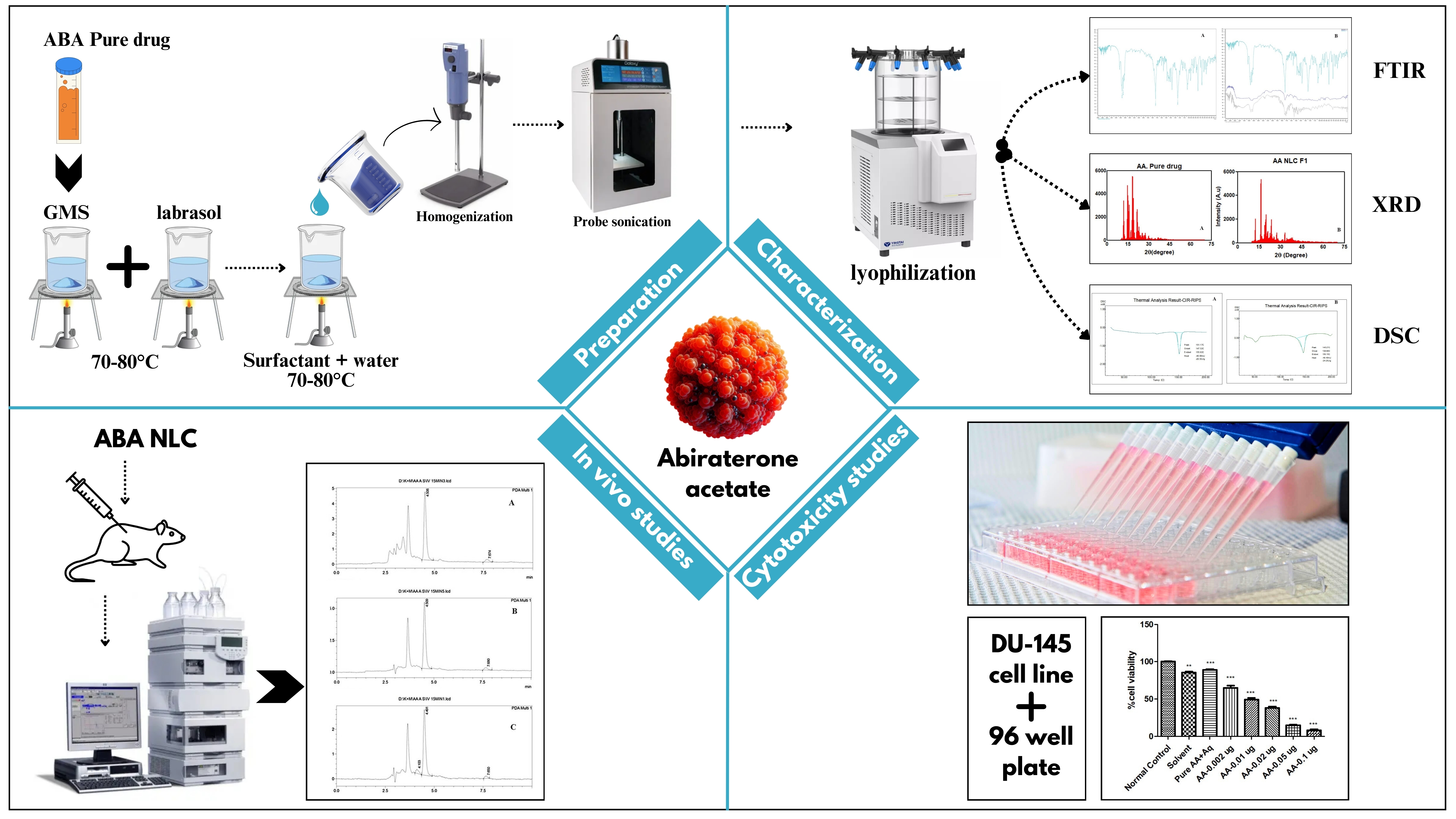
Introduction
Nanostructured lipid carriers (NLC) are novel drug delivery system as a core matrix composed by solid lipid, liquid lipid and surfactant[
1,
2]. The evolution of nanostructured lipid carrier is started after observation of some limitations of 1
st generation lipid carrier i.e. solid lipid nanocarrier (SLN)[
3]. Unlike conventional colloidal carriers including emulsions, liposomes, and polymeric micro- and nanoparticles, lipid nanocarriers offer an alternative carrier system[
4,
5]. To generate a drug-incorporated matrix, NLCs have been developed by substituting a portion of liquid lipids for solid lipids[
6]. Because of their superior supercilious formulation qualities and biocompatibility in comparison to SLNs, NLCs are being regarded potential prospective drug carriers. Despite of various types of NLC, i.e. imperfect crystal model, multiple model (consisting solid lipid, liquid lipid and surfactant), amorphous model, the multiple type model was selected due to its high drug entrapment efficiency, greater stability, improved drug release with minimizing the drug escape[
7]. ABA is being used to treat prostate cancer, was discovered through the synthesis and evaluation of steroid derivatives[
8,
9]. As abiraterone was poorly bioavailable and susceptible to hydrolysis by esterase [
9,
10] whereas ABA was esterase resistant and metabolized by deacetylation process to abiraterone in vivo CYP17 inhibition[
8,
9,
11,
12,
13]. ABA with several oral delivery challenges coming under BCS Class IV[
14]. Its high lipophilicity and low solubility result in a strong 5- to 10-fold favourable dietary impact and poor oral bioavailability (<10%)[
10,
15]. Patients consequently receive a substantial amount of ABA (1000 mg daily), which requires them to fast for at least one hour prior to and two hours after medication. The present work deals with the preparation and in vitro evaluation of colloidal formulations of ABA-loaded nano-structured lipid carrier was formulated by high sheer homogenization method. The prepared colloidal formulation of ABA was characterized by drug content estimation, particle size measurement, in vitro drug release kinetics studies. Dried samples of nano-structured lipid carrier of ABA were characterized by dynamic light scattering (DLS) method [
16]. The present work will be using the quality by design (QbD) principles, nanostructured lipid carriers (NLC) were created to increase the oral bioavailability and anticancer activity of ABA. These were assessed using in vitro, ex vivo, and in vivo tests [
17]. Equilibrium solubility analysis was used to assess the suitability of solid lipids, and the potential of surfactants and co-surfactants to create microemulsion with the chosen lipid was utilised for screening them [
18]. NLC were prepared by solvent evaporation & homogenization method using glyceryl monostearate, Compritol 888 ATO, both Transcutol P and tween 20 as the solid lipid, liquid lipid and surfactant, respectively[
19,
20,
21,
22,
23,
24,
25,
26].
Materials and methods
ABA was a gift sample from Dr. Reddy’s lab Hyderabad, India. Glyceryl monostearate was purchased from Lobachemie, Mumbai, India. Labrasol, Precirol® ATO, Compritol® 888 ATO were obtained from Gattefosse, Mumbai, India. Captex 200 P was purchased from ABITEC Corporation, Mumbai, India. Tween 80, Lactose and sodium EDTA were purchased from Himedia Laboratories Pvt, Ltd., Mumbai, India. Tween 20, Transcutol P, Concentrated HCL, NaOH pellets, Potassium dihydrogen phosphate was purchased from Merck Life Sciences Pvt. Ltd., Mumbai, India. Distilled water was used throughout the experiment. All the ingredients obtained and used in the present investigation were of analytical grade.
Saturation Solubility Study of Drug in Different Solvents.
Drug solubilized with different aqueous solvents to check the solubility like Distilled water, 0.1N HCL, 0.1N NaOH, Ethyl Acetate, Diethyl Ether, Acetonitrile, Methanol, Phosphate Buffer, Dimethyl Sulfoxide. 10 ml of solvent was taken in a volumetric flask and 10mg of drug added to the solvent and then sonicated for 10 minutes in sonicator and then visually observed.
Selection of Suitable Solid Lipid, Liquid Lipid.
To select the appropriate solid lipid, Glyceryl Monostearate (GMS), Compritol® 888 ATO, Precirol® ATO were taken of 500mg in a beaker and heated up to 80ºc till they reached their melting point. Then ABA added with continuous stirring up to saturation level. To select the appropriate liquid lipid, 2mL of Labrasol, glycerol, Captex 200 each were taken in 3 different vials and placed in a mechanical shaker with maintaining constant speed. The solutions were then taken out in an Eppendorf tube and centrifuged at 6000 rpm at 4oC for 15 minutes. 1ml from the supernatants were taken out and diluted in 0.1N HCl for quantification with the help of UV-Visible spectrophotometer (Shimadzu, UV-1800, Japan). Absorbance data was recorded and according to that the appropriate liquid lipid was chosen for NLC formulation. Similarly, Tween 20, Tween 80, Transcutol P are the surfactants taken for the screening for preparation of NLC. All 3 of them were placed in different vials and placed in a mechanical shaker with maintaining constant speed. The solutions were then taken out in an Eppendorf tube and centrifuged at 6000 rpm at 4oC for 15 minutes. 1ml from the supernatants were taken out and diluted in 0.1N HCl for quantification with the help of UV-Visible spectrophotometer. Absorbance data was recorded and according to that the appropriate liquid lipid was chosen for NLC formulation.
Percentage Transmittance
The ability of surfactants to emulsify solid-liquid lipid mixtures was taken into consideration while choosing them to produce NLCs. Using a syringe and a magnetic stirrer, 100 mg of the solid-liquid lipid combination was dissolved in 3 mL of methylene chloride and then added dropwise to 10 mL of 5% surfactant solution. Heating on a hot plate at 40°C for three to four hours eliminated the organic phase. The resulting dispersion was diluted with distilled water after being left at room temperature for one hour in a rotary evaporator to completely remove the solvent. A UV-VIS spectrophotometer set to 264 nm was used to determine the percentage transmittance of the resulting samples. Additionally, the ratio of the filtered surfactants was chosen by making placebo formulation using different ratios and by estimating stability and clarity of the prepared formulations visually.
Preparation of NLC.
ABA NLC was prepared using a high-speed homogenizer (IKA® ΜLTRA-TURRAX®, IKA, Germany). ABA was mixed with liquified GMS followed by Labrasol according to the amount with maintaining the temperature. On the other side, Transcutol P & Tween 20 were pipetted according to the amount mentioned in Table 1 and added in 20mL of distilled water with maintaining the temperature. Both the solutions were mixed with maintaining the same temperature. The formulations were homogenized at 20000 rpm for 5 minutes. The homogenized samples were gone for ultrasonication in probe sonicator (Orchid scientific and innovative India Pvt. Ltd.) under 50% power condition for 10 minutes.
Entrapment Efficiency of ABA Loaded NLC.
2mL of ABA Loaded NLC was centrifuged in a cooling micro centrifuge (Eltek refrigerated micro centrifuge RC 4815 F) at 8000 rpm at 4oc for 15 min. The supernatant solution was taken quantification with the use of UV- Visible spectrophotometer. 1ml of supernatant solution was diluted in 0.1N HCl up to 10mL. The absorbance study was carried out and the results were further analysed for calculation of Entrapment efficiency.
In Vitro Release Study:
To assess the ABA release profile from each formulation, in vitro release tests were carried out utilising a dialysis membrane and beaker on a magnetic stirrer. The dialysis membrane with pore sizes of 2.4 nm was used[
27,
28]. The specific pore diameter of the membrane was ensured before the release studies by soaking overnight in double distilled water. Dialysis membrane was mounted. 0.1N HCl (pH 6.8) used as the receptor medium (200 ml) being stirred at 150 rpm. NLC dispersion (equivalent to 10 mg of ABA) was placed in the donor compartment[
29]. During the experiments the solution in the receptor side was maintained at 37°C ± 15°C, 1 ml of the samples were withdrawn from the receiver compartment and diluted up to 10mL at predetermined time intervals (0, 1 h, 2 h, 4 h, 6 h, 8 h, 24h). The samples were analysed by the UV-VIS spectrophotometer at 264 nm[
29]. The cumulative percent drug release was calculated using the below mentioned Equation 1.
(Equation 1: Percentage drug release)
Particle Size, Polydispersity Index and Zeta Potential Analysis
Employing a Zetasizer (Anton PABAr Litesizer 500), the dynamic light scattering (DLS) experiment was used for the measurement the particle size, polydispersity index (PDI), and zeta potential (ZP) of ABA-loaded NLCs. The freeze-dried ABA Loaded NLCs were diluted with distilled water to obtain 30 – 350 kilo counts per second before analysis. The ZP value reveals the electronic charge on the NLC Surface and physical stability of these NLCs[
30].
Differential Scanning Calorimetry (DSC)
Thermal Phase transitions of the NLC were analysed using differential scanning calorimetry. ABA loaded NLC with cryoprotectant lactose of 5mg in selected sample was kept in freeze dryer for lyophilization. Lyophilized ABA NLC was transferred on to an aluminium pan sealed with a perforated lid, and the temperature is increased at 10ºc/min with a range of 100
oC to 200
oC and the transition curve was recorded. The graph plotted according to the observation of increasing temperature shows the melting point and the nature of the sample[
15].
X-Ray Diffraction (XRD)
NLC & pure drug in a glass sample tube placed into the X-Ray diffraction chamber where at a certain angle of 2Ɵ i.e. glancing angle, X-Ray was pointed on it. The diffraction of the rays plotted mark on the reader plate and later analysed and discussed the result.
In-Vivo Pharmacokinetic Study
Wistar albino rats were used in the pharmacokinetic studies was approved by Institutional Animal Ethical Committee (Regd. No. 926/PO/Re/S/06/CPCSEA). ABA loaded NLC in albino Wistar rat blood serum was estimated using UFLC method. Wistar albino rats of age 2 months and weight up to 200g were divided in 2 groups, one of NLC and another of ABA Suspension (n=6). ABA suspension was prepared by adding 123mg of ABA in 20mL of Distilled water with 20% (200mg) of Sodium CMC as suspending agent. Required amount of dose of suspension was administered through oral route to suspension group animals with the help of Oral gavage respective to their weight. ABA NLC was administered through oral route to NLC group animals with the help of Oral gavage respective to their weight. 150μl Blood was withdrawn from the lateral tail vein by dipping the tail in 40
oC water for better dilation of tail vein at various time points like 1, 4, 6, 8, 12h after dose administration. After centrifuging the plasma for 15 minutes at 5000 rpm, a certain concentration of internal standard was applied to the samples. The drug was separated from 150 μl of rat plasma using liquid-liquid extraction by adding 500 μl of ethyl acetate in a 1:4 ratio, vortexing for 10 seconds to ensure complete mixing, then centrifuging the samples for 15 minutes at 5000 rpm. After being separated into a new Eppendorf tube, the organic component of the supernatant was evaporated in a water bath set at 85 degrees Celsius. In order to estimate the levels of abiraterone and internal standard in the rat plasma, the dried components were subsequently reconstituted using mobile phase[
31,
32].
Sample Analysis by UFLC
A verified bioanalytical technique for abiraterone assessment was used to quantify the drug content in plasma in RP-UFLC (Shimadzu LC-20 AD, Japan) Fitted with photodiode array detector. A C18 column (5μm, 4.6 x 250mm) using a mobile phase of methanol and phosphate buffer 4mM (pH 3.8) (95:5 v/v) was used for the chromatographic separation of the analyte and internal standard in rat plasma. The Sample injection volume was set to 20μl. Where Samples were eluted isocratically. The Flow rate was set to be 1ml/min with the Runtime of 12min. Detection at 253nm using photodiode array detector. The calibration was conducted for ABA NLC group in the range of 2hr to 24 hr and the same was followed by ABA Suspension group. Linear regression analysis was performed to estimate value of correlation coefficient was noted. Detection of linearity was done with taking the data.
In Vitro Cytotoxicity Studies:
The cell culture studies were performed on DU-145 cell line (human grade II prostate adenocarcinoma cells) which was conducted by Indian Institute of Science Education and Research, Berhampur, Odisha. After receiving them in lyophilised vials, the cells were cultured in tissue culture flasks and maintained at 37 °C in an incubator with 52% CO2. Dulbecco's Modified Eagle's medium, foetal bovine serum, and growth media with penicillin and streptomycin were regularly given to the cells.
Cellular Cytotoxicity:
The DU-145 (10 × 100 cells/ well) were sown in 96-well plates and allowed to attach to the wells for 24 hours. The cells were then incubated in the normal culture medium with blank, drug-loaded NLC, and free drug suspension. Following a 72-hour incubation period, the cells were rinsed with phosphate buffer saline and then treated for 4 hours with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye. To dissolve the formazan crystals, the cells were treated with dimethyl sulfoxide after being once again cleaned with phosphate buffer saline. At 595 nm, the absorbance of the cells was evaluated in comparison to the control group of untreated cells. The percentage absorbance of treatment groups relative to the control was used to express the cell viability. IC50 values (μg/ mL) were calculated using GraphPad Prism software.
Results
Selection of Suitable Solid Lipid, Liquid Lipid.
The selection of solid lipid was done based on the evaluation of the maximum solubility. Figure 1 illustrates that ABA's saturation solubility data in several solid lipids showed that glyceryl monostearate had the drug's greatest solubility than in Compritol 888 ATO, and the solubility data is mentioned in table no. Higher solubility in glyceryl monostearate could be attributed to the presence of long chain triglycerides and hence used for the preparation of NLC. Figure 1B demonstrates that the liquid lipid, Labrasol exhibited maximum solubility of ABA and minimum in Captex 200. Figure 1C demonstrates that the surfactant, Transcutol P exhibited maximum solubility of ABA and minimum in Tween 80.
Percentage Transmittance
Surfactants were selected under the observation of percentage transmittance. A higher percentage transmittance correlates to the smaller sized particles. It was found that the combination of both surfactant tween 20 and Transcutol p has higher percentage transmittance (i.e. 40.65) than the individual results as mentioned in the Table 2. The combination of surfactants is always better to produce smaller sized nanoparticles. Therefore, the combination of surfactants was selected.
Entrapment Efficiency of ABA Loaded NLC.
Graph shown on Figure 2 shows that all the formulations have better entrapment efficiency i.e. more than 90 % on Table 3 which can be attributed to the proper selection of solid lipid, liquid lipid, surfactant and optimum processing conditions of homogenization time and rpm.
In Vitro Release Study:
From the graph on Figure 3, it was observed that all the formulations showed release of drug for 12h. Table 4 shows that an initial release of 20% was observed for all formulations (f1-f6) which can be due to the presence of unentrapped drugs and drugs present on the surface of NLC. Solid lipid in the concentration range of 300 to 350mg is sufficient to produce sustain release for 12h. Hence all the formulations have the potential to sustain the drug release for 12h.
Particle Size, Polydispersity Index and Zeta Potential Analysis:
From the graph in Figure 4A, it was observed that among all six formulations, f1 exhibited lowest particle size i.e. 37.92nm. Table 5 shows that a NLC formulation with Particle size less than 100 nm qualifies to be called a nano formulation. PDI value was lowest for the formulation F6 i.e. 0.209 which indicates that the particle size distribution was uniform for F6. However, PDI Value less than 0.5 can be considered as a uniform size distribution. Higher the Zeta potential either +ve or -ve, better is the stability of the NLC. In our research, formulation F1, F3 & F6 showed higher Zeta potential hence their formulation will prevent coagulation of the nanoparticles during storage[33]. Zeta potential of F1 is shown in Figure 4B.
Differential Scanning Calorimetry (DSC):
Both pure drug and lyophilized NLC were undergone differential scanning calorimetry and from the table no. it was found that ABA in NLC formulation has a melting point of 145˚C and the sharp peak with slight deviation indicates ABA is still in crystalline form. The Figure 5 shows that lyophilized NLC exhibited a nearly similar narrow range of melting with Onset and End-set temperatures of 138ºC & 150ºC respectively. The enthalpy of lyophilized NLC slightly increased from -40.9 to -48.49 mJ.
X-Ray Diffraction (XRD)
Both pure drug and Lyophilized NLC were undergone X-Ray diffraction study. From the Figure 7 it was observed that the pure drug exhibited high intensity peak at various 2Ɵ angles such as 12, 15, 17, 18, 20, 27, 32. For lyophilized NLC, i.e. F1 exhibited nearly linear intensity peaks at 2Ɵ angles like 13, 15, 17, 21, 27, 32. This suggests that there were no major changes in the crystallinity of ABA drug preparation of NLC & its lyophilization.
In-Vivo Pharmacokinetic Study:
From the Figure 8, it was observed that there was no peak of ABA obtained or observed during the UFLC method of rat serum. After completion of animal studies with blood withdrawn at 1,4,6,8,12h. Again, the experiment was repeated at same time points, but no peak was observed. Instead of ABA’s peak, there were peaks of unknown compounds observed at 4.5min in the chromatogram. So, to assure that if there is any chance of getting a peak of ABA by i.v. does a study was performed on rats by administering them ABA by i.v. route. And as per the Figure 9, it shows that there were peaks with minimal area that were obtained after the administration of ABA through i.v. route and blood withdrawn at a time point of 15min. This observation clearly shows that ABA is present in very low quantity in free form.
Cytotoxicity Study of ABA Loaded NLC:
The in vitro cytotoxicity study performed by MTT assay in Du-145 expressed higher cytotoxicity for NLC formulation which was concentration dependent. The cell viability data shown in Figure 10, reveals that NLC showed IC50 value at concentration 2ng/mL, while free drug suspension showed an IC50 value at 100 ng/mL (p < 0.001). Nearly 50-fold reduction in IC50 value of the drug abiraterone was observed from NLC indicated higher efficacy of NLC on castration resistant DU-145 prostate carcinoma cells. The p value was <0.001 at all concentrations starting at 0.002 ug to 0.1 ug. This may be explained by the increased permeability, absorption, and retention of NLC in cancer cells, which results in a high level of cytotoxic action that lowers cell survival.
Discussion
For the preparation of NLC we need a model that can deliver some characteristics such as higher drug loading, greater entrapment efficiency, enhanced drug release with minimizing the drug leakage which may lead to dose dumping. All the mentioned characteristics were found in the multiple type of Nano structured lipid carrier[
7]. So multiple type was selected over other two types of NLC i.e., imperfect crystal model and amorphous model. A mixture of solid and liquid lipids along with combined surfactant and water was prepared for multiple type NLC after the screening of all those excipients.
The screening test resulted in selection of GMS as solid lipid because of having maximum drug absorption & a greater melting point which attributed slower crystallization and larger particle size that leads to sustained release of drug [
34]. Because it dissolved a higher concentration of drug than other liquid lipids and had the potential to increase drug loading capacity in the liquid core of NLC, minimise drug ejection during manufacturing, increase solubility, and allow for controlled drug release from the matrix, Labrasol was chosen as the liquid lipid.
Percentage transmittance was conducted for the screening of surfactants, resulted in the selection of Tween 20 and Transcutol P both as combination surfactants. Because of the smaller sixed nanoparticle production, this combination contributed to a smaller particle size. Since the initial formulation was an oil-in-water emulsion, both of their HLB values helped to lower interfacial tension, which could result in a reduction in particle size at the same energy level. Likewise, the interfacial tension and particle size of NLC were associated with the zeta potential and entrapment efficiency[
35].
The ABA NLC formulation exhibited a significant release, which was sustained for 12 hours. This could be attributed to the choice made for the selection of solid lipid, liquid lipid, and surfactants of adequate concentrations. Similarly, the selection made the formulation to be grouped under nano formulation as it has particle size less than 100nm. The zeta potential in selected formulation was in higher side shows better stability and the poly dispersity index showing uniform size distribution of particles as it is less than 0.5[
36].
There is no interaction between drug and excipient observed as the DSC graph shows, negligible change in enthalpy of the lyophilized NLC which can also pursue the thermodynamic stability of the NLC[
37]. The FTIR data similarly shows no signific interaction in between the drug and excipients. And in case of XRD, the pure drug and lyophilized NLC shows no major changes in their crystallinity throughout the preparation of NLC[
15,
38].
As the ABA has a high percentage of protein binding (99%) with the Glycoprotein (89.4%) and serum albumin (95.6-99.9%) so there is a very less quantity of free drug present to detect after oral administration. An observation says from a reported article that ABA has high clearance of 2hr after i.v. Dose. That could be the probable reason for the absence of ABA NLC peaks in further chromatogram of the in vivo studies. But after a successful i.v. administration, the drug showed very few but clear peaks in the UFLC which was sufficient to prove the release and absorbance of ABA after i.v. dose but not enough to show (to obtain a Cmax & AUC plot) maximum serum obtained concentration.
DU-145 was selected for the cytotoxicity study and after a successful recovery, seeding and drug treatment, the ABA NLC showed higher cytotoxicity against all other control and blank formulation. Neary 50-fold reduction of cell viability was observed as the concentration of drug in NLC was increasing, which also represents how the cytotoxicity was concentration dependent. In the end the p value <0.001 referred that the experiment is statistically significant[
39].
Conclusion
From the above research, it may be concluded that NLC formulation of ABA has the potential to produce improved anticancer activity. Use of GMS, Labrasol, Tween 20: Transcutol p (1:1) As SL, LL, Surfactant respectively was found to be suitable for formulation of NLC with desirable particle size i.e. below 100nm and higher zeta potential -18.3 mV. The selected formulation was lyophilized successfully by using lactose as cryoprotectant for improved long-term stability. Although the in vivo study shows an insignificant result, but it refers to the higher protein binding and clearance rate of ABA. Invitro cytotoxicity study in Du-145 showed higher cytotoxicity for NLC formulation. The cytotoxicity of ABA in NLC formulation was dose dependent. Hence the process of development of NLC formulation for ABA can be considered as a suitable approach. NLC formulation can also be extended for other API of similar nature. Further research may be carried out to improve the solubility, bioavailability in the same formulation with these research findings.
Credit authorship contribution statement
Mounik Rout: Writing- Review & Editing, Visualization, Validation, Formal Analysis, Data Curation, Conceptualization. Ch. Niranjan Patra: Validation, Supervision, Project Administration, Methodology, Funding Acquisition, Conceptualization.
Acknowledgements
The authors would like to thank the Roland Institute of Pharmaceutical Sciences, Brahmapur, for supporting this research work.
Declaration of competing interest
The authors declare that they have no conflict of interests.
References
- Salvi, V.R. and P. Pawar, Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. Journal of Drug Delivery Science and Technology, 2019. 51: p. 255-267. [CrossRef]
- Pokharkar, V., A. Patil-Gadhe, and P. Palla, Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: In-vivo pharmacokinetic and toxicity study. Biomedicine & pharmacotherapy, 2017. 94: p. 150-164. [CrossRef]
- Müller, R.H., K. Mäder, and S. Gohla, Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. European journal of pharmaceutics and biopharmaceutics, 2000. 50(1): p. 161-177.
- Iqbal, M.A., et al., Nanostructured lipid carriers system: recent advances in drug delivery. Journal of drug targeting, 2012. 20(10): p. 813-830.
- Puccetti, M., et al., Engineering carrier nanoparticles with biomimetic moieties for improved intracellular targeted delivery of mRNA therapeutics and vaccines. Journal of Pharmacy and Pharmacology, 2024. 76(6): p. 592-605. [CrossRef]
- Hu, F.-Q., et al., Preparation and characteristics of monostearin nanostructured lipid carriers. International journal of pharmaceutics, 2006. 314(1): p. 83-89. [CrossRef]
- Chauhan, I., et al., Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Advanced pharmaceutical bulletin, 2020. 10(2): p. 150. [CrossRef]
- Thakur, A., et al., Abiraterone acetate in the treatment of prostate cancer. Biomedicine & Pharmacotherapy, 2018. 101: p. 211-218. [CrossRef]
- Hoy, S.M., Abiraterone acetate: a review of its use in patients with metastatic castration-resistant prostate cancer. Drugs, 2013. 73(18): p. 2077-2091. [CrossRef]
- Schultz, H.B., et al., Oral formulation strategies to improve the bioavailability and mitigate the food effect of abiraterone acetate. International journal of pharmaceutics, 2020. 577: p. 119069. [CrossRef]
- Rehman, Y. and J.E. Rosenberg, Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug design, development and therapy, 2012: p. 13-18.
- Yu, A., et al., Demonstrating Bioequivalence for Two Dose Strengths of Niraparib and Abiraterone Acetate Dual-Action Tablets Versus Single Agents: Utility of Clinical Study Data Supplemented with Modeling and Simulation. Clinical Pharmacokinetics, 2024. 63(4): p. 511-527. [CrossRef]
- Efstathiou, E., et al., Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. Journal of clinical oncology, 2012. 30(6): p. 637-643. [CrossRef]
- Zhou, H., P. Xu, and Y. Sun, Pharmacokinetics of abiraterone acetate released from a tablet based on lipid matrix in beagle dogs. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 2021. 76(7): p. 308-312. [CrossRef]
- Liu, Y., et al., Development of abiraterone acetate nanocrystal tablets to enhance oral bioavailability: formulation optimization, characterization, in vitro dissolution and pharmacokinetic evaluation. Pharmaceutics, 2022. 14(6): p. 1134. [CrossRef]
- Hoo, C.M., et al., A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. Journal of Nanoparticle Research, 2008. 10: p. 89-96. [CrossRef]
- Singh, B. and S. Beg, Quality by design in product development life cycle. Chronicle Pharmabiz, 2013. 22: p. 72-79.
- Chadha, R. and S. Bhandari, Drug–excipient compatibility screening—role of thermoanalytical and spectroscopic techniques. Journal of pharmaceutical and biomedical analysis, 2014. 87: p. 82-97. [CrossRef]
- Gardouh, A.R., et al., Influence of formulation factors on the size of nanostructured lipid carriers and nanoemulsions prepared by high shear homogenization. International journal of pharmacy and pharmaceutical sciences, 2018: p. 61-75. [CrossRef]
- Bhatt, H., et al., Development of curcumin-loaded solid lipid nanoparticles utilizing glyceryl monostearate as single lipid using QbD approach: characterization and evaluation of anticancer activity against human breast cancer cell line. Current drug delivery, 2018. 15(9): p. 1271-1283. [CrossRef]
- Sakellari, G.I., et al., Formulation design, production and characterisation of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the encapsulation of a model hydrophobic active. Food hydrocolloids for health, 2021. 1: p. 100024. [CrossRef]
- Gardouh, A.R., et al., Design and characterization of glyceryl monostearate solid lipid nanoparticles prepared by high shear homogenization. British journal of pharmaceutical research, 2013. 3(3): p. 326-346.
- Souto, E., W. Mehnert, and R. Müller, Polymorphic behaviour of Compritol® 888 ATO as bulk lipid and as SLN and NLC. Journal of microencapsulation, 2006. 23(4): p. 417-433. [CrossRef]
- Aburahma, M.H. and S.M. Badr-Eldin, Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert opinion on drug delivery, 2014. 11(12): p. 1865-1883. [CrossRef]
- Nnamani, P.O., et al., Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. International journal of pharmaceutics, 2014. 477(1-2): p. 208-217. [CrossRef]
- Shevalkar, G. and P. Vavia, Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. Journal of drug delivery science and technology, 2019. 53: p. 101211. [CrossRef]
- Dudhipala, N. and K.Y. Janga, Lipid nanoparticles of zaleplon for improved oral delivery by Box–Behnken design: optimization, in vitro and in vivo evaluation. Drug development and industrial pharmacy, 2017. 43(7): p. 1205-1214.
- Lalan, M., et al., Developmental studies of curcumin NLCs as safe alternative in management of infectious childhood dermatitis. Nanoscience & Nanotechnology-Asia, 2020. 10(4): p. 390-403. [CrossRef]
- Kudupudi, V., et al., Formulation Development and Characterization of Vancomycin Hydrochloride Colon-Targeted Tablets Using In-Situ Polyelectrolyte Complexation Technique. International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN), 2023. 16(3): p. 6533-6545. [CrossRef]
- da Fonte, P.R.M.L., Optimization of lyophilization parameters of polymeric nanoparticles for delivery of therapeutic proteins. 2016.
- Beg, S., et al., Systematic development of solid lipid nanoparticles of abiraterone acetate with improved oral bioavailability and anticancer activity for prostate carcinoma treatment. ACS omega, 2022. 7(20): p. 16968-16979. [CrossRef]
- Sarangi, D.K., et al., In vivo assessment, formulation, characterization and enhancing pharmacotherapy of encapsulated mini tablets for immediate release Sildenafil citrate and sustained release Bosentan. Results in Chemistry, 2024. 9: p. 101652. [CrossRef]
- Kamel, K.M., et al., Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: extract standardization, nanoparticle optimization, and cytotoxicity evaluation. Journal of Agricultural and Food Chemistry, 2017. 65(36): p. 7966-7981. [CrossRef]
- Viegas, C., et al., Solid lipid nanoparticles vs. nanostructured lipid carriers: a comparative review. Pharmaceutics, 2023. 15(6): p. 1593. [CrossRef]
- Konatham, S. and S. Patangay, Abiraterone acetate loaded solid lipid nanoparticles for improved oral bioavailability: Design of experiments based formulation optimization, in vitro, ex-vivo and in vivo characterization. Int. J. Appl. Pharm, 2023. 15(2): p. 131-139. [CrossRef]
- Lunardi, C.N., et al., Experimental methods in chemical engineering: Zeta potential. The Canadian Journal of Chemical Engineering, 2021. 99(3): p. 627-639. [CrossRef]
- Singh, M., S.o. Lara, and S. Tlali, Effects of size and shape on the specific heat, melting entropy and enthalpy of nanomaterials. Journal of Taibah University for Science, 2017. 11(6): p. 922-929. [CrossRef]
- Pochapski, D.J., et al., Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir, 2021. 37(45): p. 13379-13389. [CrossRef]
- Wagner, E.D. and M.J. Plewa, CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: an updated review. Journal of Environmental Sciences, 2017. 58: p. 64-76. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).