Submitted:
24 February 2025
Posted:
25 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
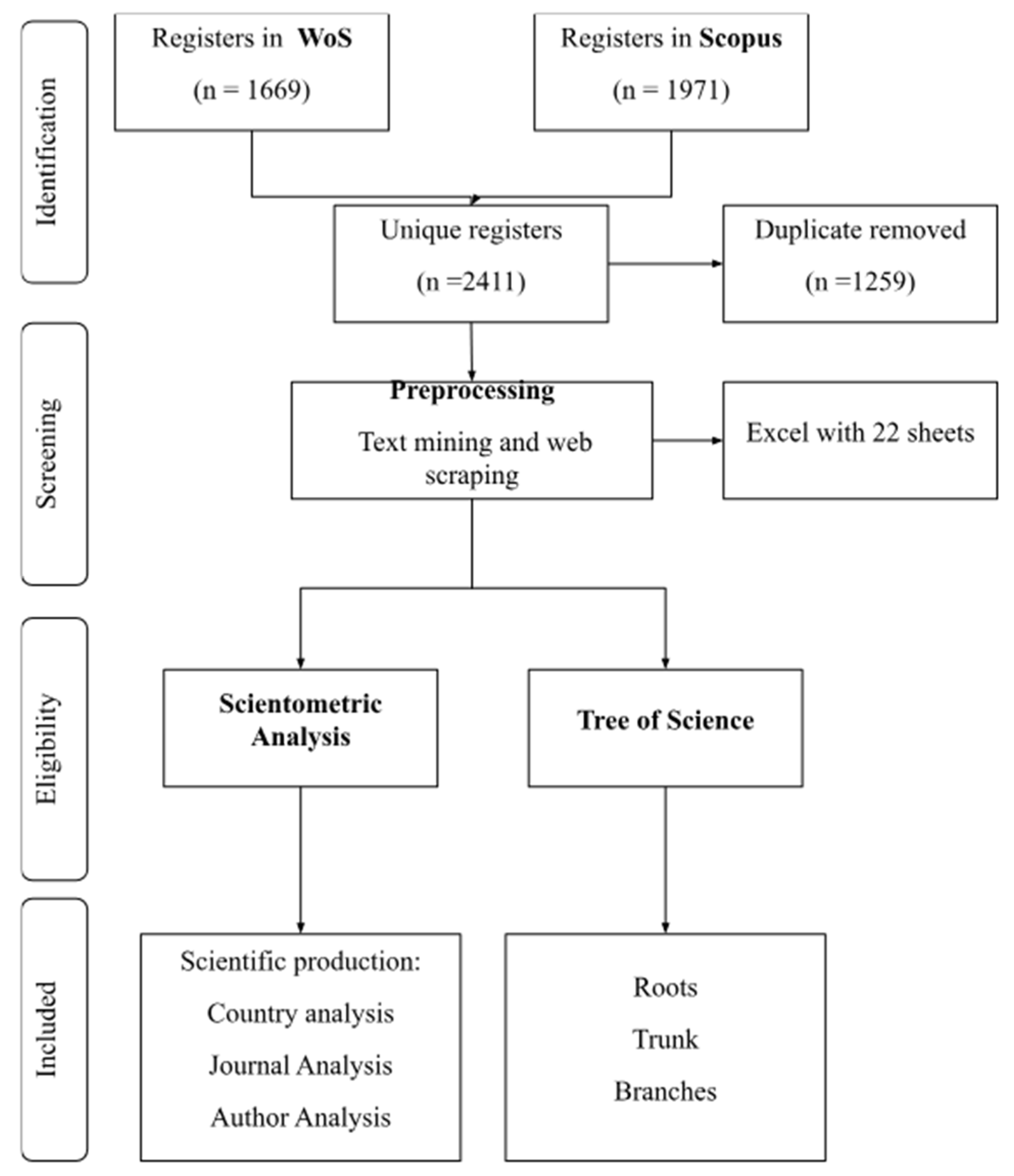
2. Methodology
3. Results
2.1. Scientometrics Analysis
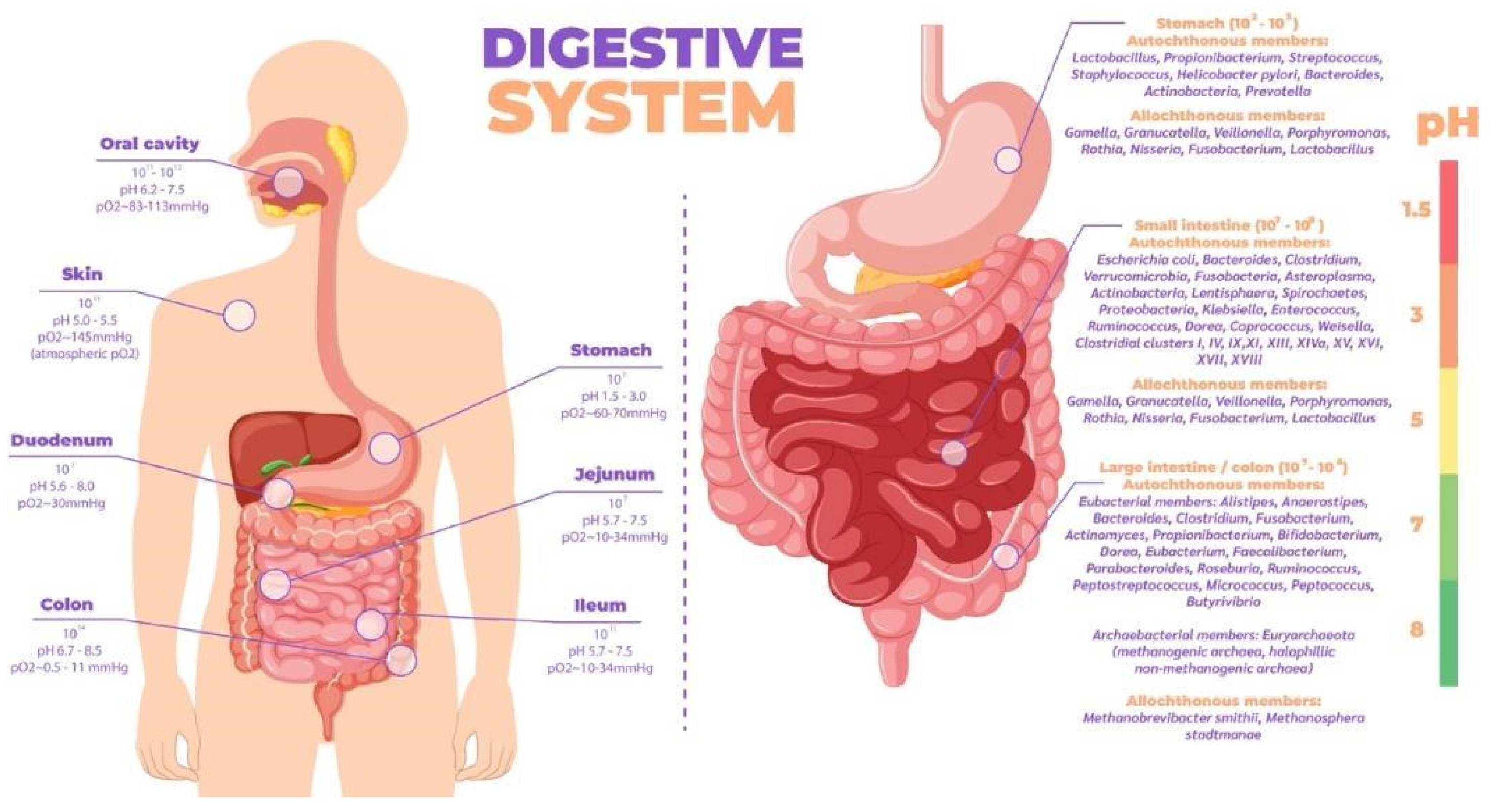
2.2. Human Microbiome and Interaction with the Host
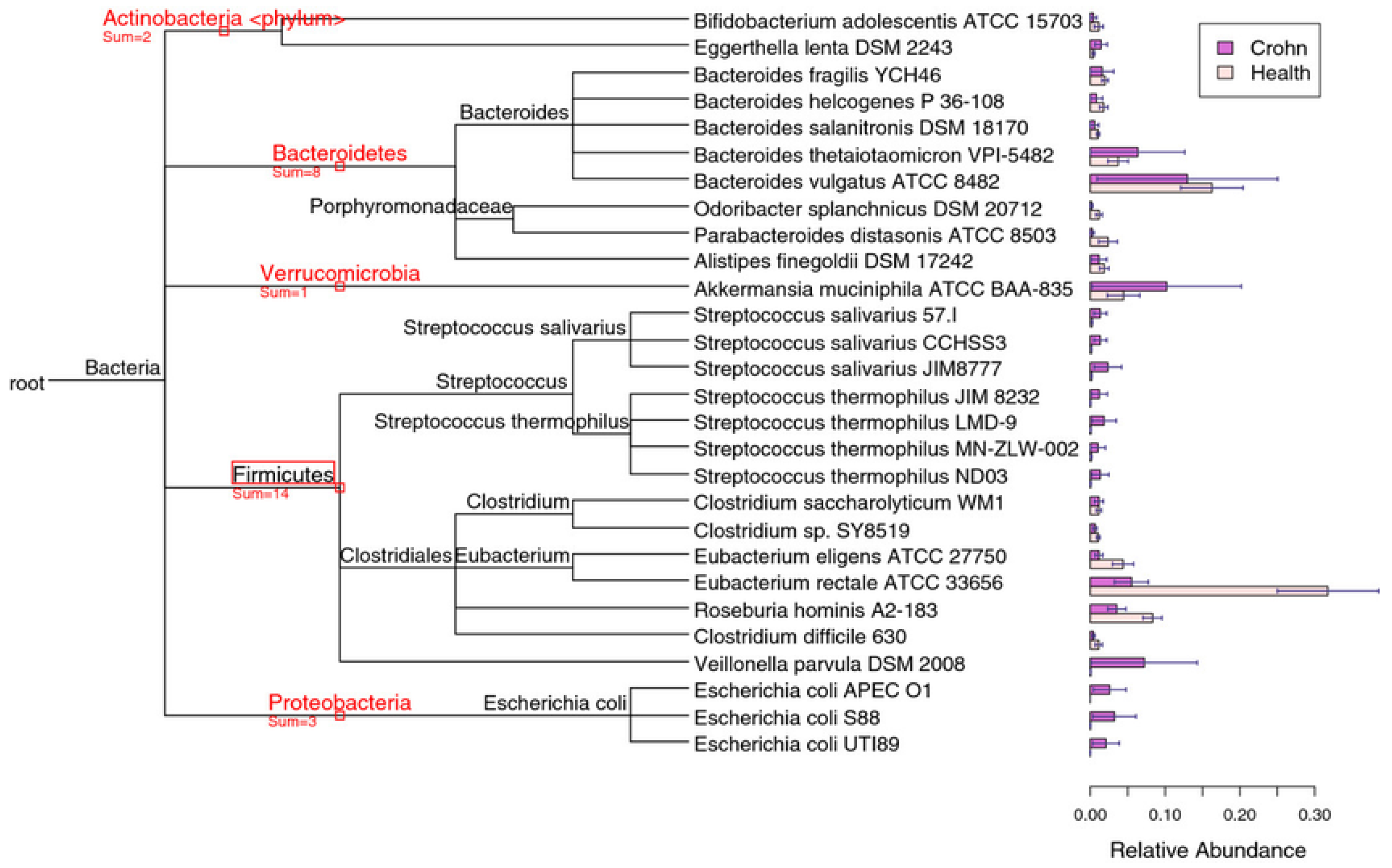
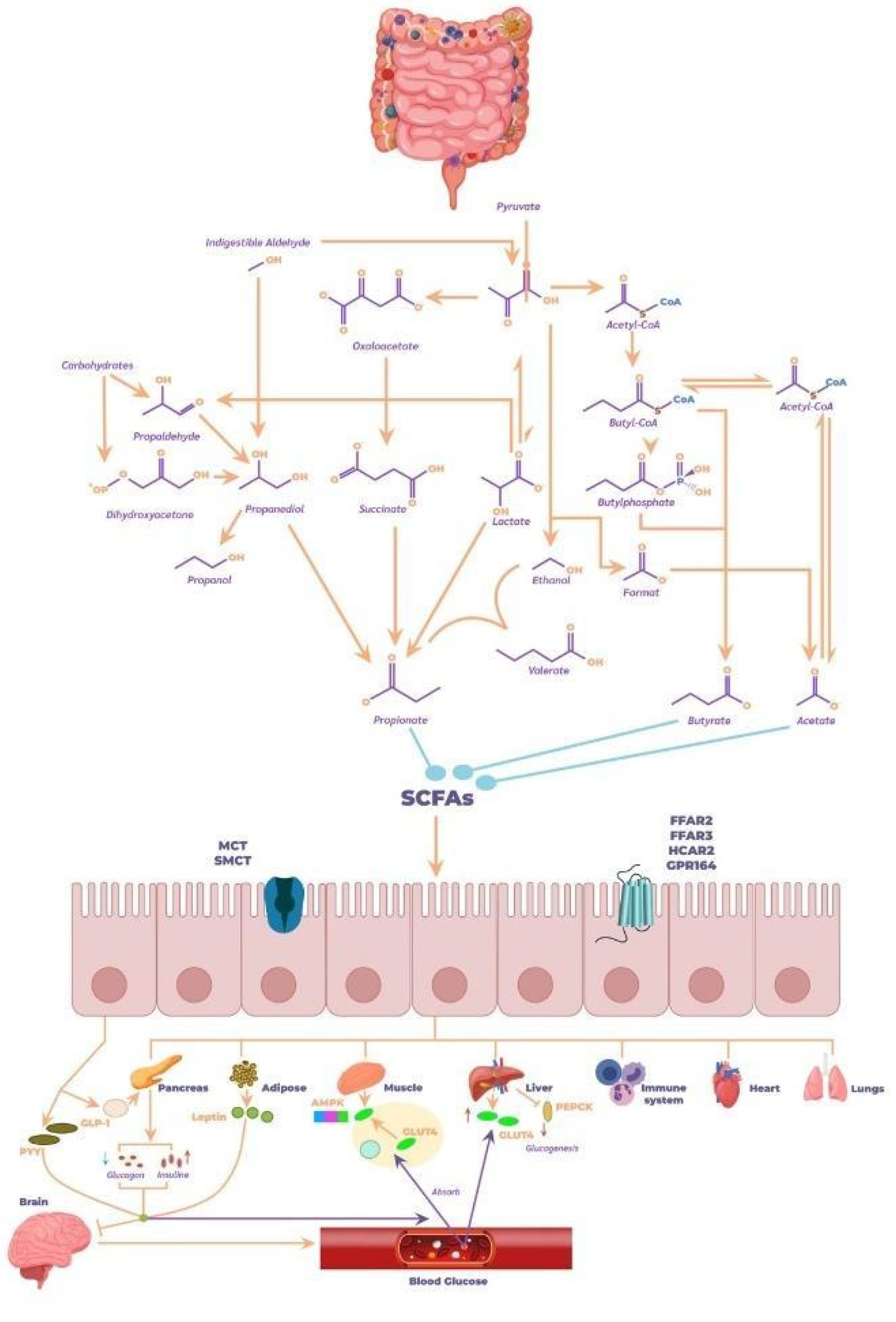
2.3. Biochemical Contribution of the Microbiome to Human Health
2.4. Emerging Therapies
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet-Gut Microbiota-Inflammation. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Arteaga-Garibay, R.I. APLICACIÓN DE SECUENCIACIÓN MASIVA PARA EL ESTUDIO Y EXPLORACIÓN DE DIVERSIDAD MICROBIANA Y SU APROVECHAMIENTO BIOTECNOLÓGICO. AP 2016, 9. [Google Scholar]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Hick, E.; Suárez, M.; Rey, A.; Mantecón, L.; Fernández, N.; Solís, G.; Gueimonde, M.; Arboleya, S. Personalized Nutrition with Banked Human Milk for Early Gut Microbiota Development: In Pursuit of the Perfect Match. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Riegelman, E.; Xue, K.S.; Wang, J.-S.; Tang, L. Gut-Brain Axis in Focus: Polyphenols, Microbiota, and Their Influence on α-Synuclein in Parkinson’s Disease. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Sochacka, K.; Kotowska, A.; Lachowicz-Wiśniewska, S. The Role of Gut Microbiota, Nutrition, and Physical Activity in Depression and Obesity-Interdependent Mechanisms/Co-Occurrence. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Missiego-Beltrán, J.; Beltrán-Velasco, A.I. The Role of Microbial Metabolites in the Progression of Neurodegenerative Diseases-Therapeutic Approaches: A Comprehensive Review. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Valencia, S.; Zuluaga, M.; Franco, A.; Osorio, M.; Betancour, S. Systematic Review and Bibliometric Analysis of the Metabolome Found in Human Breast Milk from Healthy and Gestational Diabetes Mellitus Mothers. Nova 2023, 21. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci Total Environ 2018, 631-632, 449–458. [Google Scholar] [CrossRef]
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The Microbiota-Gut-Brain Axis Participates in Chronic Cerebral Hypoperfusion by Disrupting the Metabolism of Short-Chain Fatty Acids. Microbiome 2022, 10, 62. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, X.; Fan, J.; Cheng, W.; Yan, T.-M.; Lai, Y.; Zhang, N.; Lu, Y.; Qi, J.; Huo, Z.; et al. Antibiotic-Induced Gut Microbiota Dysbiosis Modulates Host Transcriptome and m6A Epitranscriptome via Bile Acid Metabolism. Adv. Sci. (Weinh.) 2024, 11, e2307981. [Google Scholar] [CrossRef]
- Briggs, K.; Tomar, V.; Ollberding, N.; Haberman, Y.; Bourgonje, A.R.; Hu, S.; Chaaban, L.; Sunuwar, L.; Weersma, R.K.; Denson, L.A.; et al. Crohn’s Disease-Associated Pathogenic Mutation in the Manganese Transporter ZIP8 Shifts the Ileal and Rectal Mucosal Microbiota Implicating Aberrant Bile Acid Metabolism. Inflamm. Bowel Dis. 2024, 30, 1379–1388. [Google Scholar] [CrossRef]
- Obayiuwana, O.A.; Behrends, V.; Calle-Patino, Y.; Barone, M.; Turroni, S.; Brigidi, P.; Costabile, A.; Corona, G. Cooking, Digestion, and in Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. Int. J. Mol. Sci. 2023, 24, 14111. [Google Scholar] [CrossRef]
- Costabile, A.; Corona, G.; Sarnsamak, K.; Atar-Zwillenberg, D.; Yit, C.; King, A.J.; Vauzour, D.; Barone, M.; Turroni, S.; Brigidi, P.; et al. Wholegrain Fermentation Affects Gut Microbiota Composition, Phenolic Acid Metabolism and Pancreatic Beta Cell Function in a Rodent Model of Type 2 Diabetes. Front. Microbiol. 2022, 13, 1004679. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J. ’an; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J Agric Food Chem 2021, 69, 7629–7640. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma Lucidum Polysaccharides Reverses the Disturbed Gut Microbiota and Metabolism in Type 2 Diabetic Rats. Int J Biol Macromol 2020, 155, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, X.; Guan, S.; Cai, J.; Li, X.; Fang, J.; Lin, B.; Zhu, W.; Tian, J.; Jin, J.; et al. In-Depth LC-MS and in-Vitro Studies of a Triterpenoid Saponin Capilliposide-A Metabolism Modulation in Gut Microbiota of Mice. Front. Pharmacol. 2024, 15, 1361643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lo, E.K.K.; Chen, J.; Wang, K.; Felicianna; Ismaiah, M. J.; Leung, H.K.M.; Zhao, D.; Lee, J.C.-Y.; El-Nezami, H. Probiotic Mixture Ameliorates a Diet-Induced MASLD/MASH Murine Model through the Regulation of Hepatic Lipid Metabolism and the Gut Microbiome. J. Agric. Food Chem. 2024, 72, 8536–8549. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, X.; Yang, X.; Zhang, G.; Zhang, J.; Chen, L.; Niu, P.; Chen, T. Melatonin Mitigates Manganese-Induced Neural Damage via Modulation of Gut Microbiota-Metabolism in Mice. Sci. Total Environ. 2024, 923, 171474. [Google Scholar] [CrossRef]
- Zhao, S.; Li, H.; Yang, F.; Yang, Y.; Zeng, Y.; An, Z.; Li, J.; Wu, H.; Song, J.; Wu, W. Association of Short-Term PM2.5 Exposure with Airway Innate Immune Response, Microbiota and Metabolism Alterations in Human Airways. Environ. Pollut. 2024, 345, 123435. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Yuan, Q.; Hao, W.; Wang, Y.; Wu, D.; Chen, X.; Wang, S. Agaricus Bisporus Polysaccharides Ameliorate Ulcerative Colitis in Mice by Modulating Gut Microbiota and Its Metabolism. Food Funct. 2024, 15, 1191–1207. [Google Scholar] [CrossRef]
- Bai, M.; Wang, X.; Liu, D.; Xu, A.; Cheng, H.; Li, L.; Zhang, C. Tolypocladium Sinense Mycelium Polysaccharide Alleviates Obesity, Lipid Metabolism Disorder, and Inflammation Caused by High Fat Diet via Improving Intestinal Barrier and Modulating Gut Microbiota. Mol. Nutr. Food Res. 2024, 68, e2300759. [Google Scholar] [CrossRef]
- Wang, S.; Ju, D.; Zeng, X. Mechanisms and Clinical Implications of Human Gut Microbiota-Drug Interactions in the Precision Medicine Era. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus Plantarum P8 Alleviated Stress and Anxiety While Enhancing Memory and Cognition in Stressed Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Arbab Sakandar, H.; Chen, Y.; Peng, C.; Chen, X.; Imran, M.; Zhang, H. Impact of Fermentation on Antinutritional Factors and Protein Degradation of Legume Seeds: A Review. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Bai, M.; Huang, T.; Guo, S.; Wang, Y.; Wang, J.; Kwok, L.-Y.; Dan, T.; Zhang, H.; Bilige, M. Probiotic Lactobacillus Casei Zhang Improved the Properties of Stirred Yogurt. Food Biosci. 2020, 37, 100718. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Li, J.; Wu, J.; Jiang, S.; Xue, H.; Zhang, J.; Jha, R.; Wang, R. Lactic Acid Bacteria Fermentation Improves Physicochemical Properties, Bioactivity, and Metabolic Profiles of Opuntia Ficus-Indica Fruit Juice. Food Chem. 2024, 453, 139646. [Google Scholar] [CrossRef]
- Mansour, S.T.; Ibrahim, H.; Zhang, J.; Farag, M.A. Extraction and Analytical Approaches for the Determination of Post-Food Processing Major Carcinogens: A Comprehensive Review towards Healthier Processed Food. Food Chem. 2025, 464, 141736. [Google Scholar] [CrossRef]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch Microbiol 2020, 202, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Ravel, J. The Vocabulary of Microbiome Research: A Proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef]
- Prieto, P.A. Fundamentos de La Microbiota Y El Microbioma. Avances En Investigación Sobre El Microbioma Intestinal Humano. Medicina 2023, 45, 229–246. [Google Scholar] [CrossRef]
- Bragg, L.; Tyson, G.W. Metagenomics Using Next-Generation Sequencing. Environmental Microbiology 2014, 183–201. [Google Scholar] [CrossRef]
- Escalante, A.E.; Jardón Barbolla, L.; Ramírez-Barahona, S.; Eguiarte, L.E. The Study of Biodiversity in the Era of Massive Sequencing. Rev. Mex. Biodivers. 2014, 85, 1249–1264. [Google Scholar] [CrossRef]
- Odom, A.R.; Faits, T.; Castro-Nallar, E.; Crandall, K.A.; Johnson, W.E. Metagenomic Profiling Pipelines Improve Taxonomic Classification for 16S Amplicon Sequencing Data. Sci Rep 2023, 13, 13957. [Google Scholar] [CrossRef]
- Amrane, S.; Lagier, J.-C. Metagenomic and Clinical Microbiology. Hum. Microbiome J. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA Gene Sequencing for Species and Strain-Level Microbiome Analysis. Nat Commun 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed]
- Rodicio, M. del R.; Mendoza, M. del C. Identificación bacteriana mediante secuenciación del ARNr 16S: fundamento, metodología y aplicaciones en microbiología clínica. Enferm Infecc Microbiol Clin 2004, 22, 238–245. [Google Scholar] [CrossRef]
- Pflughoeft, K.J.; Versalovic, J. Human Microbiome in Health and Disease. Annu Rev Pathol 2012, 7, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Tuddenham, S.; Sears, C.L. The Intestinal Microbiome and Health. Curr Opin Infect Dis 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin Microbiol Infect 2012, 18 Suppl 4, 2–4. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front Immunol 2021, 12, 683022. [Google Scholar] [CrossRef]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of Microorganisms in the Evolution of Animals and Plants: The Hologenome Theory of Evolution. FEMS Microbiol Rev 2008, 32, 723–735. [Google Scholar] [CrossRef]
- Tiko El microbioma y su papel en el eje alimentación-intestino y salud Available online:. Available online: https://nutricionclinicaenmedicina.com/el-microbioma-y-su-papel-en-el-eje-alimentacion-intestino-y-salud/ (accessed on 15 November 2024).
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J Gastroenterol 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Del Campo-Moreno, R.; Alarcón-Cavero, T.; D’Auria, G.; Delgado-Palacio, S.; Ferrer-Martínez, M. Microbiota and Human Health: Characterization Techniques and Transference. Enferm Infecc Microbiol Clin (Engl Ed) 2018, 36, 241–245. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural Diversity, Functional Aspects and Future Therapeutic Applications of Human Gut Microbiome. Arch Microbiol 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to Host Microbiome Symbiosis in Health and Disease. Mucosal Immunol 2021, 14, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, P.; Mathew, G.S.; Selvarajan, S.; Seshadri, K.G.; Srikanth, P. Gut Microbiota and Its Mysteries. Indian J Med Microbiol 2019, 37, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol Rev 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Cani, P.D. Human Gut Microbiome: Hopes, Threats and Promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, L.; Zhai, B. The Mycobiome: Interactions with Host and Implications in Diseases. Curr Opin Microbiol 2023, 75, 102361. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb Ecol Health Dis 2015, 26, 26191. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A Review of Metabolic Potential of Human Gut Microbiome in Human Nutrition. Arch Microbiol 2018, 200, 203–217. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Metabolome-Microbiome Crosstalk and Human Disease. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Sohn, M.B.; An, L.; Pookhao, N.; Li, Q. Accurate Genome Relative Abundance Estimation for Closely Related Species in a Metagenomic Sample. BMC Bioinformatics 2014, 15, 242. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal Epithelial Cells: At the Interface of the Microbiota and Mucosal Immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e5. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Devkota, S.; McCoy, K.D.; Relman, D.A.; Yassour, M.; Young, V.B. Lessons Learned from the Prenatal Microbiome Controversy. Microbiome 2021, 9, 8. [Google Scholar] [CrossRef]
- Ardissone, A.N.; de la Cruz, D.M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W.; et al. Meconium Microbiome Analysis Identifies Bacteria Correlated with Premature Birth. PLoS One 2014, 9, e90784. [Google Scholar] [CrossRef]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am J Matern Child Nurs 2017, 42, 318–325. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Cesare Marincola, F.; Dessì, A.; Corbu, S.; Reali, A.; Fanos, V. Clinical Impact of Human Breast Milk Metabolomics. Clin Chim Acta 2015, 451, 103–106. [Google Scholar] [CrossRef]
- Maternal Factors Related to Variability in the Human Milk Microbiome; Academic Press, 2017; pp. 329–348; ISBN 9780128027257.
- Jeurink, P.V.; van Bergenhenegouwen, J.; Jiménez, E.; Knippels, L.M.J.; Fernández, L.; Garssen, J.; Knol, J.; Rodríguez, J.M.; Martín, R. Human Milk: A Source of More Life than We Imagine. Benef Microbes 2013, 4, 17–30. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front Immunol 2017, 8, 584. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Grossi, E.; Drago, L. Role of the Human Breast Milk-Associated Microbiota on the Newborns’ Immune System: A Mini Review. Front Microbiol 2017, 8, 2100. [Google Scholar] [CrossRef] [PubMed]
- Milk Bacteria and Gastrointestinal Tract: Microbial Composition of Milk. In Dietary Interventions in Gastrointestinal Diseases; Academic Press, 2019; pp. 265–275.
- Eshaghi, M.; Bibalan, M.H.; Rohani, M.; Esghaei, M.; Douraghi, M.; Talebi, M.; Pourshafie, M.R. Bifidobacterium Obtained from Mother’s Milk and Their Infant Stool; A Comparative Genotyping and Antibacterial Analysis. Microb Pathog 2017, 111, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Dasriya, V.L.; Samtiya, M.; Ranveer, S.; Dhillon, H.S.; Devi, N.; Sharma, V.; Nikam, P.; Puniya, M.; Chaudhary, P.; Chaudhary, V.; et al. Modulation of Gut-Microbiota through Probiotics and Dietary Interventions to Improve Host Health. J Sci Food Agric 2024, 104, 6359–6375. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur J Clin Nutr 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Somaratne, G.; Ferrua, M.J.; Ye, A.; Nau, F.; Floury, J.; Dupont, D.; Singh, J. Food Material Properties as Determining Factors in Nutrient Release during Human Gastric Digestion: A Review. Crit Rev Food Sci Nutr 2020, 60, 3753–3769. [Google Scholar] [CrossRef]
- Flint, H.J. The Impact of Nutrition on the Human Microbiome. Nutr Rev 2012, 70 Suppl 1, S10–S13. [Google Scholar] [CrossRef]
- Abdul Rahim, M.B.H.; Chilloux, J.; Martinez-Gili, L.; Neves, A.L.; Myridakis, A.; Gooderham, N.; Dumas, M.-E. Diet-Induced Metabolic Changes of the Human Gut Microbiome: Importance of Short-Chain Fatty Acids, Methylamines and Indoles. Acta Diabetol 2019, 56, 493–500. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, Metabolites and Host Immunity. Curr Opin Microbiol 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Rackerby, B.; Kim, H.J.; Dallas, D.C.; Park, S.H. Understanding the Effects of Dietary Components on the Gut Microbiome and Human Health. Food Sci Biotechnol 2020, 29, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Pepke, F.; Vati, S.; Chaudemanche, C.; Castelli, F.; Chollet, C.; Rué, O.; Hennequet-Antier, C.; Langella, P.; et al. In-Depth Characterization of a Selection of Gut Commensal Bacteria Reveals Their Functional Capacities to Metabolize Dietary Carbohydrates with Prebiotic Potential. mSystems 2024, 9, e0140123. [Google Scholar] [CrossRef]
- Lapébie, P.; Lombard, V.; Drula, E.; Terrapon, N.; Henrissat, B. Bacteroidetes Use Thousands of Enzyme Combinations to Break down Glycans. Nat Commun 2019, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Martens, E.C. Interrogating Gut Bacterial Genomes for Discovery of Novel Carbohydrate Degrading Enzymes. Curr Opin Chem Biol 2018, 47, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ndeh, D.; Gilbert, H.J. Biochemistry of Complex Glycan Depolymerisation by the Human Gut Microbiota. FEMS Microbiol Rev 2018, 42, 146–164. [Google Scholar] [CrossRef]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A Genomic View of the Human-Bacteroides Thetaiotaomicron Symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In Vitro Colonic Fermentation of Dietary Fibers: Fermentation Rate, Short-Chain Fatty Acid Production and Changes in Microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat Rev Gastroenterol Hepatol 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Beane, K.E.; Redding, M.C.; Wang, X.; Pan, J.H.; Le, B.; Cicalo, C.; Jeon, S.; Kim, Y.J.; Lee, J.H.; Shin, E.-C.; et al. Effects of Dietary Fibers, Micronutrients, and Phytonutrients on Gut Microbiome: A Review. Appl. Biol. Chem. 2021, 64. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol Spectr 2017, 5. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr Rev Food Sci Food Saf 2019, 18, 221–242. [Google Scholar] [CrossRef]
- Newsome, S.D.; Feeser, K.L.; Bradley, C.J.; Wolf, C.; Takacs-Vesbach, C.; Fogel, M.L. Isotopic and Genetic Methods Reveal the Role of the Gut Microbiome in Mammalian Host Essential Amino Acid Metabolism. Proc Biol Sci 2020, 287, 20192995. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A Review of the Relationship between the Gut Microbiota and Amino Acid Metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut Microbiota-Derived Metabolites in the Regulation of Host Immune Responses and Immune-Related Inflammatory Diseases. Cell Mol Immunol 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc Nutr Soc 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Iyer, A.; Russell, W.R. Impact of Protein on the Composition and Metabolism of the Human Gut Microbiota and Health. Proc Nutr Soc 2021, 80, 173–185. [Google Scholar] [CrossRef]
- Yao, Y.; Ding, L.; Huang, X. Diverse Functions of Lipids and Lipid Metabolism in Development. Small Methods 2020, 4, 1900564. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; Du, Z.-Y.; Olsen, R.E.; Ringø, E.; Tocher, D.R. The Lipids. In Fish Nutrition; Elsevier, 2022; pp. 303–467.
- He, X.; McClorry, S.; Hernell, O.; Lönnerdal, B.; Slupsky, C.M. Digestion of Human Milk Fat in Healthy Infants. Nutr Res 2020, 83, 15–29. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Cao, F.; Song, X. Gut Microbiota-Derived Fatty Acid and Sterol Metabolites: Biotransformation and Immunomodulatory Functions. Gut Microbes 2024, 16, 2382336. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life (Basel) 2024, 14. [Google Scholar] [CrossRef]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. Journal of Clinical Gastroenterology 2006, 40, 235. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel Epigenetic Therapeutic Strategies and Targets in Cancer. Biochim Biophys Acta Mol Basis Dis 2022, 1868, 166552. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Kim, C.H. Control of Lymphocyte Functions by Gut Microbiota-Derived Short-Chain Fatty Acids. Cell Mol Immunol 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-Chain Fatty Acids as Potential Regulators of Skeletal Muscle Metabolism and Function. Nat Metab 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Ganesan, R.; Suk, K.T. Therapeutic Potential of Human Microbiome-Based Short-Chain Fatty Acids and Bile Acids in Liver Disease. Livers 2022, 2, 139–145. [Google Scholar] [CrossRef]
- Golpour, F.; Abbasi-Alaei, M.; Babaei, F.; Mirzababaei, M.; Parvardeh, S.; Mohammadi, G.; Nassiri-Asl, M. Short Chain Fatty Acids, a Possible Treatment Option for Autoimmune Diseases. Biomed Pharmacother 2023, 163, 114763. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The Role of Short-Chain Fatty Acids in Immunity, Inflammation and Metabolism. Crit Rev Food Sci Nutr 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu Rev Med 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Ziaka, M.; Exadaktylos, A. Gut-Derived Immune Cells and the Gut-Lung Axis in ARDS. Crit Care 2024, 28, 220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl Res 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Horrocks, V.; King, O.G.; Yip, A.Y.G.; Marques, I.M.; McDonald, J.A.K. Role of the Gut Microbiota in Nutrient Competition and Protection against Intestinal Pathogen Colonization. Microbiology (Reading) 2023, 169. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12. [Google Scholar] [CrossRef]
- Gowen, R.; Gamal, A.; Di Martino, L.; McCormick, T.S.; Ghannoum, M.A. Modulating the Microbiome for Crohn’s Disease Treatment. Gastroenterology 2023, 164, 828–840. [Google Scholar] [CrossRef]
- Antoshina, D.V.; Balandin, S.V.; Finkina, E.I.; Bogdanov, I.V.; Eremchuk, S.I.; Kononova, D.V.; Kovrizhnykh, A.A.; Ovchinnikova, T.V. Acidocin A and Acidocin 8912 Belong to a Distinct Subfamily of Class II Bacteriocins with a Broad Spectrum of Antimicrobial Activity. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6. [Google Scholar] [CrossRef]
- Mishra, N.; Garg, A.; Ashique, S.; Bhatt, S. Potential of Postbiotics for the Treatment of Metabolic Disorders. Drug Discov Today 2024, 29, 103921. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Huang, Z.; Duraj-Thatte, A.M.; Ebert, M.P.; Zhang, F.; Burgermeister, E.; Liu, X.; Scott, B.M.; Li, G.; Zuo, T. Engineering the Gut Microbiome. Nat Rev Bioeng 2023, 1, 665–679. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Mahan, S.P.; Barnes, L., V.; Leyn, S.A.; George, C.X.; Zlamal, J.E.; Limwongyut, J.; Bazan, G.C.; Fried, J.C.; Fitzgibbons, L.N.; et al. A Broad-Spectrum Synthetic Antibiotic That Does Not Evoke Bacterial Resistance. EBioMedicine 2023, 89, 104461. [Google Scholar] [CrossRef]
- Marques, T.M.; Ganda-Mall, J.P.; Forsgård, R.; Wall, R.; Brummer, R.J.; de Vos, W.M. Correlating the Gut Microbiome to Health and Disease. In The Gut-Brain Axis; Elsevier, 2024; pp. 1–36 ISBN 9780323999717.
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin Diversity, Function, Discovery and Application as Antimicrobials. Nat Rev Microbiol 2024, 22, 556–571. [Google Scholar] [CrossRef]

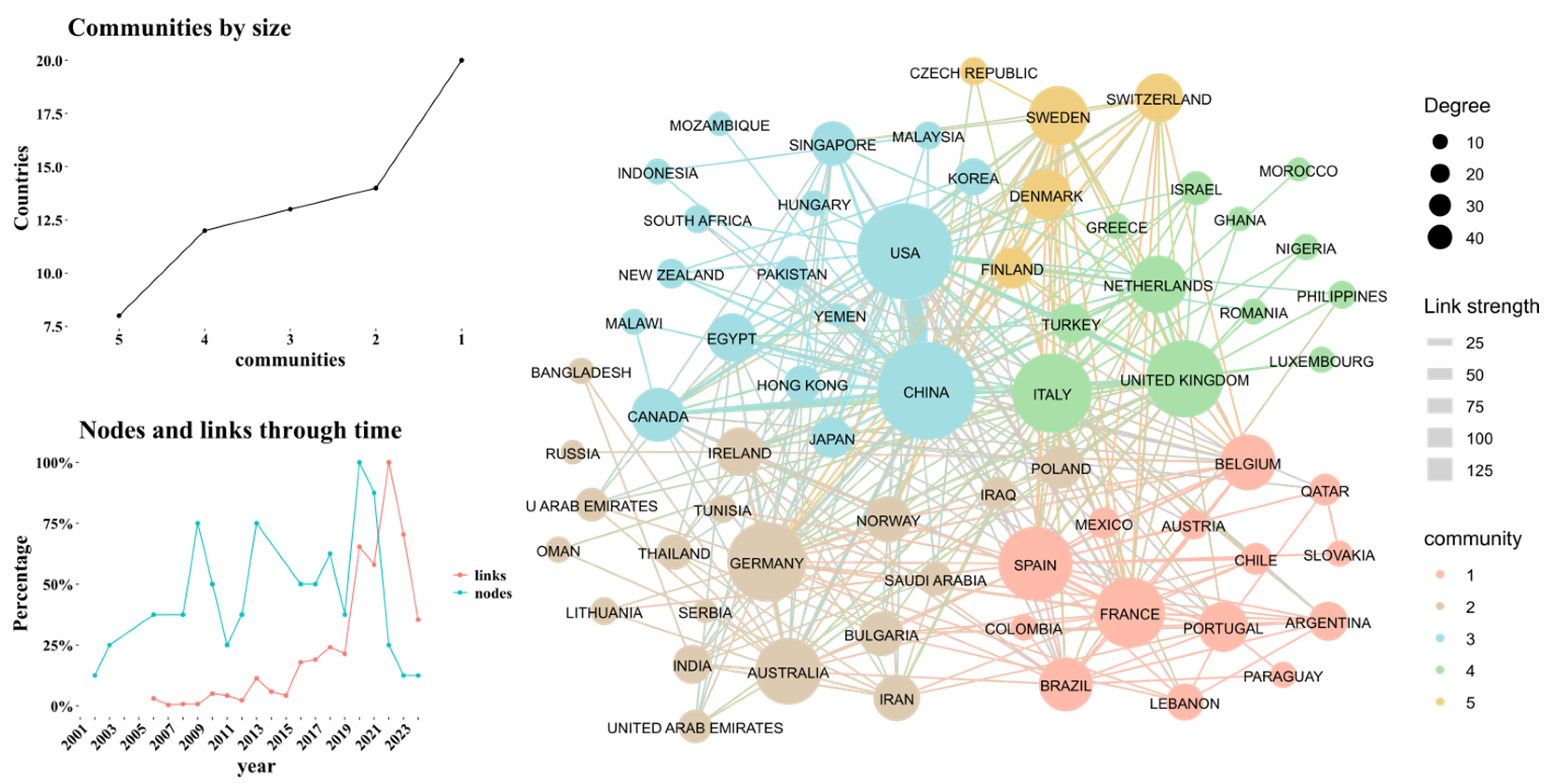
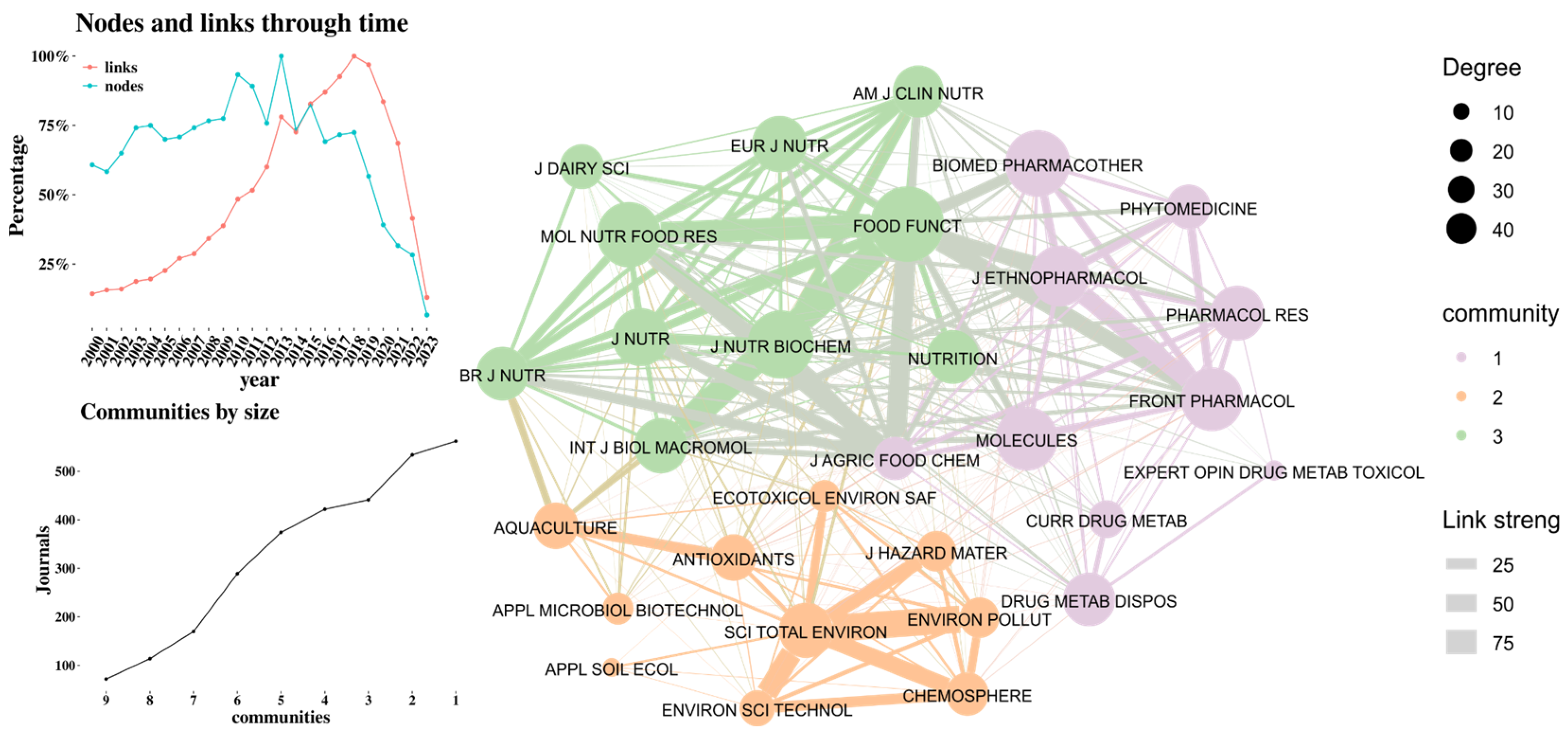
| Country | Production | Citation | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|---|
| China | 1362 (55.34%) | 24943 (23.98%) | 603 | 83 | 17 | 17 |
| USA | 243 (9.87%) | 19000 (18.26%) | 146 | 22 | 3 | 3 |
| Spain | 64 (2.6%) | 3516 (3.38%) | 43 | 4 | 3 | 0 |
| Italy | 62 (2.52%) | 3928 (3.78%) | 31 | 5 | 2 | 0 |
| Japan | 62 (2.52%) | 2106 (2.02%) | 33 | 12 | 2 | 1 |
| Germany | 53 (2.15%) | 2541 (2.44%) | 33 | 6 | 1 | 1 |
| France | 50 (2.03%) | 4567 (4.39%) | 28 | 6 | 3 | 1 |
| Korea | 45 (1.83%) | 1116 (1.07%) | 20 | 7 | 0 | 0 |
| United Kingdom | 45 (1.83%) | 5764 (5.54%) | 32 | 6 | 0 | 0 |
| Canada | 38 (1.54%) | 1222 (1.17%) | 24 | 2 | 1 | 0 |
| Journal | WoS | Scopus | H-Index | Impact Factor | Quantile |
|---|---|---|---|---|---|
| Frontiers In Microbiology | 66 | 21 | 233 | 1.07 | Q1 |
| Journal Of Agricultural And Food Chemistry | 58 | 63 | 345 | 1.11 | Q1 |
| Food And Function | 0 | 71 | 117 | 1.07 | Q1 |
| Nutrients | 46 | 54 | 209 | 1.3 | Q1 |
| Journal Of Functional Foods | 32 | 37 | 125 | 0.9 | Q1 |
| Frontiers In Pharmacology | 35 | 35 | 154 | 1.07 | Q1| |
| Molecular Nutrition And Food Research | 0 | 37 | 160 | 1.04 | Q1 |
| International Journal Of Molecular Sciences | 29 | 29 | 269 | 1.18 | Q1 |
| Frontiers In Nutrition | 25 | 27 | 77 | 0.83 | Q2 |
| International Journal Of Biological Macromolecules | 18 | 21 | 191 | 1.25 | Q1 |
| No | Researcher | Total Articles* | Scopus H-Index |
Affiliation |
|---|---|---|---|---|
| 1 | Wang Y | 148 | 17 | University of Karachi, Karachi, Pakistan |
| 2 | Zhang Y | 109 | 26 | Beijing Solidwill Sci-Tech Co. Ltd., Beijing, China |
| 3 | Li Y | 105 | 10 | The University of Texas at Dallas, Richardson, United States |
| 4 | Liu Y | 97 | 13 | First Affiliated Hospital of Zhengzhou University, Zhengzhou, China |
| 5 | Zhang J | 96 | 38 | Hainan University, Haikou, China |
| 6 | Li X | 94 | 16 | First Affiliated Hospital of Zhengzhou University, Zhengzhou, China |
| 7 | Wang J | 86 | 23 | Dongguan University of Technology, Dongguan, China |
| 8 | Wang X | 83 | 10 | Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, China |
| 9 | Chen Y | 77 | 37 | Zhejiang University School of Medicine, Hangzhou, China |
| 10 | Zhang H | 74 | 57 | Neimenggu Agricultural University, Hohhot, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





