Submitted:
21 February 2025
Posted:
21 February 2025
You are already at the latest version
Abstract
Background: Racotumomab-alum is an anti-idiotype vaccine targeting the NeuGcGM3 tumor-associated ganglioside. Clinical trials in advanced cancer patients have demonstrated low toxicity, high immunogenicity and clinical benefit. The goal of this study was to identify circulating biomarkers of clinical outcome. Methods: Eighteen patients with stage IIIb/IV non-small-cell lung cancer (NSCLC) were injected with racotumomab-alum as switch maintenance therapy after first line chemotherapy. Treatment was administered until severe performance status worsening or toxicity. The frequencies of innate and adaptive lymphocytes were assessed by flow cytometry. Circulat ing factors were measured using multi-analyte flow assay kits. Results: The median overall survival was 16.5 months. Twenty-seven percent of patients were classified as long-term survivors. Patients with lower baseline frequencies of CD4+Tregs and central memory (CM) CD8+T cells displayed longer survival rates. Furthermore, higher baseline frequencies of NKT cells and a high CD8+T/CD4+Treg ratio were associated with longer survival. Interestingly, patients with significantly lower levels of effector memory (EM) CD8+T cells survived longer. The levels of NKT cells and terminal effector memory (EMRA) CD8+T cells were higher in long–term survivors in comparison with short-term survivors at post-immune samples. As expected, the ratio CD8+T/CD4+Tregs showed significantly higher values during treatment in patients with clinical benefits. Regarding serum factors, pro-tumorigenic cytokines significantly increased during treatment in poor survivors. Conclusions: In advanced NSCLC patients receiving racotumomab-alum vaccine, longer survival is associated with a unique profile of circulating lymphocyte subsets at baseline and during treatment. Additionally, certain protumor-related cytokines increased in short-term survivors. These results should be confirmed in larger clinical trials. This clinical trial was registered in Cuban Clinical Trials Register (RPCE00000279).
Keywords:
1. Introduction
2. Materials and Methods
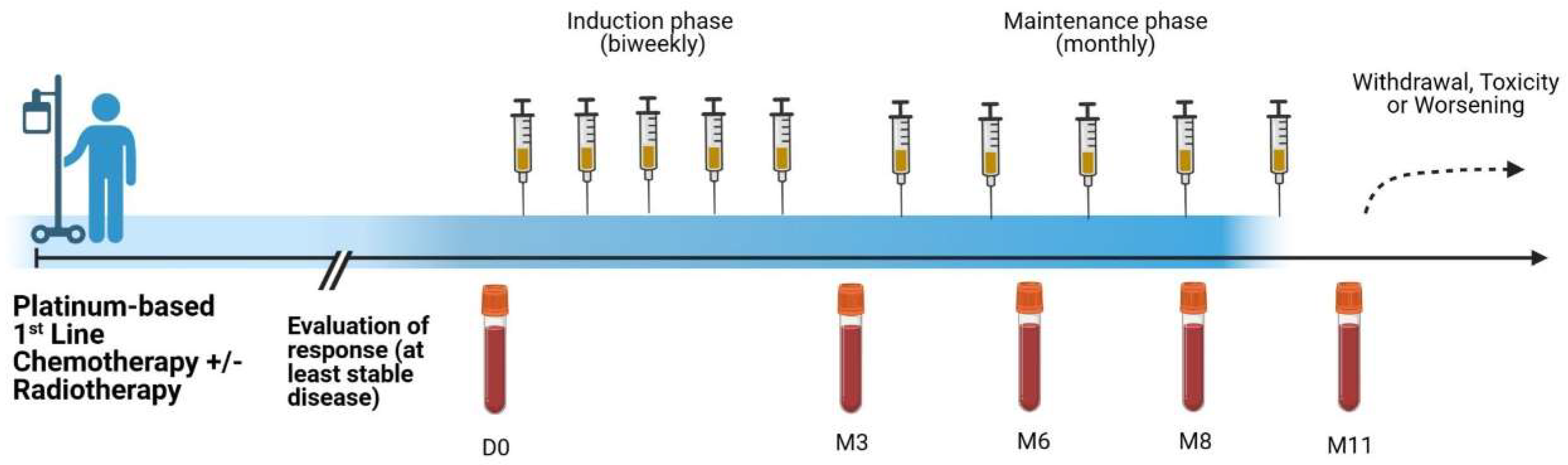
2.1. Patients and Treatment
2.2. Safety
2.3. Collection of Peripheral Blood Mononuclear Cells and Sera
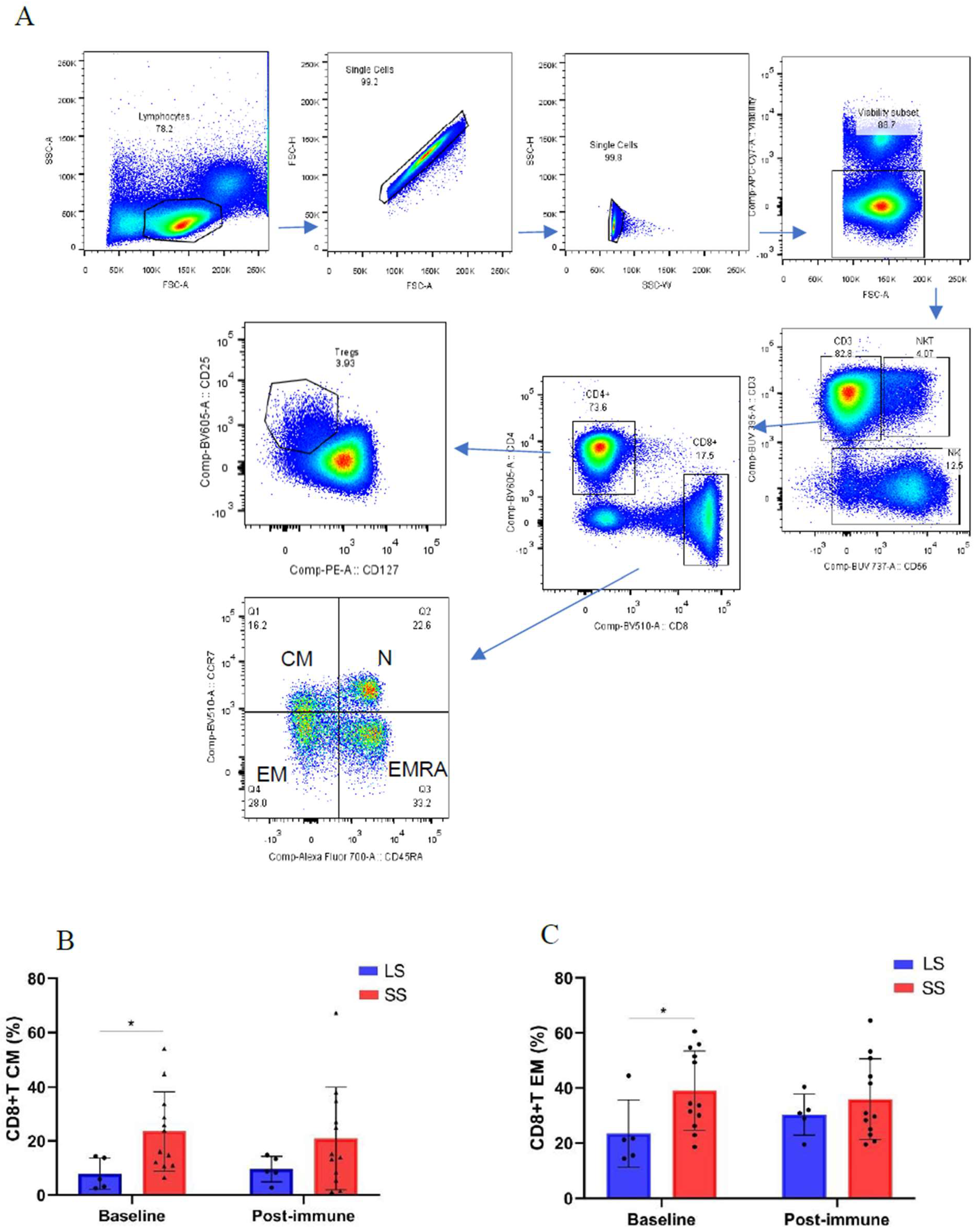
2.4. Flow Cytometry
2.5. Multiplex Assays for Soluble Factors
2.6. Statistical Analyses
3. Results
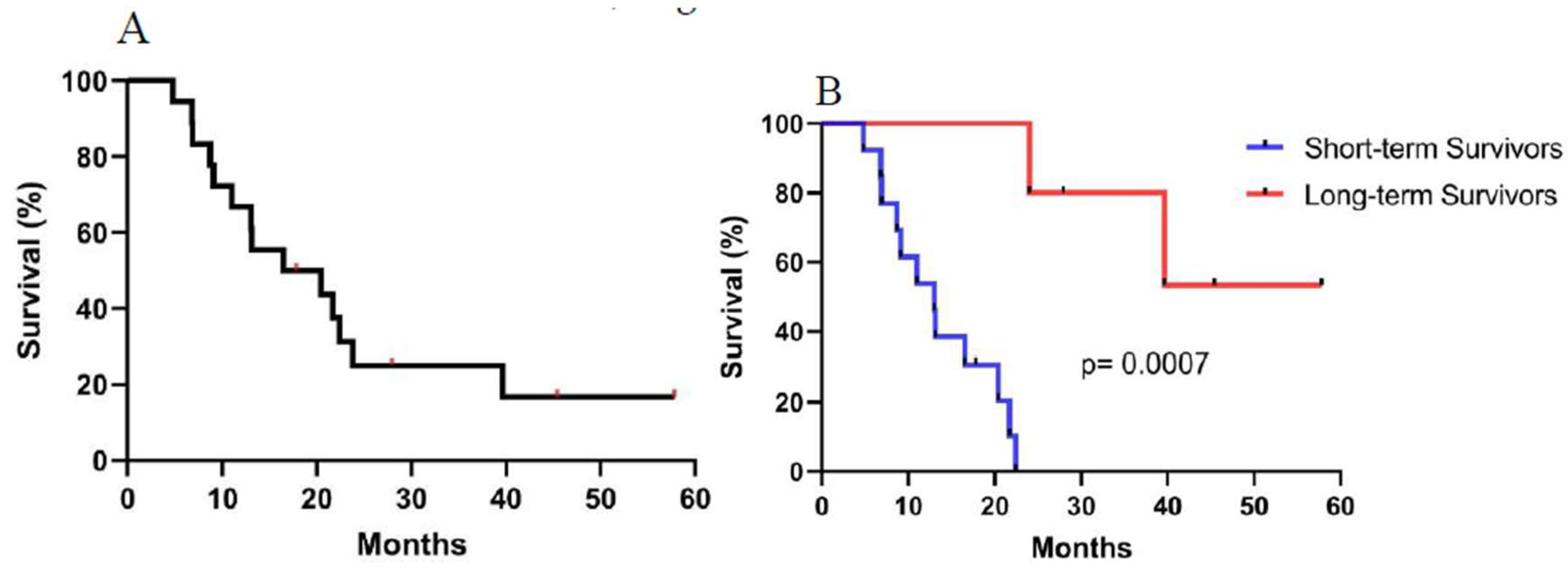
3.1. Patient Characteristics and Treatment Outcome
3.2. Safety
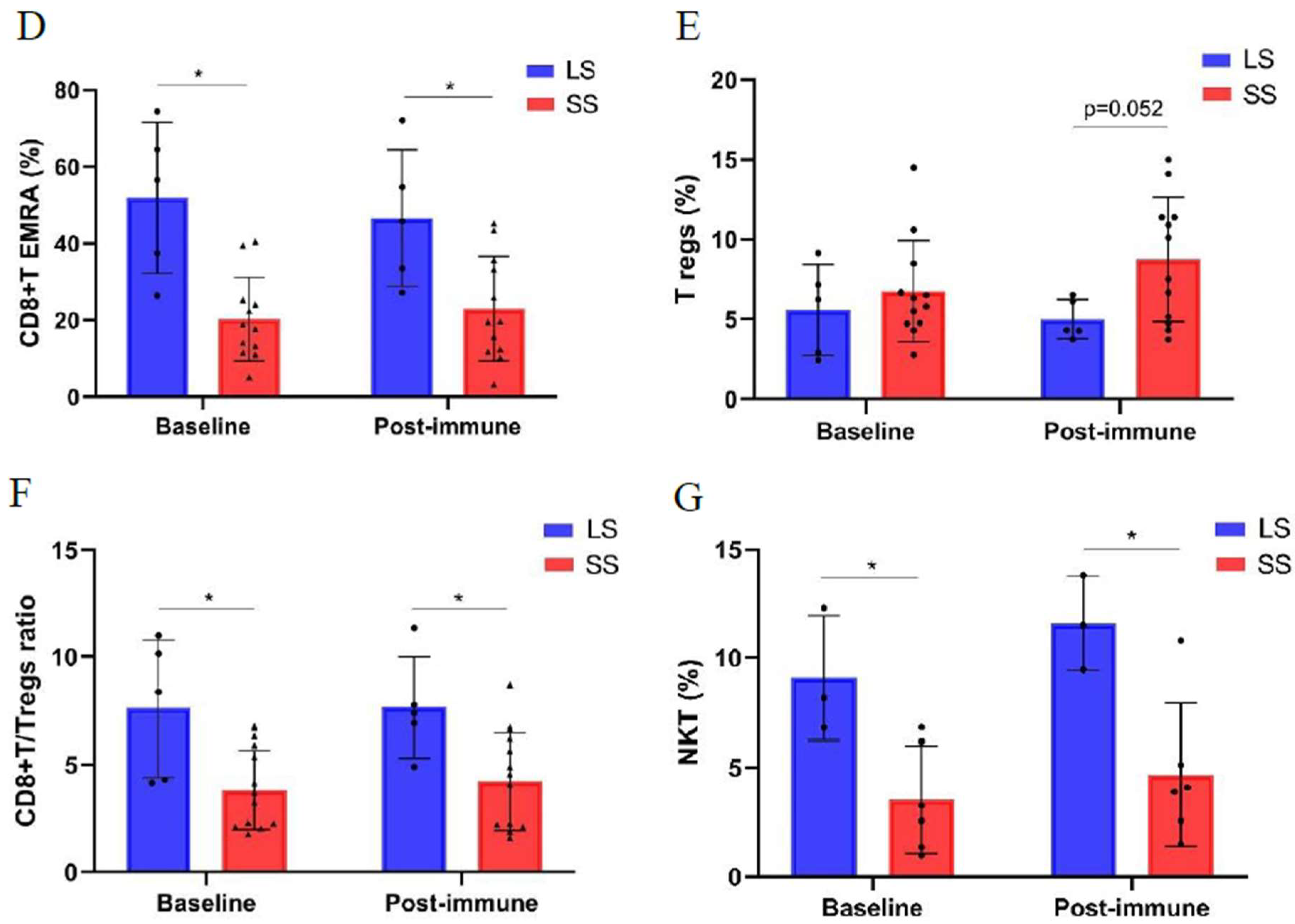
3.3. Changes of Immune Cell Populations in Short-Term and Long-Term NSCLC Survivors Treated with Racotumomab-Alum Vaccine
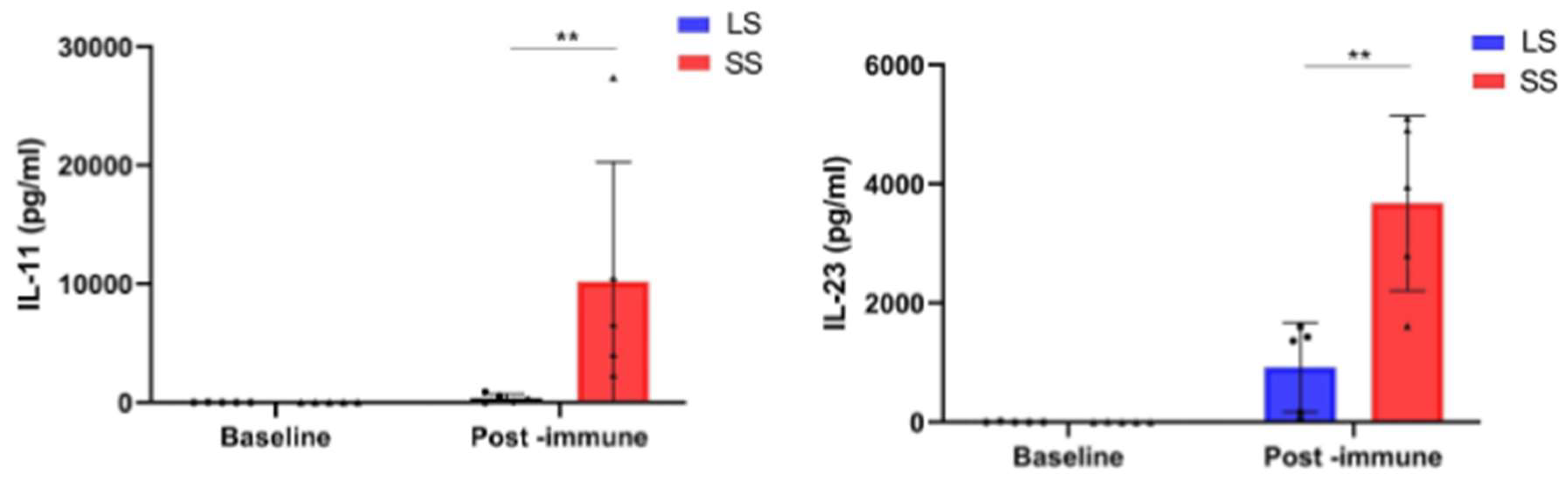
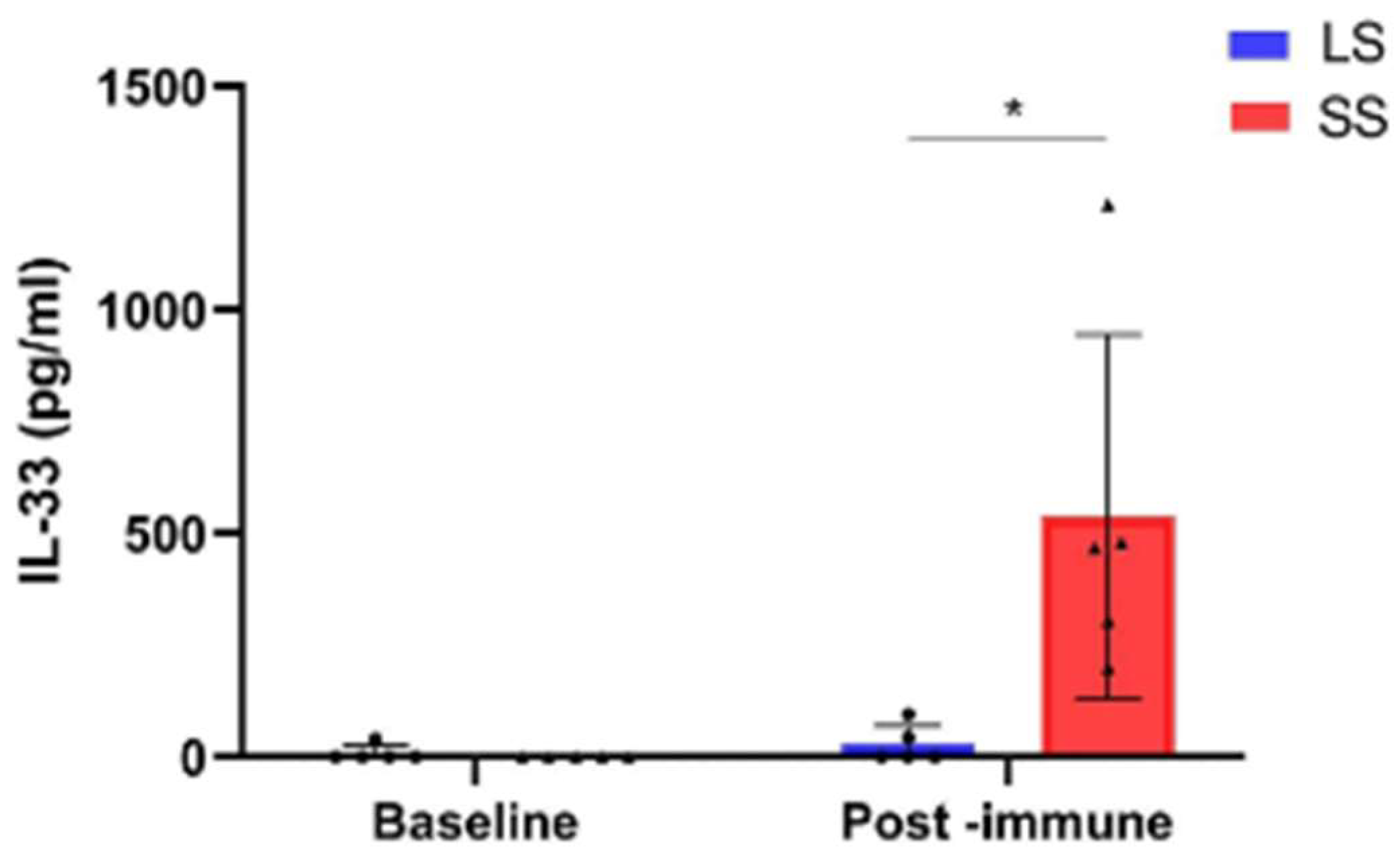
3.4. Changes in Circulating Factor Levels in NSCLC Patients During Racotumomab-Alum Vaccine Treatment
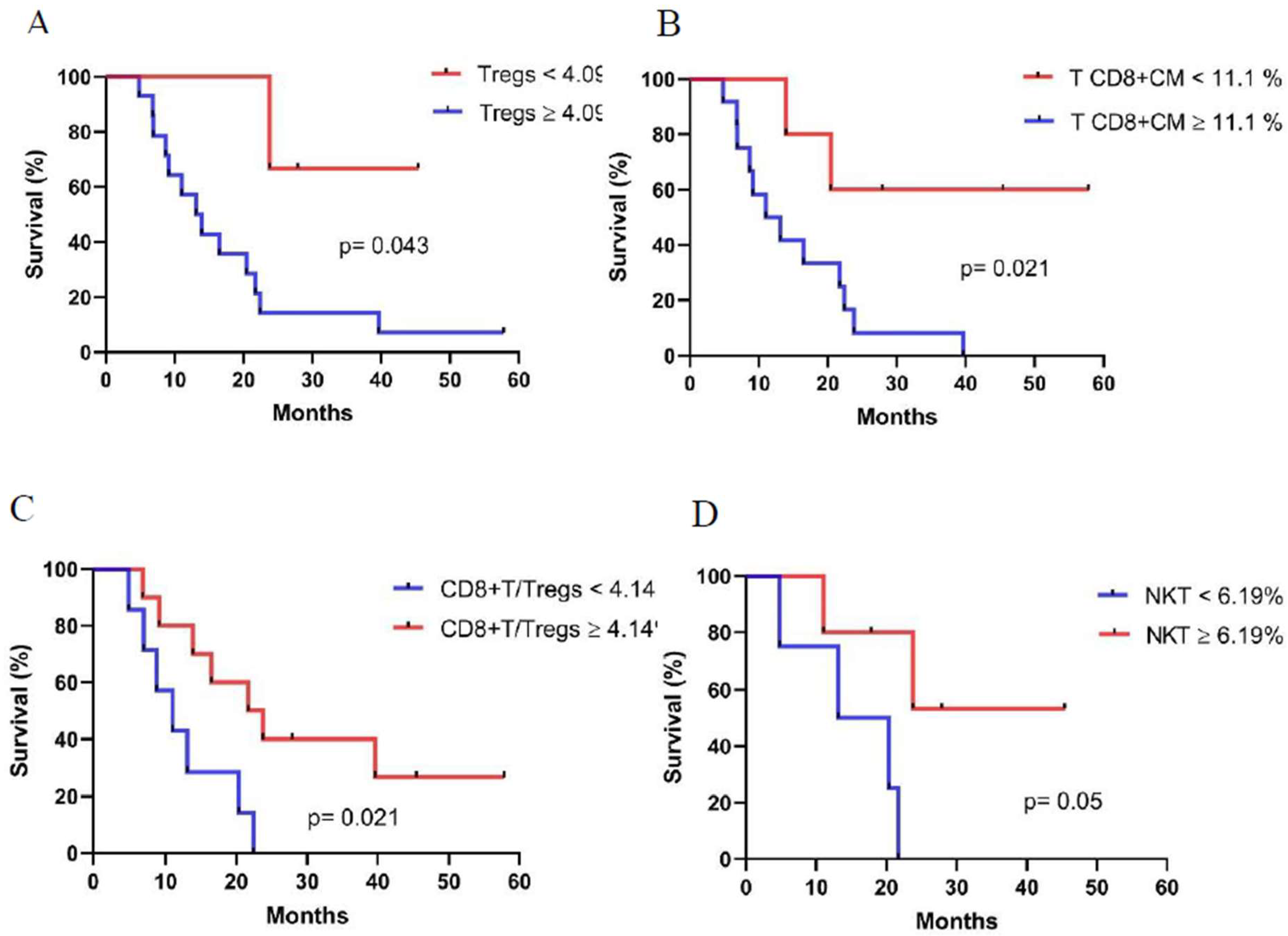
3.5. Associations Between Immune Cell Populations at Baseline and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhou, G. Tobacco, air pollution, environmental carcinogenesis, and thoughts on conquering strategies of lung cancer. Cancer Biol Med 2019, 16, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Pineros, M.; Laversanne, M.; Barrios, E.; Cancela, M.C.; de Vries, E.; Pardo, C.; Bray, F. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Health Am 2022, 13, None. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.; Routledge, T.; Pilling, J.; Scarci, M. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interact Cardiovasc Thorac Surg 2010, 10, 1015–1021. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Grimmett, E.; Al-Share, B.; Alkassab, M.B.; Zhou, R.W.; Desai, A.; Rahim, M.M.A.; Woldie, I. Cancer vaccines: past, present and future; a review article. Discov Oncol 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Shewell, L.K.; Wang, J.J.; Paton, J.C.; Paton, A.W.; Day, C.J.; Jennings, M.P. Detection of N-glycolylneuraminic acid biomarkers in sera from patients with ovarian cancer using an engineered N-glycolylneuraminic acid-specific lectin SubB2M. Biochem Biophys Res Commun 2018, 507, 173–177. [Google Scholar] [CrossRef]
- Hedlund, M.; Padler-Karavani, V.; Varki, N.M.; Varki, A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A 2008, 105, 18936–18941. [Google Scholar] [CrossRef]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A 2003, 100, 12045–12050. [Google Scholar] [CrossRef]
- Samraj, A.N.; Laubli, H.; Varki, N.; Varki, A. Involvement of a non-human sialic Acid in human cancer. Front Oncol 2014, 4, 33. [Google Scholar] [CrossRef]
- Wang, J.; Shewell, L.K.; Day, C.J.; Jennings, M.P. N-glycolylneuraminic acid as a carbohydrate cancer biomarker. Transl Oncol 2023, 31, 101643. [Google Scholar] [CrossRef]
- Vazquez, A.M.; Gabri, M.R.; Hernandez, A.M.; Alonso, D.F.; Beausoleil, I.; Gomez, D.E.; Perez, R. Antitumor properties of an anti-idiotypic monoclonal antibody in relation to N-glycolyl-containing gangliosides. Oncol Rep 2000, 7, 751–756. [Google Scholar] [CrossRef]
- Neninger, E.; Diaz, R.M.; de la Torre, A.; Rives, R.; Diaz, A.; Saurez, G.; Gabri, M.R.; Alonso, D.F.; Wilkinson, B.; Alfonso, A.M.; et al. Active immunotherapy with 1E10 anti-idiotype vaccine in patients with small cell lung cancer: report of a phase I trial. Cancer Biol Ther 2007, 6, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Alfonso, M.; Alonso, R.; Saurez, G.; Troche, M.; Catala, M.; Diaz, R.M.; Perez, R.; Vazquez, A.M. Immune responses in breast cancer patients immunized with an anti-idiotype antibody mimicking NeuGc-containing gangliosides. Clin Immunol 2003, 107, 80–89. [Google Scholar] [CrossRef]
- Alfonso, S.; Diaz, R.M.; de la Torre, A.; Santiesteban, E.; Aguirre, F.; Perez, K.; Rodriguez, J.L.; Barroso Mdel, C.; Hernandez, A.M.; Toledo, D.; et al. 1E10 anti-idiotype vaccine in non-small cell lung cancer: experience in stage IIIb/IV patients. Cancer Biol Ther 2007, 6, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Center for State Control of Medicines, E.a.M.D.C.I.H.C.f.S.C.o.M., Equipment and Medical Devices (CU); c2021. Registro. VAXIRA® (Racotumomab). Reg. No.: B-013- 001-L03C; [cited 2021 Mar 7]. Available at: https://www.cecmed.cu/registro/rcp/vaxiraracotumomab. Spanish.
- Caceres-Lavernia, H.H.; Neninger-Vinageras, E.; Varona-Rodriguez, L.M.; Olivares-Romero, Y.A.; Sanchez-Rojas, I.; Mazorra-Herrera, Z.; Basanta-Bergolla, D.; Duvergel-Calderin, D.; Torres-Cuevas, B.L.; Castillo-Carrillo, C. Racotumomab in Non-Small Cell Lung Cancer as Maintenance and Second-Line Treatment. MEDICC Rev 2021, 23, 21–28. [Google Scholar] [CrossRef]
- Sanchez, L.; Muchene, L.; Lorenzo-Luaces, P.; Viada, C.; Rodriguez, P.C.; Alfonso, S.; Crombet, T.; Neninger, E.; Shkedy, Z.; Lage, A. Differential effects of two therapeutic cancer vaccines on short- and long-term survival populations among patients with advanced lung cancer. Semin Oncol 2018, 45, 52–57. [Google Scholar] [CrossRef]
- Alfonso, S.; Valdes-Zayas, A.; Santiesteban, E.R.; Flores, Y.I.; Areces, F.; Hernandez, M.; Viada, C.E.; Mendoza, I.C.; Guerra, P.P.; Garcia, E.; et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res 2014, 20, 3660–3671. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, V.; Govaerts, E.; Nackaerts, K.; Dooms, C.; Wauters, I.; Vansteenkiste, J. Clinical factors predictive of long-term survival in advanced non-small cell lung cancer. Lung Cancer 2013, 79, 73–76. [Google Scholar] [CrossRef]
- Hsu, M.L.; Murray, J.C.; Psoter, K.J.; Zhang, J.; Barasa, D.; Brahmer, J.R.; Ettinger, D.S.; Forde, P.M.; Hann, C.L.; Lam, V.K.; et al. Clinical Features, Survival, and Burden of Toxicities in Survivors More Than One Year After Lung Cancer Immunotherapy. Oncologist 2022, 27, 971–981. [Google Scholar] [CrossRef]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017, 35, 3924–3933. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021, 16, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- von Pawel, J.; Bordoni, R.; Satouchi, M.; Fehrenbacher, L.; Cobo, M.; Han, J.Y.; Hida, T.; Moro-Sibilot, D.; Conkling, P.; Gandara, D.R.; et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: Results from the randomised phase III OAK study. Eur J Cancer 2019, 107, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Popa, X.; Martinez, O.; Mendoza, S.; Santiesteban, E.; Crespo, T.; Amador, R.M.; Fleytas, R.; Acosta, S.C.; Otero, Y.; et al. A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2016, 22, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.Y.; Chu, C.S.; Goletz, T.J.; Schlienger, K.; Yeh, H.; Coukos, G.; Rubin, S.C.; Kaiser, L.R.; June, C.H. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 2001, 61, 4766–4772. [Google Scholar]
- Yannelli, J.R.; Tucker, J.A.; Hidalgo, G.; Perkins, S.; Kryscio, R.; Hirschowitz, E.A. Characteristics of PBMC obtained from leukapheresis products and tumor biopsies of patients with non-small cell lung cancer. Oncol Rep 2009, 22, 1459–1471. [Google Scholar] [CrossRef]
- Li, L.; Chao, Q.G.; Ping, L.Z.; Xue, C.; Xia, Z.Y.; Qian, D.; Shi-ang, H. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 2009, 24, 357–367. [Google Scholar] [CrossRef]
- Koh, J.; Hur, J.Y.; Lee, K.Y.; Kim, M.S.; Heo, J.Y.; Ku, B.M.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; et al. Regulatory (FoxP3(+)) T cells and TGF-beta predict the response to anti-PD-1 immunotherapy in patients with non-small cell lung cancer. Sci Rep 2020, 10, 18994. [Google Scholar] [CrossRef]
- Borilova, S.; Grell, P.; Selingerova, I.; Gescheidtova, L.; Mlnarikova, M.; Bilek, O.; Lakomy, R.; Poprach, A.; Podhorec, J.; Kiss, I.; et al. Early changes of peripheral circulating immune subsets induced by PD-1 inhibitors in patients with advanced malignant melanoma and non-small cell lung cancer. BMC Cancer 2024, 24, 1590. [Google Scholar] [CrossRef]
- Li, P.; Qin, P.; Fu, X.; Zhang, G.; Yan, X.; Zhang, M.; Zhang, X.; Yang, J.; Wang, H.; Ma, Z. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor. Ann Palliat Med 2021, 10, 3039–3049. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Guo, Z.; Wei, W.; Wang, T.; Ouyang, R.; Yuan, X.; Xing, Y.; Wang, F.; Wu, S.; et al. Immunological profiling for short-term predictive analysis in PD-1/PD-L1 therapy for lung cancer. BMC Cancer 2024, 24, 851. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; von Scheidt, B.; McLaughlin, N.; Sparwasser, T.; Smyth, M.J. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 2010, 70, 7800–7809. [Google Scholar] [CrossRef]
- Smyth, M.J.; Ngiow, S.F.; Teng, M.W. Targeting regulatory T cells in tumor immunotherapy. Immunol Cell Biol 2014, 92, 473–474. [Google Scholar] [CrossRef]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Koh, J.; Song, S.G.; Yim, J.; Kim, M.; Keam, B.; Kim, Y.T.; Kim, J.; Chung, D.H.; Jeon, Y.K. High tumor hexokinase-2 expression promotes a pro-tumorigenic immune microenvironment by modulating CD8+/regulatory T-cell infiltration. BMC Cancer 2022, 22, 1120. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, G.; Bi, G.; Cheng, L.; Liang, J.; Li, M.; Zhang, H.; Shan, G.; Hu, Z.; Chen, Z.; et al. Unveiling chemotherapy-induced immune landscape remodeling and metabolic reprogramming in lung adenocarcinoma by scRNA-sequencing. Elife 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Lanzavecchia, A.; Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Manjarrez-Orduno, N.; Menard, L.C.; Kansal, S.; Fischer, P.; Kakrecha, B.; Jiang, C.; Cunningham, M.; Greenawalt, D.; Patel, V.; Yang, M.; et al. Circulating T Cell Subpopulations Correlate With Immune Responses at the Tumor Site and Clinical Response to PD1 Inhibition in Non-Small Cell Lung Cancer. Front Immunol 2018, 9, 1613. [Google Scholar] [CrossRef]
- Larbi, A.; Fulop, T. From "truly naive" to "exhausted senescent" T cells: when markers predict functionality. Cytometry A 2014, 85, 25–35. [Google Scholar] [CrossRef]
- Lee, S.W.; Choi, H.Y.; Lee, G.W.; Kim, T.; Cho, H.J.; Oh, I.J.; Song, S.Y.; Yang, D.H.; Cho, J.H. CD8(+) TILs in NSCLC differentiate into TEMRA via a bifurcated trajectory: deciphering immunogenicity of tumor antigens. J Immunother Cancer 2021, 9. [Google Scholar] [CrossRef]
- Crosby, E.J.; Hobeika, A.C.; Niedzwiecki, D.; Rushing, C.; Hsu, D.; Berglund, P.; Smith, J.; Osada, T.; Gwin Iii, W.R.; Hartman, Z.C.; et al. Long-term survival of patients with stage III colon cancer treated with VRP-CEA(6D), an alphavirus vector that increases the CD8+ effector memory T cell to Treg ratio. J Immunother Cancer 2020, 8. [Google Scholar] [CrossRef]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol 2008, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Q.; Xiao, W.; Wang, L.; He, Y.L. Type I natural killer T cells: naturally born for fighting. Acta Pharmacol Sin 2010, 31, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Krovi, S.H.; Gapin, L. Invariant Natural Killer T Cell Subsets-More Than Just Developmental Intermediates. Front Immunol 2018, 9, 1393. [Google Scholar] [CrossRef]
- Motohashi, S.; Kobayashi, S.; Ito, T.; Magara, K.K.; Mikuni, O.; Kamada, N.; Iizasa, T.; Nakayama, T.; Fujisawa, T.; Taniguchi, M. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer 2002, 102, 159–165. [Google Scholar] [CrossRef]
- Bricard, G.; Cesson, V.; Devevre, E.; Bouzourene, H.; Barbey, C.; Rufer, N.; Im, J.S.; Alves, P.M.; Martinet, O.; Halkic, N.; et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol 2009, 182, 5140–5151. [Google Scholar] [CrossRef]
- Tachibana, T.; Onodera, H.; Tsuruyama, T.; Mori, A.; Nagayama, S.; Hiai, H.; Imamura, M. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res 2005, 11, 7322–7327. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.M.; Cheng, O.; Shaulov, A.; Koezuka, Y.; Bubley, G.J.; Wilson, S.B.; Balk, S.P.; Exley, M.A. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol 2001, 167, 4046–4050. [Google Scholar] [CrossRef]
- Molling, J.W.; Kolgen, W.; van der Vliet, H.J.; Boomsma, M.F.; Kruizenga, H.; Smorenburg, C.H.; Molenkamp, B.G.; Langendijk, J.A.; Leemans, C.R.; von Blomberg, B.M.; et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer 2005, 116, 87–93. [Google Scholar] [CrossRef]
- Gentilini, M.V.; Perez, M.E.; Fernandez, P.M.; Fainboim, L.; Arana, E. The tumor antigen N-glycolyl-GM3 is a human CD1d ligand capable of mediating B cell and natural killer T cell interaction. Cancer Immunol Immunother 2016, 65, 551–562. [Google Scholar] [CrossRef]
- Dockry, E.; O'Leary, S.; Gleeson, L.E.; Lyons, J.; Keane, J.; Gray, S.G.; Doherty, D.G. Epigenetic induction of CD1d expression primes lung cancer cells for killing by invariant natural killer T cells. Oncoimmunology 2018, 7, e1428156. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Onate, C.; Perez, G.; Alfaro, C.; Martin-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 2017, 28, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Wakui, H.; Utsumi, H.; Sagawa, Y.; Hosomi, Y.; Kuwano, K.; Homma, S. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin Lung Cancer 2018, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Julie, C.; Dumenil, C.; Helias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 2018, 7, e1452581. [Google Scholar] [CrossRef]
- Xu, D.H.; Zhu, Z.; Wakefield, M.R.; Xiao, H.; Bai, Q.; Fang, Y. The role of IL-11 in immunity and cancer. Cancer Lett 2016, 373, 156–163. [Google Scholar] [CrossRef]
- Leung, J.H.; Ng, B.; Lim, W.W. Interleukin-11: A Potential Biomarker and Molecular Therapeutic Target in Non-Small Cell Lung Cancer. Cells 2022, 11. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Wang, R.; Pan, Y.; He, Z.; Chu, X.; Song, H.; Chen, L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci Rep 2016, 6, 38408. [Google Scholar] [CrossRef]
- Langrish, C.L.; McKenzie, B.S.; Wilson, N.J.; de Waal Malefyt, R.; Kastelein, R.A.; Cua, D.J. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004, 202, 96–105. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Yan, J.; Smyth, M.J.; Teng, M.W.L. Interleukin (IL)-12 and IL-23 and Their Conflicting Roles in Cancer. Cold Spring Harb Perspect Biol 2018, 10. [Google Scholar] [CrossRef]
- Liu, D.; Xing, S.; Wang, W.; Huang, X.; Lin, H.; Chen, Y.; Lan, K.; Chen, L.; Luo, F.; Qin, S.; et al. Prognostic value of serum soluble interleukin-23 receptor and related T-helper 17 cell cytokines in non-small cell lung carcinoma. Cancer Sci 2020, 111, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tian, C.; Zhang, C.; Xiang, M. The Controversial Role of IL-33 in Lung Cancer. Front Immunol 2022, 13, 897356. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, Y.; Luo, G.; Wang, Z.; Yu, P.; Zheng, L. [Expression and clinical significance of IL-33 in patients with non-small cell lung cancer]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 808–811. [Google Scholar] [PubMed]
- Li, A.; Herbst, R.H.; Canner, D.; Schenkel, J.M.; Smith, O.C.; Kim, J.Y.; Hillman, M.; Bhutkar, A.; Cuoco, M.S.; Rappazzo, C.G.; et al. IL-33 Signaling Alters Regulatory T Cell Diversity in Support of Tumor Development. Cell Rep 2019, 29, 2998–3008. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, H.Q.; Li, H.; Zhao, Y.; Lui, V.W.Y.; Chen, L.; Wu, X.M.; Sun, W.; Wen, W.P. Stromal interleukin-33 promotes regulatory T cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol Immunother 2019, 68, 221–232. [Google Scholar] [CrossRef]
| Variable | Categories | N (%) |
| Age | < 60 y | 6 (33.3) |
| ≥ 60 y | 12 (66.6) | |
| Gender | Female | 9 (50) |
| Male | 9 (50) | |
| ECOG PS | 0 | 8 (44.4) |
| 1 | 9 (50) | |
| 2 | 1 (5.5) | |
| Smoking history | Current smoker | 13 (72.2) |
| Former smoker | 2 (11.1) | |
| Non-smoker | 3 (16.6) | |
| Disease Stage | IIIA | 4 (22.2) |
| IIIB | 6 (33.3) | |
| IV | 8 (44.4) | |
| Tumor histology | Adenocarcinoma | 5 (27.7) |
| Squamous cell carcinoma | 6 (33.3) | |
| NSCLC (NOS) | 7 (38.8) | |
| First-line treatment | Chemotherapy | 2 (11.1) |
| Radio + chemotherapy | 16 (88.8) | |
| Response to first-line treatment | CR | 1 (5.5) |
| PR | 6 (33.3) | |
| SD | 9 (50) | |
| PD | 2 (11.1) | |
| Other chemotherapy lines | Yes | 14 (77.7) |
| No | 4 (22.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





