1. Introduction
Cytochrome P450 enzymes (CYPs) are a superfamily of heme-containing monooxygenases that play a key role in metabolizing endogenous and exogenous compounds, including drugs, steroids, and toxins [
1]. They also contribute to natural product biosynthesis by catalyzing regio- and stereoselective oxidation reactions such as hydroxylation [
2], epoxidation [
3], decarboxylation [
4], nitration [
5], and C–C bond cleavage [
6]. These transformations enhance molecular complexity and bioactivity, facilitating the production of antibiotics, alkaloids, and other secondary metabolites. The broad substrate specificity and catalytic versatility of CYPs make them valuable in pharmaceutical synthesis, fine chemical production, and biocatalysis, offering environmentally friendly alternatives to traditional chemical processes.
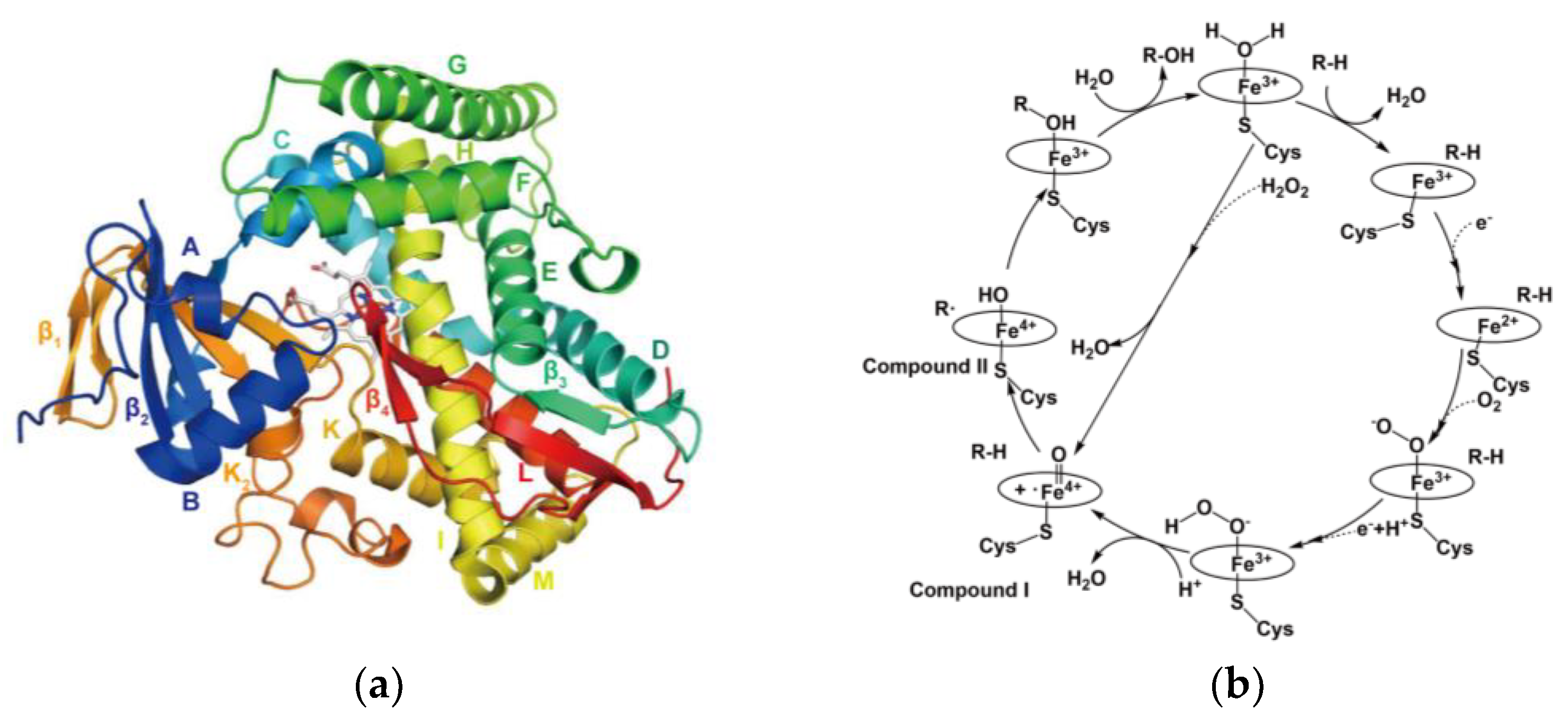
Cytochrome P450 enzymes share a conserved structural fold with a heme-binding domain that facilitates oxygen activation and catalysis [
7]. Their active site architecture varies to accommodate diverse substrates, with key structural elements such as the I-helix, F-G loop region, and heme-thiolate ligand playing crucial roles in catalysis [
7] (
Figure 1(
a)). This structural diversity enables their broad substrate specificity and functional adaptability in metabolism and biosynthesis. The catalytic cycle of most CYPs begins with substrate binding, inducing a conformational change and facilitating electron transfer from NAD(P)H via cytochrome P450 reductase [
8]. Molecular oxygen then binds to the heme iron, followed by a second electron transfer, forming a highly reactive iron-oxo species that drives oxidation reactions such as hydroxylation and epoxidation [
8]. The oxidized product is then released, restoring the enzyme to its resting state for the next catalytic cycle [
8] (
Figure 1(
b)).
The application of CYPs in synthetic biology requires enzyme engineering to overcome the limitations of wild-type variants, such as low catalytic efficiency, narrow substrate specificity, and poor stability under industrial conditions. Many natural CYPs exhibit low expression, dependence on specific redox partners, and insufficient regio- and stereoselectivity for targeted transformations. Their susceptibility to inhibition and degradation further restricts their practical use [
9]. Protein engineering approaches, including directed evolution and rational design, enhance stability, activity, and substrate scope to address these challenges [
10]. Optimized P450 variants will enable more efficient and selective biotransformations, expanding their applications in pharmaceuticals, fine chemicals, and biofuel production.
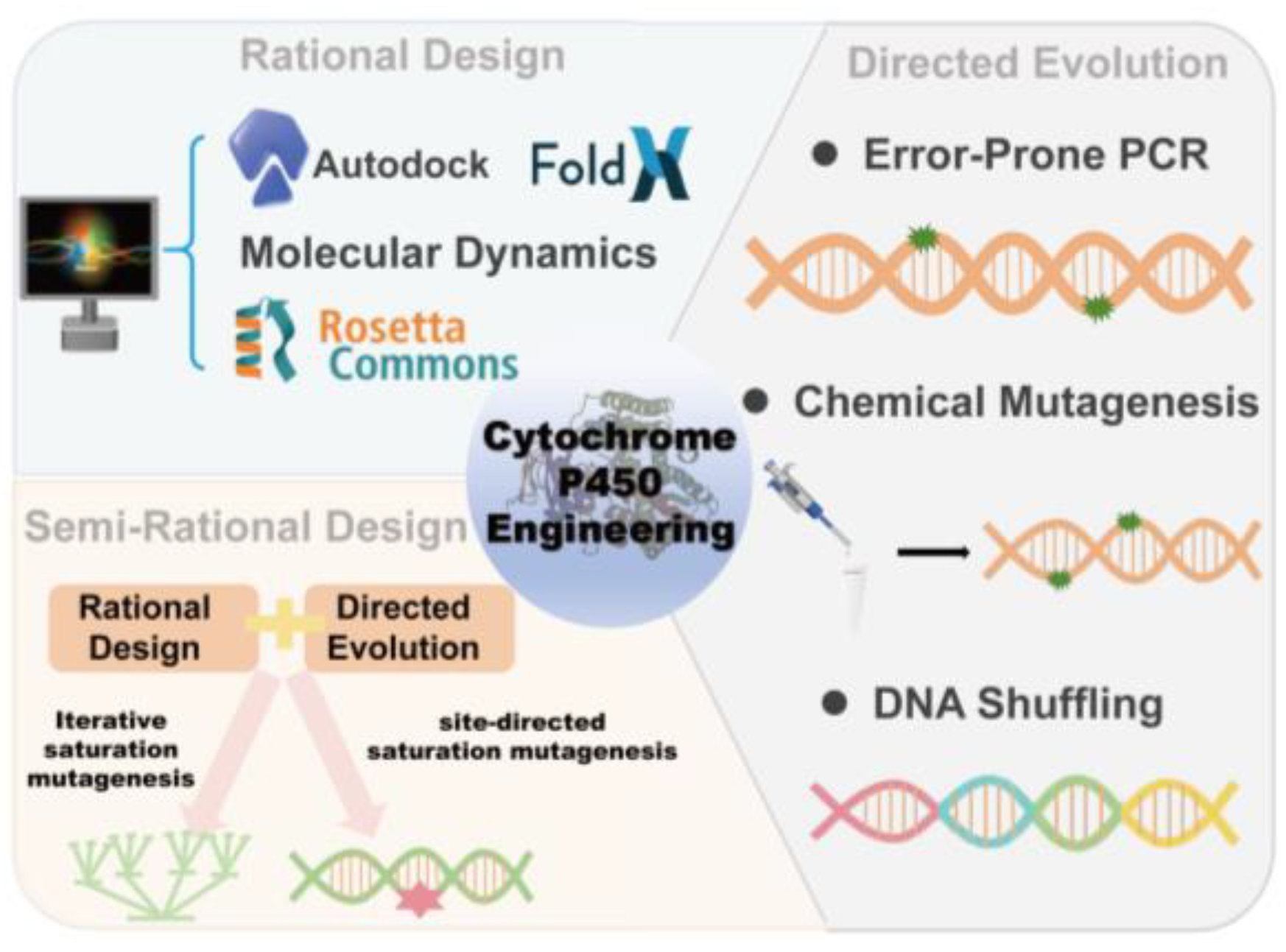
2. Engineering Approaches for Cytochrome P450 Enzymes
CYPs engineering employs rational design, semi-rational design, and directed evolution to optimize enzyme function (
Figure 2). These strategies have been pivotal in enhancing P450 enzyme properties for applications in biocatalysis, synthetic chemistry, and pharmaceutical development.
2.1 Rational Design
Rational design in protein engineering is a strategy that applies structural and mechanistic insights to guide the introduction of targeted mutations, thereby enhancing the function, stability, or specificity of proteins [
11]. This approach integrates structural analysis, computational modeling, site-directed mutagenesis, and biochemical assessments to predict and implement beneficial modifications. By utilizing detailed knowledge of enzyme structure, active site dynamics, and molecular interactions, rational design enables precise optimization of protein properties, facilitating their application in various biotechnological and industrial contexts.
Steck
et al. employed rational design to engineer CYP enzymes with the aim of enhancing C–H amination efficiency [
12] (
Figure 3(
a)). This was achieved by disrupting the native proton relay network and modifying conserved structural elements. Targeted mutations in key proton transfer residues—namely Thr268, His266, Glu267, and Thr438—suppressed unproductive pathways, thereby improving catalytic performance. Furthermore, alterations to the heme environment, particularly within the loop surrounding the heme-ligating cysteine, optimized nitrene transfer activity. This mechanism-guided strategy successfully repurposed P450 enzymes for abiological transformations, highlighting the potential of rational design to expand enzyme functionality in complex chemical processes.
In a related study, Ellis
et al. applied rational design to engineer the GcoA enzyme from the GcoAB P450 system, aiming to improve oxidative demethylation of lignin-derived aromatic aldehydes, which naturally exhibit limited activity towards o- and p-vanillin [
13] (
Figure 3(
b)). Structural and computational analyses identified key active site residues—F169 and T296—whose mutations (F169S and T296S) enhanced substrate binding and catalytic efficiency. These single-point mutations reduced steric hindrance and introduced hydrogen bond donors, improving the demethylation of aldehyde substrates. The engineered variants demonstrated enhanced catalytic activity in vitro and successfully functioned in
Pseudomonas putida, thereby expanding the potential of P450 enzymes for lignin valorization.
Further, Gao
et al. employed a structure-guided approach to engineer CYP154C2 with the goal of enhancing 2α-hydroxylation efficiency of steroids [
14] (
Figure 3(
c)). By analyzing the crystal structure of testosterone-bound CYP154C2, key active site residues were identified, leading to the design of multiple mutants, including L88F, M191F, and V285L. Among these, the L88F/M191F and M191F/V285L mutants exhibited significant improvements in catalytic efficiency, with up to a 46.5-fold increase in androstenedione conversion, alongside high regio- and stereoselectivity. Structural modifications enhanced substrate binding affinity and facilitated the displacement of iron-coordinated water, optimizing proton delivery and catalytic turnover. These findings underscore the effectiveness of structure-guided rational engineering in expanding the biocatalytic potential of P450 enzymes for steroid hydroxylation.
Zhang
et al. optimized CYP DoxA to enhance its hydroxylation activity at the C-14 position of daunorubicin, a critical step in doxorubicin biosynthesis [
15] (
Figure 3(
d)). Through molecular docking and structural modeling, they identified crucial substrate-binding residues and constructed a mutant library to improve binding stability. The DoxA(P88Y) mutant demonstrated a 56% increase in catalytic efficiency, attributed to enhanced hydrophobic interactions with daunorubicin. Molecular dynamics simulations confirmed increased binding stability and structural rigidity, contributing to higher enzymatic activity. This work further exemplifies the power of rational design in optimizing P450 enzymes for efficient drug biosynthesis.
Figure 3.
Representative Examples of Rational Design of P450 Enzyme-Catalyzed.
Figure 3.
Representative Examples of Rational Design of P450 Enzyme-Catalyzed.
Reactions:
(
a) CYP was engineered to enhance their C–H ami nation efficiency [
12]. (
b) GcoA was engineered to catalyze the oxidative demethylation of lignin-derived aromatic aldehydes [
13]. (
c) CYP154C2 was engineered to catalyze the 2α-hydroxylation of steroids [
14]. (
d) CYP DoxA was engineered to catalyze the hydroxylation at the C-14 position of daunorubicin [
15]. (
e) CYP105AS1 was engineered to improve the stereoselectivity in the biosynthesis of pravastatin [
17]. (
f) CYP102A1 was engineered for the stereoselective metabolism of the proton pump inhibitor omeprazole (OMP) [
18].
Computational-aided rational design leverages structural and sequence data to predict and optimize protein functions by targeting key residues involved in substrate binding, catalysis, and stability [
16]. Techniques such as molecular docking, molecular dynamics simulations, and free energy calculations allow for the modeling of protein-ligand interactions, assessment of conformational changes, and prediction of mutation effects on enzyme activity. By integrating these computational tools with experimental validation, researchers can design proteins with enhanced specificity, stability, and catalytic efficiency, thereby accelerating the development of biocatalysts for both industrial and therapeutic applications.
Ashworth
et al. utilized a computational-aided rational design strategy to enhance the stereoselectivity of CYP105AS1, a CYP enzyme from
Amycolatopsis orientalis, for pravastatin biosynthesis [
17] (
Figure 3(
e)). Using the Rosetta CoupledMoves protocol, they generated a virtual library of mutants optimized for compactin binding in a pro-pravastatin orientation. Computational screening and molecular dynamics simulations identified key mutations that correlated with experimental stereoselectivity. The optimized CYP105AS1 variant exhibited >99% selective hydroxylation of compactin to pravastatin, completely eliminating the undesired 6-epi-pravastatin diastereomer. This study exemplifies the synergy of computational modeling and experimental validation in precisely engineering P450 enzymes to improve catalytic efficiency and selectivity.
Huang
et al. employed UniDesign, a computational framework for enzyme engineering, to optimize CYP102A1 for the stereoselective metabolism of omeprazole (OMP), a proton pump inhibitor [
18] (
Figure 3(
f)). Using a nonstereoselective triple mutant (A82F/F87V/L188Q) as the starting point, computational analysis identified mutations at three active site positions (75, 264, and 328) to enhance the transition state for (R)-OMP while destabilizing (S)-OMP. The engineered variants (UD1, UD2, and UD3) exhibited high turnover rates and enantiomeric excess values up to 92% for (R)-OMP hydroxylation. This study highlights the effectiveness of UniDesign in guiding the engineering of P450 enzymes for targeted drug metabolism.
2.2. Semi-Rational Design
Semi-rational design for enzyme engineering integrates computational predictions with experimental mutagenesis to optimize enzyme performance [
19]. This approach utilizes structural and mechanistic insights to identify potential mutation sites, followed by directed mutagenesis to experimentally explore these sites. In contrast to fully rational design, which relies exclusively on structural or computational models, semi-rational design allows for iterative refinement. This flexibility incorporates both predicted and previously unforeseen beneficial mutations, increasing the likelihood of achieving the desired enzyme properties. By combining the efficiency of computational tools with the adaptability of experimental validation, semi-rational design enhances the potential for achieving substantial improvements in enzyme function. This strategy has been particularly effective in enhancing enzyme stability, activity, and specificity, making it an invaluable tool in biocatalysis and industrial applications.
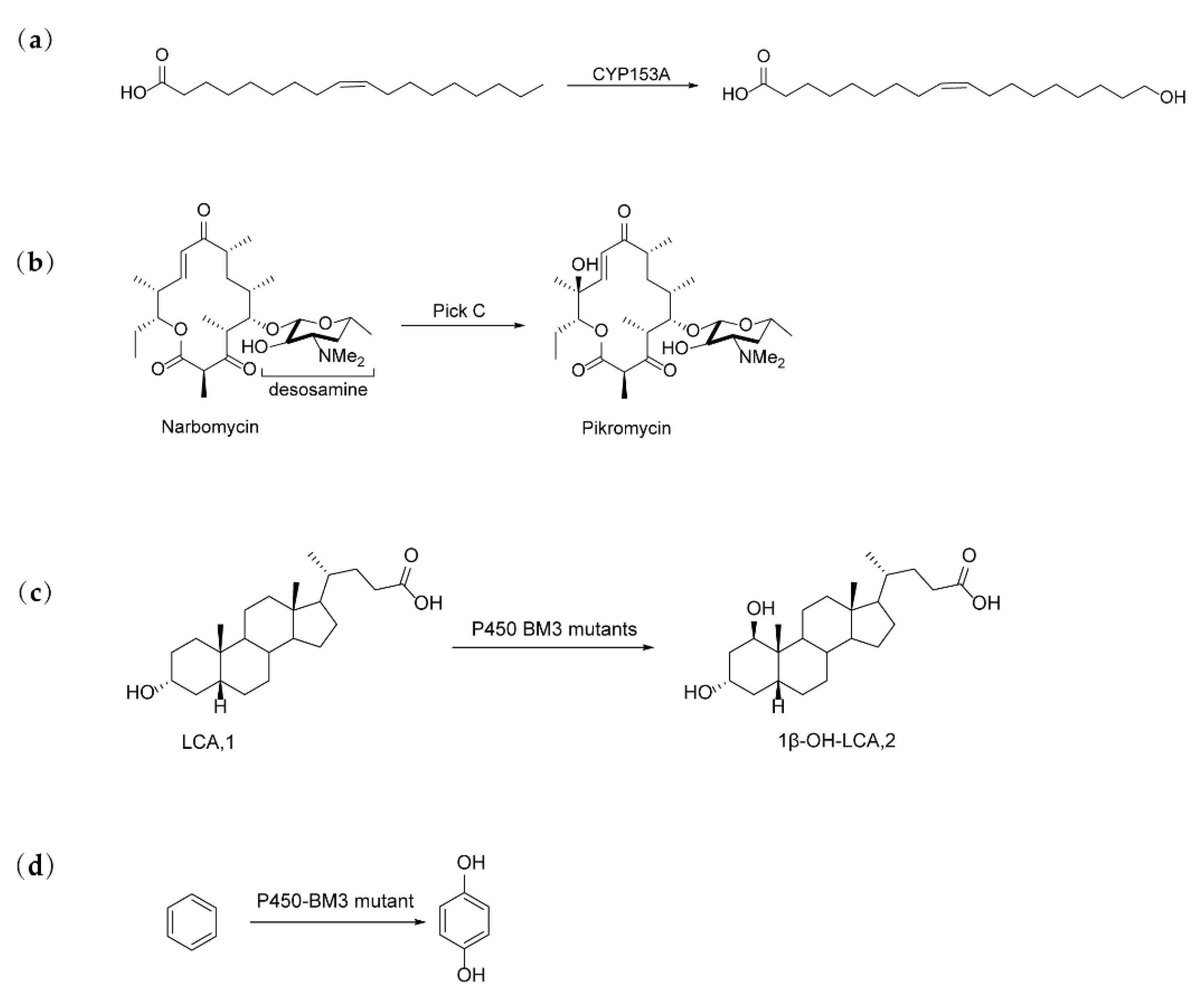
Duan et al. demonstrated the utility of a semi-rational approach by engineering a hybrid CYP153A/putidaredoxin (Pdx)/putidaredoxin reductase (Pdr) system to improve the ω-hydroxylation of oleic acid [
20] (
Figure 4(
a)). By combining site-directed saturation mutagenesis (SDSM) with iterative saturation mutagenesis (ISM), they identified a key variant (Variant II) with mutations at the Pdx-CYP153A interface (S120 and P165) and within the substrate binding pocket (S453). This mutant exhibited a 2.7-fold increase in in vitro activity and a 2.0-fold improvement in whole-cell transformation. Furthermore, a 96-well screening format was developed to facilitate efficient enzyme evaluation. This study underscores the importance of electron transfer between cytochrome P450 enzymes and non-native redox partners in optimizing the performance of hybrid P450 systems.
Non-canonical amino acids (ncAAs) are increasingly employed in protein engineering to introduce novel chemical functionalities, enhance enzyme stability, and broaden the catalytic potential of enzymes, including cytochrome P450 enzymes (CYPs) [
21]. In the context of CYPs, ncAAs enable fine-tuning of substrate specificity, enhanced catalytic efficiency, and the activation of new reactions, thus expanding their applications in biocatalysis and synthetic biology. Pan et al. applied a semi-rational approach to engineer the P450 enzyme PikC, which is involved in pikromycin biosynthesis [
22] (
Figure 4(
b)). By incorporating ncAAs at the His238 position, they enabled the enzyme to recognize macrolactone precursors without a sugar appendix. The key mutant, PikCH238pAcF, which carried p-acetylphenylalanine, demonstrated aglycone hydroxylating activity and reprogrammed the biosynthetic pathway when combined with a promiscuous glycosyltransferase. Structural analysis of both the substrate-free and enzyme-substrate complexes provided crucial insights into substrate binding and catalytic selectivity, highlighting the potential of semi-rational mutagenesis to expand the functional versatility of P450 enzymes.
Li et al. employed a semi-rational approach to engineer the cytochrome P450 monooxygenase CYP102A1 (P450 BM3) from Bacillus megaterium to improve regio- and stereo-selectivity for the 1β-hydroxylation of lithocholic acid (LCA) [
23] (
Figure 4(
c)). A mutation library was generated and refined over four rounds of mutagenesis, leading to the identification of W72 as a key residue that influences selectivity at the C1 position of LCA. The quadruple mutant (G87A/W72T/A74L/L181M) achieved 99.4% selectivity and 68.1% substrate conversion, resulting in a 21.5-fold increase in 1β-OH-LCA production compared to the wild-type enzyme. Molecular docking studies revealed that the introduction of hydrogen bonds at W72 significantly improved both selectivity and catalytic efficiency, offering structural insights into the enhanced activation of Csp3-H bonds.
Zhou et al. applied a semi-rational strategy to engineer cytochrome P450-BM3 to catalyze the bioconversion of benzene to hydroquinone (HQ), addressing the wild-type enzyme’s inability to accept benzene as a substrate [
24] (
Figure 4(
d)). Through this semi-rational approach, they identified highly active mutants that efficiently converted benzene to phenol, followed by regioselective oxidation to HQ, avoiding overoxidation. Computational analysis of the P450-BM3 variant A82F/A328F revealed selective oxidation of phenol, generating a reactive epoxide intermediate that fragments in aqueous medium to form HQ. This biocatalytic process was successfully integrated into an engineered Escherichia coli system, facilitating the cascade transformation of benzene to arbutin, a compound with significant anti-inflammatory and antibacterial properties.
2.3. Directed Evolution
Directed evolution is a sophisticated protein engineering technique that mimics natural selection to develop proteins with specific, desirable traits. The process involves introducing genetic variability through random mutagenesis, followed by high-throughput screening or selection to identify variants exhibiting improved functionality. These variants are then subjected to successive rounds of mutation and selection, progressively optimizing the protein's properties for the desired application [
25]. The pioneering work of Frances Arnold in directed evolution established crucial methodologies, particularly in random mutagenesis and efficient screening strategies. These innovations have facilitated the design of enzymes with enhanced catalytic efficiency, stability, and selectivity [
26]. Her contributions have significantly advanced biocatalysis, facilitating breakthroughs in enzyme design for industrial applications, including green chemistry and pharmaceutical synthesis [
27].
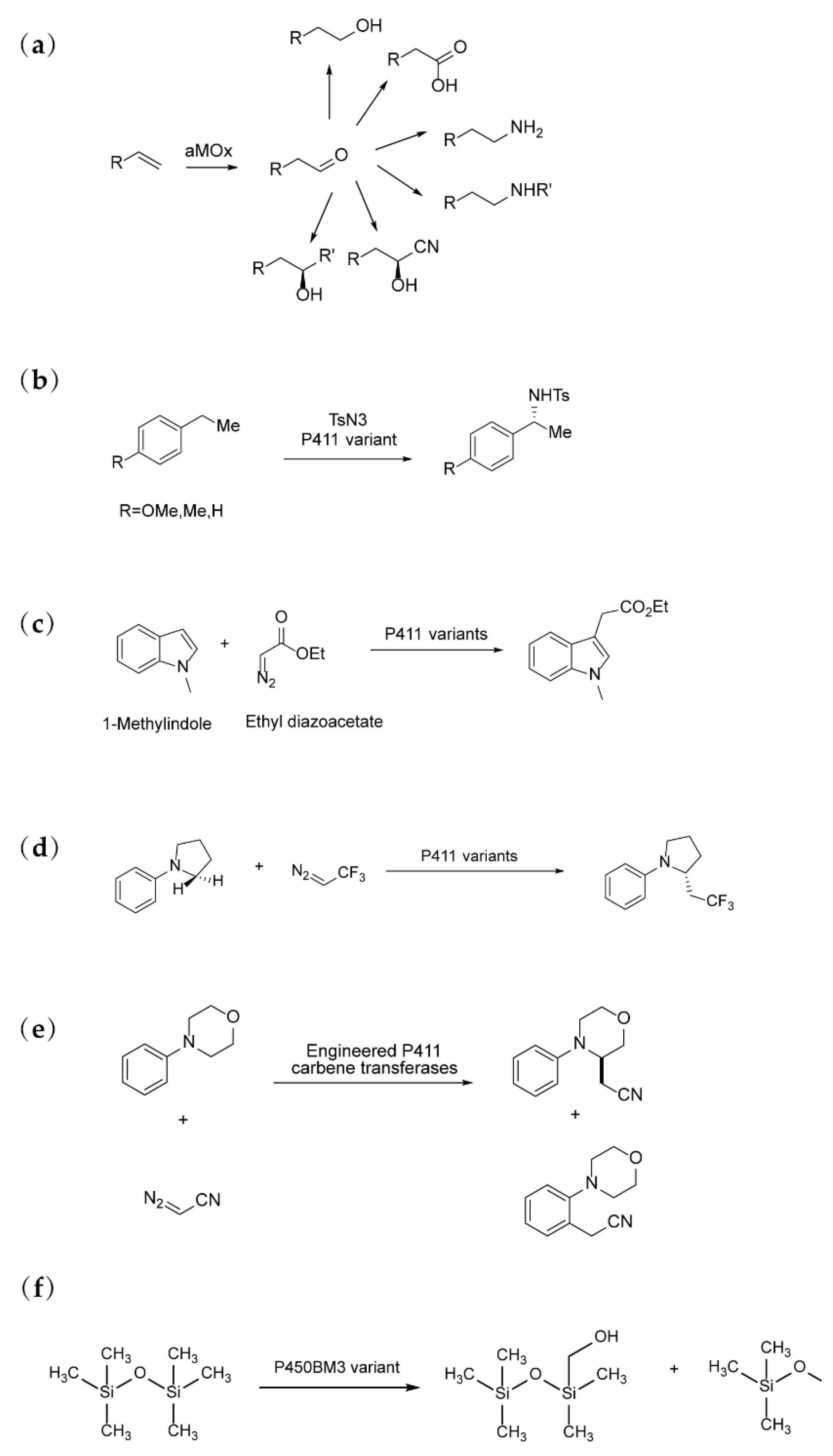
In 2017, Arnold’s group applied directed evolution to engineer a CYP capable of catalyzing the anti-Markovnikov oxidation of styrenes using a metal-oxo-mediated mechanism [
28] (
Figure 5(
a)). This engineered enzyme selectively promoted anti-Markovnikov oxidation, which is typically less favored than alkene epoxidation, by stabilizing high-energy intermediates and facilitating oxo transfer, including enantioselective 1,2-hydride migration. The evolved P450 enzyme showed significant potential for integration with other catalysts in synthetic pathways, enabling the efficient functionalization of styrenes in anti-Markovnikov reactions, thus providing a valuable tool for simplifying the synthesis of complex molecules.
In the same year, Arnold’s team engineered the CYP monooxygenase P411CHA to catalyze the highly enantioselective, intermolecular amination of benzylic C–H bonds, a reaction that had previously been considered a major challenge in synthetic chemistry [
29] (
Figure 5(
b)). The evolved enzyme demonstrated excellent selectivity and achieved up to 1,300 turnovers using an iron-heme cofactor, which is typically inert in C–H amination reactions. This work showcased how the protein framework could enhance the reactivity of the iron cofactor, facilitating non-natural transformations without relying on expensive precious metal catalysts. Furthermore, by evolving P411s for simpler sulfimidation reactions, the enzyme acquired promiscuous activity for C–H amination, illustrating the potential of stepwise evolution to expand enzyme functionality. These findings underscore the ability of cytochrome P450 enzymes to serve as versatile platforms for the development of catalysts capable of mediating a broad spectrum of C–H functionalization reactions.
In 2019, Arnold's group engineered a cytochrome P450 variant capable of efficiently catalyzing carbene transfer to heterocycles and cyclic alkenes [
30] (
Figure 5(
c)). By employing a high-throughput screening assay for indole C3-alkylation, they were able to analyze thousands of variants generated through random and targeted mutagenesis. This led to the identification of a highly active variant with 11 amino acid substitutions and a significant deletion in the non-catalytic reductase domain, achieving up to 470 turnovers per minute. The enzyme was further optimized through parallel evolution to catalyze more challenging carbene functionalizations, such as regioselective pyrrole alkylation and enantioselective indole alkylation. These P450 variants, utilizing an earth-abundant iron cofactor, demonstrated high efficiency and selectivity, providing a sustainable alternative to traditional carbene transfer reactions and expanding the catalytic repertoire of carbene transferases.
In a related 2019 study, Arnold's team extended their carbene transfer strategy to engineer cytochrome P450 enzymes capable of selectively inserting fluoroalkyl groups into α-amino C(sp3)−H bonds, thus enabling the enantiodivergent synthesis of fluoroalkyl-containing molecules [
31] (
Figure 5(
d)). The engineered enzymes achieved total turnovers (TTN) of up to 4,070 and enantiomeric excess (ee) of up to 99% under mild conditions. The directed evolution approach allowed for the complete inversion of product enantioselectivity, demonstrating the power of enzyme optimization. This work illustrates the potential of genetically encoded catalytic platforms for synthesizing chiral organofluorine compounds, a task that traditional small-molecule catalysts cannot easily achieve. The study significantly broadens the scope of enzymatic C−H alkylation, opening new avenues for the synthesis of fluorinated bioactive molecules.
In 2023, Arnold's team demonstrated the selective functionalization of both α-amino C(sp3)–H and ortho-arene C(sp2)–H bonds through carbene transfer reactions, further expanding the catalytic capabilities of cytochrome P450 enzymes [
32] (
Figure 5(
e)). By introducing just nine mutations, they achieved precise control over site-selectivity for cyanomethylation, illustrating the utility of directed evolution in fine-tuning enzyme specificity. The engineered enzymes exhibited divergent reaction mechanisms, with X-ray crystal structures revealing a previously unobserved helical disruption in the active site, which contributed to the selectivity. This work significantly expands the catalytic scope of P450 enzymes, demonstrating their potential as "generalist" enzymes for developing highly selective catalysts for targeted C–H functionalizations.
In 2024, Arnold’s group engineered a bacterial cytochrome P450BM3 variant to catalyze the cleavage of silicon-carbon (Si–C) bonds in volatile methylsiloxanes (VMS) [
33] (
Figure 5(
f)). The engineered enzyme catalyzed two tandem oxidations of a siloxane methyl group, followed by a [
1,
2]-Brook rearrangement and hydrolysis, resulting in Si–C bond cleavage. This discovery of a siloxane oxidase provides a promising approach for the biodegradation of VMS, a class of compounds that have traditionally been considered non-biodegradable. The study highlights how directed evolution can amplify even trace enzymatic activities to create biocatalysts capable of performing non-natural transformations, offering potential solutions for the biodegradation of previously persistent environmental pollutants such as siloxanes.
Together, these studies underscore the power of directed evolution in expanding the catalytic capabilities of enzymes, demonstrating its potential for creating biocatalysts that can perform complex and non-natural transformations with high efficiency, selectivity, and sustainability.
Figure 5.
Representative Examples of Directed evolution of P450 Enzyme-Cat.
Figure 5.
Representative Examples of Directed evolution of P450 Enzyme-Cat.
alyzed Reactions:
(
a) aMOx was engineered to catalyze the anti-Markovnikov oxidation of styrenes [
28]. (
b) P411CHA was engineered for the enantioselective intermolecular amination of benzylic C–H bonds [
29]. (
c) P441 was engineered to catalyze carbene transfer to heterocycles and cyclic alkenes [
30]. (
d) P411 was engineered to insert fluoroalkyl groups [
31]. (
e) P411 was engineered to catalyze cyanomethylation [
32]. (
f) P450BM3 was engineered to catalyze the cleavage of silicon-carbon (Si–C) bonds in volatile methylsiloxanes (VMS) [
33].
3. Challenges and Future Directions for the Engineering of Cytochrome P450 Enzymes
The structural complexity of cytochrome P450 enzymes presents significant challenges in their engineering. High-resolution structural data is often difficult to obtain due to the enzymes’ flexible active sites and conformational variability, which complicates the design of precise mutants [
33]. Moreover, P450 enzymes exhibit diverse substrate specificity, making it challenging to predict how structural modifications will affect substrate binding and product formation [
34]. These factors collectively hinder the rational design of P450 mutants for specific reactions, emphasizing the need for advanced techniques to overcome these structural and functional limitations.
A major challenge in the engineering of cytochrome P450 lies in optimizing catalytic efficiency, which requires balancing enzyme activity with substrate turnover and stability. While increasing enzymatic activity is essential for efficient catalysis, it often comes at the cost of reduced stability, as more active forms of the enzyme may be prone to denaturation or degradation [
35]. Conversely, enhancing stability can sometimes lead to lower substrate turnover rates [
36], as the enzyme's active site may become less flexible or accessible. Achieving an optimal balance between these factors is critical for developing P450 enzymes that are not only highly active but also stable enough for industrial and pharmaceutical applications, making this a key hurdle in enzyme engineering.
Challenges in cost and scalability are significant obstacles in the practical application of cytochrome P450 enzymes for biocatalysis. Large-scale production of engineered P450s often faces difficulties related to high production costs, including the need for expensive cofactors, specialized reagents, and sophisticated purification processes [
37]. Additionally, achieving consistent enzyme activity and stability at industrial scales is complex, as the enzymes may lose efficiency or degrade under non-laboratory conditions [
38]. Cost-effective biocatalysis remains a major hurdle, as optimizing the enzymes for large-scale applications while maintaining cost efficiency requires advances in enzyme stability, production methods, and reactor design [
39]. Overcoming these challenges is essential for making P450-based processes viable for commercial use in industries such as pharmaceuticals, biofuels, and fine chemicals.
Future directions in optimizing P450s for high-throughput screening (HTS) focus on developing more efficient methods for screening large enzyme libraries. As the need for highly specific and efficient P450 variants increases, advancements in automation, miniaturization, and real-time monitoring of enzyme activity are crucial for accelerating the screening process. Innovative techniques, such as microfluidic systems [
40], fluorescence-based assays [
41], and next-generation sequencing [
42], hold promise for rapidly evaluating vast libraries of P450 mutants with high sensitivity and throughput. Additionally, the integration of computational tools, including machine learning and artificial intelligence [
43,
44], could further enhance the prediction of enzyme behavior, enabling more targeted library designs. These advancements will enable the identification of optimized P450 variants more quickly and efficiently, opening new possibilities for their application in industrial and pharmaceutical processes.
Expanding the substrate range of P450 enzymes is a key future direction in P450 engineering, with the goal of enabling their use in a broader array of industrial and pharmaceutical applications. Innovations in directed evolution, rational design, and computational modeling are driving efforts to create P450 variants capable of metabolizing a wider variety of substrates, including those with complex or non-natural structures [
45]. Advances in understanding the enzyme-substrate interactions at a molecular level, combined with high-throughput screening and machine learning tools, will facilitate the design of P450s with enhanced specificity and versatility [
46]. This expansion of substrate range could significantly improve the efficiency of biocatalytic processes in areas such as drug synthesis, biofuel production, and the manufacture of specialty chemicals, making P450 enzymes even more valuable in industrial biotechnology.
The future direction of integrating P450 enzymes with other biocatalysts also includes developing strategies to combine P450s with complementary enzymes for multi-step biotransformations. By coupling P450s with enzymes such as reductases [
47,
48], dehydrogenases [
49,
50], or transferases [
51], researchers aim to create more efficient and streamlined catalytic processes for complex reactions that require multiple enzymatic steps. Advances in enzyme engineering and metabolic pathway design will allow for the seamless integration of P450s into cascades, facilitating the synthesis of valuable compounds with high specificity and yield. This approach could significantly improve the efficiency of biocatalysis while also reducing the need for harsh chemicals or expensive reagents typically used in chemical synthesis.
4. Conclusions
In conclusion, recent advances in the engineering of cytochrome P450 enzymes have significantly enhanced their potential for diverse industrial and pharmaceutical applications. Improved understanding of enzyme structure and function, alongside innovations in computational modeling, high-throughput screening, and directed evolution, has led to the development of P450 variants with enhanced activity, stability, and substrate specificity. Despite ongoing challenges, such as optimizing catalytic efficiency, overcoming substrate promiscuity, and ensuring scalability, these enzymes are increasingly being engineered for complex biotransformations and multi-step reactions. Looking ahead, continued progress in integrating P450s with other biocatalysts, expanding their substrate range, and improving screening methods will further broaden their utility. As these challenges are addressed, cytochrome P450 enzymes are set to become even more indispensable in the fields of green chemistry, sustainable manufacturing, and the production of bio-based pharmaceuticals and specialty chemicals.