Submitted:
24 February 2025
Posted:
25 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
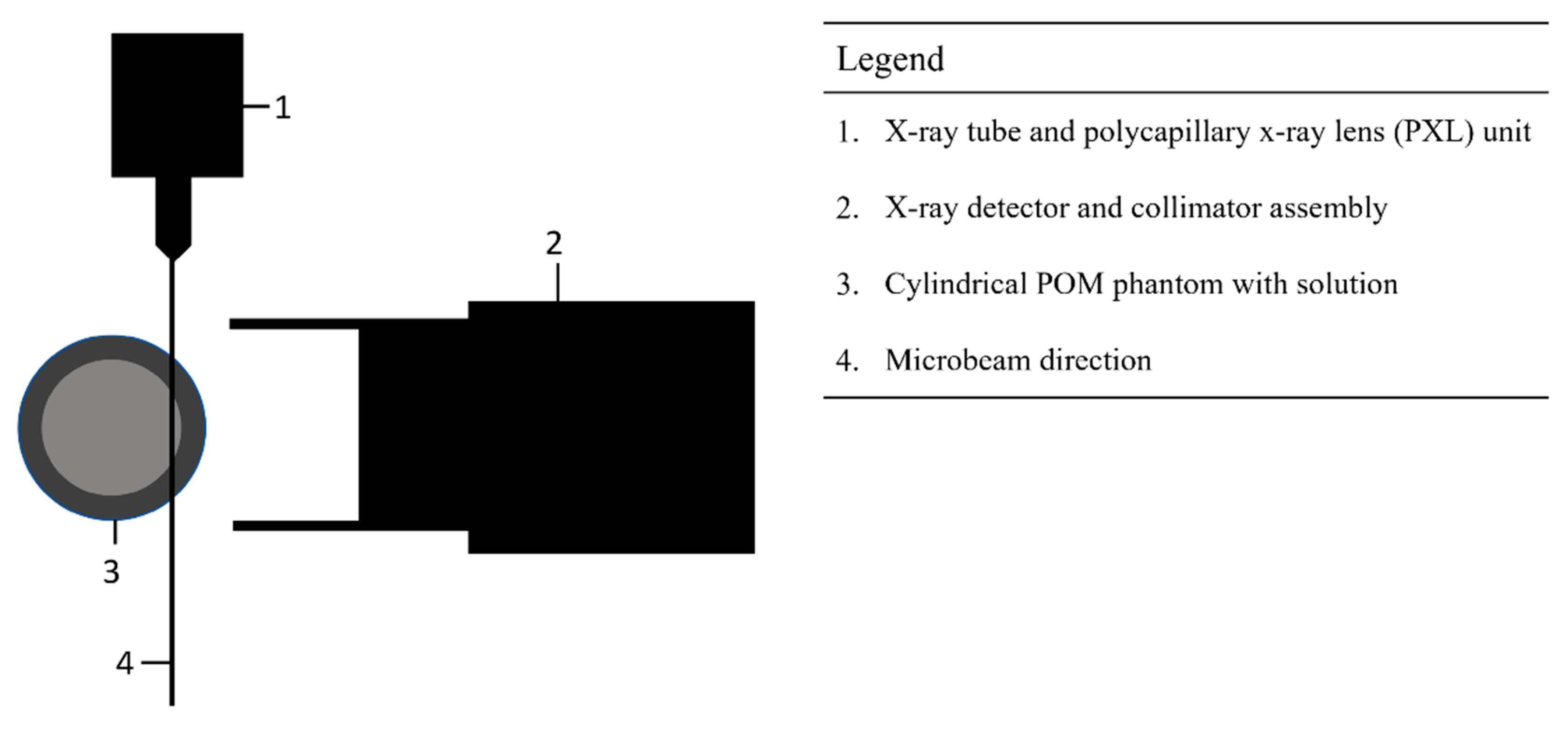
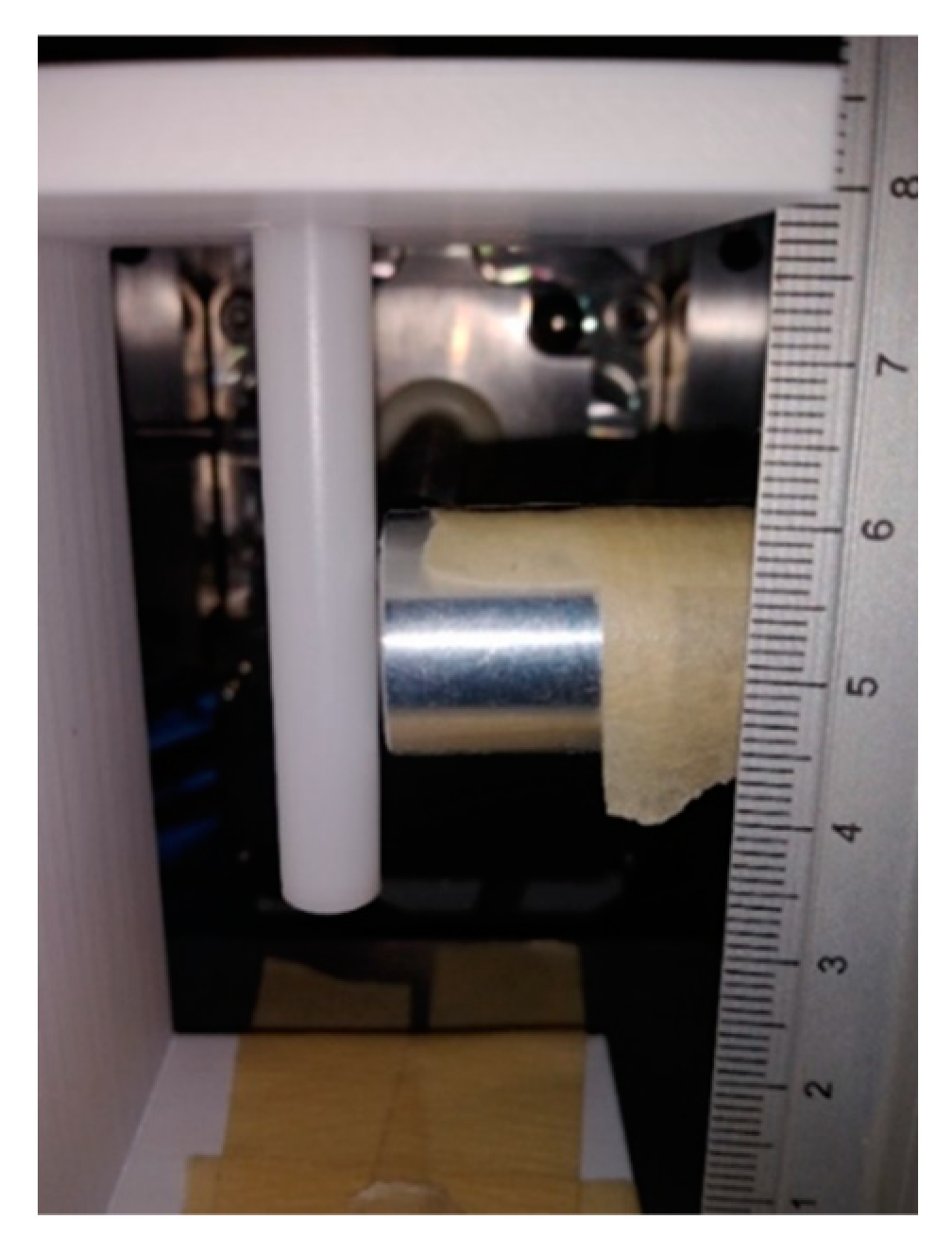
2.1. XRF Experimental Setup
2.2. Standard Solutions and POM Phantoms
2.3. XRF Experimental Procedures
2.4. Data Analysis
2.5. Radiation Dose Calculations
3. Results
3.1. Calibration Lines and Detection Limits
3.2. Radiation Dose Estimates
4. Discussion
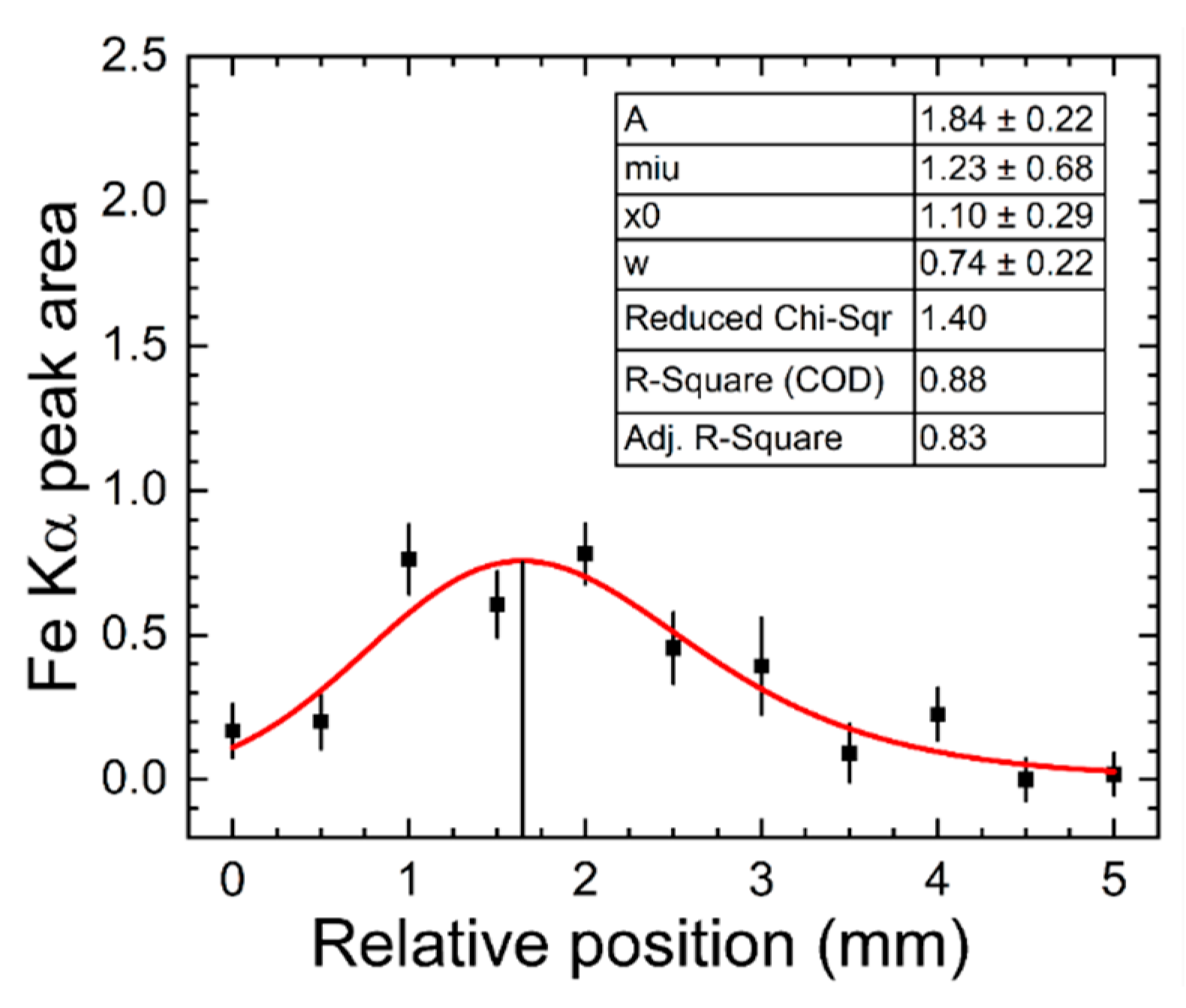
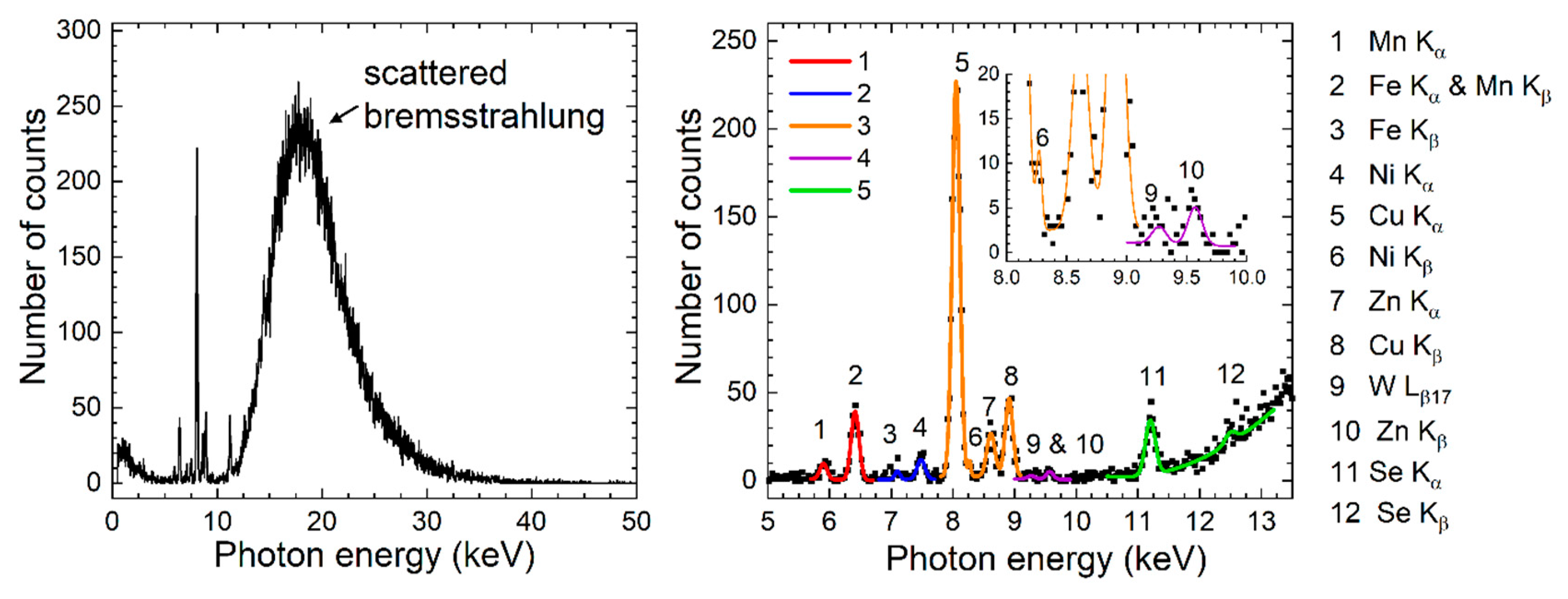
4.1. Spectral Issues
4.2. Measured Ratio Analysis
4.3. In Vivo Measurements Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallee, Bert, L. The Function of Trace Elements in Biology. The Scientific Monthly 1951, 72, 368–376. [Google Scholar]
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. Journal of Inorganic Biochemistry 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, B.G.; Neilands, J.B. Metalloproteins. Annu. Rev. Biochem. 1964, 33, 331–354. [Google Scholar] [CrossRef]
- Haraguchi, H. Metallomics as Integrated Biometal Science. J. Anal. At. Spectrom. 2004, 19, 5. [Google Scholar] [CrossRef]
- Lu, Y.; Yeung, N.; Sieracki, N.; Marshall, N.M. Design of Functional Metalloproteins. Nature 2009, 460, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Neve, J. Clinical Implications of Trace Elements in Endocrinology. Biol Trace Elem Res 1992, 32, 173–185. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy Metals and Neurodegenerative Diseases: An Observational Study. Biol Trace Elem Res 2014, 161, 151–160. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, Essentiality and Toxicity of Trace Elements in Human Health. Molecular Aspects of Medicine 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Calderón Guzmán, D.; Juárez Olguín, H.; Osnaya Brizuela, N.; Hernández Garcia, E.; Lindoro Silva, M. The Use of Trace and Essential Elements in Common Clinical Disorders: Roles in Assessment of Health and Oxidative Stress Status. Nutrition and Cancer 2019, 71, 13–20. [Google Scholar] [CrossRef]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium Intake, Stroke, and Cardiovascular Disease. Journal of the American College of Cardiology 2011, 57, 1210–1219. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N Engl J Med 2015, 372, 1832–1843. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, C.; Liao, M.; He, H.; Liu, Y.; Jing, C.; Zeng, F.; Chen, Q. Admission Serum Sodium and Potassium Levels Predict Survival among Critically Ill Patients with Acute Kidney Injury: A Cohort Study. BMC Nephrol 2019, 20, 311. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.L.; Beard, J.D.; Umbach, D.M.; Allen, K.; Keller, J.; Mariosa, D.; Sandler, D.P.; Schmidt, S.; Fang, F.; Ye, W.; et al. Blood Levels of Trace Metals and Amyotrophic Lateral Sclerosis. NeuroToxicology 2016, 54, 119–126. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Mancini, C.; Abete, M.C.; Brusco, A. Altered Homeostasis of Trace Elements in the Blood of SCA2 Patients. Journal of Trace Elements in Medicine and Biology 2018, 47, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; DiSilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A Comparison of Zinc Metabolism, Inflammation, and Disease Severity in Critically Ill Infected and Noninfected Adults Early after Intensive Care Unit Admission. The American Journal of Clinical Nutrition 2011, 93, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Florea, D.; Molina-López, J.; Hogstrand, C.; Lengyel, I.; De La Cruz, A.P.; Rodríguez-Elvira, M.; Planells, E. Changes in Zinc Status and Zinc Transporters Expression in Whole Blood of Patients with Systemic Inflammatory Response Syndrome (SIRS). Journal of Trace Elements in Medicine and Biology 2018, 49, 202–209. [Google Scholar] [CrossRef]
- Bowman, A.B.; Kwakye, G.F.; Herrero Hernández, E.; Aschner, M. Role of Manganese in Neurodegenerative Diseases. Journal of Trace Elements in Medicine and Biology 2011, 25, 191–203. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P.; Schapira, A.H.V.; Marsden, C.D. ; The Royal Kings and Queens Parkinson’s Disease Research Group Alterations in Levels of Iron, Ferritin, and Other Trace Metals in Neurodegenerative Diseases Affecting the Basal Ganglia. Ann Neurol. 1992, 32, S94–S100. [Google Scholar] [CrossRef]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N Engl J Med 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. The Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Addison, G.M.; Beamish, M.R.; Hales, C.N.; Hodgkins, M.; Jacobs, A.; Llewellin, P. An Immunoradiometric Assay for Ferritin in the Serum of Normal Subjects and Patients with Iron Deficiency and Iron Overload. J Clin Pathol 1972, 25, 326–329. [Google Scholar] [CrossRef]
- Cook, J.D. Defining Optimal Body Iron. Proc. Nutr. Soc. 1999, 58, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Finkelstein, J.L.; O’Dell, D.; Erickson, D.; Mehta, S. Rapid Diagnostics for Point-of-Care Quantification of Soluble Transferrin Receptor. EBioMedicine 2019, 42, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Serhan, M.; Jackemeyer, D.; Abi Karam, K.; Chakravadhanula, K.; Sprowls, M.; Cay-Durgun, P.; Forzani, E. A Novel Vertical Flow Assay for Point of Care Measurement of Iron from Whole Blood. Analyst 2021, 146, 1633–1641. [Google Scholar] [CrossRef]
- Ammann, A.A. Inductively Coupled Plasma Mass Spectrometry (ICP MS): A Versatile Tool. J. Mass Spectrom. 2007, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkani, M.F. Guideline of Inductively Coupled Plasma Mass Spectrometry “ICP–MS”: Fundamentals, Practices, Determination of the Limits, Quality Control, and Method Validation Parameters. SN Appl. Sci. 2019, 1, 791. [Google Scholar] [CrossRef]
- Bornhorst, J.; Kipp, A.P.; Haase, H.; Meyer, S.; Schwerdtle, T. The Crux of Inept Biomarkers for Risks and Benefits of Trace Elements. TrAC Trends in Analytical Chemistry 2018, 104, 183–190. [Google Scholar] [CrossRef]
- Seregina, I.F.; Osipov, K.; Bol’shov, M.A.; Filatova, D.G.; Lanskaya, S.Yu. Matrix Interference in the Determination of Elements in Biological Samples by Inductively Coupled Plasma–Mass Spectrometry and Methods for Its Elimination. J Anal Chem 2019, 74, 182–191. [Google Scholar] [CrossRef]
- Chettle, D.R. In Vivo Applications of X-Ray Fluorescence in Human Subjects. Pramana - J Phys 2011, 76, 249–259. [Google Scholar] [CrossRef]
- Chettle, D.R.; McNeill, F.E. Elemental Analysis in Living Human Subjects Using Biomedical Devices. Physiol. Meas. 2019, 40, 12TR01. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Gould, R.W.; Gedcke, D. Quantitative X-Ray Spectrometry; Practical spectroscopy; Dekker: New York, 1995; ISBN 978-0-8247-9554-2. [Google Scholar]
- Fleming, D.E.B.; Gherase, M.R. A Rapid, High Sensitivity Technique for Measuring Arsenic in Skin Phantoms Using a Portable x-Ray Tube and Detector. Phys. Med. Biol. 2007, 52, N459–N465. [Google Scholar] [CrossRef]
- Fleming, D.E.B. The Measurement of Trace Elements in Human Nails and Nail Clippings Using Portable X-ray Fluorescence: A Review. X-Ray Spectrometry 2022, 51, 328–337. [Google Scholar] [CrossRef]
- Gherase, M.R.; Al-Hamdani, S. A Microbeam Grazing-Incidence Approach to L-Shell x-Ray Fluorescence Measurements of Lead Concentration in Bone and Soft Tissue Phantoms. Physiol. Meas. 2018, 39, 035007. [Google Scholar] [CrossRef]
- Prange, A.; Böddeker, H.; Michaelis, W. Multi-Element Determination of Trace Elements in Whole Blood and Blood Serum by TXRF. Z. Anal. Chem. 1989, 335, 914–918. [Google Scholar] [CrossRef]
- Ayala, R.E.; Alvarez, E.M.; Wobrauschek, P. Direct Determination of Lead in Whole Human Blood by Total Reflection X-Ray Fluorescence Spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy 1991, 46, 1429–1432. [Google Scholar] [CrossRef]
- Marco, L.M.; Greaves, E.D.; Alvarado, J. Analysis of Human Blood Serum and Human Brain Samples by Total Reflection X-Ray FLuorescence Spectrometry Applying Compton Peak Standardizationଝ. Atomic Spectroscopy 1999. [Google Scholar] [CrossRef]
- Khuder, A.; Bakir, M.A.; Karjou, J.; Sawan, M.Kh. XRF and TXRF Techniques for Multi-Element Determination of Trace Elements in Whole Blood and Human Hair Samples. J Radioanal Nucl Chem 2007, 273, 435–442. [Google Scholar] [CrossRef]
- Stosnach, H.; Mages, M. Analysis of Nutrition-Relevant Trace Elements in Human Blood and Serum by Means of Total Reflection X-Ray Fluorescence (TXRF) Spectroscopy. Spectrochimica Acta Part B: Atomic Spectroscopy 2009, 64, 354–356. [Google Scholar] [CrossRef]
- Majewska, U.; Łyżwa, P.; Łyżwa, K.; Banaś, D.; Kubala-Kukuś, A.; Wudarczyk-Moćko, J.; Stabrawa, I.; Braziewicz, J.; Pajek, M.; Antczak, G.; et al. Determination of Element Levels in Human Serum: Total Reflection X-Ray Fluorescence Applications. Spectrochimica Acta Part B: Atomic Spectroscopy 2016, 122, 56–61. [Google Scholar] [CrossRef]
- Lahmar, L.; Benamar, M.E.A.; Melzi, M.A.; Melkaou, C.H.; Mabdoua, Y. Determination of Trace Elements Fe, Cu and Zn in the Algerian Cancerous Plasma Using X-ray Fluorescence (XRF). X-Ray Spectrometry 2020, 49, 313–321. [Google Scholar] [CrossRef]
- Gherase, M.R.; Serna, B.; Kroeker, S. A Novel Calibration for L-Shell x-Ray Fluorescence Measurements of Bone Lead Concentration Using the Strontium Kβ/Kα Ratio. Physiol. Meas. 2021, 42, 045011. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values: ICRP Publication 89: Approved by the Commission in September 2001. Ann ICRP 2002, 32, 1–277. [Google Scholar] [CrossRef]
- Farquharson, M.J.; Bradley, D.A. The Feasibility of a Sensitive Low-Dose Method for the in Vivo Evaluation of Fe in Skin Using K-Shell x-Ray Fluorescence (XRF). Phys. Med. Biol. 1999, 44, 955–965. [Google Scholar] [CrossRef]
- Molokhia, A.; Dyer, A.; Portnoy, B. Simultaneous Determination of Eight Trace Elements in Human Skin by Instrumental Neutron-Activation Analysis. Analyst 1981, 106, 1168. [Google Scholar] [CrossRef]
- Malmqvist, K.G.; Carlsson, L.-E.; Forslind, B.; Roomans, G.M.; Akselsson, K.R. Proton and Electron Microprobe Analysis of Human Skin. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 1984, 3, 611–617. [Google Scholar] [CrossRef]
- Gorodetsky, R.; Sheskin, J.; Weinreb, A. Iron, Copper, and Zinc Concentrations in Normal Skin and in Various Nonmalignant and Malignant Lesions. Int J Dermatology 1986, 25, 440–445. [Google Scholar] [CrossRef]
- Hollands, R.; Spyrou, N.M.; Vijh, V.; Scales, J.T. Elemental Composition of Skin Tissue by PIXE and INA Analyses. J Radioanal Nucl Chem 1997, 217, 185–187. [Google Scholar] [CrossRef]
- Warner, R.R.; Myers, M.C.; Taylor, D.A. Electron Probe Analysis of Human Skin: Element Concentration Profiles. Journal of Investigative Dermatology 1988, 90, 78–85. [Google Scholar] [CrossRef]
- Forslind, B. The Skin Barrier: Analysis of Physiologically Important Elements and Trace Elements. Acta Dermato-Venereologica 2000, 80, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Desouza, E.D.; Atiya, I.A.; Al-Ebraheem, A.; Wainman, B.C.; Fleming, D.E.B.; McNeill, F.E.; Farquharson, M.J. Characterization of the Depth Distribution of Ca, Fe and Zn in Skin Samples, Using Synchrotron Micro-x-Ray Fluorescence (SμXRF) to Help Quantify in-Vivo Measurements of Elements in the Skin. Applied Radiation and Isotopes 2013, 77, 68–75. [Google Scholar] [CrossRef]
- Bagshaw, A.P.; Farquharson, M.J. Simultaneous Determination of Iron, Copper and Zinc Concentrations in Skin Phantoms Using XRF Spectrometry. X-Ray Spectrometry 2002, 31, 47–52. [Google Scholar] [CrossRef]
- Molokhia, A.; Portnoy, B.; Dyer, A. Neutron Activation Analysis of Trace Elements in Skin.: VIII. SELENIUM IN NORMAL SKIN. Br J Dermatol 1979, 101, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Gherase, M.R.; Fleming, D.E.B. A Calibration Method for Proposed XRF Measurements of Arsenic and Selenium in Nail Clippings. Phys. Med. Biol. 2011, 56, N215–N225. [Google Scholar] [CrossRef]
- Shehab, H.; Desouza, E.D.; O’Meara, J.; Pejović-Milić, A.; Chettle, D.R.; Fleming, D.E.B.; McNeill, F.E. Feasibility of Measuring Arsenic and Selenium in Human Skin Using in Vivo x-Ray Fluorescence (XRF)—a Comparison of Methods. Physiol. Meas. 2016, 37, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Yedomon, B.; Menudier, A.; Etangs, F.L.D.; Anani, L.; Fayomi, B.; Druet-Cabanac, M.; Moesch, C. Biomonitoring of 29 Trace Elements in Whole Blood from Inhabitants of Cotonou (Benin) by ICP-MS. Journal of Trace Elements in Medicine and Biology 2017, 43, 38–45. [Google Scholar] [CrossRef]
- Heitland, P.; Köster, H.D. Biomonitoring of 37 Trace Elements in Blood Samples from Inhabitants of Northern Germany by ICP–MS. Journal of Trace Elements in Medicine and Biology 2006, 20, 253–262. [Google Scholar] [CrossRef]
- Bocca, B.; Madeddu, R.; Asara, Y.; Tolu, P.; Marchal, J.A.; Forte, G. Assessment of Reference Ranges for Blood Cu, Mn, Se and Zn in a Selected Italian Population. Journal of Trace Elements in Medicine and Biology 2011, 25, 19–26. [Google Scholar] [CrossRef]
- Harrington, J.M.; Young, D.J.; Essader, A.S.; Sumner, S.J.; Levine, K.E. Analysis of Human Serum and Whole Blood for Mineral Content by ICP-MS and ICP-OES: Development of a Mineralomics Method. Biol Trace Elem Res 2014, 160, 132–142. [Google Scholar] [CrossRef]
- Hoet, P.; Jacquerye, C.; Deumer, G.; Lison, D.; Haufroid, V. Reference Values of Trace Elements in Blood and/or Plasma in Adults Living in Belgium. Clinical Chemistry and Laboratory Medicine (CCLM) 2021, 59, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Komarova, T.; McKeating, D.; Perkins, A.V.; Tinggi, U. Trace Element Analysis in Whole Blood and Plasma for Reference Levels in a Selected Queensland Population, Australia. IJERPH 2021, 18, 2652. [Google Scholar] [CrossRef]
- Harvey, L.J.; Ashton, K.; Hooper, L.; Casgrain, A.; Fairweather-Tait, S.J. Methods of Assessment of Copper Status in Humans: A Systematic Review. The American Journal of Clinical Nutrition 2009, 89, 2009S–2024S. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fekete, K.; Decsi, T. Methods of Assessment of Zinc Status in Humans: A Systematic Review. The American Journal of Clinical Nutrition 2009, 89, 2040S–2051S. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. The Journal of Nutrition 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of Assessment of Selenium Status in Humans: A Systematic Review. The American Journal of Clinical Nutrition 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef]
- Simić, A.; Hansen, A.F.; Syversen, T.; Lierhagen, S.; Ciesielski, T.M.; Romundstad, P.R.; Midthjell, K.; Åsvold, B.O.; Flaten, T.P. Trace Elements in Whole Blood in the General Population in Trøndelag County, Norway: The HUNT3 Survey. Science of The Total Environment 2022, 806, 150875. [Google Scholar] [CrossRef]
- Bárány, E.; Bergdahl, I.A.; Bratteby, L.-E.; Lundh, T.; Samuelson, G.; Schütz, A.; Skerfving, S.; Oskarsson, A. Trace Element Levels in Whole Blood and Serum from Swedish Adolescents. Science of The Total Environment 2002, 286, 129–141. [Google Scholar] [CrossRef]
- Syversen, T.; Evje, L.; Wolf, S.; Flaten, T.P.; Lierhagen, S.; Simic, A. Trace Elements in the Large Population-Based HUNT3 Survey. Biol Trace Elem Res 2021, 199, 2467–2474. [Google Scholar] [CrossRef]
- Nisse, C.; Tagne-Fotso, R.; Howsam, M.; Richeval, C.; Labat, L.; Leroyer, A. Blood and Urinary Levels of Metals and Metalloids in the General Adult Population of Northern France: The IMEPOGE Study, 2008–2010. International Journal of Hygiene and Environmental Health 2017, 220, 341–363. [Google Scholar] [CrossRef]
- Taylor, John R Introduction to Error Analysis. The Study of Uncertainties in Physical Measurements; 2nd ed.; University Science Books: 55D Gate Five Road, Sausalito, CA, USA, 94965, 1997; ISBN 978-0-935702-75-X. [Google Scholar]
- Braverman, I.M. The Cutaneous Microcirculation. Journal of Investigative Dermatology Symposium Proceedings 2000, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deslattes, R.D.; Kessler, E.G.; Indelicato, P.; De Billy, L.; Lindroth, E.; Anton, J. X-Ray Transition Energies: New Approach to a Comprehensive Evaluation. Rev. Mod. Phys. 2003, 75, 35–99. [Google Scholar] [CrossRef]
- Seltzer, S. XCOM-Photon Cross Sections Database, NIST Standard Reference Database 8 1987.
- Tissue Substitutes in Radiation Dosimetry and Measurement; International Commission on Radiation Units and Measurements, Ed. ; ICRU report; International Commission on Radiation Units and Measurements: Bethesda, Md., U.S.A, 1989; ISBN 978-0-913394-38-0. [Google Scholar]
- Gherase, M.R.; Al-Hamdani, S. Improvements and Reproducibility of an Optimal Grazing-Incidence Position Method to L-Shell x-Ray Fluorescence Measurements of Lead in Bone and Soft Tissue Phantoms. Biomed. Phys. Eng. Express 2018, 4, 065024. [Google Scholar] [CrossRef] [PubMed]
- Gherase, M.R. A Two-Dimensional K-Shell X-Ray Fluorescence (2D-KXRF) Model for Soft Tissue Attenuation Corrections of Strontium Measurements in a Cortical Lamb Bone Sample. Metrology 2023, 3, 325–346. [Google Scholar] [CrossRef]
- Somervaille, L.J.; Chettle, D.R.; Scott, M.C. In Vivo Measurement of Lead in Bone Using X-Ray Fluorescence. Phys. Med. Biol. 1985, 30, 929–943. [Google Scholar] [CrossRef]
- Wielopolski, L.; Rosen, J.F.; Slatkin, D.N.; Zhang, R.; Kalef-Ezra, J.A.; Rothman, J.C.; Maryanski, M.; Jenks, S.T. In Vivo Measurement of Cortical Bone Lead Using Polarized x Rays. Medical Physics 1989, 16, 521–528. [Google Scholar] [CrossRef]
- Nie, H.; Chettle, D.; Luo, L.; O’Meara, J. Dosimetry Study for a New in Vivo X-Ray Fluorescence (XRF) Bone Lead Measurement System. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2007, 263, 225–230. [Google Scholar] [CrossRef]
- Gherase, M.R.; Feng, R.; Fleming, D.E.B. Optimization of L-shell X-ray Fluorescence Detection of Lead in Bone Phantoms Using Synchrotron Radiation. X-Ray Spectrometry 2017, 46, 537–547. [Google Scholar] [CrossRef]
- Ertuğral, B.; Apaydın, G.; Çevik, U.; Ertuğrul, M.; Kobya, A.İ. K/K X-Ray Intensity Ratios for Elements in the Range 16⩽Z⩽92 Excited by 5.9, 59.5 and 123.6 keV Photons. Radiation Physics and Chemistry 2007, 76, 15–22. [Google Scholar] [CrossRef]
- McCollough, C.H.; Schueler, B.A. Calculation of Effective Dose. Medical Physics 2000, 27, 828–837. [Google Scholar] [CrossRef]
- Todd, A.C.; McNeill, F.E.; Palethorpe, J.E.; Peach, D.E.; Chettle, D.R.; Tobin, M.J.; Strosko, S.J.; Rosen, J.C. In Vivo X-Ray Fluorescence of Lead in Bone Using K X-Ray Excitation with 109Cd Sources: Radiation Dosimetry Studies. Environmental Research 1992, 57, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Gherase, M.R.; Mader, J.E.; Fleming, D.E.B. The Radiation Dose from a Proposed Measurement of Arsenic and Selenium in Human Skin. Phys. Med. Biol. 2010, 55, 5499–5514. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A.; Mahesh, M.; Bhargavan-Chatfield, M.; Chambers, C.E.; Elee, J.G.; Frush, D.P.; Miller, D.L.; Royal, H.D.; Milano, M.T.; Spelic, D.C.; et al. Patient Exposure from Radiologic and Nuclear Medicine Procedures in the United States: Procedure Volume and Effective Dose for the Period 2006–2016. Radiology 2020, 295, 418–427. [Google Scholar] [CrossRef]
- The 2007 Recommendations of the International Commission on Radiological Protection; Valentin, J. , International Commission on Radiological Protection, Eds.; ICRP publication; Elsevier: Oxford, 2007; ISBN 978-0-7020-3048-2. [Google Scholar]
- Piorek, S. Field-Portable X-Ray Fluorescence Spectrometry: Past, Present, and Future. Field Analyt. Chem. Technol. 1997, 1, 317–329. [Google Scholar] [CrossRef]
- Richmond, C.R. ICRP Publication 23. 1985; 48. [Google Scholar] [CrossRef]
- Sherman, J. The Theoretical Derivation of Fluorescent X-Ray Intensities from Mixtures. Spectrochimica Acta 1955, 7, 283–306. [Google Scholar] [CrossRef]
- De Boer, D.K.G.; Borstrok, J.J.M.; Leenaers, A.J.G.; Van Sprang, H.A.; Brouwer, P.N. How Accurate Is the Fundamental Parameter Approach? XRF Analysis of Bulk and Multilayer Samples. X-Ray Spectrometry 1993, 22, 33–38. [Google Scholar] [CrossRef]
- Szalóki, I.; Gerényi, A.; Radócz, G.; Lovas, A.; De Samber, B.; Vincze, L. FPM Model Calculation for Micro X-Ray Fluorescence Confocal Imaging Using Synchrotron Radiation. J. Anal. At. Spectrom. 2017, 32, 334–344. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, R.K. Optical Coherence Tomography Based Angiography [Invited]. Biomed. Opt. Express 2017, 8, 1056. [Google Scholar] [CrossRef]
- Lv, J.; Ai, P.; Lei, S.; Zhou, F.; Chen, S.; Zhang, Y. Selenium Levels and Skin Diseases: Systematic Review and Meta-Analysis. Journal of Trace Elements in Medicine and Biology 2020, 62, 126548. [Google Scholar] [CrossRef]
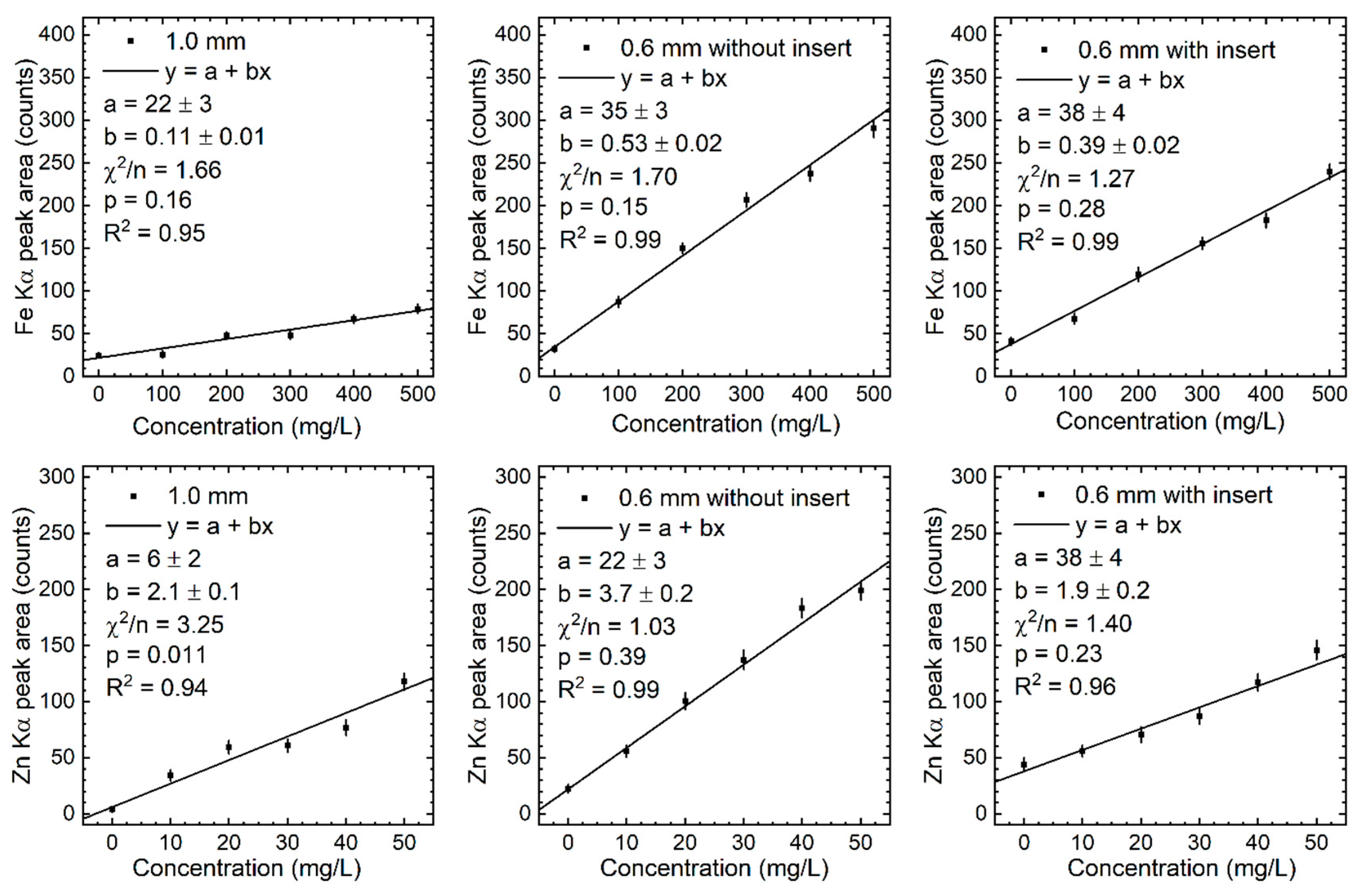
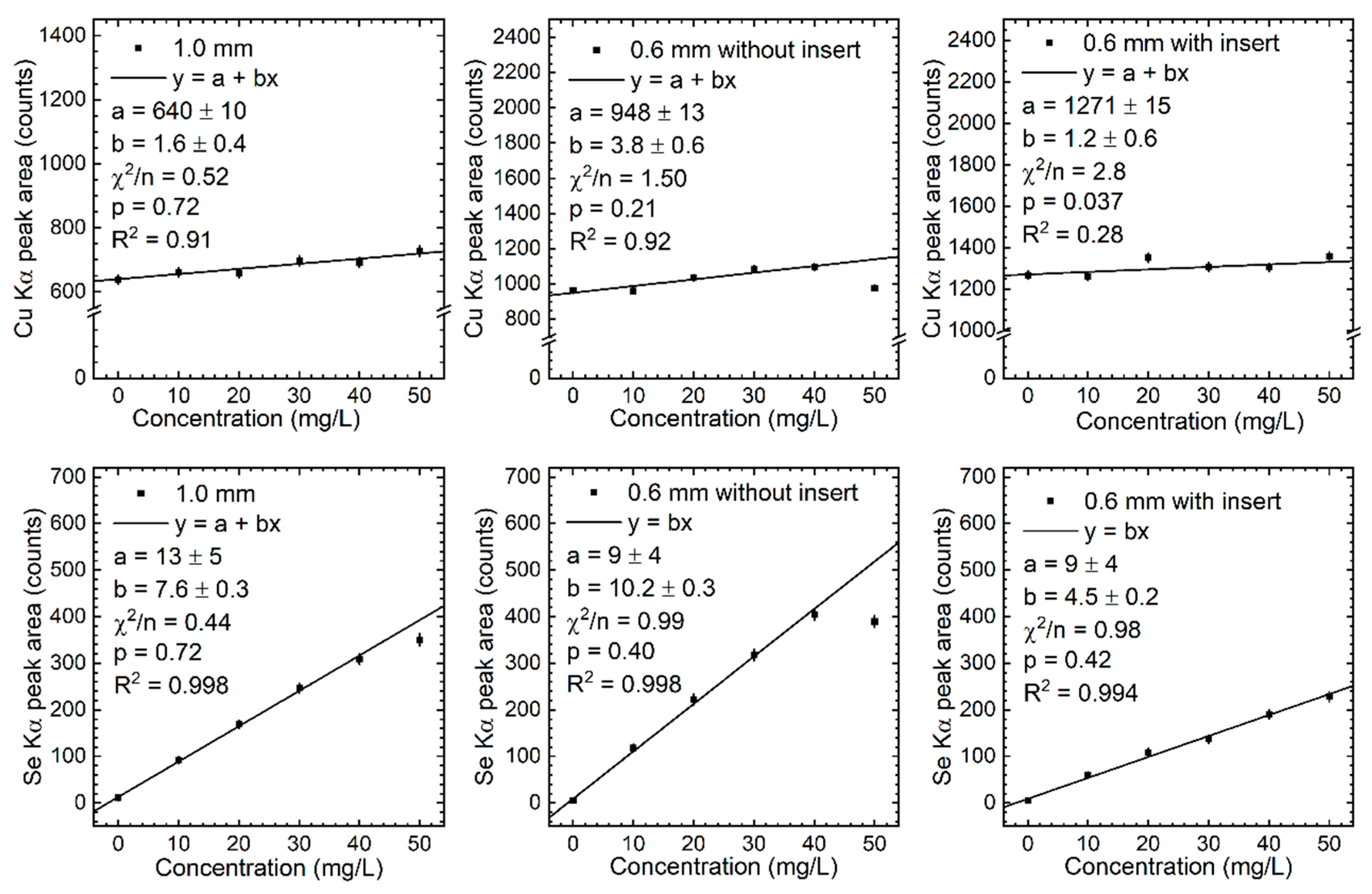
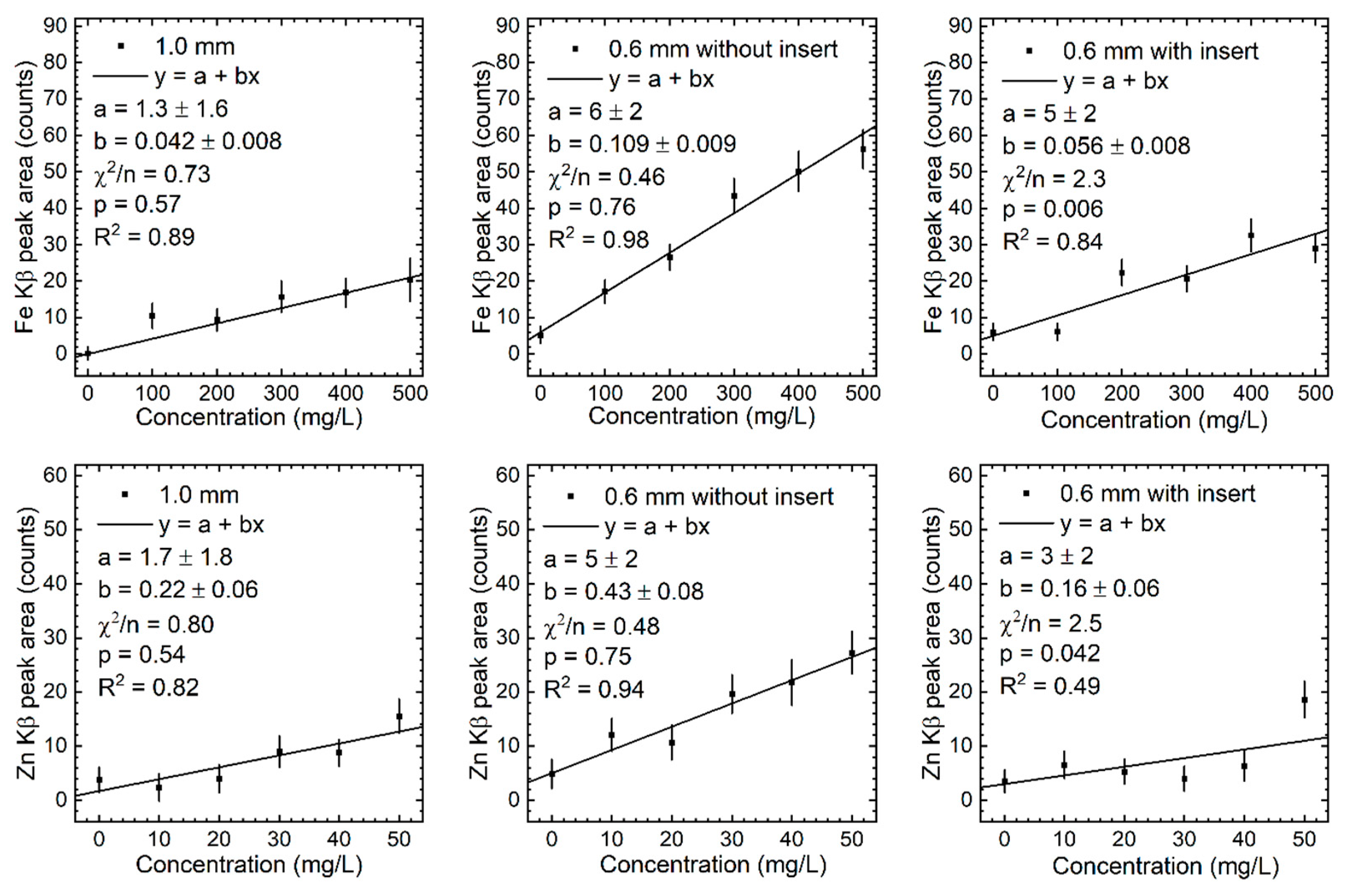
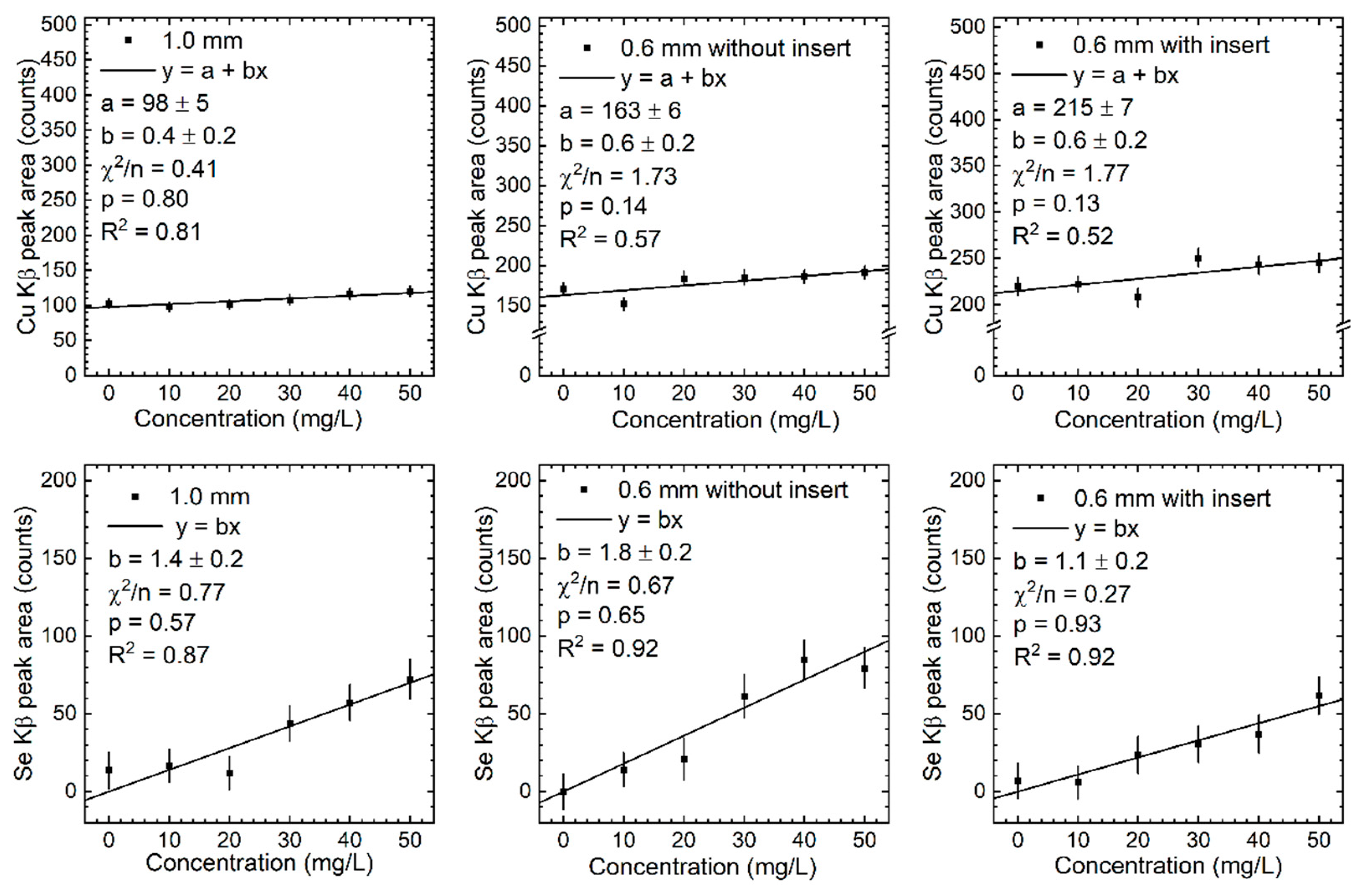
| Element | Z | Range | Arithmetic Mean | Geometric Mean | Median | Ref. | |
| Min | Max | ||||||
| Fe | 26 | 387 | 554 | 472 | 469 | 476 | [58] |
| 468 | 631 | 541 | [68] | ||||
| Cu | 29 | 0.610 | 1.900 | 0.920 | [69] | ||
| 0.720 | 1.800 | 1.042 | 1.020 | [59] | |||
| 0.776 | 1.495 | 1.036 | 1.011 | [60] | |||
| 0.720 | 1.020 | 0.875 | 0.870 | 0.873 | [58] | ||
| 0.580 | 1.590 | 0.795 | [62] | ||||
| 0.650 | 1.420 | 0.840 | [63] | ||||
| 0.676 | 1.837 | 1.078 | 1.040 | [70] | |||
| 0.820 | 1.270 | 1.010 | [68] | ||||
| Zn | 30 | 6.100 | 3.100 | 9.800 | [69] | ||
| 4.686 | 8.585 | 6.418 | 6.387 | [60] | |||
| 3.684 | 6.668 | 4.938 | 4.845 | 4.863 | [58] | ||
| 4.770 | 7.272 | 5.876 | 5.805 | 5.844 | [71] | ||
| 3.700 | 7.250 | 5.477 | [62] | ||||
| 4.620 | 9.250 | 6.750 | [63] | ||||
| 4.424 | 17.152 | 8.085 | 7.629 | [70] | |||
| 5.900 | 9.100 | 7.500 | [68] | ||||
| Se | 34 | 0.110 | 0.055 | 0.180 | [69] | ||
| 0.085 | 0.182 | 0.133 | 0.132 | [59] | |||
| 0.106 | 0.185 | 0.140 | 0.138 | [60] | |||
| 0.080 | 0.155 | 0.110 | [62] | ||||
| 0.118 | 0.224 | 0.141 | [63] | ||||
| 0.061 | 0.201 | 0.115 | 0.113 | [70] | |||
| 0.075 | 0.137 | 0.100 | [68] | ||||
| Element | Solvent c(HNO3) |
(mg/L) |
(mg/L) |
(mL) |
(mL) |
(mg) |
(mg) |
|---|---|---|---|---|---|---|---|
| Fe | 2% w/w | 1001 | 4 | 2 | 0.002 | 2.002 | 0.008 |
| Cu | 2% w/w | 1001 | 4 | 0.2 | 0.002 | 0.2002 | 0.0008 |
| Zn | 2% w/w | 1001 | 4 | 0.2 | 0.002 | 0.2002 | 0.0008 |
| Se | 0.5 mol/L | 1001 | 4 | 0.2 | 0.002 | 0.2002 | 0.0008 |
| Solution number | (mL) |
(mL) |
(mg/L) |
(mg/L) |
||||||
| Fe | Cu | Zn | Se | Fe | Cu | Zn | Se | |||
| 1 | 17.4 | 0.102 | 100 | 10 | 10 | 10 | 1 | 0.1 | 0.1 | 0.1 |
| 2 | 7.4 | 0.102 | 200 | 20 | 20 | 20 | 2 | 0.3 | 0.3 | 0.3 |
| 3 | 4.1 | 0.002 | 299 | 30 | 30 | 30 | 1 | 0.1 | 0.1 | 0.1 |
| 4 | 2.4 | 0.004 | 400 | 40 | 40 | 40 | 2 | 0.2 | 0.2 | 0.2 |
| 5 | 1.4 | 0.002 | 501 | 50 | 50 | 50 | 2 | 0.2 | 0.2 | 0.2 |
| POM sample |
Outer diameter (mm) |
Inner diameter (mm) |
Wall thickness (mm) |
Length (cm) |
| 1.0 mm wall cup | 7.5 | 5.5 | 1.0 | 5.0 |
| 0.6-mm wall cup | 7.5 | 6.3 | 0.6 | 5.0 |
| cylindrical insert | 5.3 | – | – | 4.9 |
| X-ray linear attenuation coefficient | (mm−1) | |||
| Photon energy (keV) | 5 | 10 | 15 | 20 |
| Water solution 5 (1.00 g/cm3) | 4.27 × 100 | 5.44 × 10−1 | 1.71 × 10−1 | 8.28 × 10−2 |
| Human blood (1.06 g/cm3) | 4.49 × 100 | 5.85 × 10−1 | 1.85 × 10−1 | 8.93 × 10−2 |
| POM (1.40 g/cm3) | 4.65 × 100 | 5.81 × 10−1 | 1.86 × 10−1 | 9.28 × 10−2 |
| Human skin (1.09 g/cm3) | 4.56 × 100 | 5.39 × 10−1 | 1.71 × 10−1 | 8.37 × 10−2 |
| No. | XRF peaks (Siegbahn nomenclature) |
Origin of observed XRF peaks | |
|---|---|---|---|
| 1 | 2 | Mn Kα, Fe Kα | solution & metallic parts of XRF system |
| 2 | 2 | Ni Kα, Fe Kβ | solution & metallic parts of XRF system |
| 3 | 4 | Cu Kα, Ni Kβ, Zn Kα, Cu Kβ | solution & metallic parts of XRF system |
| 4 | 2 | W Lβ17, Zn Kβ | solution & x-ray tube anode material |
| 5 | 2 | Se Kα, Se Kβ | solution |
| Peak | W. av. | |||||||||||
| Quantity | ||||||||||||
| Unit | counts mg−1 L | counts | mg/L | counts mg−1 L | counts | mg/L | mg/L | |||||
| Fe | 0.11 | 0.01 | 3.351 | 91 | 8 | 0.042 | 0.008 | 1.749 | 125 | 24 | 95 | 8 |
| Cu | 1.6 | 0.4 | 13.942 | 26 | 7 | 0.4 | 0.2 | 6.284 | 47 | 24 | 28 | 6 |
| Zn | 2.1 | 0.1 | 2.478 | 3.5 | 0.2 | 0.22 | 0.06 | 2.341 | 32 | 9 | 3.6 | 0.2 |
| Se | 7.6 | 0.3 | 5.413 | 2.14 | 0.08 | 0.035 | 0.004 | 0.299 | 26 | 3 | 2.2 | 0.1 |
| Peak | W. av. | |||||||||||
| Quantity | ||||||||||||
| Unit | counts mg−1 L | counts | mg/L | counts mg−1 L | counts | mg/L | mg/L | |||||
| Fe | 0.53 | 0.02 | 4.02471 | 22.8 | 0.9 | 0.109 | 0.009 | 2.300 | 63 | 5 | 24 | 1 |
| Cu | 3.80 | 0.6 | 16.494 | 13 | 2 | 0.6 | 0.2 | 7.730 | 39 | 13 | 14 | 2 |
| Zn | 3.7 | 0.2 | 3.805 | 3.1 | 0.2 | 0.43 | 0.08 | 2.697 | 19 | 4 | 3.1 | 0.2 |
| Se | 10.2 | 0.3 | 4.893 | 1.44 | 0.04 | 1.8 | 0.2 | 11.498 | 19 | 2 | 1.45 | 0.04 |
| Peak | W. av. | |||||||||||
| Quantity | ||||||||||||
| Unit | counts mg−1 L | counts | mg/L | counts mg−1 L | counts | mg/L | mg/L | |||||
| Fe | 0.39 | 0.02 | 4.583 | 35 | 2 | 0.056 | 0.008 | 2.339 | 125 | 18 | 36 | 2 |
| Cu | 1.2 | 0.6 | 20.176 | 50 | 25 | 0.6 | 0.2 | 9.322 | 47 | 16 | 48 | 13 |
| Zn | 1.9 | 0.2 | 5.973 | 9 | 1 | 0.16 | 0.06 | 2.113 | 40 | 15 | 10 | 1 |
| Se | 4.5 | 0.2 | 5.036 | 3.4 | 0.1 | 1.1 | 0.2 | 11.281 | 31 | 6 | 3.4 | 0.1 |
| Element | 1.0 mm wall cup | 0.6 mm wall cup without insert | 0.6 mm wall cup with insert | Human blood concentration range |
| Fe | 95 8 | 24 1 | 36 2 | 387 – 631 |
| Cu | 28 6 | 14 2 | 48 13 | 0.580– 0.90 |
| Zn | 3.6 0.2 | 3.1 0.2 | 10 1 | 3.684 – 17.152 |
| Se | 2.2 0.1 | 1.45 0.04 | 3.4 0.1 | 0.055 – 0.224 |
| Phantom configuration | (mm) | (mm) | (g) | (g) | (keV) | Dose rate (mGy/s) | Dose (mGy) |
| 1 mm POM | 5.50 | 1.00 | 0.012 | 0.006 | 8.669 | 0.26 | 47 |
| 0.6 mm POM without insert | 6.30 | 0.60 | 0.014 | 0.004 | 8.545 | 0.27 | 48 |
| 0.6 mm POM with insert | 1.00 | 3.25 | 0.002 | 0.021 | 9.350 | 0.23 | 42 |
| Phantom configuration | ||||||
| Fe | Cu | |||||
| 1.0 mm cup | 0.44(9) | 0.132(5) | 3.4(7) | 0.3(1) | 0.136(3) | 1.8(9) |
| 0.6 mm cup w/o insert | 0.20(2) | 1.5(1) | 0.15((5) | 1.1(4) | ||
| 0.6 mm cup with insert | 0.15(2) | 1.1(2) | 0.4(2) | 3(1) | ||
| Zn | Se | |||||
| 1.0 mm cup | 0.13(2) | 0.138(5) | 0.9(2) | 0.19(2) | 0.161(5) | 1.2(2) |
| 0.6 mm cup w/o insert | 0.11(2) | 0.8(2) | 0.19(2) | 1.2(1) | ||
| 0.6 mm cup with insert | 0.4(2) | 0.6(3) | 0.26(4) | 1.6(2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





