Submitted:
23 February 2025
Posted:
24 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.1. Congestion Is a Distinctive Mark of Heart Failure
2.1.1. Clinical Profile of Patients and Symptomatology
| Classification System | Criteria and Categories | Clinical Implications |
|---|---|---|
| Universal Classification |
Stage A: At risk (no structural disease or symptoms) Stage B: Pre-HF (structural disease, abnormal function, elevated biomarkers, no symptoms) Stage C: Symptomatic HF (structural/functional abnormality with past or current symptoms) Stage D: Advanced HF (persistent symptoms despite optimal treatment, requiring specialized care) |
Identifies patients at risk and guides early intervention strategies to prevent progression. |
| ACC/AHA Stages |
Stage A:
At risk for HF Stage B: Pre-HF (structural disease, no symptoms) Stage C: Symptomatic HF Stage D: Advanced HF (persistent symptoms despite optimal therapy) |
Provides a framework for staging HF, aiding in risk stratification and treatment escalation. |
| NYHA Functional Classification |
Class I:
No limitation on physical activity Class II: Mild symptoms with ordinary activity Class III: Marked limitation in daily activities Class IV: Symptoms present at rest and worsened by any activity |
Assesses functional impairment, guiding therapy intensity, and predicting prognosis. |
2.2. Pathophysiology of Congestion in HF
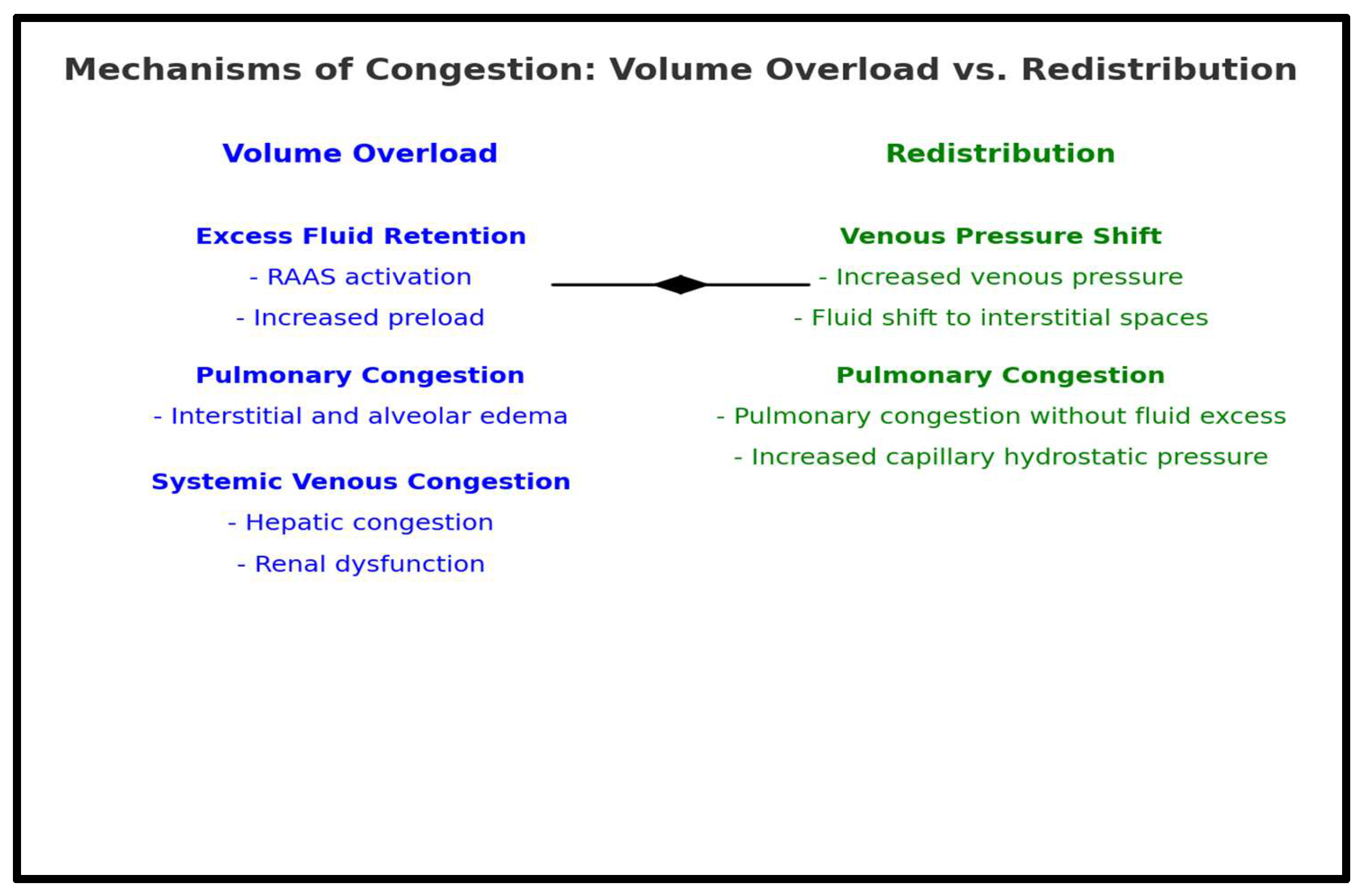
3. Congestion and Organ Response
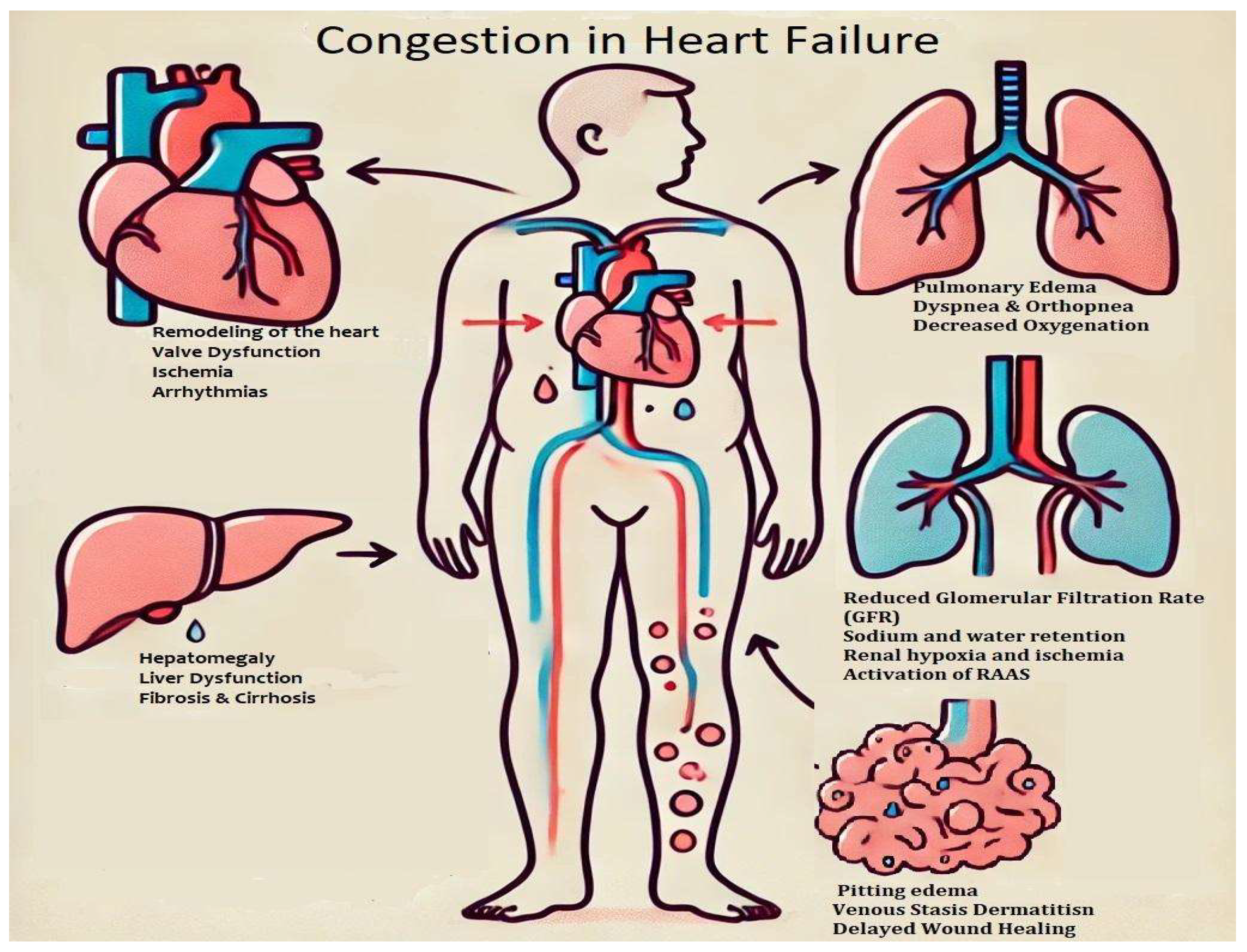
3.1. Effects of Congestion on the Heart
3.1.1. Congestion and Heart Remodeling
- The Weak Heart: Systolic dysfunction with LV dilation and reduced ejection fraction (HFrEF).
- The Big Heart: Remodeling from hypertension or cardiomyopathies with LV dilation and mitral regurgitation.
- The Noisy Heart: Mitral regurgitation due to LV distortion in dilated or ischemic cardiomyopathy.
- The Stiff Heart: Diastolic dysfunction with elevated filling pressures.
- The Wet Heart: Pulmonary congestion and edema, visible on imaging [29].
3.1.2. Valves Disfunction
3.1.3. Ischemia and Arrhythmias
3.1.4. The Right Ventricle Dysfunction
3.1.5. The Need for Early Intervention and Treatment
3.2. Effects of Congestion in the Lungs
- Respiratory Complication: Pulmonary congestion reduces lung compliance, increasing breathing effort and causing hypoxemia and respiratory distress [39]. Impaired mucociliary clearance heightens pneumonia risk, while ventilation-perfusion mismatch worsens oxygen exchange, triggering compensatory mechanisms such as increased respiratory rate and sympathetic activation [26,40,41]. Many HF patients also experience sleep-disordered breathing, including Cheyne-Stokes respiration and central sleep apnea, contributing to nocturnal hypoxia, fatigue, and cognitive decline [39].
- Right Heart Strain and Pulmonary Hypertension: Persistent pulmonary congestion raises pulmonary vascular resistance, increasing right ventricular workload and leading to hypertrophy and eventual right-sided heart failure. In severe cases, pulmonary hypertension develops, further impairing gas exchange and reducing exercise tolerance [37,38,42].
3.2.4. Diagnostic and Therapeutic Approaches
3.3. Interdependence Between Renal Function and Heart Failure
3.3.1. Kidney and Congestion
3.3.2. Assessing and Managing Renal Congestion in Heart Failure
3.4. Effects of Congestion on the Digestive System
3.4.2. Liver and Congestion
3.4.3. Intestinal and Congestion
3.4.4. Assessment and Management of Digestive Congestion in HF
3.5. Effects of Congestion in Heart Failure on the Neurovascular System
3.6. Anemia as a Consequence of Congestion in Heart Failure
3.7. Musculoskeletal System and Skeletal Muscle Dysfunction
3.8. Endocrine System and Metabolic Dysregulation
3.9. Immune System and Chronic Inflammation
3.10. Skin and Peripheral Edema
3.10. Hepatosplenic and Lymphatic System Dysfunction
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| HF | Heart failure |
References
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; Gheorghiade, M. The Global Health and Economic Burden of Hospitalizations for Heart Failure. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Hummel, A.; Empen, K.; Dörr, M.; Felix, S.B. De Novo Acute Heart Failure and Acutely Decompensated Chronic Heart Failure. Dtsch. Ärztebl. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical Epidemiology of Heart Failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Rosano, G. The Heart and the Gut. Eur. Heart J. 2014, 35, 426–430. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; Drazner, M.H.; Felker, G.M.; Filippatos, G.; Fonarow, G.C.; Fiuzat, M.; Gomez-Mesa, J.; Heidenreich, P.; Imamura, T.; Januzzi, J.; Jankowska, E.A.; Khazanie, P.; Kinugawa, K.; Lam, C.S.P.; Matsue, Y.; Metra, M.; Ohtani, T.; Francesco Piepoli, M.; Ponikowski, P.; Rosano, G.M.C.; Sakata, Y.; SeferoviĆ, P.; Starling, R.C.; Teerlink, J.R.; Vardeny, O.; Yamamoto, K.; Yancy, C.; Zhang, J.; Zieroth, S. Universal Definition and Classification of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Follath, F.; Harjola, V.-P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L.; Ponikowski, P.; Tavazzi, L.; on behalf of the EuroHeart Survey Investigators. EuroHeart Failure Survey II (EHFS II): A Survey on Hospitalized Acute Heart Failure Patients: Description of Population. Eur. Heart J. 2006, 27, 2725–2736. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; Jessup, M.; Linde, C.; Nihoyannopoulos, P.; Parissis, J.T.; Pieske, B.; Riley, J.P.; Rosano, G.M.C.; Ruilope, L.M.; Ruschitzka, F.; Rutten, F.H.; Van Der Meer, P. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Harjola, V.; Follath, F.; Nieminen, M.S.; Brutsaert, D.; Dickstein, K.; Drexler, H.; Hochadel, M.; Komajda, M.; Lopez-Sendon, J.L.; Ponikowski, P.; Tavazzi, L. Characteristics, Outcomes, and Predictors of Mortality at 3 Months and 1 Year in Patients Hospitalized for Acute Heart Failure. Eur. J. Heart Fail. 2010, 12, 239–248. [Google Scholar] [CrossRef]
- Ammar, K.A.; Jacobsen, S.J.; Mahoney, D.W.; Kors, J.A.; Redfield, M.M.; Burnett, J.C.; Rodeheffer, R.J. Prevalence and Prognostic Significance of Heart Failure Stages: Application of the American College of Cardiology/American Heart Association Heart Failure Staging Criteria in the Community. Circulation 2007, 115, 1563–1570. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Solomon, S.D. Classification of Heart Failure According to Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 3217–3225. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; Fang, J.C.; Fedson, S.E.; Fonarow, G.C.; Hayek, S.S.; Hernandez, A.F.; Khazanie, P.; Kittleson, M.M.; Lee, C.S.; Link, M.S.; Milano, C.A.; Nnacheta, L.C.; Sandhu, A.T.; Stevenson, L.W.; Vardeny, O.; Vest, A.R.; Yancy, C.W. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Mullens, W. How to Tackle Congestion in Acute Heart Failure. Korean J. Intern. Med. 2018, 33, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Christersson, M.; Gustafsson, S.; Lampa, E.; Almstedt, M.; Cars, T.; Bodegård, J.; Arefalk, G.; Sundström, J. Usefulness of Heart Failure Categories Based on Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2024, 13, e032257. [Google Scholar] [CrossRef] [PubMed]
- Mocan, D.; Lala, R.I.; Puschita, M.; Pilat, L.; Darabantiu, D.A.; Pop-Moldovan, A. The Congestion “Pandemic” in Acute Heart Failure Patients. Biomedicines 2024, 12, 951. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically Mediated Changes in Capacitance: Redistribution of the Venous Reservoir as a Cause of Decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Fudim, M.; Hernandez, A.F.; Felker, G.M. Role of Volume Redistribution in the Congestion of Heart Failure. J. Am. Heart Assoc. 2017, 6, e006817. [Google Scholar] [CrossRef]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Tang, W.H.W. Renal Sodium Avidity in Heart Failure: From Pathophysiology to Treatment Strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef]
- Kumric, M.; Kurir, T.T.; Bozic, J.; Slujo, A.B.; Glavas, D.; Miric, D.; Lozo, M.; Zanchi, J.; Borovac, J.A. Pathophysiology of Congestion in Heart Failure: A Contemporary Review. Card. Fail. Rev. 2024, 10, e13. [Google Scholar] [CrossRef]
- Cotter, G.; Metra, M.; Milo-Cotter, O.; Dittrich, H.C.; Gheorghiade, M. Fluid Overload in Acute Heart Failure — Re-distribution and Other Mechanisms beyond Fluid Accumulation. Eur. J. Heart Fail. 2008, 10, 165–169. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ. Heart Fail. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.C.; Jorde, U.P. The Active Role of Venous Congestion in the Pathophysiology of Acute Decompensated Heart Failure. Rev. Esp. Cardiol. Engl. Ed. 2010, 63, 5–8. [Google Scholar] [CrossRef]
- Udani, S.M.; Koyner, J.L. The Effects of Heart Failure on Renal Function. Cardiol. Clin. 2010, 28, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-D.; Katz, S.D. Anemia in Chronic Heart Failure: Prevalence, Etiology, Clinical Correlates, and Treatment Options. Circulation 2006, 113, 2454–2461. [Google Scholar] [CrossRef]
- Dupont, M.; Mullens, W.; Tang, W.H.W. Impact of Systemic Venous Congestion in Heart Failure. Curr. Heart Fail. Rep. 2011, 8, 233–241. [Google Scholar] [CrossRef]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in Heart Failure: A Contemporary Look at Physiology, Diagnosis and Treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Verma, A.; Meris, A.; Skali, H.; Ghali, J.K.; Arnold, J.M.O.; Bourgoun, M.; Velazquez, E.J.; McMurray, J.J.V.; Kober, L.; Pfeffer, M.A.; Califf, R.M.; Solomon, S.D. Prognostic Implications of Left Ventricular Mass and Geometry Following Myocardial Infarction. JACC Cardiovasc. Imaging 2008, 1, 582–591. [Google Scholar] [CrossRef]
- Lieb, W.; Gona, P.; Larson, M.G.; Aragam, J.; Zile, M.R.; Cheng, S.; Benjamin, E.J.; Vasan, R.S. The Natural History of Left Ventricular Geometry in the Community. JACC Cardiovasc. Imaging 2014, 7, 870–878. [Google Scholar] [CrossRef]
- Ciampi, Q.; Villari, B. Role of Echocardiography in Diagnosis and Risk Stratification in Heart Failure with Left Ventricular Systolic Dysfunction. Cardiovasc. Ultrasound 2007, 5, 34. [Google Scholar] [CrossRef]
- Cammalleri, V.; Antonelli, G.; De Luca, V.M.; Carpenito, M.; Nusca, A.; Bono, M.C.; Mega, S.; Ussia, G.P.; Grigioni, F. Functional Mitral and Tricuspid Regurgitation across the Whole Spectrum of Left Ventricular Ejection Fraction: Recognizing the Elephant in the Room of Heart Failure. J. Clin. Med. 2023, 12, 3316. [Google Scholar] [CrossRef]
- Dietz, M.F.; Prihadi, E.A.; Van Der Bijl, P.; Goedemans, L.; Mertens, B.J.A.; Gursoy, E.; Van Genderen, O.S.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Remodeling and Function in Patients With Significant Secondary Tricuspid Regurgitation. Circulation 2019, 140, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; Vriz, O.; Dini, F.L.; on behalf of all investigators. Different Correlates but Similar Prognostic Implications for Right Ventricular Dysfunction in Heart Failure Patients with Reduced or Preserved Ejection Fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef]
- Kobayashi, M.; Gargani, L.; Palazzuoli, A.; Ambrosio, G.; Bayés-Genis, A.; Lupon, J.; Pellicori, P.; Pugliese, N.R.; Reddy, Y.N.V.; Ruocco, G.; Duarte, K.; Huttin, O.; Rossignol, P.; Coiro, S.; Girerd, N. Association between Right-Sided Cardiac Function and Ultrasound-Based Pulmonary Congestion on Acutely Decompensated Heart Failure: Findings from a Pooled Analysis of Four Cohort Studies. Clin. Res. Cardiol. 2021, 110, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. The Role of Echocardiography in Heart Failure. J. Nucl. Med. 2015, 56 (Supplement 4), 31S–38S. [Google Scholar] [CrossRef] [PubMed]
- Fromm, R.E.; Varon, J.; Gibbs, L.R. Congestive Heart Failure and Pulmonary Edema for the Emergency Physician. J. Emerg. Med. 1995, 13, 71–87. [Google Scholar] [CrossRef]
- Girerd, N.; Seronde, M.-F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; Fillieux, L.; Abraham, W.T.; Januzzi, J.; Sebbag, L.; Zannad, F.; Mebazaa, A.; Rossignol, P. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Pirrotta, F.; Mazza, B.; Gennari, L.; Palazzuoli, A. Pulmonary Congestion Assessment in Heart Failure: Traditional and New Tools. Diagnostics 2021, 11, 1306. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef]
- Valika, A.; Advocate Medical Group – Midwest Heart Specialists, Advocate Heart Institute, Oak Brook, IL, USA; Costanzo, M.R.; Advocate Medical Group – Midwest Heart Specialists, Advocate Heart Institute, Oak Brook, IL, USA. Sleep-Disordered Breathing During Congestive Heart Failure: To Intervene or Not to Intervene? Card. Fail. Rev. 2017, 3, 134. [Google Scholar] [CrossRef]
- Waldman, S.; Pelner, L. The Relationship of Upper Respiratory Infections to Congestive Heart-Failure in Patients With Heart Disease. Postgrad. Med. 1956, 19, 451–458. [Google Scholar] [CrossRef]
- Melenovsky, V.; Andersen, M.J.; Andress, K.; Reddy, Y.N.; Borlaug, B.A. Lung Congestion in Chronic Heart Failure: Haemodynamic, Clinical, and Prognostic Implications. Eur. J. Heart Fail. 2015, 17, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, E.; Bauer, F.; Eicher, J.; Flécher, E.; Gellen, B.; Guihaire, J.; Guijarro, D.; Roul, G.; Salvat, M.; Tribouilloy, C.; Zores, F.; Lamblin, N.; De Groote, P.; Damy, T. Pulmonary Hypertension in Chronic Heart Failure: Definitions, Advances, and Unanswered Issues. ESC Heart Fail. 2018, 5, 755–763. [Google Scholar] [CrossRef]
- Kobayashi, M.; Bercker, M.; Huttin, O.; Pierre, S.; Sadoul, N.; Bozec, E.; Chouihed, T.; Ferreira, J.P.; Zannad, F.; Rossignol, P.; Girerd, N. Chest X-Ray Quantification of Admission Lung Congestion as a Prognostic Factor in Patients Admitted for Worsening Heart Failure from the ICALOR Cohort Study. Int. J. Cardiol. 2020, 299, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Brito, D.; Vaqar, S.; Chhabra, L.; Doerr, C. Congestive Heart Failure (Nursing). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Damman, K.; Valente, M.A.E.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal Impairment, Worsening Renal Function, and Outcome in Patients with Heart Failure: An Updated Meta-Analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Fabiani, I.; Conte, L.; Nesti, L.; Masi, S.; Natali, A.; Colombo, P.C.; Pedrinelli, R.; Dini, F.L. Persistent Congestion, Renal Dysfunction and Inflammatory Cytokines in Acute Heart Failure: A Prognosis Study. J. Cardiovasc. Med. 2020, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Coats, A.J.; Chioncel, O.; Spoletini, I.; Rosano, G. Renal Function, Electrolytes, and Congestion Monitoring in Heart Failure. Eur. Heart J. Suppl. 2019, 21 (Supplement_M), M25–M31. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Demissei, B.G.; Ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Givertz, M.M.; Bloomfield, D.M.; Dittrich, H.; Damman, K.; Pérez-Calvo, J.I.; Voors, A.A. Prevalence, Predictors and Clinical Outcome of Residual Congestion in Acute Decompensated Heart Failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef]
- Clark, A.L.; Kalra, P.R.; Petrie, M.C.; Mark, P.B.; Tomlinson, L.A.; Tomson, C.R. Change in Renal Function Associated with Drug Treatment in Heart Failure: National Guidance. Heart 2019, 105, 904–910. [Google Scholar] [CrossRef]
- Al-Naher, A.; Wright, D.; Devonald, M.A.J.; Pirmohamed, M. Renal Function Monitoring in Heart Failure – What Is the Optimal Frequency? A Narrative Review. Br. J. Clin. Pharmacol. 2018, 84, 5–17. [Google Scholar] [CrossRef]
- Hillege, H.L.; Nitsch, D.; Pfeffer, M.A.; Swedberg, K.; McMurray, J.J.V.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Östergren, J.; Cornel, J.H.; De Zeeuw, D.; Pocock, S.; Van Veldhuisen, D.J. Renal Function as a Predictor of Outcome in a Broad Spectrum of Patients With Heart Failure. Circulation 2006, 113, 671–678. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Blankstein, R.; Bakris, G.L. Renal Hemodynamic Changes in Heart Failure. Heart Fail. Clin. 2008, 4, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Ortiz, A.; Covic, A.; Solak, Y.; Goldsmith, D.; Kanbay, M. Focus on Renal Congestion in Heart Failure. Clin. Kidney J. 2016, 9, 39–47. [Google Scholar] [CrossRef]
- Aronson, D. The Interstitial Compartment as a Therapeutic Target in Heart Failure. Front. Cardiovasc. Med. 2022, 9, 933384. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Ali, M.A.; Wang, A.Y.-M.; Jha, V. Acute Kidney Injury in Acute Heart Failure–When to Worry and When Not to Worry? Nephrol. Dial. Transplant. 2024, 40, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; Ronco, C.; Tang, W.H.W.; McCullough, P.A.; on behalf of the American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139. [Google Scholar] [CrossRef]
- Pellicori, P.; Kallvikbacka-Bennett, A.; Dierckx, R.; Zhang, J.; Putzu, P.; Cuthbert, J.; Boyalla, V.; Shoaib, A.; Clark, A.L.; Cleland, J.G.F. Prognostic Significance of Ultrasound-Assessed Jugular Vein Distensibility in Heart Failure. Heart 2015, 101, 1149–1158. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Sundaram, V.; Fang, J.C. Gastrointestinal and Liver Issues in Heart Failure. Circulation 2016, 133, 1696–1703. [Google Scholar] [CrossRef]
- Møller, S.; Bernardi, M. Interactions of the Heart and the Liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Auer, J. What Does the Liver Tell Us about the Failing Heart? Eur. Heart J. 2013, 34, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, V.M.; Damman, K.; Hillege, H.L.; Van Beek, A.P.; Van Veldhuisen, D.J.; Voors, A.A. Abnormal Liver Function in Relation to Hemodynamic Profile in Heart Failure Patients. J. Card. Fail. 2010, 16, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J.; Fuhrmann, V.; Lainscak, M.; Lassus, J.; Legrand, M.; Masip, J.; Mueller, C.; Papp, Z.; Parissis, J.; Platz, E.; Rudiger, A.; Ruschitzka, F.; Schäfer, A.; Seferovic, P.M.; Skouri, H.; Yilmaz, M.B.; Mebazaa, A. Organ Dysfunction, Injury and Failure in Acute Heart Failure: From Pathophysiology to Diagnosis and Management. A Review on Behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef]
- Myers, R.P.; Cerini, R.; Sayegh, R.; Moreau, R.; Degott, C.; Lebrec, D.; Lee, S.S. Cardiac Hepatopathy: Clinical, Hemodynamic, and Histologic Characteristics and Correlations. Hepatology 2003, 37, 393–400. [Google Scholar] [CrossRef]
- Krack, A.; Sharma, R.; Figulla, H.R.; Anker, S.D. The Importance of the Gastrointestinal System in the Pathogenesis of Heart Failure. Eur. Heart J. 2005, 26, 2368–2374. [Google Scholar] [CrossRef]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; Von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; Poole-Wilson, P.; Volk, H.-D.; Lochs, H.; Anker, S.D. Altered Intestinal Function in Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Macerola, N.; Favuzzi, A.M.; Nicolazzi, M.A.; Gasbarrini, A.; Montalto, M. The Gut in Heart Failure: Current Knowledge and Novel Frontiers. Med. Princ. Pract. 2022, 31, 203–214. [Google Scholar] [CrossRef]
- Aspromonte, N.; Fumarulo, I.; Petrucci, L.; Biferali, B.; Liguori, A.; Gasbarrini, A.; Massetti, M.; Miele, L. The Liver in Heart Failure: From Biomarkers to Clinical Risk. Int. J. Mol. Sci. 2023, 24, 15665. [Google Scholar] [CrossRef]
- Rajeev, V.; Chai, Y.L.; Poh, L.; Selvaraji, S.; Fann, D.Y.; Jo, D.-G.; De Silva, T.M.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V.; Chen, C.P.; Lai, M.K.P. Chronic Cerebral Hypoperfusion: A Critical Feature in Unravelling the Etiology of Vascular Cognitive Impairment. Acta Neuropathol. Commun. 2023, 11, 93. [Google Scholar] [CrossRef]
- Goyal, P.; Didomenico, R.J.; Pressler, S.J.; Ibeh, C.; White-Williams, C.; Allen, L.A.; Gorodeski, E.Z.; Albert, N.; Fudim, M.; Lekavich, C.; Watson, K.; Gulati, S.; Kalogeropoulos, A.; Lewsey, S. Cognitive Impairment in Heart Failure: A Heart Failure Society of America Scientific Statement. J. Card. Fail. 2024, 30, 488–504. [Google Scholar] [CrossRef]
- Ovsenik, A.; Podbregar, M.; Fabjan, A. Cerebral Blood Flow Impairment and Cognitive Decline in Heart Failure. Brain Behav. 2021, 11, e02176. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Cohn, J.N. The Autonomic Nervous System and Heart Failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Rowell, L.B. Reflex Control of Regional Circulations in Humans. J. Auton. Nerv. Syst. 1984, 11, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; Zito, M.C.; Oppedisano, F.; Mollace, R.; Paone, S.; Palma, E.; Muscoli, C.; Mollace, V. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef]
- Nägele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; Von Eckardstein, A.; Enseleit, F.; Lüscher, T.F.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Retinal Microvascular Dysfunction in Heart Failure. Eur. Heart J. 2018, 39, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Wexler, D.; Blum, M.; Keren, G.; Sheps, D.; Leibovitch, E.; Brosh, D.; Laniado, S.; Schwartz, D.; Yachnin, T.; Shapira, I.; Gavish, D.; Baruch, R.; Koifman, B.; Kaplan, C.; Steinbruch, S.; Iaina, A. The Use of Subcutaneous Erythropoietin and Intravenous Iron for the Treatment of the Anemia of Severe, Resistant Congestive Heart Failure Improves Cardiac and Renal Function and Functional Cardiac Class, and Markedly Reduces Hospitalizations. J. Am. Coll. Cardiol. 2000, 35, 1737–1744. [Google Scholar] [CrossRef]
- Esteves, A.F.; Gonçalves, S.; Duarte, T.; Ferreira, J.; Coelho, R.; Quintal, J.; Pohle, C.; Fonseca, N.; Caria, R. Iron Deficiency in Acute Coronary Syndromes: Prevalence and Prognostic Impact. Porto Biomed. J. 2025, 10. [Google Scholar] [CrossRef]
- McCullough, P.A. Anemia of Cardiorenal Syndrome. Kidney Int. Suppl. 2021, 11, 35–45. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Gallotta, M.; Iovine, F.; Nuti, R.; Silverberg, D.S. Anaemia in Heart Failure: A Common Interaction with Renal Insufficiency Called the Cardio-Renal Anaemia Syndrome: Anaemia and Heart Failure. Int. J. Clin. Pract. 2007, 62, 281–286. [Google Scholar] [CrossRef]
- Kumar, U.; Wettersten, N.; Garimella, P.S. Cardiorenal Syndrome. Cardiol. Clin. 2019, 37, 251–265. [Google Scholar] [CrossRef]
- Jelani, Q.; Katz, S.D. Treatment of Anemia in Heart Failure: Potential Risks and Benefits of Intravenous Iron Therapy in Cardiovascular Disease. Cardiol. Rev. 2010, 18, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, M.; Parissis, J.T.; Akiyama, E.; Mebazaa, A. Understanding Acute Heart Failure: Pathophysiology and Diagnosis. Eur. Heart J. Suppl. 2016, 18 (suppl G), G11–G18. [Google Scholar] [CrossRef]
- Lena, A.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure—The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Turrise, S.; MacLaughlin, E.J.; Chang, P.-S.; Clair, W.K.; Lewis, E.F.; Forman, D.E.; Goodlin, S.J.; on behalf of the American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Stroke Council. Preventing and Managing Falls in Adults With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Binu, A.; Cherian, K.; Kapoor, N.; Chacko, S.; George, O.; Paul, T. The Heart of the Matter: Cardiac Manifestations of Endocrine Disease. Indian J. Endocrinol. Metab. 2017, 21, 919. [Google Scholar] [CrossRef]
- Aroor, A.R.; Mandavia, C.H.; Sowers, J.R. Insulin Resistance and Heart Failure. Heart Fail. Clin. 2012, 8, 609–617. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; Iovino, M.; Zupo, R.; Guastamacchia, E.; De Pergola, G.; Iacoviello, M.; Triggiani, V. Endocrine System Dysfunction and Chronic Heart Failure: A Clinical Perspective. Endocrine 2022, 75, 360–376. [Google Scholar] [CrossRef]
- Kopkan, L.; Hosková, L.; Melenovský, V.; Husková, Z.; Cervenka, L. Abstract P421: The Effects of Dual Angiotensin Receptor and Neprilysin Inhibitor on Organoprotection in Experimental Model of Chronic Heart Failure. Hypertension 2017, 70 (suppl_1), AP421. [Google Scholar] [CrossRef]
- Perrone-Filardi, P.; Paolillo, S.; Agostoni, P.; Basile, C.; Basso, C.; Barilla, F.; Correale, M.; Curcio, A.; Mancone, M.; Merlo, M.; Metra, M. Renin-angiotensin-aldosterone system inhibition in patients affected by heart failure: efficacy, mechanistic effects and practical use of sacubitril/valsartan. Position Paper of the Italian Society of Cardiology. European Journal of Internal Medicine 2022, 102, 8–16. [Google Scholar] [CrossRef]
- Di Lodovico, E.; Facondo, P.; Delbarba, A.; Pezzaioli, L.C.; Maffezzoni, F.; Cappelli, C.; Ferlin, A. Testosterone, hypogonadism, and heart failure. Circulation: Heart Failure 2022, 15, e008755. [Google Scholar] [CrossRef] [PubMed]
- Martucci, G.; Pappalardo, F.; Subramanian, H.; Ingoglia, G.; Conoscenti, E.; Arcadipane, A. Endocrine Challenges in Patients with Continuous-Flow Left Ventricular Assist Devices. Nutrients 2021, 13, 861. [Google Scholar] [CrossRef]
- Alevroudis, I.; Kotoulas, S.-C.; Tzikas, S.; Vassilikos, V. Congestion in Heart Failure: From the Secret of a Mummy to Today’s Novel Diagnostic and Therapeutic Approaches: A Comprehensive Review. J. Clin. Med. 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Felker, G.M. Biomarkers of Congestion. JACC Heart Fail. 2020, 8, 398–400. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation – Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Hofmann, U.; Frantz, S. How can we cure a heart "in flame"? A translational view on inflammation in heart failure. Basic Res Cardiol. 2013, 108, 356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wrigley, B.J.; Shantsila, E.; Tapp, L.D.; Lip, G.Y.H. CD 14++ CD 16+ Monocytes in Patients with Acute Ischaemic Heart Failure. Eur. J. Clin. Invest. 2013, 43, 121–130. [Google Scholar] [CrossRef]
- Kologrivova, I.; Shtatolkina, M.; Suslova, T.; Ryabov, V. Cells of the Immune System in Cardiac Remodeling: Main Players in Resolution of Inflammation and Repair After Myocardial Infarction. Front Immunol. 2021, 12, 664457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howerton, E.; Tarzami, S.T. Tumor Necrosis Factor-Alpha and Inflammation-Mediated Cardiac Injury. J Cell Sci Ther 2017, 8, 268. [Google Scholar] [CrossRef]
- Prokopidis, K.; Irlik, K.; Hendel, M.; Piaśnik, J.; Lip, G.Y.H.; Nabrdalik, K. Prognostic Impact and Prevalence of Cachexia in Patients With Heart Failure: A Systematic Review and Meta-Analysis. J Cachexia Sarcopenia Muscle 2024, 15, 2536–2543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yosipovitch, G.; Nedorost, S.T.; Silverberg, J.I.; Friedman, A.J.; Canosa, J.M.; Cha, A. Stasis Dermatitis: An Overview of Its Clinical Presentation, Pathogenesis, and Management. Am. J. Clin. Dermatol. 2023, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L. Care of the lower extremities in patients with acute decompensated heart failure. Crit Care Nurse 2011, 31, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Boehmer, J.P.; Blaha, C.; Lucking, R.; Kunselman, A.R.; Sinoway, L.I. Chronic heart failure does not attenuate the total activity of sympathetic outflow to skin during whole-body heating. Circ Heart Fail. 2013, 6, 271–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hiraiwa, H.; Okumura, T.; Murohara, T. The Cardiosplenic Axis: The Prognostic Role of the Spleen in Heart Failure. Heart Fail. Rev. 2022, 27, 2005–2015. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Iwakiri, Y. The Lymphatic Vascular System in Liver Diseases: Its Role in Ascites Formation. Clin. Mol. Hepatol. 2013, 19, 99. [Google Scholar] [CrossRef]
| Effect | Description |
|---|---|
| Acute Kidney Injury (AKI) | Chronic congestion reduces renal perfusion, making the renal medulla hypoxic and predisposing it to acute tubular necrosis (ATN). |
| Fibrosis and Chronic Kidney Disease (CKD) | Persistent renal venous hypertension promotes inflammation, oxidative stress, and tubulointerstitial fibrosis, progressively leading to structural nephron loss and worsening renal function. |
| Altered Drug Metabolism and Toxicity | Decreased renal clearance in congested kidneys impairs the excretion of medications such as loop diuretics, ACE inhibitors/ARBs, and Digoxin. |
| Electrolyte and Acid-Base Imbalances |
Hyperkalemia due to impaired potassium excretion, exacerbated by RAAS inhibitors; Hyponatremia Metabolic acidosis |
| Systemic Inflammation and Endothelial Dysfunction |
Release of pro-inflammatory cytokines (TNF-α, IL-6), contributing to vascular endothelial dysfunction. Accumulation of uremic toxins promotes vascular calcification, oxidative stress, and worsening myocardial remodeling. |
| Anemia and Erythropoietin Deficiency | Impaired erythropoietin (EPO) production in congested kidneys leads to anemia of chronic disease, exacerbating fatigue, hypoxia, and cardiac strain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




