis," (in eng), Front Pharmacol, vol. 14, p. 1189389, 2023. [CrossRef]
- C. ROBINSON, "C. ROBINSON et al., “Achieving a High Level of Protection from Pesticides in Europe: Problems with the Current Risk Assessment Procedure and Solutions,” European Journal of Risk Regulation, vol. 11, no. 3, pp. 450–480, 2020." ed. [CrossRef]
- G. Jacob, ",Vincent Gear Thomas , Zapata Demi , Barron Ileana G. , Zapata Isain Comprehensive assessment of pesticide use patterns and increased cancer risk Frontiers in Cancer Control and Society VOLUME2, 2024" ed. [CrossRef]
- F. S. C. o. Japan, "Mepanipyrim (Pesticides)," (in eng), Food Saf (Tokyo), vol. 4, no. 1, pp. 28-29, Mar 2016. [CrossRef]
- J. Zweigle, A. Schmidt, B. Bugsel, C. Vogel, F. Simon, and C. Zwiener, "Perfluoroalkyl acid precursor or weakly fluorinated organic compound? A proof of concept for oxidative fractionation of PFAS and organofluorines," (in eng), Anal Bioanal Chem, vol. 416, no. 29, pp. 6799-6808, Dec 2024. [CrossRef]
- G. C. C. Weis et al., "Immunomodulatory effect of mancozeb, chlorothalonil, and thiophanate methyl pesticides on macrophage cells," (in eng), Ecotoxicol Environ Saf, vol. 182, p. 109420, Oct 30 2019. [CrossRef]
- T. W. Jabusch and R. S. Tjeerdema, "Partitioning of penoxsulam, a new sulfonamide herbicide," (in eng), J Agric Food Chem, vol. 53, no. 18, pp. 7179-83, Sep 07 2005. [CrossRef]
- D. M. Patel et al., "Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort," (in eng), Environ Int, vol. 143, p. 105955, Oct 2020. [CrossRef]
- S. O. Abarikwu et al., "Influence of triazines and lipopolysaccharide coexposure on inflammatory response and histopathological changes in the testis and liver of BalB/c mice," (in eng), Heliyon, vol. 10, no. 2, p. e24431, Jan 30 2024. [CrossRef]
- C. A. Laetz, D. H. Baldwin, T. K. Collier, V. Hebert, J. D. Stark, and N. L. Scholz, "The synergistic toxicity of pesticide mixtures: implications for risk assessment and the conservation of endangered Pacific salmon," (in eng), Environ Health Perspect, vol. 117, no. 3, pp. 348-53, Mar 2009. [CrossRef]
- N. Washuck, M. Hanson, and R. Prosser, "Yield to the data: some perspective on crop productivity and pesticides," (in eng), Pest Manag Sci, vol. 78, no. 5, pp. 1765-1771, May 2022. [CrossRef]
- H. M. Berman et al., "The Protein Data Bank," (in eng), Nucleic Acids Res, vol. 28, no. 1, pp. 235-42, Jan 01 2000. [CrossRef]
- A. Gaulton et al., "ChEMBL: a large-scale bioactivity database for drug discovery," (in eng), Nucleic Acids Res, vol. 40, no. Database issue, pp. D1100-7, Jan 2012. [CrossRef]
- S. Kim et al., "PubChem 2023 update," (in eng), Nucleic Acids Res, vol. 51, no. D1, pp. D1373-D1380, Jan 06 2023. [CrossRef]
- M. M. Mysinger, M. Carchia, J. J. Irwin, and B. K. Shoichet, "Directory of useful decoys, enhanced (DUD-E): better ligands and decoys for better benchmarking," (in eng), J Med Chem, vol. 55, no. 14, pp. 6582-94, Jul 26 2012. [CrossRef]
- G. Poli, T. Seidel, and T. Langer, "Conformational Sampling of Small Molecules With iCon: Performance Assessment in Comparison With OMEGA," (in eng), Front Chem, vol. 6, p. 229, 2018. [CrossRef]
- G. Wolber and T. Langer, "LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters," (in eng), J Chem Inf Model, vol. 45, no. 1, pp. 160-9, 2005. [CrossRef]
- T. Kaserer, K. R. Beck, M. Akram, A. Odermatt, and D. Schuster, "Pharmacophore Models and Pharmacophore-Based Virtual Screening: Concepts and Applications Exemplified on Hydroxysteroid Dehydrogenases," (in eng), Molecules, vol. 20, no. 12, pp. 22799-832, Dec 19 2015. [CrossRef]
- A. Vuorinen and D. Schuster, "Methods for generating and applying pharmacophore models as virtual screening filters and for bioactivity profiling," (in eng), Methods, vol. 71, pp. 113-34, Jan 2015. [CrossRef]
- S. Labadie et al., "Structure-based discovery of C-2 substituted imidazo-pyrrolopyridine JAK1 inhibitors with improved selectivity over JAK2," (in eng), Bioorg Med Chem Lett, vol. 22, no. 24, pp. 7627-33, Dec 15 2012. [CrossRef]
- Q. Su et al., "Discovery of (2," (in eng), J Med Chem, vol. 63, no. 9, pp. 4517-4527, May 14 2020. [CrossRef]
- T. Siu et al., "The Discovery of 3-((4-Chloro-3-methoxyphenyl)amino)-1-((3R,4S)-4-cyanotetrahydro-2H-pyran-3-yl)-1H-pyrazole-4-carboxamide, a Highly Ligand Efficient and Efficacious Janus Kinase 1 Selective Inhibitor with Favorable Pharmacokinetic Properties," (in eng), J Med Chem, vol. 60, no. 23, pp. 9676-9690, Dec 14 2017. [CrossRef]
- M. L. Arwood et al., "New scaffolds for type II JAK2 inhibitors overcome the acquired G993A resistance mutation," (in eng), Cell Chem Biol, vol. 30, no. 6, pp. 618-631.e12, Jun 15 2023. [CrossRef]
- S. Jaime-Figueroa et al., "Discovery of a series of novel 5H-pyrrolo[2,3-b]pyrazine-2-phenyl ethers, as potent JAK3 kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 23, no. 9, pp. 2522-6, May 01 2013. [CrossRef]
- A. Thorarensen et al., "Design of a Janus Kinase 3 (JAK3) Specific Inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) Allowing for the Interrogation of JAK3 Signaling in Humans," (in eng), J Med Chem, vol. 60, no. 5, pp. 1971-1993, Mar 09 2017. [CrossRef]
- M. Forster et al., "Selective JAK3 Inhibitors with a Covalent Reversible Binding Mode Targeting a New Induced Fit Binding Pocket," (in eng), Cell Chem Biol, vol. 23, no. 11, pp. 1335-1340, Nov 17 2016. [CrossRef]
- J. E. Chrencik et al., "Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6," (in eng), J Mol Biol, vol. 400, no. 3, pp. 413-33, Jul 16 2010. [CrossRef]
- V. Tsui et al., "A new regulatory switch in a JAK protein kinase," (in eng), Proteins, vol. 79, no. 2, pp. 393-401, Feb 2011. [CrossRef]
- D. Giordano, C. Biancaniello, M. A. Argenio, and A. Facchiano, "Drug Design by Pharmacophore and Virtual Screening Approach," (in eng), Pharmaceuticals (Basel), vol. 15, no. 5, May 23 2022. [CrossRef]
- "Christopher Adams, David J. Aldous, Shelley Amendola, Paul Bamborough, Colin Bright, Sarah Crowe, Paul Eastwood, Garry Fenton, Martyn Foster, Trevor K.P. Harrison, Sue King, Justine Lai, Christopher Lawrence, Jean-Philippe Letallec, Clive McCarthy, Neil Moorcroft, Kenneth Page, Sudha Rao, Jane Redford, Shazia Sadiq, Keith Smith, John E. Souness, Sukanthini Thurairatnam, Mark Vine, Barry Wyman, ;Mapping the kinase domain of janus kinase 3,;Bioorganic & Medicinal Chemistry Letters,;Volume 13, Issue 18, 2003, pages 3105-3110." ed. [CrossRef]
- C. J. Burns et al., "Phenylaminopyrimidines as inhibitors of Janus kinases (JAKs)," (in eng), Bioorg Med Chem Lett, vol. 19, no. 20, pp. 5887-92, Oct 15 2009. [CrossRef]
- A. G. Cole et al., "2-Benzimidazolyl-9-(chroman-4-yl)-purinone derivatives as JAK3 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 19, no. 23, pp. 6788-92, Dec 01 2009. [CrossRef]
- M. Gerspacher et al., "2-Amino-aryl-7-aryl-benzoxazoles as potent, selective and orally available JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 20, no. 5, pp. 1724-7, Mar 01 2010. [CrossRef]
- C. Pissot-Soldermann et al., "Discovery and SAR of potent, orally available 2,8-diaryl-quinoxalines as a new class of JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 20, no. 8, pp. 2609-13, Apr 15 2010. [CrossRef]
- S. D. Fidanze et al., "Imidazo[2,1-b]thiazoles: multitargeted inhibitors of both the insulin-like growth factor receptor and members of the epidermal growth factor family of receptor tyrosine kinases," (in eng), Bioorg Med Chem Lett, vol. 20, no. 8, pp. 2452-5, Apr 15 2010. [CrossRef]
- M. L. Dart et al., "Homogeneous Assay for Target Engagement Utilizing Bioluminescent Thermal Shift," (in eng), ACS Med Chem Lett, vol. 9, no. 6, pp. 546-551, Jun 14 2018. [CrossRef]
- S. T. S. R. I. M. S. C. R. D. National Center for Biotechnology Information (2024). PubChem Bioassay Record for AID 1699, 2024 from https://pubchem.ncbi.nlm.nih.gov/bioassay/1699 .
- P. Xu et al., "Janus kinases (JAKs): The efficient therapeutic targets for autoimmune diseases and myeloproliferative disorders," (in eng), Eur J Med Chem, vol. 192, p. 112155, Apr 15 2020. [CrossRef]
- D. B. Belanger et al., "Discovery of orally bioavailable imidazo[1,2-a]pyrazine-based Aurora kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 20, no. 22, pp. 6739-43, Nov 15 2010. [CrossRef]
- T. Siu et al., "The discovery of tricyclic pyridone JAK2 inhibitors. Part 1: hit to lead," (in eng), Bioorg Med Chem Lett, vol. 20, no. 24, pp. 7421-5, Dec 15 2010. [CrossRef]
- J. Wityak et al., "Discovery and initial SAR of 2-amino-5-carboxamidothiazoles as inhibitors of the Src-family kinase p56(Lck)," (in eng), Bioorg Med Chem Lett, vol. 13, no. 22, pp. 4007-10, Nov 17 2003. [CrossRef]
- M. H. U. F. S. V. G. S. M. A. H. G. D. J. A. G. D. B. (GB), "US-8592415-B2," United States, 2009/02/11.
- X. Ren et al., "Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway," (in eng), ACS Med Chem Lett, vol. 1, no. 9, pp. 454-9, Dec 09 2010. [CrossRef]
- M. E. Flanagan et al., "Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection," (in eng), J Med Chem, vol. 53, no. 24, pp. 8468-84, Dec 23 2010. [CrossRef]
- S. Ioannidis et al., "Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the Jak/Stat pathway," (in eng), J Med Chem, vol. 54, no. 1, pp. 262-76, Jan 13 2011. [CrossRef]
- M. E. McDonnell et al., "Anilino-monoindolylmaleimides as potent and selective JAK3 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 24, no. 4, pp. 1116-21, Feb 15 2014. [CrossRef]
- L. S. Harikrishnan et al., "Pyrrolo[1,2-f]triazines as JAK2 inhibitors: achieving potency and selectivity for JAK2 over JAK3," (in eng), Bioorg Med Chem Lett, vol. 21, no. 5, pp. 1425-8, Mar 01 2011. [CrossRef]
- M. H. Kim et al., "Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells," (in eng), Bioorg Med Chem, vol. 19, no. 6, pp. 1915-23, Mar 15 2011. [CrossRef]
- J. R. Medina et al., "Aminoindazole PDK1 Inhibitors: A Case Study in Fragment-Based Drug Discovery," (in eng), ACS Med Chem Lett, vol. 1, no. 8, pp. 439-42, Nov 11 2010. [CrossRef]
- T. Wang et al., "In vitro and in vivo evaluation of 6-aminopyrazolyl-pyridine-3-carbonitriles as JAK2 kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 21, no. 10, pp. 2958-61, May 15 2011. [CrossRef]
- M. Thomas et al., "Discovery of 5-(arenethynyl) hetero-monocyclic derivatives as potent inhibitors of BCR-ABL including the T315I gatekeeper mutant," (in eng), Bioorg Med Chem Lett, vol. 21, no. 12, pp. 3743-8, Jun 15 2011. [CrossRef]
- L. Shu et al., "Design, synthesis, and pharmacological evaluation of 4- or 6-phenyl-pyrimidine derivatives as novel and selective Janus kinase 3 inhibitors," (in eng), Eur J Med Chem, vol. 191, p. 112148, Apr 01 2020. [CrossRef]
- Q. Qiu et al., "Exploration of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Bispecific Inhibitors Based on the Moiety of Fedratinib for Treatment of Both Hematologic Malignancies and Solid Cancers," (in eng), J Med Chem, vol. 66, no. 8, pp. 5753-5773, Apr 27 2023. [CrossRef]
- J. Lim et al., "Discovery of 1-amino-5H-pyrido[4,3-b]indol-4-carboxamide inhibitors of Janus kinase 2 (JAK2) for the treatment of myeloproliferative disorders," (in eng), J Med Chem, vol. 54, no. 20, pp. 7334-49, Oct 27 2011. [CrossRef]
- F. Giraud, P. Marchand, D. Carbonnelle, M. Sartor, F. Lang, and M. Duflos, "Synthesis of N-aryl-3-(indol-3-yl)propanamides and their immunosuppressive activities," (in eng), Bioorg Med Chem Lett, vol. 20, no. 17, pp. 5203-6, Sep 01 2010. [CrossRef]
- C. Gao et al., "Selectivity data: assessment, predictions, concordance, and implications," (in eng), J Med Chem, vol. 56, no. 17, pp. 6991-7002, Sep 12 2013. [CrossRef]
- M. E. Liosi et al., "Selective Janus Kinase 2 (JAK2) Pseudokinase Ligands with a Diaminotriazole Core," (in eng), J Med Chem, vol. 63, no. 10, pp. 5324-5340, May 28 2020. [CrossRef]
- A. Poulsen et al., "Structure-based design of PDK1 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 22, no. 1, pp. 305-7, Jan 01 2012. [CrossRef]
- C. A. Zificsak et al., "Optimization of a novel kinase inhibitor scaffold for the dual inhibition of JAK2 and FAK kinases," (in eng), Bioorg Med Chem Lett, vol. 22, no. 1, pp. 133-7, Jan 01 2012. [CrossRef]
- L. B. Schenkel et al., "Discovery of potent and highly selective thienopyridine Janus kinase 2 inhibitors," (in eng), J Med Chem, vol. 54, no. 24, pp. 8440-50, Dec 22 2011. [CrossRef]
- Y. A. Sonawane, M. A. Taylor, J. V. Napoleon, S. Rana, J. I. Contreras, and A. Natarajan, "Cyclin Dependent Kinase 9 Inhibitors for Cancer Therapy," (in eng), J Med Chem, vol. 59, no. 19, pp. 8667-8684, Oct 13 2016. [CrossRef]
- A. D. William et al., "Discovery of kinase spectrum selective macrocycle (16E)-14-methyl-20-oxa-5,7,14,26-tetraazatetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8(27),9,11,16,21,23-decaene (SB1317/TG02), a potent inhibitor of cyclin dependent kinases (CDKs), Janus kinase 2 (JAK2), and fms-like tyrosine kinase-3 (FLT3) for the treatment of cancer," (in eng), J Med Chem, vol. 55, no. 1, pp. 169-96, Jan 12 2012. [CrossRef]
- M. Menichincheri et al., "Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor," (in eng), J Med Chem, vol. 59, no. 7, pp. 3392-408, Apr 14 2016. [CrossRef]
- Z. Liu et al., "Drug Discovery Targeting Bromodomain-Containing Protein 4," (in eng), J Med Chem, vol. 60, no. 11, pp. 4533-4558, Jun 08 2017. [CrossRef]
- M. Hoemann et al., "Synthesis and optimization of furano[3,2-d]pyrimidines as selective spleen tyrosine kinase (Syk) inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 22, pp. 5562-5567, Nov 15 2016. [CrossRef]
- V. Oza et al., "Synthesis and evaluation of triazolones as checkpoint kinase 1 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 22, no. 6, pp. 2330-7, Mar 15 2012. [CrossRef]
- E. G. Yang et al., "Design and Synthesis of Janus Kinase 2 (JAK2) and Histone Deacetlyase (HDAC) Bispecific Inhibitors Based on Pacritinib and Evidence of Dual Pathway Inhibition in Hematological Cell Lines," (in eng), J Med Chem, vol. 59, no. 18, pp. 8233-62, Sep 22 2016. [CrossRef]
- B. J. Dugan et al., "A selective, orally bioavailable 1,2,4-triazolo[1,5-a]pyridine-based inhibitor of Janus kinase 2 for use in anticancer therapy: discovery of CEP-33779," (in eng), J Med Chem, vol. 55, no. 11, pp. 5243-54, Jun 14 2012. [CrossRef]
- M.-F. M. J. U. B. P. J. U. B. M. J. U. B. J. W. U. G. A. J. U. K. S. G. U. M. D. WAYNE, "Pyrrolo[2,3-D]pyrimidine compounds," United States, 2008/08/20.
- T. Yogo et al., "Structure-Based Design and Synthesis of 3-Amino-1,5-dihydro-4H-pyrazolopyridin-4-one Derivatives as Tyrosine Kinase 2 Inhibitors," (in eng), J Med Chem, vol. 59, no. 2, pp. 733-49, Jan 28 2016. [CrossRef]
- M. K. Kim et al., "Benzimidazole Derivatives as Potent JAK1-Selective Inhibitors," (in eng), J Med Chem, vol. 58, no. 18, pp. 7596-602, Sep 24 2015. [CrossRef]
- X. Chen, L. J. Wilson, R. Malaviya, R. L. Argentieri, and S. M. Yang, "Virtual screening to successfully identify novel janus kinase 3 inhibitors: a sequential focused screening approach," (in eng), J Med Chem, vol. 51, no. 21, pp. 7015-9, Nov 13 2008. [CrossRef]
- O. Fedorov et al., "A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases," (in eng), Proc Natl Acad Sci U S A, vol. 104, no. 51, pp. 20523-8, Dec 18 2007. [CrossRef]
- E. F. DiMauro et al., "Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: synthesis, SAR, and in vivo anti-inflammatory activity," (in eng), J Med Chem, vol. 49, no. 19, pp. 5671-86, Sep 21 2006. [CrossRef]
- T. Wang et al., "Discovery of Disubstituted Imidazo[4,5-b]pyridines and Purines as Potent TrkA Inhibitors," (in eng), ACS Med Chem Lett, vol. 3, no. 9, pp. 705-9, Sep 13 2012. [CrossRef]
- M. Liu, X. Ju, J. Zou, J. Shi, and G. Jia, "Recent researches for dual Aurora target inhibitors in antitumor field," (in eng), Eur J Med Chem, vol. 203, p. 112498, Oct 01 2020. [CrossRef]
- H. R. Lawrence et al., "Development of o-chlorophenyl substituted pyrimidines as exceptionally potent aurora kinase inhibitors," (in eng), J Med Chem, vol. 55, no. 17, pp. 7392-7416, Sep 13 2012. [CrossRef]
- E. J. Hanan et al., "Discovery of potent and selective pyrazolopyrimidine janus kinase 2 inhibitors," (in eng), J Med Chem, vol. 55, no. 22, pp. 10090-107, Nov 26 2012. [CrossRef]
- A. D. William et al., "Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma," (in eng), J Med Chem, vol. 54, no. 13, pp. 4638-58, Jul 14 2011. [CrossRef]
- T. Forsyth et al., "SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors," (in eng), Bioorg Med Chem Lett, vol. 22, no. 24, pp. 7653-8, Dec 15 2012. [CrossRef]
- J. J. Chen et al., "Development of pyrimidine-based inhibitors of Janus tyrosine kinase 3," (in eng), Bioorg Med Chem Lett, vol. 16, no. 21, pp. 5633-8, Nov 01 2006. [CrossRef]
- X. Liang et al., "Design, synthesis and preliminary biological evaluation of 4-aminopyrazole derivatives as novel and potent JAKs inhibitors," (in eng), Bioorg Med Chem, vol. 24, no. 12, pp. 2660-72, Jun 15 2016. [CrossRef]
- R. N. Yu et al., "Structure-based design and synthesis of pyrimidine-4,6-diamine derivatives as Janus kinase 3 inhibitors," (in eng), Bioorg Med Chem, vol. 27, no. 8, pp. 1646-1657, Apr 15 2019. [CrossRef]
- M. S. A. Elsayed et al., "Application of Sequential Palladium Catalysis for the Discovery of Janus Kinase Inhibitors in the Benzo[ c]pyrrolo[2,3- h][1,6]naphthyridin-5-one (BPN) Series," (in eng), J Med Chem, vol. 61, no. 23, pp. 10440-10462, Dec 13 2018. [CrossRef]
- F. M. Uckun et al., "Anti-breast cancer activity of LFM-A13, a potent inhibitor of Polo-like kinase (PLK)," (in eng), Bioorg Med Chem, vol. 15, no. 2, pp. 800-14, Jan 15 2007. [CrossRef]
- S. Van Epps et al., "Design and synthesis of tricyclic cores for kinase inhibition," (in eng), Bioorg Med Chem Lett, vol. 23, no. 3, pp. 693-8, Feb 01 2013. [CrossRef]
- M. Soth et al., "3-Amido pyrrolopyrazine JAK kinase inhibitors: development of a JAK3 vs JAK1 selective inhibitor and evaluation in cellular and in vivo models," (in eng), J Med Chem, vol. 56, no. 1, pp. 345-56, Jan 10 2013. [CrossRef]
- S. M. Yang et al., "Simplified staurosporine analogs as potent JAK3 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 17, no. 2, pp. 326-31, Jan 15 2007. [CrossRef]
- S. M. Lynch et al., "Strategic use of conformational bias and structure based design to identify potent JAK3 inhibitors with improved selectivity against the JAK family and the kinome," (in eng), Bioorg Med Chem Lett, vol. 23, no. 9, pp. 2793-800, May 01 2013. [CrossRef]
- H. Guan et al., "Discovery of novel Jak2-Stat pathway inhibitors with extended residence time on target," (in eng), Bioorg Med Chem Lett, vol. 23, no. 10, pp. 3105-10, May 15 2013. [CrossRef]
- T. H. Marsilje et al., "Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials," (in eng), J Med Chem, vol. 56, no. 14, pp. 5675-90, Jul 25 2013. [CrossRef]
- M. W. Martin et al., "Discovery of novel 2,3-diarylfuro[2,3-b]pyridin-4-amines as potent and selective inhibitors of Lck: synthesis, SAR, and pharmacokinetic properties," (in eng), Bioorg Med Chem Lett, vol. 17, no. 8, pp. 2299-304, Apr 15 2007. [CrossRef]
- M. Siu et al., "2-Amino-[1,2,4]triazolo[1,5-a]pyridines as JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 23, no. 17, pp. 5014-21, Sep 01 2013. [CrossRef]
- M. G. Brasca et al., "Discovery of NMS-E973 as novel, selective and potent inhibitor of heat shock protein 90 (Hsp90)," (in eng), Bioorg Med Chem, vol. 21, no. 22, pp. 7047-63, Nov 15 2013. [CrossRef]
- E. F. DiMauro et al., "Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: development of an expedient and divergent synthetic route and preliminary SAR," (in eng), Bioorg Med Chem Lett, vol. 17, no. 8, pp. 2305-9, Apr 15 2007. [CrossRef]
- B. Côté et al., "Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 17, no. 24, pp. 6816-20, Dec 15 2007. [CrossRef]
- J. P. Seerden et al., "Synthesis and structure-activity relationships of 4-fluorophenyl-imidazole p38α MAPK, CK1δ and JAK2 kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 24, no. 15, pp. 3412-8, Aug 01 2014. [CrossRef]
- Y. Zhong et al., "Small-Molecule Fms-like Tyrosine Kinase 3 Inhibitors: An Attractive and Efficient Method for the Treatment of Acute Myeloid Leukemia," (in eng), J Med Chem, vol. 63, no. 21, pp. 12403-12428, Nov 12 2020. [CrossRef]
- H. Zhao and A. Caflisch, "Discovery of ZAP70 inhibitors by high-throughput docking into a conformation of its kinase domain generated by molecular dynamics," (in eng), Bioorg Med Chem Lett, vol. 23, no. 20, pp. 5721-6, Oct 15 2013. [CrossRef]
- Q. Su et al., "Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2 inhibitors," (in eng), J Med Chem, vol. 57, no. 1, pp. 144-58, Jan 09 2014. [CrossRef]
- T. Siu et al., "The discovery of reverse tricyclic pyridone JAK2 inhibitors. Part 2: lead optimization," (in eng), Bioorg Med Chem Lett, vol. 24, no. 6, pp. 1466-71, Mar 15 2014. [CrossRef]
- Q. Huang et al., "Design of potent and selective inhibitors to overcome clinical anaplastic lymphoma kinase mutations resistant to crizotinib," (in eng), J Med Chem, vol. 57, no. 4, pp. 1170-87, Feb 27 2014. [CrossRef]
- Shen et al., "Dual-target Janus kinase (JAK) inhibitors: Comprehensive review on the JAK-based strategies for treating solid or hematological malignancies and immune-related diseases," (in eng), Eur J Med Chem, vol. 239, p. 114551, Sep 05 2022. [CrossRef]
- S. Wang et al., "Design, synthesis, and biological evaluation of 2,4-diamino pyrimidine derivatives as potent FAK inhibitors with anti-cancer and anti-angiogenesis activities," (in eng), Eur J Med Chem, vol. 222, p. 113573, Oct 15 2021. [CrossRef]
- A. M. Haidle et al., "Thiophene carboxamide inhibitors of JAK2 as potential treatments for myleoproliferative neoplasms," (in eng), Bioorg Med Chem Lett, vol. 24, no. 8, pp. 1968-73, Apr 15 2014. [CrossRef]
- A. Costales et al., "2-Amino-7-substituted benzoxazole analogs as potent RSK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 24, no. 6, pp. 1592-6, Mar 15 2014. [CrossRef]
- E. Park et al., "Discovery and Biological Evaluation of," (in eng), J Med Chem, vol. 64, no. 2, pp. 958-979, Jan 28 2021. [CrossRef]
- J. Ren et al., "Design and synthesis of boron-containing diphenylpyrimidines as potent BTK and JAK3 dual inhibitors," (in eng), Bioorg Med Chem, vol. 28, no. 2, p. 115236, Jan 15 2020. [CrossRef]
- M. G. Brasca et al., "Pyrrole-3-carboxamides as potent and selective JAK2 inhibitors," (in eng), Bioorg Med Chem, vol. 22, no. 17, pp. 4998-5012, Sep 01 2014. [CrossRef]
- E. J. Hanan et al., "Discovery of selective and noncovalent diaminopyrimidine-based inhibitors of epidermal growth factor receptor containing the T790M resistance mutation," (in eng), J Med Chem, vol. 57, no. 23, pp. 10176-91, Dec 11 2014. [CrossRef]
- N. C. Goodwin et al., "Discovery of a Type III Inhibitor of LIM Kinase 2 That Binds in a DFG-Out Conformation," (in eng), ACS Med Chem Lett, vol. 6, no. 1, pp. 53-7, Jan 08 2015. [CrossRef]
- J. J. Duan et al., "Discovery of pyrrolo[1,2-b]pyridazine-3-carboxamides as Janus kinase (JAK) inhibitors," (in eng), Bioorg Med Chem Lett, vol. 24, no. 24, pp. 5721-5726, Dec 15 2014. [CrossRef]
- J. L. Henderson et al., "Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor," (in eng), J Med Chem, vol. 58, no. 1, pp. 419-32, Jan 08 2015. [CrossRef]
- M. G. Brasca et al., "Novel pyrrole carboxamide inhibitors of JAK2 as potential treatment of myeloproliferative disorders," (in eng), Bioorg Med Chem, vol. 23, no. 10, pp. 2387-407, May 15 2015. [CrossRef]
- H. Wan et al., "Discovery of a Highly Selective JAK2 Inhibitor, BMS-911543, for the Treatment of Myeloproliferative Neoplasms," (in eng), ACS Med Chem Lett, vol. 6, no. 8, pp. 850-5, Aug 13 2015. [CrossRef]
- K. Zimmermann et al., "9H-Carbazole-1-carboxamides as potent and selective JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 25, no. 14, pp. 2809-12, Jul 15 2015. [CrossRef]
- H. Yamagishi et al., "Discovery of 3,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-2(1H)-one derivatives as novel JAK inhibitors," (in eng), Bioorg Med Chem, vol. 23, no. 15, pp. 4846-4859, Aug 01 2015. [CrossRef]
- Y. Nakajima et al., "Synthesis and evaluation of novel 1H-pyrrolo[2,3-b]pyridine-5-carboxamide derivatives as potent and orally efficacious immunomodulators targeting JAK3," (in eng), Bioorg Med Chem, vol. 23, no. 15, pp. 4871-4883, Aug 01 2015. [CrossRef]
- W. D. Jang et al., "Discovery of Tyk2 inhibitors via the virtual site-directed fragment-based drug design," (in eng), Bioorg Med Chem Lett, vol. 25, no. 18, pp. 3947-52, Sep 15 2015. [CrossRef]
- Liu et al., "Design and synthesis of carbazole carboxamides as promising inhibitors of Bruton's tyrosine kinase (BTK) and Janus kinase 2 (JAK2)," (in eng), Bioorg Med Chem Lett, vol. 25, no. 19, pp. 4265-9, Oct 01 2015. [CrossRef]
- A. C. Hart et al., "Structure-Based Design of Selective Janus Kinase 2 Imidazo[4,5-d]pyrrolo[2,3-b]pyridine Inhibitors," (in eng), ACS Med Chem Lett, vol. 6, no. 8, pp. 845-9, Aug 13 2015. [CrossRef]
- M. Gehringer and S. A. Laufer, "Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology," (in eng), J Med Chem, vol. 62, no. 12, pp. 5673-5724, Jun 27 2019. [CrossRef]
- ed. [CrossRef]
- J. S. Choi et al., "Highly potent and selective pyrazolylpyrimidines as Syk kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 25, no. 20, pp. 4441-6, Oct 15 2015. [CrossRef]
- M. A. U. D. B. D. U. D. B. J. U. G. D. E. U. M. E. F. U. M. K. L. (US), "US-8633173-B2," United States,.
- Jones et al., "Design and Synthesis of a Pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin," (in eng), J Med Chem, vol. 60, no. 2, pp. 767-786, Jan 26 2017. [CrossRef]
- A. Casimiro-Garcia et al., "Identification of Cyanamide-Based Janus Kinase 3 (JAK3) Covalent Inhibitors," (in eng), J Med Chem, vol. 61, no. 23, pp. 10665-10699, Dec 13 2018. [CrossRef]
- B. S. M. U. P. A. (US), "Inhibitors of protein kinases," United States, 2008/04/16.
- P. A. V. U. B. D. G. U. L. Q. U. M. H. U. Z. K. (US), "US-8815840-B2," United States, 2008/12/19.
- A. S. P. U. C. M. U. D. C. U. J. J. U. P. Q. U. R. A. U. S. M. E. U. W. D. J. (US)WOO HYUN CHONG (US) CASH BRANDON (US), "Pyrrolopyrimidines as janus kinase inhibitors," United States, 2011/12/06.
- M. Forster, M. Gehringer, and S. A. Laufer, "Recent advances in JAK3 inhibition: Isoform selectivity by covalent cysteine targeting," (in eng), Bioorg Med Chem Lett, vol. 27, no. 18, pp. 4229-4237, Sep 15 2017. [CrossRef]
- P. M. A. U. X. J. U. A. B. A. U. H. S. J. U. W. S. G. U. H. H.-C. U. J. E. J. (US), "US-8633206-B2," United States, 2009/10/15.
- M. F. U. F. A. E. U. F. M. E. U. G. A. U. J. T. A. U. K. N. U. M.-F. M. J. (US)STROHBACH, "Pyrrolo[2,3-D]pyrimidine derivatives," United States, 2013/02/22.
- M. A. A. C. M. M. C. S. S. I. S. S. J. (IN), "Bicyclic compounds and their uses as dual c-SRC/JAK inhibitors," United States, 2010/02/17.
- A. U. I. S. U. L. M. U. S. M. U. W. T. U. Z. H.-J. (US), "9-(pyrazol-3-yl)-9H-purine-2-amine and 3-(pyrazol-3-yl) -3H-imidazo[4,5-B] pyridin-5-amine derivatives and their use for the treatment of cancer," United States, 2007/05/04.
- V. F. J. U. H. J. C. U. L. R. U. L. H. U. L. A. J. U. S. E. B. U. S. M. (US), "US-8618103-B2," United States, 2009/09/10.
- W. S. T. U. B. G. D. U. D. L. M. U. D. J. U. G. J. U. H. J. U. J. B. U. K. J. (US)LIN SHUQUN (US)LU ZHONGHUI (US)SPERGEL STEVEN H (US)TOKARSKI JOHN S (US)WU HONG (US)YANG BINGWEI VERA (US), "US-8921368-B2," United States, 2011/07/27.
- J. Hynes et al., "Discovery of potent and efficacious pyrrolopyridazines as dual JAK1/3 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 27, no. 14, pp. 3101-3106, Jul 15 2017. [CrossRef]
- W. S. T. U. D. J. U. D. J. U. G. J. U. H. J. U. J. B. U. K. J. U. L. S. U. L. Z. (US)PITTS WILLIAM J (US)SPERGEL STEVEN H (US)WU HONG (US)YANG BINGWEI VERA (US), "Pyrrolopyridazine JAK3 inhibitors and their use for the treatment of inflammatory and autoimmune diseases," United States, 2011/03/17.
- A. S. U. A. S. W. U. C. K. R. U. H. J. U. H. L. U. J. Y. U. K. T. U. S. J. (US), "US-8791123-B2," United States, 2009/07/09.
- N. S. J. S. M. J. M. T. J. H. Y. J. Y. H. J. M. K. J. H. A. J. I. M. J. H. Y. (JP), "US-8609647-B2," United States, 2009/07/31.
- C. A. P. U. S. R. B. U. Y. E. W. T. U. F. H. U. B. M. J. U. Z. W. (US), "Macrocyclic compounds and their use as kinase inhibitors," United States 2009/01/23.
- B. J. U. C. J. T. U. J. J. U. M. M. U. W. C. U. W. K. U. Y. J. R. U. Z. H. (US), "Acyclic cyanoethylpyrazoles as janus kinase inhibitors," United States, 2011/09/22.
- B. B. Hansen et al., "Fragment-Based Discovery of Pyrazolopyridones as JAK1 Inhibitors with Excellent Subtype Selectivity," (in eng), J Med Chem, vol. 63, no. 13, pp. 7008-7032, Jul 09 2020. [CrossRef]
- M. M. Vasbinder et al., "Identification of azabenzimidazoles as potent JAK1 selective inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 1, pp. 60-7, Jan 01 2016. [CrossRef]
- F. Stauffer, S. W. Cowan-Jacob, C. Scheufler, and P. Furet, "Identification of a 5-[3-phenyl-(2-cyclic-ether)-methylether]-4-aminopyrrolo[2,3-d]pyrimidine series of IGF-1R inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 8, pp. 2065-7, Apr 15 2016. [CrossRef]
- T. Wang et al., "Design, synthesis and evaluation of pyrrolo[2,3-d]pyrimidine-phenylamide hybrids as potent Janus kinase 2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 12, pp. 2936-2941, Jun 15 2016. [CrossRef]
- H. Kim, M. K. Kim, H. Choo, and Y. Chong, "Novel JAK1-selective benzimidazole inhibitors with enhanced membrane permeability," (in eng), Bioorg Med Chem Lett, vol. 26, no. 14, pp. 3213-3215, Jul 15 2016. [CrossRef]
- V. J. Cee et al., "Alkynylpyrimidine amide derivatives as potent, selective, and orally active inhibitors of Tie-2 kinase," (in eng), J Med Chem, vol. 50, no. 4, pp. 627-40, Feb 22 2007. [CrossRef]
- M. W. Martin et al., "Novel 2-aminopyrimidine carbamates as potent and orally active inhibitors of Lck: synthesis, SAR, and in vivo antiinflammatory activity," (in eng), J Med Chem, vol. 49, no. 16, pp. 4981-91, Aug 10 2006. [CrossRef]
- O. Phuangsawai et al., "Evaluation of the anti-malarial activity and cytotoxicity of 2,4-diamino-pyrimidine-based kinase inhibitors," (in eng), Eur J Med Chem, vol. 124, pp. 896-905, Nov 29 2016. [CrossRef]
- W. Hou, Y. Ren, Z. Zhang, H. Sun, Y. Ma, and B. Yan, "Novel quinazoline derivatives bearing various 6-benzamide moieties as highly selective and potent EGFR inhibitors," (in eng), Bioorg Med Chem, vol. 26, no. 8, pp. 1740-1750, May 01 2018. [CrossRef]
- S. Narayan et al., "ASR352, A potent anticancer agent: Synthesis, preliminary SAR, and biological activities against colorectal cancer bulk, 5-fluorouracil/oxaliplatin resistant and stem cells," (in eng), Eur J Med Chem, vol. 161, pp. 456-467, Jan 01 2019. [CrossRef]
- V. H. U. Y. V. U. G. P. U. A. R. P. (US), "Substituted pyrrolo[2,3-b]pyridines as ITK and JAK inhibitors," United States, 2013/04/18.
- Y. Wang et al., "Identification of 4-(2-furanyl)pyrimidin-2-amines as Janus kinase 2 inhibitors," (in eng), Bioorg Med Chem, vol. 25, no. 1, pp. 75-83, Jan 01 2017. [CrossRef]
- B. D. G. U. B. M. B. U. D. G. U. G. M. A. U. K. S. S. U. L. C. M. U. L. Q. U. S. QING, "Substituted tetrahydrocarbazole and carbazole carboxamide compounds," United States, 2013/06/25.
- H. K. J. W. T. J. T. K. J. K. J. J. M. M. J. U. M. J. N. M. (JP), "Substituted pyrrolo[2,3-h][1,6]naphthyridines and compositions thereof as JAK inhibitors," United States, 2011/08/12.
- Y. B. V. U. B. G. D. U. G. A. K. I. P. W. J. (US), "Imidazopyridazine JAK3 inhibitors and their use for the treatment of inflammatory and autoimmune diseases," United States, 2012/09/06.
- V. H. (US) and Y. V. I. G. P. I. A. R. P. (US), "Substituted pyrrolo[2,3-b]pyrazines and substituted pyrazolo[3,4-b]pyridines as ITK and JAK kinase inhibitors," United States, 2013/04/18.
- P. Czodrowski et al., "Structure-Based Optimization of Potent, Selective, and Orally Bioavailable CDK8 Inhibitors Discovered by High-Throughput Screening," (in eng), J Med Chem, vol. 59, no. 20, pp. 9337-9349, Oct 27 2016. [CrossRef]
- M. P. Clark et al., "Development of new pyrrolopyrimidine-based inhibitors of Janus kinase 3 (JAK3)," (in eng), Bioorg Med Chem Lett, vol. 17, no. 5, pp. 1250-3, Mar 01 2007. [CrossRef]
- M.-F. M. J. U. B. P. J. U. B. M. J. U. B. J. W. U. G. A. J. U. K. S. G. U. M. D. WAYNE, "Pyrrolo[2,3-d]pyrimidine compounds," United States, 2008/08/20.
- J. B. Smaill et al., "Tyrosine Kinase Inhibitors. 20. Optimization of Substituted Quinazoline and Pyrido[3,4-d]pyrimidine Derivatives as Orally Active, Irreversible Inhibitors of the Epidermal Growth Factor Receptor Family," (in eng), J Med Chem, vol. 59, no. 17, pp. 8103-24, Sep 08 2016. [CrossRef]
- H. T. U. X. C.-B. U. L. H.-Y. U. L. Q. (US), "US-8765734-B2," United States, 2010/03/10.
- T. K. J. W. T. J. H. K. J. K. K. J. N. T. J. Y. A. J. N. T. J. K. T. J. H. Y. (JP), "Tricyclic pyrrolopyridine compound, and JAK inhibitor," United States, 2013/02/08.
- X. N. U. L. M. C. H. H. C. D. W. (CN), "Substituted heteroaryl compounds and methods of use," United States, 2014/03/28.
- G. D. M. U. B. K. A. U. V. E. (US), "Azaindole derivatives as tyrosine kinase inhibitors," United States, 2011/05/17.
- Y. Ge et al., "Discovery of Novel Bruton's Tyrosine Kinase (BTK) Inhibitors Bearing a," (in eng), ACS Med Chem Lett, vol. 7, no. 12, pp. 1050-1055, Dec 08 2016. [CrossRef]
- L. Y.-L. U. R. J. D. (US), "3-[4-(7H-pyrrolo[2,3-D]pyrimidin-4-yl)-1H-pyrazol-1-yl]octane—or heptane-nitrile as JAK inhibitors," United States, 2009/05/22.
- S. W.-G. C. D. W. C. L. J. C. J. J. (CN), "Pyrrolopyrimidine compounds and uses thereof," United States, 2010/08/20.
- B. J. U. C. M. L. U. C. M. U. C. J. T. U. K. J. D. U. J. J. U. P. S. U. S. P. (US)SIU TONY (US)SMITH GRAHAM FRANK (US)TORRES LUIS E (US)WOO HYUN CHONG (US)YOUNG JONATHAN R (US) ZHANG HONGJUN (US), "Cyanomethylpyrazole carboxamides as janus kinase inhibitors," United States, 2011/09/22.
- R. E. P. U. R. M. V. R. (US), "Substituted pyrido[2,3-d]pyrimidin-7(8H)-ones and therapeutic uses thereof," United States, 2009/12/18.
- B. Lam et al., "Discovery of TAK-659 an orally available investigational inhibitor of Spleen Tyrosine Kinase (SYK)," (in eng), Bioorg Med Chem Lett, vol. 26, no. 24, pp. 5947-5950, Dec 15 2016. [CrossRef]
- P. Blomgren et al., "Discovery of Lanraplenib (GS-9876): A Once-Daily Spleen Tyrosine Kinase Inhibitor for Autoimmune Diseases," (in eng), ACS Med Chem Lett, vol. 11, no. 4, pp. 506-513, Apr 09 2020. [CrossRef]
- A. F. Abdel-Magid, "Janus-Associated Kinase 1 (JAK1) Inhibitors as Potential Treatment for Immune Disorders," (in eng), ACS Med Chem Lett, vol. 8, no. 6, pp. 598-600, Jun 08 2017. [CrossRef]
- J. Kempson et al., "Discovery of highly potent, selective, covalent inhibitors of JAK3," (in eng), Bioorg Med Chem Lett, vol. 27, no. 20, pp. 4622-4625, Oct 15 2017. [CrossRef]
- E. J. Hanan, J. Liang, X. Wang, R. A. Blake, N. Blaquiere, and S. T. Staben, "Monomeric Targeted Protein Degraders," (in eng), J Med Chem, vol. 63, no. 20, pp. 11330-11361, Oct 22 2020. [CrossRef]
- K. A. Brameld et al., "Discovery of the Irreversible Covalent FGFR Inhibitor 8-(3-(4-Acryloylpiperazin-1-yl)propyl)-6-(2,6-dichloro-3,5-dimethoxyphenyl)-2-(methylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (PRN1371) for the Treatment of Solid Tumors," (in eng), J Med Chem, vol. 60, no. 15, pp. 6516-6527, Aug 10 2017. [CrossRef]
- <div data-v-0a50e552="" style="background-repeat: no-repeat; box-sizing: inherit; padding: 0px; margin: 0px;">SGC Frankfurt donated chemical probe project: TP-030-2 was donated by Takeda. Website: https://www.sgc-ffm.uni-frankfurt.de/#!specificprobeoverview/TP-030-2. Control: TP-030n. References: 1. Yoshikawa, Masato, Morihisa Saitoh, Taisuke Katoh, Tomohiro Seki, Simone V. Bigi, Yuji Shimizu, Tsuyoshi Ishii, Takuro Okai, Masako Kuno, Harumi Hattori, Etsuro Watanabe, Kumar S. Saikatendu, Hua Zou, Masanori Nakakariya, Takayuki Tatamiya, Yoshihisa Nakada, and Takatoshi Yogo. 2018. ‘Discovery of 7-Oxo-2,4,5,7-Tetrahydro-6 H-Pyrazolo[3,4- c]Pyridine Derivatives as Potent, Orally Available, and Brain-Penetrating Receptor Interacting Protein 1 (RIP1) Kinase Inhibitors: Analysis of Structure-Kinetic Relationships’. Journal of Medicinal Chemistry />" ed. [CrossRef]
- L. Yao et al., "Design and Synthesis of Ligand Efficient Dual Inhibitors of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Based on Ruxolitinib and Vorinostat," (in eng), J Med Chem, vol. 60, no. 20, pp. 8336-8357, Oct 26 2017. [CrossRef]
- T. Fischer et al., "Discovery of novel substituted benzo-anellated 4-benzylamino pyrrolopyrimidines as dual EGFR and VEGFR2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 27, no. 12, pp. 2708-2712, Jun 15 2017. [CrossRef]
- M. L. Vazquez et al., "Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases," (in eng), J Med Chem, vol. 61, no. 3, pp. 1130-1152, Feb 08 2018. [CrossRef]
- B. J. U. C. J. U. S. T. U. S. G. F. U. T. L. E. U. W. H. C. U. Y. J. R. U. W. Z. (CN)SHI FENG (CN), "Pyrazole carboxamides as Janus kinase inhibitors," United States, 2011/09/22.
- L. Juillerat-Jeanneret, J. D. Aubert, J. Mikulic, and D. Golshayan, "Fibrogenic Disorders in Human Diseases: From Inflammation to Organ Dysfunction," (in eng), J Med Chem, vol. 61, no. 22, pp. 9811-9840, Nov 21 2018. [CrossRef]
- Y. Huang, G. Dong, H. Li, N. Liu, W. Zhang, and C. Sheng, "Discovery of Janus Kinase 2 (JAK2) and Histone Deacetylase (HDAC) Dual Inhibitors as a Novel Strategy for the Combinational Treatment of Leukemia and Invasive Fungal Infections," (in eng), J Med Chem, vol. 61, no. 14, pp. 6056-6074, Jul 26 2018. [CrossRef]
- N. P. Grimster et al., "Discovery and Optimization of a Novel Series of Highly Selective JAK1 Kinase Inhibitors," (in eng), J Med Chem, vol. 61, no. 12, pp. 5235-5244, Jun 28 2018. [CrossRef]
- C. Chough, M. Joung, S. Lee, J. Lee, J. H. Kim, and B. M. Kim, "Development of selective inhibitors for the treatment of rheumatoid arthritis: (R)-3-(3-(Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)-3-oxopropanenitrile as a JAK1-selective inhibitor," (in eng), Bioorg Med Chem, vol. 26, no. 8, pp. 1495-1510, May 01 2018. [CrossRef]
- T. Yu et al., "Discovery of a highly potent orally bioavailable imidazo-[1, 2-a]pyrazine Aurora inhibitor," (in eng), Bioorg Med Chem Lett, vol. 28, no. 8, pp. 1397-1403, May 01 2018. [CrossRef]
- M. I. El-Gamal, S. K. Al-Ameen, D. M. Al-Koumi, M. G. Hamad, N. A. Jalal, and C. H. Oh, "Recent Advances of Colony-Stimulating Factor-1 Receptor (CSF-1R) Kinase and Its Inhibitors," (in eng), J Med Chem, vol. 61, no. 13, pp. 5450-5466, Jul 12 2018. [CrossRef]
- Y. Wang et al., "Discovery of potent anti-inflammatory 4-(4,5,6,7-tetrahydrofuro[3,2-c]pyridin-2-yl) pyrimidin-2-amines for use as Janus kinase inhibitors," (in eng), Bioorg Med Chem, vol. 27, no. 12, pp. 2592-2597, Jun 15 2019. [CrossRef]
- J. Zheng, J. Wu, X. Ding, H. C. Shen, and G. Zou, "Small molecule approaches to treat autoimmune and inflammatory diseases (Part I): Kinase inhibitors," (in eng), Bioorg Med Chem Lett, vol. 38, p. 127862, Apr 15 2021. [CrossRef]
- L. Yao, P. M. Ramanujulu, A. Poulsen, S. Ohlson, and B. W. Dymock, "Merging of ruxolitinib and vorinostat leads to highly potent inhibitors of JAK2 and histone deacetylase 6 (HDAC6)," (in eng), Bioorg Med Chem Lett, vol. 28, no. 15, pp. 2636-2640, Aug 15 2018. [CrossRef]
- Q. Liu et al., "Conversion of carbazole carboxamide based reversible inhibitors of Bruton's tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series," (in eng), Bioorg Med Chem Lett, vol. 28, no. 18, pp. 3080-3084, Oct 01 2018. [CrossRef]
- A. C. Pippione et al., "-Acetyl-3-aminopyrazoles block the non-canonical NF-kB cascade by selectively inhibiting NIK," (in eng), Medchemcomm, vol. 9, no. 6, pp. 963-968, Jun 01 2018. [CrossRef]
- L. Yin et al., "A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in gliobastoma therapy," (in eng), Eur J Med Chem, vol. 144, pp. 1-28, Jan 20 2018. [CrossRef]
- C. Chough, S. Lee, M. Joung, J. Lee, J. H. Kim, and B. M. Kim, "Design, synthesis and evaluation of (" (in eng), Medchemcomm, vol. 9, no. 3, pp. 477-489, Mar 01 2018. [CrossRef]
- Y. Y. Chu-Farseeva et al., "Design and synthesis of potent dual inhibitors of JAK2 and HDAC based on fusing the pharmacophores of XL019 and vorinostat," (in eng), Eur J Med Chem, vol. 158, pp. 593-619, Oct 05 2018. [CrossRef]
- K. Zhang et al., "Development and Therapeutic Implications of Tyrosine Kinase 2 Inhibitors," (in eng), J Med Chem, vol. 66, no. 7, pp. 4378-4416, Apr 13 2023. [CrossRef]
- S. P. Henry and W. L. Jorgensen, "Progress on the Pharmacological Targeting of Janus Pseudokinases," (in eng), J Med Chem, vol. 66, no. 16, pp. 10959-10990, Aug 24 2023. [CrossRef]
- Y. Ge et al., "Identification of highly potent BTK and JAK3 dual inhibitors with improved activity for the treatment of B-cell lymphoma," (in eng), Eur J Med Chem, vol. 143, pp. 1847-1857, Jan 01 2018. [CrossRef]
- R. Moslin et al., "Identification of imidazo[1,2-," (in eng), Medchemcomm, vol. 8, no. 4, pp. 700-712, Apr 01 2017. [CrossRef]
- S. Noji et al., "Discovery of a Janus Kinase Inhibitor Bearing a Highly Three-Dimensional Spiro Scaffold: JTE-052 (Delgocitinib) as a New Dermatological Agent to Treat Inflammatory Skin Disorders," (in eng), J Med Chem, vol. 63, no. 13, pp. 7163-7185, Jul 09 2020. [CrossRef]
- Y. Li et al., "Discovery of 4-piperazinyl-2-aminopyrimidine derivatives as dual inhibitors of JAK2 and FLT3," (in eng), Eur J Med Chem, vol. 181, p. 111590, Nov 01 2019. [CrossRef]
- "10.6019/CHEMBL5465560," ed.
- Q. Miao, K. Ma, D. Chen, X. Wu, and S. Jiang, "Targeting tropomyosin receptor kinase for cancer therapy," (in eng), Eur J Med Chem, vol. 175, pp. 129-148, Aug 01 2019. [CrossRef]
- R. Moslin et al., "Identification of," (in eng), J Med Chem, vol. 62, no. 20, pp. 8953-8972, Oct 24 2019. [CrossRef]
- M. C. Bryan and N. S. Rajapaksa, "Kinase Inhibitors for the Treatment of Immunological Disorders: Recent Advances," (in eng), J Med Chem, vol. 61, no. 20, pp. 9030-9058, Oct 25 2018. [CrossRef]
- C. Zhang et al., "Discovery of 3-(4-(2-((1," (in eng), J Med Chem, vol. 64, no. 4, pp. 1966-1988, Feb 25 2021. [CrossRef]
- C. Shi et al., "Discovery of 6-(2-(dimethylamino)ethyl)-N-(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole-6-yl)pyrimidin-2-yl)-5,6,7,8-tetrahydro-1,6-naphthyridin-2-amine as a highly potent cyclin-dependent kinase 4/6 inhibitor for treatment of cancer," (in eng), Eur J Med Chem, vol. 178, pp. 352-364, Sep 15 2019. [CrossRef]
- X. Liang et al., "Discovery of Novel Janus Kinase (JAK) and Histone Deacetylase (HDAC) Dual Inhibitors for the Treatment of Hematological Malignancies," (in eng), J Med Chem, vol. 62, no. 8, pp. 3898-3923, Apr 25 2019. [CrossRef]
- X. Liang et al., "Design, Synthesis, and Antitumor Evaluation of 4-Amino-(1," (in eng), ACS Med Chem Lett, vol. 7, no. 10, pp. 950-955, Oct 13 2016. [CrossRef]
- J. Bach et al., "Identification of 2-Imidazopyridine and 2-Aminopyridone Purinones as Potent Pan-Janus Kinase (JAK) Inhibitors for the Inhaled Treatment of Respiratory Diseases," (in eng), J Med Chem, vol. 62, no. 20, pp. 9045-9060, Oct 24 2019. [CrossRef]
- G. Tang, L. Liu, X. Wang, and Z. Pan, "Discovery of 7H-pyrrolo[2,3-d]pyrimidine derivatives as selective covalent irreversible inhibitors of interleukin-2-inducible T-cell kinase (Itk)," (in eng), Eur J Med Chem, vol. 173, pp. 167-183, Jul 01 2019. [CrossRef]
- S. Antonysamy et al., "Fragment-based discovery of JAK-2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 19, no. 1, pp. 279-82, Jan 01 2009. [CrossRef]
- N. S. Garton et al., "Optimisation of a novel series of potent and orally bioavailable azanaphthyridine SYK inhibitors," (in eng), Bioorg Med Chem Lett, vol. 26, no. 19, pp. 4606-4612, Oct 01 2016. [CrossRef]
- K. A. Leonard et al., "Discovery of a Gut-Restricted JAK Inhibitor for the Treatment of Inflammatory Bowel Disease," (in eng), J Med Chem, vol. 63, no. 6, pp. 2915-2929, Mar 26 2020. [CrossRef]
- L. Shi, Z. Zhong, X. Li, Y. Zhou, and Z. Pan, "Discovery of an Orally Available Janus Kinase 3 Selective Covalent Inhibitor," (in eng), J Med Chem, vol. 62, no. 2, pp. 1054-1066, Jan 24 2019. [CrossRef]
- S. National Center for Biotechnology Information (2024). PubChem Substance Record for SID 395763535, Source: PATENTSCOPE (WIPO). Retrieved December 18, 2024 from https://pubchem.ncbi.nlm.nih.gov/substance/395763535 .
- X. Yuan, H. Wu, H. Bu, J. Zhou, and H. Zhang, "Targeting the immunity protein kinases for immuno-oncology," (in eng), Eur J Med Chem, vol. 163, pp. 413-427, Feb 01 2019. [CrossRef]
- A. Ritzén et al., "Fragment-Based Discovery of 6-Arylindazole JAK Inhibitors," (in eng), ACS Med Chem Lett, vol. 7, no. 6, pp. 641-6, Jun 09 2016. [CrossRef]
- A. Fensome et al., "Dual Inhibition of TYK2 and JAK1 for the Treatment of Autoimmune Diseases: Discovery of (( S)-2,2-Difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841)," (in eng), J Med Chem, vol. 61, no. 19, pp. 8597-8612, Oct 11 2018. [CrossRef]
- S. F. TP-030-2, "10.6019/CHEMBL4507326,".
- T. Yang et al., "Discovery of Potent and Orally Effective Dual Janus Kinase 2/FLT3 Inhibitors for the Treatment of Acute Myelogenous Leukemia and Myeloproliferative Neoplasms," (in eng), J Med Chem, vol. 62, no. 22, pp. 10305-10320, Nov 27 2019. [CrossRef]
- N. S. F. J. National Center for Biotechnology Information (2024). PubChem Bioassay Record for AID 1791642, Source: ChEMBL. Retrieved December 25, 2024 from https://pubchem.ncbi.nlm.nih.gov/bioassay/1791642 . ed.
- S. M. Lee et al., "The discovery of 2,5-isomers of triazole-pyrrolopyrimidine as selective Janus kinase 2 (JAK2) inhibitors versus JAK1 and JAK3," (in eng), Bioorg Med Chem, vol. 24, no. 21, pp. 5036-5046, Nov 01 2016. [CrossRef]
- Z. Luo et al., "Discovery and optimization of selective RET inhibitors via scaffold hopping," (in eng), Bioorg Med Chem Lett, vol. 47, p. 128149, Sep 01 2021. [CrossRef]
- A. Egyed, D. Bajusz, and G. M. Keserű, "The impact of binding site waters on the activity/selectivity trade-off of Janus kinase 2 (JAK2) inhibitors," (in eng), Bioorg Med Chem, vol. 27, no. 8, pp. 1497-1508, Apr 15 2019. [CrossRef]
- Y. Chen et al., "Optimization of Pyrimidine Compounds as Potent JAK1 Inhibitors and the Discovery of R507 as a Clinical Candidate," (in eng), ACS Med Chem Lett, vol. 13, no. 11, pp. 1805-1811, Nov 10 2022. [CrossRef]
- L. Liu et al., "Structure-based design of novel class II c-Met inhibitors: 2. SAR and kinase selectivity profiles of the pyrazolone series," (in eng), J Med Chem, vol. 55, no. 5, pp. 1868-97, Mar 08 2012. [CrossRef]
- A. Schlapbach et al., "Pyrrolo-pyrimidones: a novel class of MK2 inhibitors with potent cellular activity," (in eng), Bioorg Med Chem Lett, vol. 18, no. 23, pp. 6142-6, Dec 01 2008. [CrossRef]
- D. Bauer et al., "Evaluation of indazole-based compounds as a new class of potent KDR/VEGFR-2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 18, no. 17, pp. 4844-8, Sep 01 2008. [CrossRef]
- H. Zhou et al., "Discovery and optimization of heteroaryl piperazines as potent and selective PI3Kδ inhibitors," (in eng), Bioorg Med Chem Lett, vol. 30, no. 1, p. 126715, Jan 01 2020. [CrossRef]
- R. R. Shah et al., "Hi-JAK-ing the ubiquitin system: The design and physicochemical optimisation of JAK PROTACs," (in eng), Bioorg Med Chem, vol. 28, no. 5, p. 115326, Mar 01 2020. [CrossRef]
- Y. Zhu et al., "Synthesis and biological activity of thieno[3,2-d]pyrimidines as potent JAK3 inhibitors for the treatment of idiopathic pulmonary fibrosis," (in eng), Bioorg Med Chem, vol. 28, no. 2, p. 115254, Jan 15 2020. [CrossRef]
- Y. Sasaki et al., "Efficient synthesis of tert-butyl 3-cyano-3-cyclopropyl-2-oxopyrrolidine-4-carboxylates: Highly functionalized 2-pyrrolidinone enabling access to novel macrocyclic Tyk2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 30, no. 5, p. 126963, Mar 01 2020. [CrossRef]
- K. Lu et al., "Discovery of triazolo [1,5-a] pyridine derivatives as novel JAK1/2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 30, no. 14, p. 127225, Jul 15 2020. [CrossRef]
- Y. Zhu et al., "Identification of," (in eng), J Med Chem, vol. 63, no. 13, pp. 6748-6773, Jul 09 2020. [CrossRef]
- X. Wang, G. Xue, and Z. Pan, "Design, synthesis and structure-activity relationship of indolylindazoles as potent and selective covalent inhibitors of interleukin-2 inducible T-cell kinase (ITK)," (in eng), Eur J Med Chem, vol. 187, p. 111918, Feb 01 2020. [CrossRef]
- B. S. Gerstenberger et al., "Discovery of Tyrosine Kinase 2 (TYK2) Inhibitor (PF-06826647) for the Treatment of Autoimmune Diseases," (in eng), J Med Chem, vol. 63, no. 22, pp. 13561-13577, Nov 25 2020. [CrossRef]
- M. Zak et al., "Discovery of a class of highly potent Janus Kinase 1/2 (JAK1/2) inhibitors demonstrating effective cell-based blockade of IL-13 signaling," (in eng), Bioorg Med Chem Lett, vol. 29, no. 12, pp. 1522-1531, Jun 15 2019. [CrossRef]
- D. J. Baillache and A. Unciti-Broceta, "Recent developments in anticancer kinase inhibitors based on the pyrazolo[3,4-," (in eng), RSC Med Chem, vol. 11, no. 10, pp. 1112-1135, Oct 01 2020. [CrossRef]
- G. Thoma et al., "Syk inhibitors with high potency in presence of blood," (in eng), Bioorg Med Chem Lett, vol. 24, no. 10, pp. 2278-82, May 15 2014. [CrossRef]
- L. Luo et al., "Synthesis and anticancer activity evaluation of naphthalene-substituted triazole spirodienones," (in eng), Eur J Med Chem, vol. 213, p. 113039, Mar 05 2021. [CrossRef]
- T. Yang et al., "-(Pyrimidin-2-yl)-1,2,3,4-tetrahydroisoquinolin-6-amine Derivatives as Selective Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms," (in eng), J Med Chem, vol. 63, no. 23, pp. 14921-14936, Dec 10 2020. [CrossRef]
- C. J. Matheson et al., "2-Arylamino-6-ethynylpurines are cysteine-targeting irreversible inhibitors of Nek2 kinase," (in eng), RSC Med Chem, vol. 11, no. 6, pp. 707-731, Jun 01 2020. [CrossRef]
- C. Liu et al., "Discovery of BMS-986202: A Clinical Tyk2 Inhibitor that Binds to Tyk2 JH2," (in eng), J Med Chem, vol. 64, no. 1, pp. 677-694, Jan 14 2021. [CrossRef]
- Y. G. Zheng et al., "Design, synthesis, biological activity evaluation of 3-(4-phenyl-1H-imidazol-2-yl)-1H-pyrazole derivatives as potent JAK 2/3 and aurora A/B kinases multi-targeted inhibitors," (in eng), Eur J Med Chem, vol. 209, p. 112934, Jan 01 2021. [CrossRef]
- K. Sanachai et al., "Discovery of novel JAK2 and EGFR inhibitors from a series of thiazole-based chalcone derivatives," (in eng), RSC Med Chem, vol. 12, no. 3, pp. 430-438, Mar 01 2021. [CrossRef]
- L. Wu et al., "Discovery of Pemigatinib: A Potent and Selective Fibroblast Growth Factor Receptor (FGFR) Inhibitor," (in eng), J Med Chem, vol. 64, no. 15, pp. 10666-10679, Aug 12 2021. [CrossRef]
- P. Xu et al., "Discovery of imidazopyrrolopyridines derivatives as novel and selective inhibitors of JAK2," (in eng), Eur J Med Chem, vol. 218, p. 113394, Jun 05 2021. [CrossRef]
- D. Rao et al., "Discovery of a potent, selective, and covalent ZAP-70 kinase inhibitor," (in eng), Eur J Med Chem, vol. 219, p. 113393, Jul 05 2021. [CrossRef]
- S. Howard et al., "Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity," (in eng), J Med Chem, vol. 52, no. 2, pp. 379-88, Jan 22 2009. [CrossRef]
- J. R. Pollard and M. Mortimore, "Discovery and development of aurora kinase inhibitors as anticancer agents," (in eng), J Med Chem, vol. 52, no. 9, pp. 2629-51, May 14 2009. [CrossRef]
- T. Yang et al., "Identification of a Novel 2,8-Diazaspiro[4.5]decan-1-one Derivative as a Potent and Selective Dual TYK2/JAK1 Inhibitor for the Treatment of Inflammatory Bowel Disease," (in eng), J Med Chem, vol. 65, no. 4, pp. 3151-3172, Feb 24 2022. [CrossRef]
- X. Liang et al., "Discovery of Novel Pyrrolo[2,3-," (in eng), J Med Chem, vol. 65, no. 2, pp. 1243-1264, Jan 27 2022. [CrossRef]
- C. R. Wellaway et al., "Investigation of Janus Kinase (JAK) Inhibitors for Lung Delivery and the Importance of Aldehyde Oxidase Metabolism," (in eng), J Med Chem, vol. 65, no. 1, pp. 633-664, Jan 13 2022. [CrossRef]
- S. Wu, M. Liao, M. Li, M. Sun, N. Xi, and Y. Zeng, "Structure-based discovery of potent inhibitors of Axl: design, synthesis, and biological evaluation," (in eng), RSC Med Chem, vol. 13, no. 10, pp. 1246-1264, Oct 19 2022. [CrossRef]
- W. Mao et al., "Synthesis and evaluation of hydrazinyl-containing pyrrolo[2,3-d]pyrimidine series as potent, selective and oral JAK1 inhibitors for the treatment of rheumatoid arthritis," (in eng), Bioorg Med Chem Lett, vol. 74, p. 128905, Oct 15 2022. [CrossRef]
- S. Cascioferro et al., "An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds," (in eng), Eur J Med Chem, vol. 142, pp. 328-375, Dec 15 2017. [CrossRef]
- V. Asati, A. Anant, P. Patel, K. Kaur, and G. D. Gupta, "Pyrazolopyrimidines as anticancer agents: A review on structural and target-based approaches," (in eng), Eur J Med Chem, vol. 225, p. 113781, Dec 05 2021. [CrossRef]
- O. M. Soltan, M. E. Shoman, S. A. Abdel-Aziz, A. Narumi, H. Konno, and M. Abdel-Aziz, "Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy," (in eng), Eur J Med Chem, vol. 225, p. 113768, Dec 05 2021. [CrossRef]
- H. Yamagishi et al., "Discovery of tricyclic dipyrrolopyridine derivatives as novel JAK inhibitors," (in eng), Bioorg Med Chem, vol. 25, no. 20, pp. 5311-5326, Oct 15 2017. [CrossRef]
- R. Kiss et al., "Identification of a novel inhibitor of JAK2 tyrosine kinase by structure-based virtual screening," (in eng), Bioorg Med Chem Lett, vol. 19, no. 13, pp. 3598-601, Jul 01 2009. [CrossRef]
- L. J. Wilson et al., "Synthetic staurosporines via a ring closing metathesis strategy as potent JAK3 inhibitors and modulators of allergic responses," (in eng), Bioorg Med Chem Lett, vol. 19, no. 12, pp. 3333-8, Jun 15 2009. [CrossRef]
- S. Ioannidis et al., "Discovery of pyrazol-3-ylamino pyrazines as novel JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 19, no. 23, pp. 6524-8, Dec 01 2009. [CrossRef]
- M. W. Ledeboer et al., "2-Aminopyrazolo[1,5-a]pyrimidines as potent and selective inhibitors of JAK2," (in eng), Bioorg Med Chem Lett, vol. 19, no. 23, pp. 6529-33, Dec 01 2009. [CrossRef]
- T. Wang et al., "A novel chemotype of kinase inhibitors: Discovery of 3,4-ring fused 7-azaindoles and deazapurines as potent JAK2 inhibitors," (in eng), Bioorg Med Chem Lett, vol. 20, no. 1, pp. 153-6, Jan 01 2010. [CrossRef]
- "CHEMBL104466," ed.
- M. H. U. F. S. V. G. S. M. A. H. G. D. J. A. G. D. B. (GB),.
- R. A. M. Serafim et al., "Discovery of a Potent Dual SLK/STK10 Inhibitor Based on a Maleimide Scaffold," (in eng), J Med Chem, vol. 64, no. 18, pp. 13259-13278, Sep 23 2021. [CrossRef]
- R. Ruel et al., "Discovery and preclinical studies of 5-isopropyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(2-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine (BMS-645737), an in vivo active potent VEGFR-2 inhibitor," (in eng), Bioorg Med Chem Lett, vol. 18, no. 9, pp. 2985-9, May 01 2008. [CrossRef]
- J. Das et al., "Pyrazolo-pyrimidines: a novel heterocyclic scaffold for potent and selective p38 alpha inhibitors," (in eng), Bioorg Med Chem Lett, vol. 18, no. 8, pp. 2652-7, Apr 15 2008. [CrossRef]
- M. H. U. F. S. V. G. S. M. A. H. G. D. J. A. G. D. B. (GB), "Selective kinase inhibitors," United States, 2009/02/11.
- R. S. Bhide et al., "Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor," (in eng), J Med Chem, vol. 49, no. 7, pp. 2143-6, Apr 06 2006. [CrossRef]
- R. M. Borzilleri et al., "Discovery and evaluation of N-cyclopropyl- 2,4-difluoro-5-((2-(pyridin-2-ylamino)thiazol-5- ylmethyl)amino)benzamide (BMS-605541), a selective and orally efficacious inhibitor of vascular endothelial growth factor receptor-2," (in eng), J Med Chem, vol. 49, no. 13, pp. 3766-9, Jun 29 2006. [CrossRef]
- C. Liu et al., "5-Cyanopyrimidine derivatives as a novel class of potent, selective, and orally active inhibitors of p38alpha MAP kinase," (in eng), J Med Chem, vol. 48, no. 20, pp. 6261-70, Oct 06 2005. [CrossRef]
- Y. Sugimoto et al., "Novel pyrrolopyrimidines as Mps1/TTK kinase inhibitors for breast cancer," (in eng), Bioorg Med Chem, vol. 25, no. 7, pp. 2156-2166, Apr 01 2017. [CrossRef]

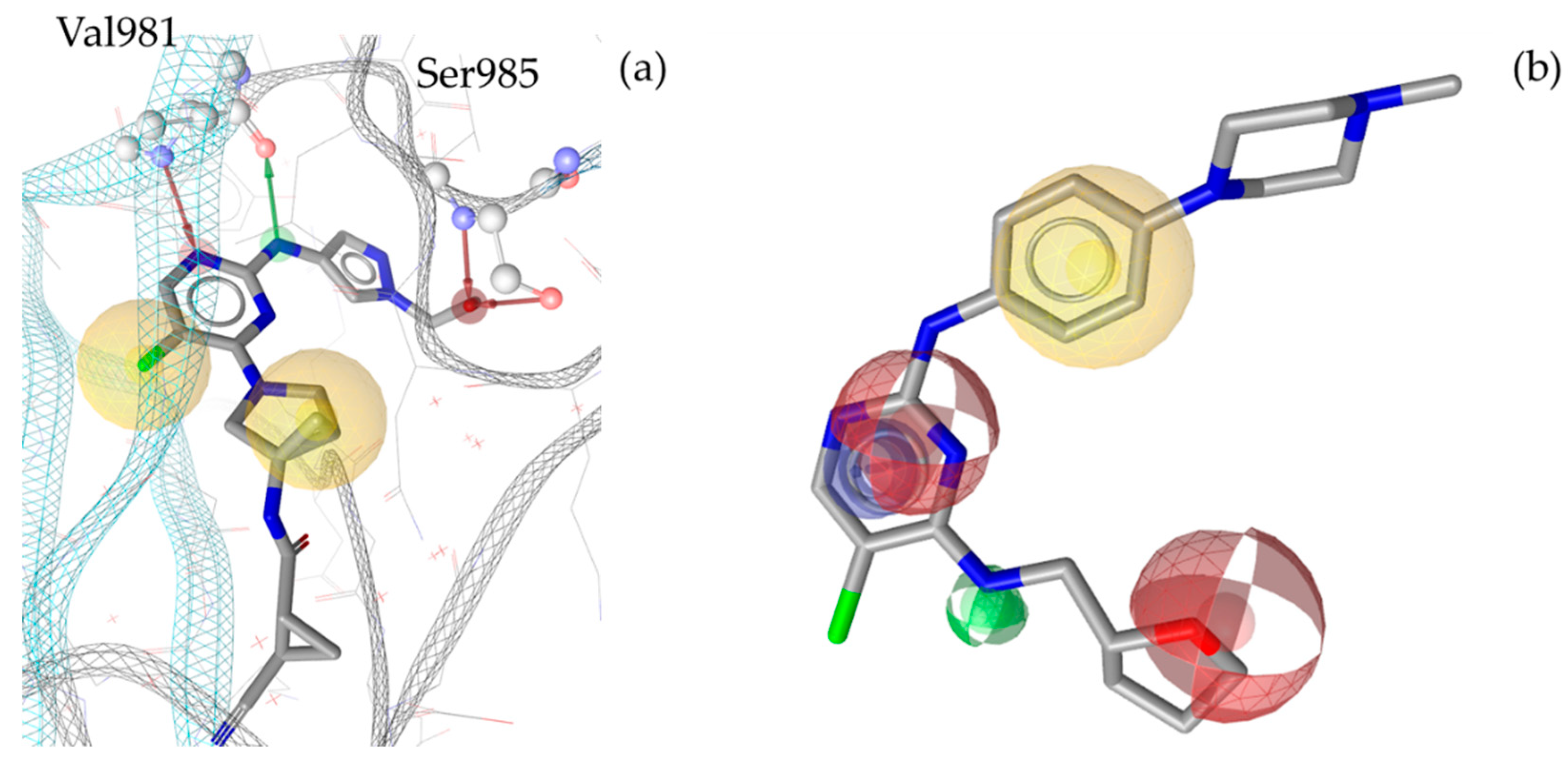
| Overall evaluation | JAK1 TOTAL (8 models) |
JAK2 TOTAL (10 models) |
JAK3 TOTAL (10 models) |
TYK2 TOTAL (9 models) |
|---|---|---|---|---|
| active hits / TPs | 95 | 167 | 116 | 68 |
| inactive hits | 0 | 15 | 2 | 40 |
| decoy hits | 79 | 75 | 292 | 136 |
| FPs | 79 | 90 | 294 | 176 |
| TNs | 3232 | 2799 | 4247 | 2935 |
| number of ACs in database |
105 | 185 | 129 | 75 |
| number of IAs in database |
48 | 49 | 42 | 61 |
| number of DCs in database |
3263 | 2840 | 4499 | 3050 |
| Model Accuracy | 0.97 | 0.96 | 0.93 | 0.94 |
| YoA | 0.55 | 0.65 | 0.28 | 0.28 |
| EF | 17.76 | 10.80 | 10.24 | 11.84 |
| sensitivity | 0.90 | 0.90 | 0.86 | 0.91 |
| specificity | 1.00 | 0.99 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





