1. Introduction
Food allergy is a major public health problem, with prevalence rates increasing over the past twenty to thirty years. According to community-based EuroPrevall surveys, the prevalence of self-reported among adults in Europe ranges from 2% to 37% [
1].
Nearly half of adults with food allergies have experienced at least one adult-onset reaction, and 38% have reported at least one food allergy-related visit to an emergency department during their lifetime [
2]. There are many challenges in estimating food allergy prevalence [
2,
3] A recent systematic review reported a lifetime prevalence of any food allergy diagnosed by a physician of 9.3% in children and 5% in adults [
4].
Citrus fruits, particularly oranges (
Citrus sinensis), belongs to the family
Rutaceae. Orange consumption have been linked to allergic reactions, including oral allergy symptoms such as itching and swelling of the lips, tongue and throat [
5,
6].
Oranges are consumed worldwide. Despite their widespread use both as food and in flavouring, reports of allergies to arrange, lemon or mandarin are relatively rare, with orange allergies accounting for only 4.4% of all food allergies [
2] reported by Lyons in 2020.
Four primary allergens have been described in orange, labelled Cit s 1 through Cit s 4, with the major allergens being Cit s 1 (a germin-like protein) and Cit s 2 (profilin) [
7].
Cit s 1, a 24-kDa glycoprotein [
7], is also found in lemon peel [
8]. Its biological role is still under investigation but is considered one of the main orange allergens [
7].
Cit s 2, a 14-kDa profilin, is another major orange allergen[
7,
9]. Like other profilins, its biological activity is related to the structural organisation of actin filaments and germination [
10].
Cit s 3, a 9.46 kDa lipid transfer protein (LTP) type 1, belongs to the LTP family of panallergens. Despite its LTP classification, it is considered a minor allergen with a prevalence of 34% [
11]. Like most LTP, it is most abundant in the peel but is also present in the pulp [
12].
Cit s 7, a 6.951-kDa gibberellin-regulated protein[
13], was tested for allergenicity in a group of 14 patients with orange allergies, with 85.7% of patients [
14] testing positive for it through ELISA, Prick or basophil activation tests. Cross-reactivity between orange and lemon allergens and LTP of peach (
Pru p 3) has also been described [
15].
Regarding lemon (
Citrus limon), one allergen has been described: Cit l 3, a 9.6-kDa lipid-transfer protein type 1. Among 27 sera analysed, specific IgE to the purified allergens was found in 54% for
nCit l 3, 48% for
nCit s 3, 46% for
rCit s 3 and in 37% for
rPru p 3. Members of the LTP allergen family are involved in allergy to oranges, displaying positive in vitro and in vivo reactions seen in 30-50% of patients studied. Both orange and lemon allergens show cross-reactivity with the major peach allergen,
Pru p 3 [
16].
In mandarin (
Citrus reticulata), the main allergen identified is
Cit r 3, a 9-kDa lipid-transfer protein type 1, found in a patient with mandarin-induced anaphylaxis [
17]. This case, which occurred in Northern and Central Europe, was unusual, as fruit-induced anaphylaxis is rare in this region. It also highlighted the importance of sensitisation to LTP and the consequent predisposition to severe allergic reactions [
17].
Germins are cell wall proteins expressed during the early stages of germination in response in response to physical or chemical stress [
18].
This is a case-report study focused on the unusual occurrence of orange allergy in five patients. Our aim was to identify new allergens in orange, lemon and mandarin different from LTP.
2. Material and Methods
We report five cases of patients -four women and one man- aged between 31 and 44 years, who first attended our allergy clinic with suspected orange allergy. The characteristics of the patients and the diagnostic procedures performed are detailed below.
All five patients developed immediate allergic reactions following orange ingestion, presenting with symptoms such as sneezing, nasal congestion, erythema over the chest, and shortness of breath, along with generalized urticaria and facial angioedema. The reactions wire classified as ordinal Food Allergy Severity Score (oFASS) grade 4, indicating severe food allergy [
1].
2.1. Skin Prick Test
Skin prick tests (SPT) were performed using commercial extracts of common aeroallergens, including pollen, house dust mite, molds, animal dander and panallergens, such as peach non-specific lipid transfer proteins (nsLTP) (Pru p 3) and pollen profilins.
SPT were conducted for the following allergens:
Pollens: Phleum pratense, Cynodon dactylon, Olea europaea, Cupressus arizonica, Platanus acerifolia, Artemisia vulgaris, Plantago lanceolata.
Animal dander: dog and cat.
Molds: Alternaria alternata, Aspergillus fumigatus
Panallergens: Peach nsLTP (Pru p 3) (Diater ®; 30 µg/mL) and pollen profilin (Pho d 2)
Additionally, SPT for food allergens were performed using commercial extracts for cocoa, hazelnut, peanut, almond, sunflower seed, pistachio, walnut, peach, apple, pear, kiwi, banana, melon, strawberry, pineapple, tomato, celery, and paprika (Diater ® Laboratories).
2.2. Prick-by-Prick Test
The prick-by-prick test was performed using fresh samples of food.
Orange (Citrus sinensis): peel and pulp
Lemon (Citrus limon): peel and pulp
Mandarin (Citrus reticulata): peel and pulp
Citrus seeds
2.3. Anaphylaxis Score and Immunological Tests
The anaphylaxis severity score was assessed using the oFASS-5 classification. Additionally, the following immunological tests were performed:
Total IgE (kU/L)
Serum-specific IgE (sIgE) to nsLTP (Pru p 3, Pru p 7) (Thermo Fisher Scientific Inc., Phadia, AB, Uppsala, Sweden)
Microarray-based IgE profiling (ALEX) [
19]
Food Allergy Quality of Life Questionnaire Adult Form (FAQLQ-AF) (3,4)
2.4. Preparation of Allergenic Extracts
Allergenic extracts were prepared from orange (Citrus sinensis) peel and pulp, lemon (Citrus limon) peel and pulp, and mandarin (Citrus reticulata) peel and pulp. Samples were washed with deionised water, then weighed and homogenised in a beaker with Björksten Buffer (NaH2PO4 0.14% (w/v), NaH2PO4 x 2 H2O 0.18% (w/v), Polyvinylpolypyrrolidone 2% (w/v), EDTA 0.07% (w/v) and Diethyldithiocarbonate 0.07% in deionised water). The mixture was blender until a homogeneous suspension was obtained under gentle mixing.
The protein concentration was standardized to 10 mg /ml of buffer. This buffer was used for the extraction and stabilisation of proteins in fruit and vegetables. The pH of the extracts was adjusted to 7.7-8.5 by adding 1M NaOH. This was followed by extraction under gentle mixing in a cold chamber for protein extraction and stabilization in fruit and vegetable samples. The pH was adjusted to 7.7–8.5 using 1M NaOH, followed by extraction under gentle mixing at 4°C for 2 hours using a magnetic stirrer (Thermo Fisher). The extract was then centrifuged at 9,000 rpm for 30 minutes at 4°C.
The supernatant was filtered through a series of clarification filters with pore sizes of 25–30 µm, 10–13 µm, and 2 µm, sequentially removing impurities, sediments, and suspended particles.
2.5. Protein Content
The total protein concentration was determined by the Bradford method [
5], using the BIO-RAD (protein assay) reagent at a ratio of 1:5 with pure water. A BSA (bovine serum albumin) standard solution was prepared at 1 mg/ml, followed by serial dilutions to generate a standard curve. A negative control (blank) was also included.
After adding 900 µl of Bradford reagent, the samples were vortexed and incubated for five minutes. Then, 200 µl per well was loaded in triplicate into a 96-well plate to be read in the PowerWave XS2 spectrophotometer (Biotek) with Gen 5 software, following the Bradford protocol at a wavelength of 595 nm. A correlation coefficient ≥0.99 was required for the standard curve. The protein concentration of the extracts was determined by interpolating the absorbance values against the standard curve.
2.6. SDS PAGE and Immunoblotting
After determining the protein concentration in the allergenic extracts, the dilution formula (C1 x V1 = C2 x V2) was used to bring the samples to 0.05 mg/ml under reducing conditions (optimal concentration for SDS-PAGE and immunoblotting [western blot]).
For reducing conditions, the samples were mixed with 5x Sample Buffer, composed of
- -
0.5 M Tris-HCL (pH 6.8) at 12% (v/v),
- -
glycerol at 25% (v/v),
- -
SDS at 10% (w/v) in a 20% concentration (v/v),
- -
2-β-mercaptoethanol at 5% (v/v),
- -
Bromophenol blue at 0.5% (w/v) in a 20% concentration (v/v)
- -
and deionized water at 18% (v/v).
Samples were denatured by heating at 99°C for 10 min.
SDS-PAGE Analysis
Denatured samples were loaded at 0.5 µg per lane onto 15% acrylamide/bisacrylamide gels under denaturing conditions. Electrophoresis was conducted using the PowerPac 300 BASIC (BIO-RAD) following the Laemmli method (6) at
- -
200 V, 400 mA for 45 minutes
- -
Electrophoretic cuvette filled with 10 x Tris/Glycine/SDS electrophoresis buffer (BIO-RAD).
For molecular weight determination, Precision Plus Dual Color Protein Standards (BIO-RAD) (ranging from 250 kDa to <10 kDa) were used at a 1:5 dilution ratio with 1x Sample buffer.
Proteins were visualized using the PierceTM Silver Stain Kit (Thermo Scientific), following the manufacturer’s protocol. Molecular weights were analyzed using a BIO-RAD transilluminator and Image Lab software.
Western Blotting
To assess sIgE sensitization to citrus allergens, extracts were subjected to Western blot analysis. Proteins were separated by SDS-PAGE (15% gels, 0.5 per lane) under reducing conditions, using the PowerPac 300 BASIC system (BIO-RAD) with the Laemmli method (1) at 200 V, 400 mA, for 45 minutes.
Following electrophoresis, proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Trans-Blot Turbo Transfer Pack, BIO-RAD, Germany) using the Trans-Blot Turbo system (BIO-RAD).
The membrane was blocked for 1 hour at room temperature (RT) with phosphate-buffered saline with phosphate buffered saline (PBS, pH 7.4) containing 0.5% Tween and 3% milk powder.
For IgE detection, the membrane was incubated overnight with patient serum (primary antibody) at a 1:5 dilution in PBS-Tween (0.5%) and 3% milk powder. Following incubation, the membrane underwent five washes with 0.5% PBS-Tween.
The primary antibody was detected using horseradish peroxidase (HRP)-conjugated Mouse Anti-Human IgE Fc-HRP (SouthernBiothech), followed by Western Lightining Plus-ECL (PerkinElmer, Inc.) chemiluminescence detection, according to the manufacturer’s protocol.
2.7. Protein MALDI-TOF/TOF Identification
To identify IgE-binding proteins from lemon, mandarin and orange, protein bands of interest were excised from the SDS-PAGE gel and submitted to the Proteomics Department of the Faculty of Pharmacy of Universidad Complutense de Madrid (UCM) [Madrid Complutense University] for analysis via peptide mass fingerprinting (MALDI-TOF/TOF).
In this method, the target protein undergoes enzymatic digestion, typically using trypsin, which hydrolyzes the protein into peptides at specific cleavage sites. The resulting peptide fragments are then analyzed using a mass spectrometer equipped with an appropriate ionization source, such as Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) or Electrospray Ionization Time-of-Flight (ESI-TOF). The absolute peptide masses obtained are used to generate a peptide mass fingerprint enabling protein identification.
3. Results
3.1. Skin Prick Test. Prick-by-Prick Tests
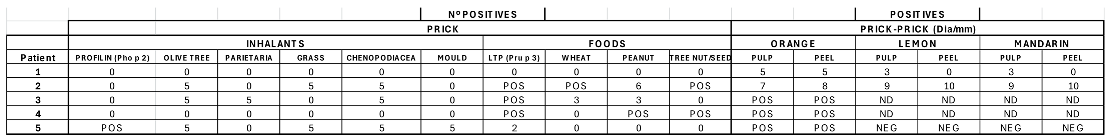
Patient 1:
Skin prick test (SPT to commercial aeroallergen extracts: Negative.
SPT to commercial food extracts: Negative.
-
Prick-by-prick test:
- ○
Orange: positive for pulp and peel (5 mm major diameter papule)
- ○
Lemon: positive for pulp (3 mm major diameter papule), negative for peel.
- ○
Mandarin: positive for pulp (3 mm major diameter papule), negative for peel.
The patient tolerated an oral lemon and mandarin challenge.
Patient 2:
SPT to aeroallergens: positive for cat and dog dander, Phleum pratense, Cynodon dactylon, Olea europaea, Salsola kali, Chenopodium album.
SPT to foods: positive to peach nsLTP (Pru p3), hazelnut, almond, peanut, and pistachio nut.
-
Prick-by-prick:
- ○
Orange: positive for pulp (7 mm major diameter papule) and peel (8 mm)
- ○
Lemon: positive for pulp (9 mm major diameter papule) and peel (10 mm papule)
- ○
Mandarin: positive for pulp (9 mm major diameter papule) and peel (10 mm papule)
- ○
Lemon seed: positive (7 mm major diameter papule)
- ○
Mandarin seed: negative. Orange seed not tested.
Patient 3:
SPT to aeroallergens: positive for Olea europaea, Parietaria judaica, Artemisia vulgaris, Salsola kali, and Chenopodium album.
SPT to foods: positive for peach nsLTP (Pru p 3), corn flour, soy flour, hazelnut, walnut, sunflower seeds and tomato.
-
Prick-by-prick:
- ○
Orange: positive for pulp and peel. Diameter not available.
- ○
Lemon and mandarin: not tested.
Patient 4:
SPT to commercial aeroallergen extracts: Negative.
SPT to foods: positive for hazelnut, almond, peanut, walnut, pistachio, apple, strawberry and peach nsLTP (Pru p 3).
-
Prick-by-prick:
- ○
Orange: positive for pulp (6 mm) and peel (6 mm).
- ○
Lemon and mandarin: not tested.
Patient 5:
SPT to commercial aeroallergen extracts: positive for Alternaria alternata, Artemisia vulgaris, Chenopodium album, Salsola kali, Phleum pratense, Cupressus arizonica, Olea europaea, and profilin (Pho p 2).
SPT to foods: positive for peach, watermelon and melon.
-
Prick-by-prick:
- ○
Positive for orange pulp (5 mm) and peel (5 mm),
- ○
Positive for apple pulp and peel
- ○
Negative for lemon and mandarin.
Orange (pulp and peel): positive in all five patients.
3.2. Molecular Diagnosis and Symptom Scoring
Total IgE and LTP-specific IgE levels varied among patients.
Only Patient 1 showed positive IgE reactivity to all tested recombinant allergens.
The remaining four patients recognized only 4, 4, 1 and 0 recombinant allergens, respectively.
Food Allergy Quality of Life Questionnaire (FAQLQ) scores ranged from 99 to 166 points.
Table 2. Orange sensitisation, molecular allergens (kUA/L), rhinitis/asthma presence, symptom scores (FAQLQ), and tryptase levels. nsLTP allergens are highlighted in black.
Table 3. Clinical characteristics of the first allergic episode.
3.3. Characterisation of Relevant Proteins.
After extract preparation, protein concentrations (mg/ml) were determined as follows
Orange peel: 0.17
Orange pulp: 0.06
Lemon peel: 0.06
Lemon pulp: 0.03
Mandarin peel: 0.12
Mandarin pulp: 0.04
Electrophoretic Analysis
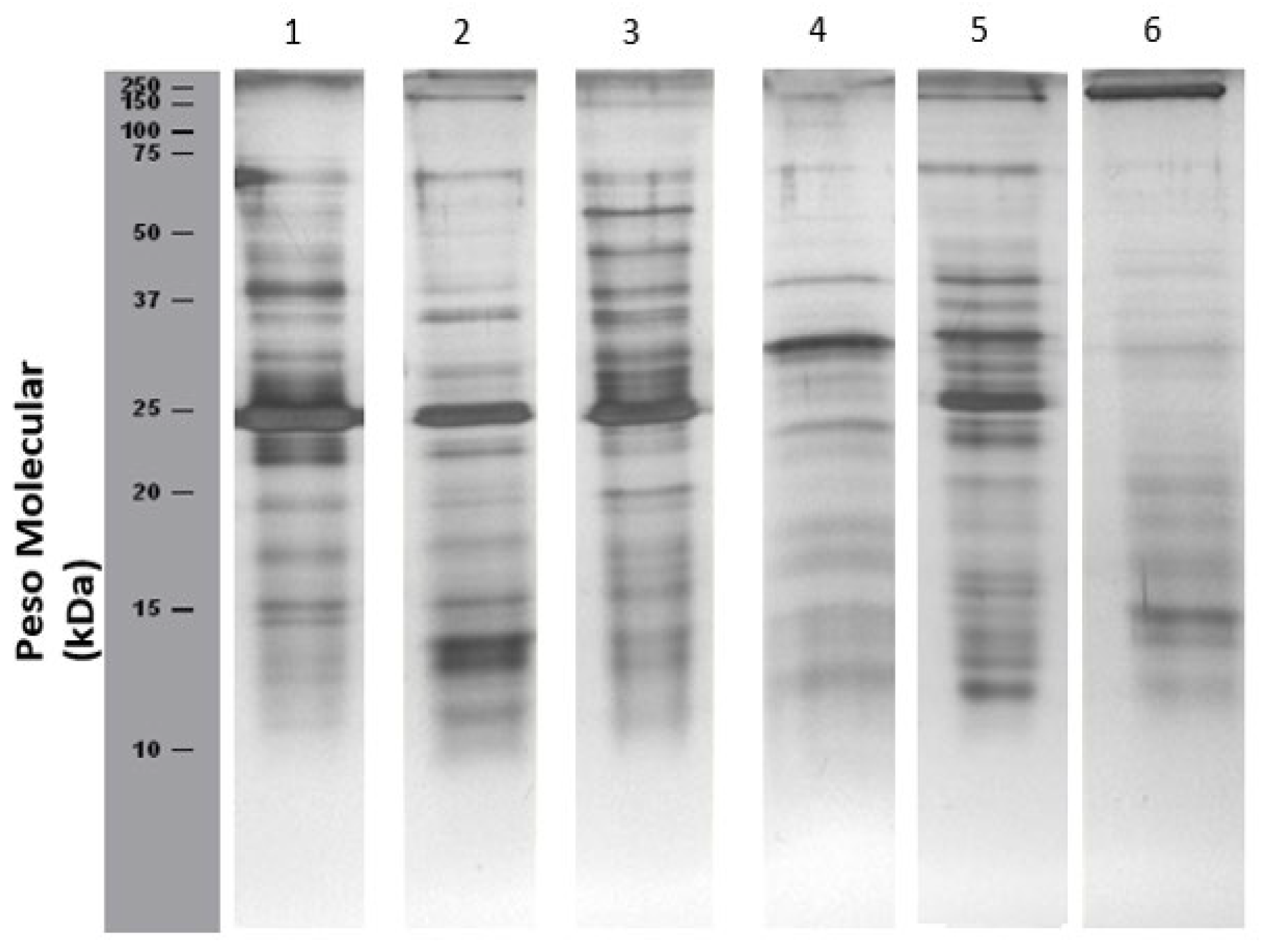
Figure 1 presents SDS-PAGE profiles of citrus fruit peel and pulp extracts:
-
Orange (lane 1-2):
- ○
Bands corresponding to profilin (Cit s 2, 14 kDa) are detected in both peel and pulp.
- ○
A protein approximately 23 kDa, possibly corresponding to germin, is also present.
- ○
No bands below 10 kDa (e.g., Cit s 3, nsLTP, 9.46 kDa; Cit s 7, gibberellin, 8 kDa) were detected, likely due to low protein concentration. However, their presence cannot be ruled out.
- ○
Additional undocumented bands not listed by International Union of Immunological Societies (IUIS) were also observed.
-
Lemon (lanes 3-4):
- ○
A faint band ~10 kDa, potential Cit s 3, (an nsLTP identified at 9.6 kDa), was detected in both peel and pulp.
- ○
Additional proteins with molecular weights ranging from 15-75 kDa were also observed.
-
Mandarin (lanes 5-6):
- ○
A faint band slightly above 10 kDa was detected, which may correspond to Cit r 3, an nsLTP at 9 kDa.
- ○
The number of detected proteins was higher in the peel extract (lane 5) than in the pulp extract (lane 6).
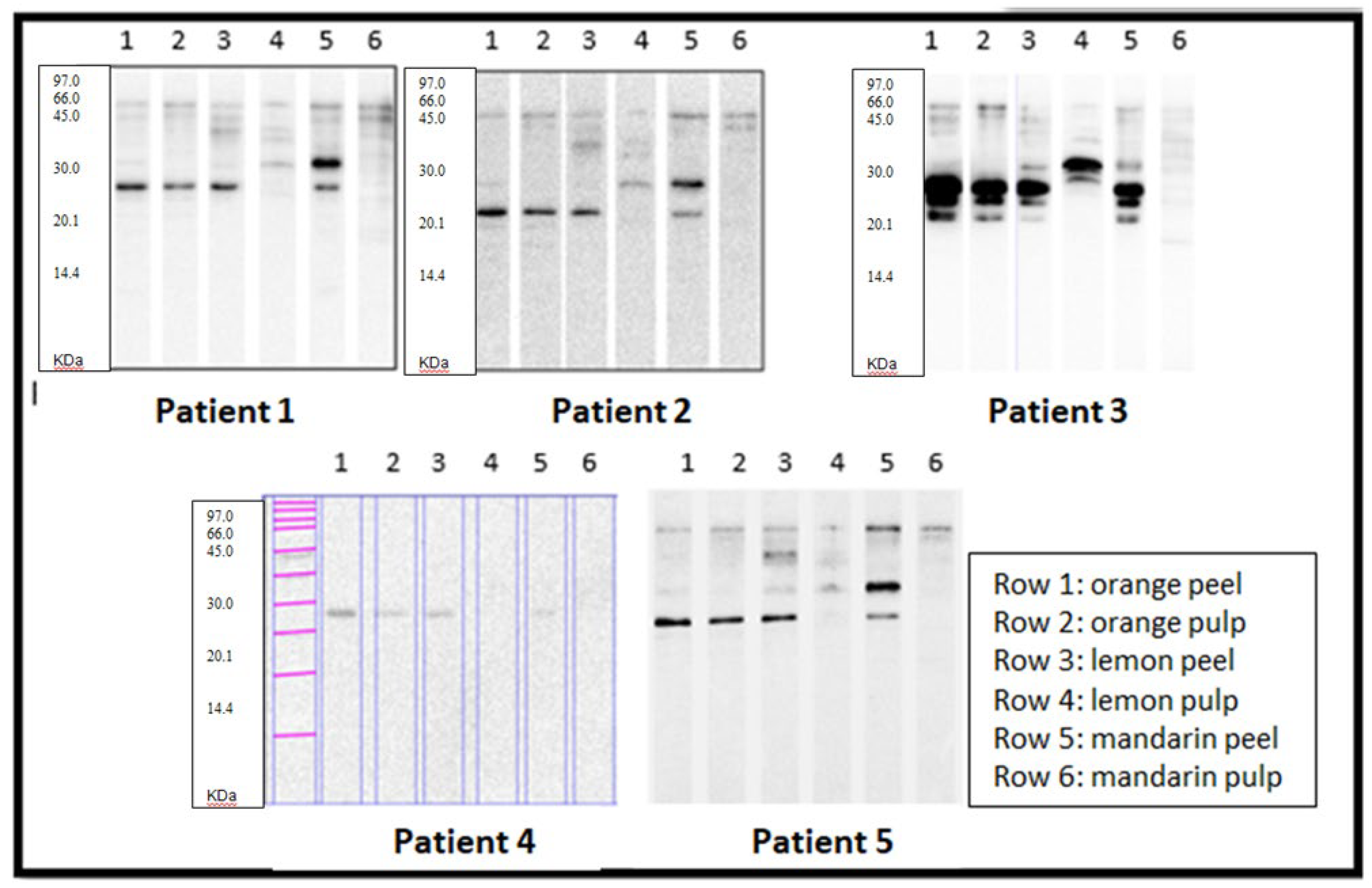
Once the extracts were characterised, they were tested against the sera of the five selected patients (view
Figure 2).
Strikingly, all patients recognised a protein of around 23 kDa present in orange peel and pulp, lemon peel and mandarin peel. In contrast, recognition of lemon and mandarin pulp extracts was much lower in most patients, except for patient 3, who strongly recognised a protein at about 30 kDa (above) in the lemon pulp extract.
3.4. Allergen Sequences
To confirm specific allergen binding in Western blotting across all five patients, we selected the 23 kDa band, which was strongly recognised by all of them. This band, detected in orange (pulp and peel), lemon(peel), and mandarin (peel and pulp), has already been annotated in IUIS in the case of Citrus sinensis (orange) as a germin (Cit s 1).
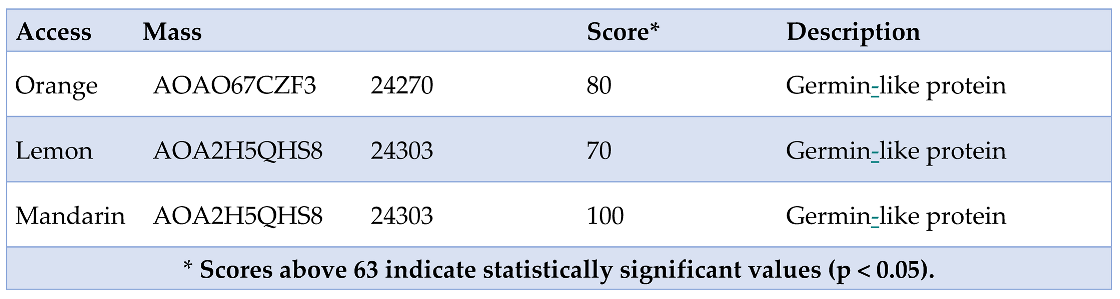
To perform peptide fingerprinting, SDS-PAGE (15% and 10%) was carried out for the three citrus fruits. As a positive control, the 23 kDa band from orange-compatible with Cit s 1 (germin) and already annotated in IUIS—was selected. The 23 kDa bands from orange, lemon, and mandarin peel were excised and submitted for peptide fingerprint analysis, yielding the following sequences (
Table 4).
The specific values for each of the citrus fruits were as follows.
- -
Orange - Score: 80; Expect: 0.0013; Monoisotopic mass (Mr): 24270 and Calculated pI:5.76.
- -
Lemon - Score: 70; Expect: 0.012; Monoisotopic mass (Mr): 24303 and Calculated pI:6.06.
- -
Mandarin - Score: 100; Expect: 1.2e-005; Monoisotopic mass (Mr): 24303 and Calculated pI:6.06.
4. Discussion
Although several citrus allergens have been described, and oranges are considered allergenic sources to be taken into account (
www.foodallergyitalia.org), sensitisation to citrus does not usually involve manifestations as obvious allergic as those caused by other foods[
16]. Only one-third of reactions to citrus fruits have been confirmed by oral challenge tests[
20].
To date, the IUIS database lists the following allergens for orange: Cit s 1 (germin, 23 kDa), Cit s 2 (profilin, 14 kDa), Cit s 3 (nsLTP, 9.46 kDa) and Cit s 7 (gibberellin, 8 kDa). However, for lemon and mandarin, only Cit l 3 and Cit r 3, both LTP of 9.6 and 9 kDa, respectively, have been annotated.
Regarding citrus fruit extracts in general and in our study, the protein concentrations obtained were low, with averages of 0.11 mg/ml for peel and 0.04 mg/ml for pulp. These low concentrations resulted in very small working amounts (0.05 mg/ml) for electrophoresis and Western blotting, limiting antigen availability for visualisation and recognition.
The patients studied showed similar sensitisation patterns in terms of the number of proteins recognised and the intensity of recognition for orange peel and pulp. However, in the case of lemon and mandarin, they recognised more bands, with grater intensity, in the peel. None of the five patients recognised orange profilin nor nsLTPs. The gibberellin-binding zone, described at 8 kDa for orange, was also negative. On the other hand, four out of five patients showed IgE-reactive bands with molecular weights of approximately 50-75 kDa, present in both peel and pulp.
For mandarin, specifically in its peel, four of the five patients recognised two proteins: one at approximately 32 kDa, which was analysed in this study, and another at a slightly lower molecular weight (around 27-28 kDa), which could be interests for further research. In patient 4, the bands were faint, but clearly distinguishable from the lanes where no recognition was observed.
In general, no greater reactivity was observed for the peel than for the pulp, ruling out a predominance of LTP in the reactions. In terms of the prick-by-prick tests with orange pulp and peel, all patients tested positive, with no evidence of larger diameters for peel. All patients were positive for prick-by-prick testing with all three citrus fruits, except for two negative cases: patient 1 for lemon and mandarin peel and patient 5 for lemon and mandarin. The prick-by-prick test remains essential for confirming sensitisation in allergic reactions to suspected food when standard prick tests yield negative results.
In most cases, prior reactions to other foods involved nuts and peach. In these patients, citrus-related symptoms triggered anaphylaxis.
A 23 kDa IgE-reactive band, identified as a germin-like protein (Cit s 1), was observed in all five patients for orange peel and pulp, but only in the peel of lemon and mandarin.
Previous studies have shown that Cit s 1 (germin-like protein) and Cit s 2 (profilin) are heat-resistant and maintain their allergenic properties in processed juices [
12,
13]. Therefore, the presence of germin in lemon and mandarin becomes even more significant.
The presence of IgG in the patients’ sera cannot be ruled out. However, the Western blot images obtained (revealed with anti-human IgE) and the detection of specific IgE to Citrus sinensis at class 3 (3.5-17.5 kU/L) in two patients and class 2 (0.70-3.5 kU/L) in two others, indicate the predominant role of an IgE-mediated response in patients 1,2,3 and 5. Regarding patient 4, whose specific IgE to Citrus sinesis was 0.15 kU/L (normal levels), the 6 mm wheal observed in the prick test suggests an IgE-mediated mechanism.
5. Conclusions
In our study, a 23 kDa band (germin-like protein) was consistently detected in orange, lemon and mandarin peel. This protein (already annotated in IUIS as germin in orange), had not been previously described in IUIS for lemon and mandarin.
Peptide fingerprinting confirmed that its sequences correspond to germin by mass spectrometry. The localisation of germin was verified in the peel of all three citrus fruits and in the pulp of orange, but not in the pulp of lemon or mandarin.
References
- Fernández-Rivas, M.; Gómez García, I.; Gonzalo-Fernández, A.; Fuentes Ferrer, M.; Dölle-Bierke, S.; Marco-Martín, G.; et al. Development and validation of the food allergy severity score. Allergy. mayo de 2022, 77, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Clausen, M.; Knulst, A.C.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J Allergy Clin Immunol Pract. septiembre de 2020, 8, 2736–2746.e9. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. diciembre de 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Spolidoro, G.C.I.; Amera, Y.T.; Ali, M.M.; Nyassi, S.; Lisik, D.; Ioannidou, A.; et al. Frequency of food allergy in Europe: An updated systematic review and meta-analysis. Allergy. febrero de 2023, 78, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Zepeda, C.; Pérez-Allegue, I.; Pastor-Vargas, C.; Bartolomé-Zavala, B.; González-de-Olano, D. Allergy to orange with cystatine-like protein as one of its allergens. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. agosto de 2021, 127, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Inomata, N.; Miyakawa, M.; Ikeda, N.; Oda, K.; Aihara, M. Identification of gibberellin-regulated protein as a new allergen in orange allergy. Clin Exp Allergy J Br Soc Allergy Clin Immunol. noviembre de 2018, 48, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.F.; Retzek, M.; Foetisch, K.; Sierra-Maestro, E.; Cid-Sanchez, A.B.; Pascual, C.Y.; et al. Germin-like protein Cit s 1 and profilin Cit s 2 are major allergens in orange (Citrus sinensis) fruits. Mol Nutr Food Res. marzo de 2006, 50, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.A.; Bernardo, L.; Spadafora, A.; Faccioli, P.; Canton, C.; Mazzuca, S. The Citrus clementina putative allergens: from proteomic analysis to structural features. J Agric Food Chem. 18 de septiembre de 2013, 61, 8949–8958. [Google Scholar] [CrossRef] [PubMed]
- López-Torrejón, G.; Ibáñez, M.D.; Ahrazem, O.; Sánchez-Monge, R.; Sastre, J.; Lombardero, M.; et al. Isolation, cloning and allergenic reactivity of natural profilin Cit s 2, a major orange allergen. Allergy. noviembre de 2005, 60, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Moens, P.D.J. Structure and functions of profilins. Biophys Rev. julio de 2009, 1, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Ibáñez, M.D.; López-Torrejón, G.; Sánchez-Monge, R.; Sastre, J.; Lombardero, M.; et al. Orange germin-like glycoprotein Cit s 1: an equivocal allergen. Int Arch Allergy Immunol. 2006, 139, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Ibáñez, M.D.; López-Torrejón, G.; Sánchez-Monge, R.; Sastre, J.; Lombardero, M.; et al. Lipid transfer proteins and allergy to oranges. Int Arch Allergy Immunol. julio de 2005, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Inomata, N. Gibberellin-regulated protein allergy: Clinical features and cross-reactivity. Allergol Int Off J Jpn Soc Allergol. enero de 2020, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Inomata, N. Gibberellin-regulated protein allergy: Clinical features and cross-reactivity. Allergol Int [Internet]. 1 de enero de 2020 [citado 17 de febrero de 2025];69, 11–18. Disponible en: https://www.sciencedirect.com/science/article/pii/S1323893019301716.
- Ahrazem, O.; Ibáñez, M.D.; López-Torrejón, G.; Sánchez-Monge, R.; Sastre, J.; Lombardero, M.; et al. Lipid transfer proteins and allergy to oranges. Int Arch Allergy Immunol. julio de 2005, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Ibáñez, M.D.; López-Torrejón, G.; Sánchez-Monge, R.; Sastre, J.; Lombardero, M.; et al. Lipid transfer proteins and allergy to oranges. Int Arch Allergy Immunol. julio de 2005, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Ahrazem, O.; Lopez-Torrejon, G.; Bridts, C.H.; Salcedo, G.; Stevens, W.J. Anaphylaxis from mandarin (Citrus reticulata): identification of potential responsible allergens. Int Arch Allergy Immunol. 2007, 144, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Jarolim, E.; Schmid, B.; Bernier, F.; Berna, A.; Kinaciyan, T.; Focke, M.; et al. Allergologic exploration of germins and germin-like proteins, a new class of plant allergens. Allergy. septiembre de 2002, 57, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.; Diaz-Perales, A.; Escribese, M.M.; Kleine-Tebbe, J.; Matricardi, P.M.; Ollert, M.; et al. Molecular allergology and its impact in specific allergy diagnosis and therapy. Allergy. diciembre de 2021, 76, 3642–3658. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, C.; Ispano, M.; Pastorello, E.A.; Ansaloni, R.; Magri, G.C. Comparison of results of skin prick tests (with fresh foods and commercial food extracts) and RAST in 100 patients with oral allergy syndrome. J Allergy Clin Immunol. marzo de 1989, 83, 683–690. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).