Submitted:
25 February 2025
Posted:
26 February 2025
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Adverse Events Modeling
- (I)
- (II)
Results
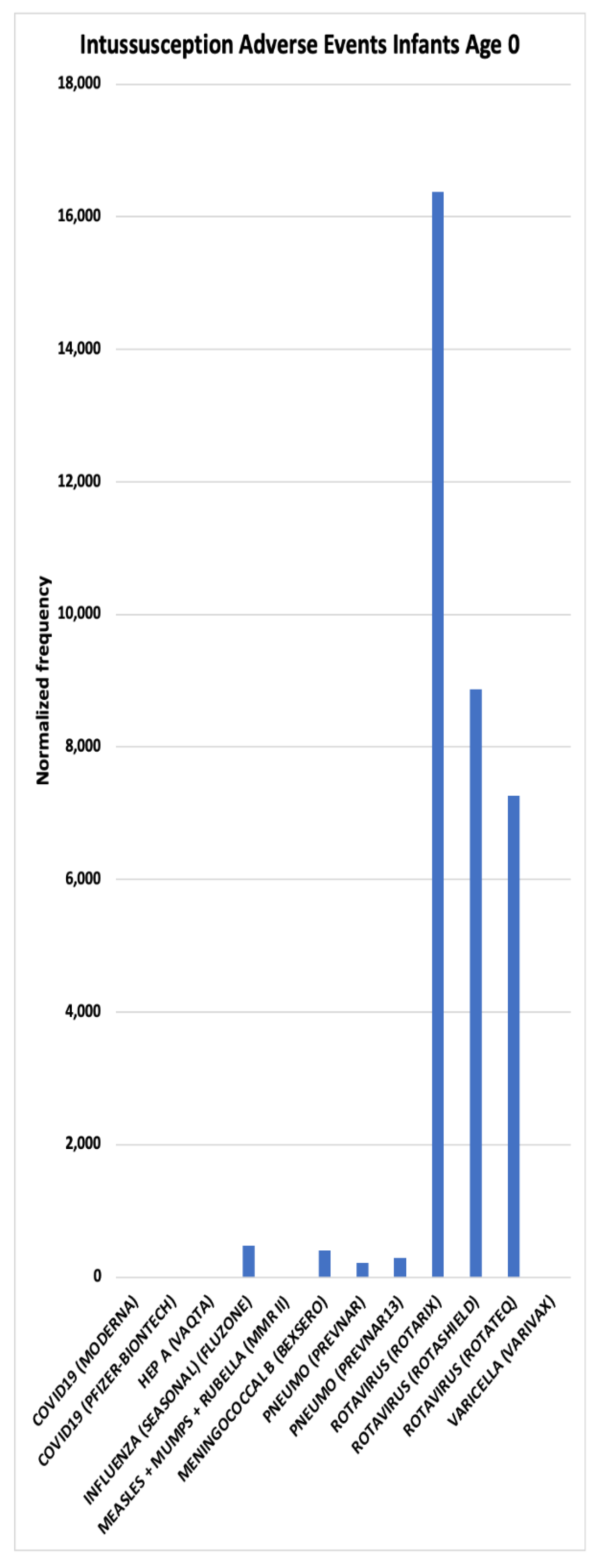
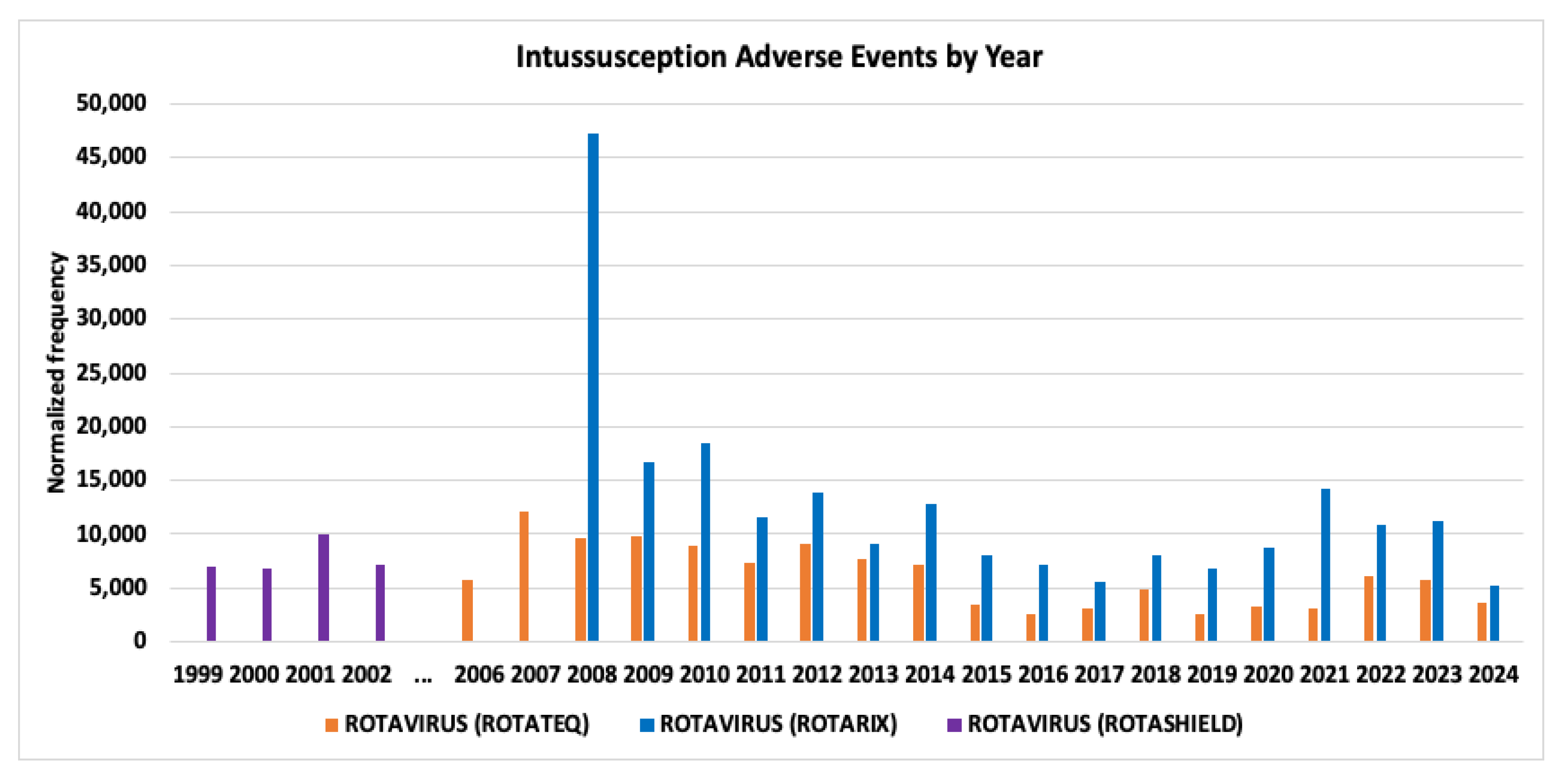
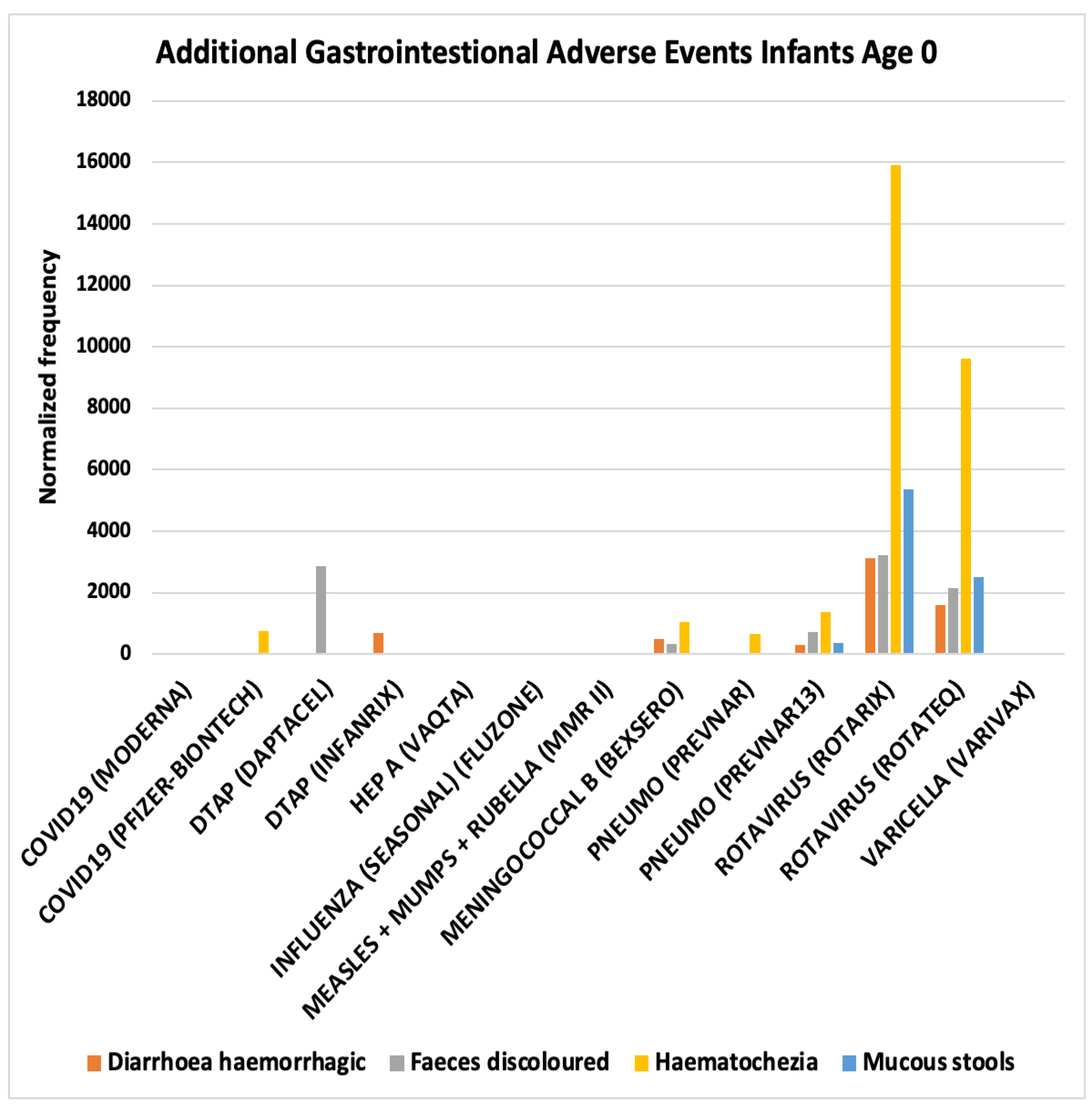
| Concomitant Vaccines | Intussusception AEFIs Infants Age 0 normalized frequency |
| DTAP (ACEL-IMUNE)+HIB + HEP B (COMVAX) & POLIO VIRUS, INACT. (POLIOVAX) & ROTAVIRUS (ROTASHIELD) |
9,375 |
| DTAP (DAPTACEL) & HEP B (RECOMBIVAX HB) & HIB (ACTHIB) & PNEUMO (PREVNAR) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
14,706 |
| DTAP (DAPTACEL) & HEP B (RECOMBIVAX HB) & HIB (ACTHIB) & PNEUMO (PREVNAR13) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
20,833 |
| DTAP (DAPTACEL) & HIB (ACTHIB) & PNEUMO (PREVNAR) & POLIO VIRUS, INACT. (IPOL) |
671 |
| DTAP (DAPTACEL) & HIB (ACTHIB) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
25,714 |
| DTAP (DAPTACEL) & HIB (ACTHIB) & PNEUMO (PREVNAR13) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
14,286 |
| DTAP (DAPTACEL) & HIB (ACTHIB) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
14,286 |
| DTAP (DAPTACEL) & HIB (ACTHIB) & ROTAVIRUS (ROTATEQ) |
12,500 |
| DTAP (DAPTACEL) & HIB + HEP B (COMVAX) & PNEUMO (PREVNAR) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
12,397 |
| DTAP (DAPTACEL) & PNEUMO (PREVNAR) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
23,077 |
| DTAP (INFANRIX) & HIB (ACTHIB) & PNEUMO (PREVNAR13) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
18,182 |
| DTAP (INFANRIX) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTARIX) |
17,391 |
| DTAP (INFANRIX) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
8,571 |
| DTAP (TRIPEDIA) & HIB (ACTHIB) & PNEUMO (PREVNAR) & POLIO VIRUS, INACT. (IPOL) & ROTAVIRUS (ROTATEQ) |
10,256 |
| DTAP + HEPB + IPV (PEDIARIX) & HIB (ACTHIB) & INFLUENZA (SEASONAL) (FLUZONE) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
15,625 |
| DTAP + HEPB + IPV (PEDIARIX) & HIB (ACTHIB) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
9,305 |
| DTAP + HEPB + IPV (PEDIARIX) & HIB (ACTHIB) & PNEUMO (PREVNAR13) |
1,271 |
| DTAP + HEPB + IPV (PEDIARIX) & HIB (ACTHIB) & ROTAVIRUS (ROTATEQ) |
17,241 |
| DTAP + HEPB + IPV (PEDIARIX) & HIB (PEDVAXHIB) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
10,062 |
| DTAP + HEPB + IPV (PEDIARIX) & INFLUENZA (SEASONAL) (FLUZONE) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
16,667 |
| DTAP + HEPB + IPV (PEDIARIX) & PNEUMO (PREVNAR) |
1,695 |
| DTAP + HEPB + IPV (PEDIARIX) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
19,608 |
| DTAP + IPV + HIB (INFANRIX QUINTA) & PNEUMO (SYNFLORIX) & ROTAVIRUS (ROTATEQ) |
17,500 |
| DTAP + IPV + HIB (PENTACEL) & HEP B (RECOMBIVAX HB) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
7,613 |
| DTAP + IPV + HIB (PENTACEL) & INFLUENZA (SEASONAL) (FLUZONE) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
10,204 |
| DTAP + IPV + HIB (PENTACEL) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
10,384 |
| DTAP + IPV + HIB (PENTACEL) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
9,264 |
| DTAP+IPV+HEPB+HIB (INFANRIX HEXA) & PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
11,200 |
| DTAP+IPV+HEPB+HIB (INFANRIX HEXA) & PNEUMO (PREVNAR13) |
619 |
| DTAP+IPV+HEPB+HIB (INFANRIX HEXA) & ROTAVIRUS (ROTATEQ) |
10,000 |
| DTAP+IPV+HIB+HEPB (VAXELIS) & PNEUMO (VAXNEUVANCE) & ROTAVIRUS (ROTATEQ) |
8,108 |
| HIB (ACTHIB) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTARIX) |
32,258 |
| HIB (ACTHIB) & PNEUMO (PREVNAR13) & ROTAVIRUS (ROTATEQ) |
8,333 |
| PNEUMO (PREVNAR) & ROTAVIRUS (ROTATEQ) |
7,692 |
| Vaccine | Dose 1 | Dose 2 | Dose 3 |
| ROTAVIRUS (ROTARIX) | 56.1% | 42.6% | 1.2% |
| ROTAVIRUS (ROTASHIELD) | 66.7% | 19.4% | 13.9% |
| ROTAVIRUS (ROTATEQ) | 36.8% | 36.0% | 27.2% |
Discussion
Study Limitations
Study Recommendations
Conclusions
Author contributions
Conflicts of interest
Ethical approval
Consent to participate
Consent to publication
Availability of data and materials
Funding: Distribution Statement
Abbreviations
| AEFI | adverse event following immunization |
| CDC | Center for Disease Control |
| COVID19 | coronavirus disease 2019 vaccine |
| DTAP | diphtheria, tetanus, and acellular pertussis vaccine |
| HEP A | hepatitis A vaccine |
| HEP B | hepatitis B vaccine |
| HIB | Haemophilus influenzae type b vaccine |
| ICH | International Council for Harmonisation |
| IPV | inactivated polio virus vaccine |
| MedDRA | Medical Dictionary for Regulatory Activities |
| MMR | measles, mumps, and rubella vaccine |
| PNEUMO | pneumococcal vaccine |
| RV1 | Rotatix rotavirus vaccine |
| RV5 | RotaTeq rotavirus vaccine |
| RVGE | rotavirus gastroenteritis |
| SAE | serious adverse event |
| VAERS | Vaccine Adverse Event Reporting System |
References
- Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211-28. [CrossRef]
- Ruiz-Palacios Guillermo M, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SueAnn C, et al. Safety and Efficacy of an Attenuated Vaccine against Severe Rotavirus Gastroenteritis. N Engl J Med. 354:11-22. [CrossRef]
- Chandran A, Santosham M. RotaTeq™: a three-dose oral pentavalent reassortant rotavirus vaccine. Expert Rev Vaccines. 2008;7:1475-80. [CrossRef]
- Macías-Parra M, Vidal-Vázquez P, Reyna-Figueroa J, Rodríguez-Weber MÁ, Moreno-Macías H, Hernández-Benavides I, et al. Immunogenicity of RV1 and RV5 vaccines administered in standard and interchangeable mixed schedules: a randomized, double-blind, non-inferiority clinical trial in Mexican infants. Front Public Health. 2024;12. [CrossRef]
- Sun Z-W, Fu Y, Lu H-L, Yang R-X, Goyal H, Jiang Y, et al. Association of Rotavirus Vaccines With Reduction in Rotavirus Gastroenteritis in Children Younger Than 5 Years: A Systematic Review and Meta-analysis of Randomized Clinical Trials and Observational Studies. JAMA Pediatr. 2021;175:e210347-e. [CrossRef]
- Buyse H, Vinals C, Karkada N, Han HH. The human rotavirus vaccine Rotarix™ in infants. Hum Vaccin Immunother. 2014;10:19-24. [CrossRef]
- O’Ryan, M. Rotarix™ (RIX4414): an oral human rotavirus vaccine. Expert Rev Vaccines. 2007;6:11-9. [CrossRef]
- Zhu J, Cheng W, Xu Y, Guo Y, Shi L. Two cases of small bowel necrosis due to intussusception secondary to abnormal proliferation of intestinal Peyer’s patches in infants after MMR vaccination. BMC Pediatr. 2024;24:147. [CrossRef]
- Patel Manish M, López-Collada Vesta R, Bulhões Marília M, De Oliveira Lucia H, Márquez Aurora B, Flannery B, et al. Intussusception Risk and Health Benefits of Rotavirus Vaccination in Mexico and Brazil. N Engl J Med. 364:2283-92. [CrossRef]
- Vesikari T, Matson David O, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and Efficacy of a Pentavalent Human–Bovine (WC3) Reassortant Rotavirus Vaccine. N Engl J Med. 354:23-33. [CrossRef]
- VAERS. VAERS Data Sets 2024 [cited 2024 June 28, 2024]. Available from: https://vaers.hhs.gov/data/datasets.html.
- MedDRA. Medical Dictionary for Regulatory Archives 2024 [cited 2024 July 1, 2024]. Available from: www.meddra.org.
- Ricke DO. VAERS-Tools 2022 [cited 2024 July 1, 2024]. Available from: https://github.com/doricke/VAERS-Tools.
- Ricke DO, Smith N. VAERS Vasculitis Adverse Events Retrospective Study: Etiology Model of Immune Complexes Activating Fc Receptors in Kawasaki Disease and Multisystem Inflammatory Syndromes. Life. 2024;14. [CrossRef]
- CDC. Child and Adolescent Immunization Schedule by Age 2024 [cited 2024 June 28, 2024]. Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- Moser CA, Dolfi DV, Di Vietro ML, Heaton PA, Offit PA, Clark HF. Hypertrophy, Hyperplasia, and Infectious Virus in Gut-Associated Lymphoid Tissue of Mice after Oral Inoculation with Simian-Human or Bovine-Human Reassortant Rotaviruses. J Infect Dis. 2001;183:1108-11. [CrossRef]
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception Risk and Disease Prevention Associated With Rotavirus Vaccines in Australia's National Immunization Program. Clin Infect Dis. 2013;57:1427-34. [CrossRef]
- Murphy Trudy V, Gargiullo Paul M, Massoudi Mehran S, Nelson David B, Jumaan Aisha O, Okoro Catherine A, et al. Intussusception among Infants Given an Oral Rotavirus Vaccine. N Engl J Med. 344:564-72. [CrossRef]
- Ricke, DO. Intussusception Adverse Events Post Vaccination. In. Harvard Dataverse; 2025.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





