1. Introduction
Increases in circulating interleukin-6 (IL-6) and its soluble receptor indicate the transition from acute to chronic inflammation [
1]. Determination of IL-6 in human plasma or serum has been a long-standing tool in the diagnosis of chronic inflammatory diseases such as rheumatoid arthritis [
1]. By now, it also plays a role in the clinical management of various other etiologies, including cancer [
2], cardiovascular disease and atherosclerosis [
3], Covid-19 progression [
4,
5], and the post-acute Covid-19 vaccination syndrome [
6]. In these and other inflammatory diseases, sensitivity, specificity and the diagnostic value of IL-6 is considered superior to more conventional inflammation markers such as C-reactive protein or acute phase proteins. The growing demand on IL-6 determination in routine health care has prompted the development of new assays suitable for point of care testing and high throughput measurements [
7,
8]. Despite the widespread implementation of IL-6 measurements in medical laboratories, pre-analytic interferences and disturbances of IL-6 measurements are poorly defined.
The few available publications on IL-6 pre-analytics provide rather ambiguous information. It has been reported that IL-6 is stable in human serum at ambient temperature for up to 11 days [
9]. While reassuring, this observation does not address interferences acting between the acquisition of a blood sample and the separation of liquid phase from cellular components. That time period commonly addressed as pre-centrifugation delay is the least well controlled segment of the analytical cycle. Published observations indicate, that prolonged pre-centrifugation delay encompassing the exposure of the uncentrifuged blood sample to ambient temperature for several hours will alter the results of IL-6 determinations significantly. However, it is unclear in which manner the results are thereby affected. On the one hand, it has been reported that IL-6 values were lowered, when serum samples were kept at 20 °C for three hours before centrifugation [
10]. On the other hand, it has been reported, that IL-6 levels were increased upon preincubation (25 °C, 4 h) before centrifugation [
11]. Interestingly, the latter effect was observed in serum but only to a lesser degree in ethylenediamine tetraacetic acid (EDTA) plasma [
11].
To sum up, prolonged pre-centrifugation delay could be a significant confounder of IL-6 measurements, possibly engendering false-high or false-low values. It remains unclear which of these opposing effects prevails. It could be that not all blood specimen are similarly susceptible to interference by pre-centrifugation delay with IL-6 determination. However, heparin plasma, the blood specimen most commonly used in Germany in health care of critical ill patients, has not been investigated in this respect. Here, we validate the impact of pre-centrifugation delay on IL-6 determinations in heparin plasma and other blood specimen commonly used in routine laboratory diagnostic.
2. Materials and Methods
2.1. Sample Collection
Left-overs of heparin plasma, serum, EDTA-plasma and glycolysis-stabilized EDTA-plasma (containing 1 mM NaF) submitted to the laboratory for routine medical diagnostics were used for all experiments and analyses. Based on case records, patient samples were classified as critical ill with sepsis (N = 3 females, N = 6 males, mean/ median age = 61/ 58 years), or critical ill without sepsis (N = 16 females, N = 30 males, mean/ median age = 61/ 65 years). Patients were assumed critically ill when subjected to intensive care. These cases included recovery from cardiac (N = 36) or abdominal surgery (N=6), end-stage cancer excluding leukemia (N = 11), acute infection and severe autoimmune-disease (one each). Sepsis was diagnosed according to the ICD-10 classification based on repeated positive blood cultures. Left-overs of diagnostic samples from healthy blood donors (N = 29 females, N = 12 males, mean/ median age = 39/35,5 years) submitted to laboratory during blood donation served as controls. All samples were anonymized before inclusion. Clinical trial protocols were approved by the local ethics board of Heinrich-Heine University Düsseldorf (study number 2023-2681). The investigation conforms with the principles outlined in the World´s Medical Association Declaration of Helsinki. The study reflects a quality control study according to DIN EN ISO 12189 stipulated by the German accreditation agency (DAkks).
2.2. Laboratory Procedures
Blood samples were collected by cubital vein puncture using the Vacutainer® system (Becton Dickinson GmbH, Heidelberg, Germany). The following types of blood specimen were tested: Serum, heparin-plasma, EDTA-plasma, and EDTA-plasma containing 1mM NaF. All samples were subjected to centrifugation (4000 x g, 10 min) within 30 min, and IL-6 was measured in the supernatant by an accredited routine laboratory diagnostic procedure (Elecsys IL-6) implemented on a Cobas 8000 analyzer (both Roche Diagnostics, Mannheim, Germany). Before centrifugation, samples were facultatively subjected to cell lysis by ultra sound treatment using a Bandelin Sonoplus HD 2070 Ultraschall-Homogenisator (1 2 GmbH & Co. KG, Berlin, Germany) or to preincubation (20 °C, 24 - 72 h). Preincubation was done with and without addition of lipopolysaccharide (LPS) from Escherichia coli strain O55:B5 or phytohemagglutinin (PHA) from Phaseolus vulgaris (both SIGMA-ALDRICH, St. Louis, USA). Stock solutions of LPS and PHA were diluted with phosphate buffered saline (GIBCO/Fisher Scientific, Schwerte, Germany).
2.3. Statistics
Graph Pad Prism 9 (Graph Pad Software, Inc., San Diego, California, USA, Graph Pad Prism 9 for Apple Macintosh, released 2020) was used for analysis. Normal distribution was tested by the Shapiro-Wilk test. Non-normally distributed data are presented by median values and interquartile-ranges. Differences between groups were analyzed by the Mann-Whitney-U test (two-tailed). For all tests statistical significance was assumed at p < 0.01.
3. Results
3.1. Pre-Incubation of Uncentrifuged Heparin Plasma at 20 °C Can Increase IL-6 Several-Fold
Based on the interleukin-8 paradigm [
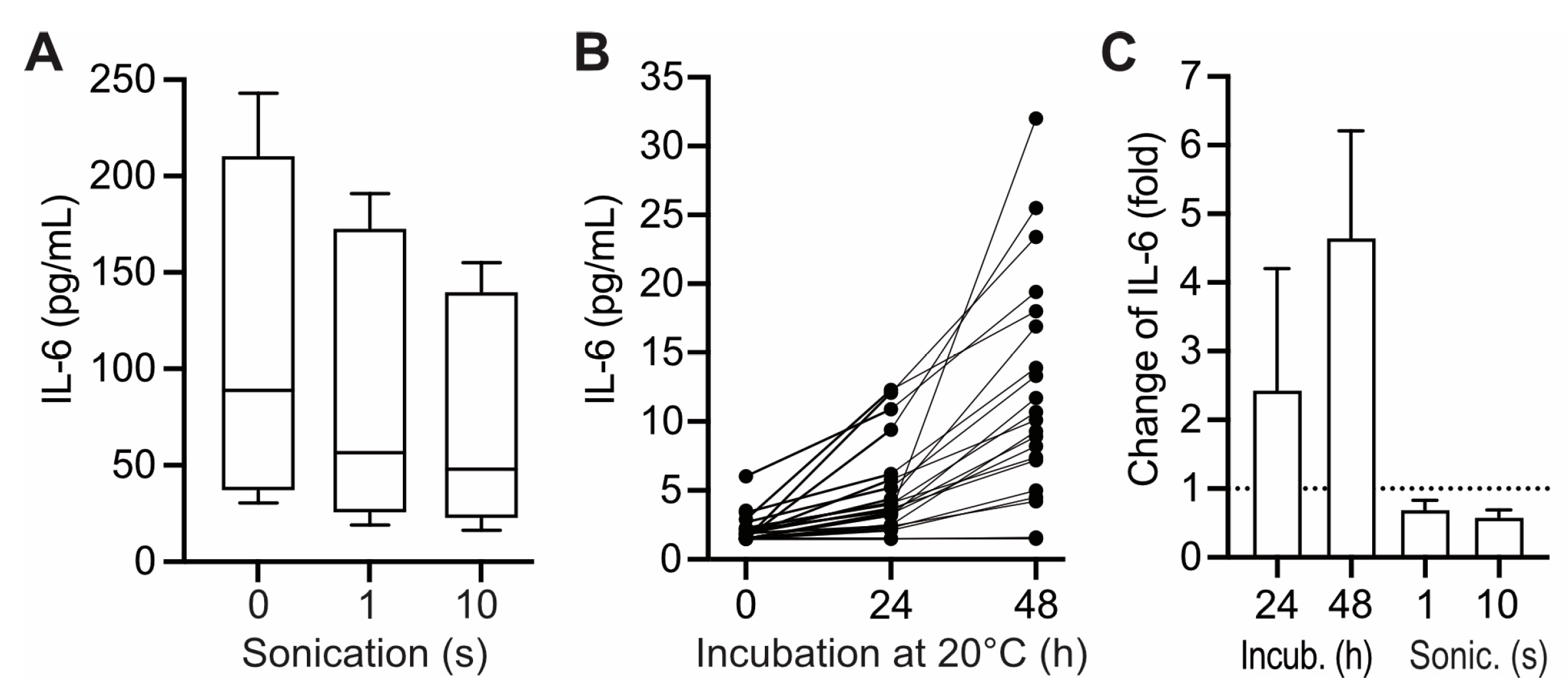
12], we envisioned that upon prolonged pre-centrifugation delay IL-6 possibly increases in the liquid phase of blood samples due to release from cell binding in the course of hemolysis. To test this hypothesis, hemolysis was induced in uncentrifuged heparin plasma by ultrasound treatment. To increase sensitivity, the experiment was carried out with samples of critical ill patients exhibiting increased IL-6 values. Induction of hemolysis was confirmed by increase in potassium and lactate dehydrogenase and decrease of haptoglobine (data not shown). However, hemolysis was not correlated to significant increases in IL-6 in the supernatant. On the contrary, IL-6 levels dropped slightly upon prolonged cell disruptive treatment (
Figure 1 A,
1C). Hemolysis-induced release of IL-6 from cell binding was thus excluded as a mechanism interfering with IL-6 measurements in human blood samples.
Alternatively, we imagined that IL-6 levels could become increased during prolonged pre-centrifugation delay due to synthesis and incretion by lymphocytes present in the sample [
13]. To test that hypothesis, heparin plasma was incubated at 20 °C before centrifugation and IL-6 was subsequently determined in the supernatant. The test was carried out on heparin plasma from healthy controls in order to exclude possible interference with the results by disease-related pre-conditioning of lymphocytes [
14,
15]. In most samples IL-6 values increased upon preincubation in a manner correlated to the duration of preincubation (effects up to 48 h shown in
Figure 1B and C). The size of that effect exhibited considerable inter-individual variance: Following a pre-centrifugation delay of 24 h (considered relevant for clinical settings), 19 of 21 tested samples exhibited significant (1.5- to 4.2-fold) increases in IL-6 (
Figure 1C). However, in three of the tested samples, pre-incubation for more than 24 h was required to obtain relevant effects on subsequent IL-6 measurements, while in two samples IL-6 measurement was unaffected by preincubation for up to 48 h (
Figure 1B).
3.2. IL-6 Is Stimulated in Uncentrifuged Heparin Plasma by Addition of LPS and PHA
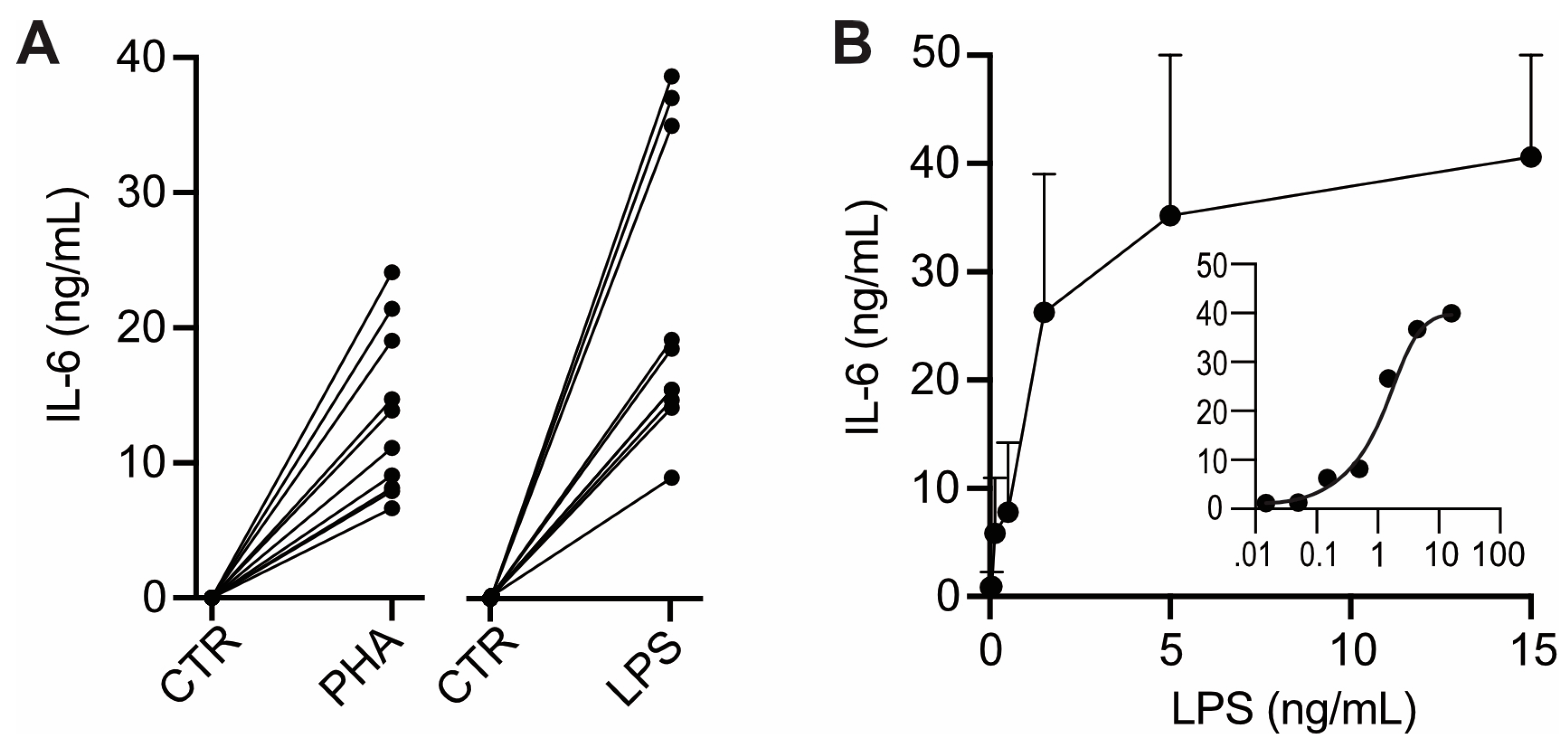
IL-6 production can be stimulated in isolated human white blood cells by experimental exposure to pro-inflammatory bacterial toxins such as LPS and PHA [
13,
16]. We used that approach to test whether the lymphocytes present in un-centrifuged heparin plasma are similarly capable of responding to experimental exposure to pro-inflammatory stimuli. Upon addition of PHA or LPS to uncentrifuged heparin plasma IL-6 increased considerably (
Figure 2A). These IL-6 increases were about 1000-fold higher than those following pre-incubation without pro-inflammatory stimuli (compare
Figure 2A with
Figure 1B, please note that in
Figure 2 IL-6 is given in ng/mL). The ED
50 of LPS in heparin plasma (≅ 2 ng/mL) (
Figure 2B, insert) was similar to that previously determined in isolated white blood cells [
16]. These observations indicate that lymphocytes present in heparin plasma exhibit a normal IL-6 response to pro-inflammatory stimuli. Therefore, in vitro synthesis and incretion is a plausible source of the IL-6 increases inducible by preincubation of uncentrifuged heparin plasma (
Figure 1B).
3.3. Critical Illness or Sepsis Is Not Correlated to IL-6 Increases Inducible by Preincubation
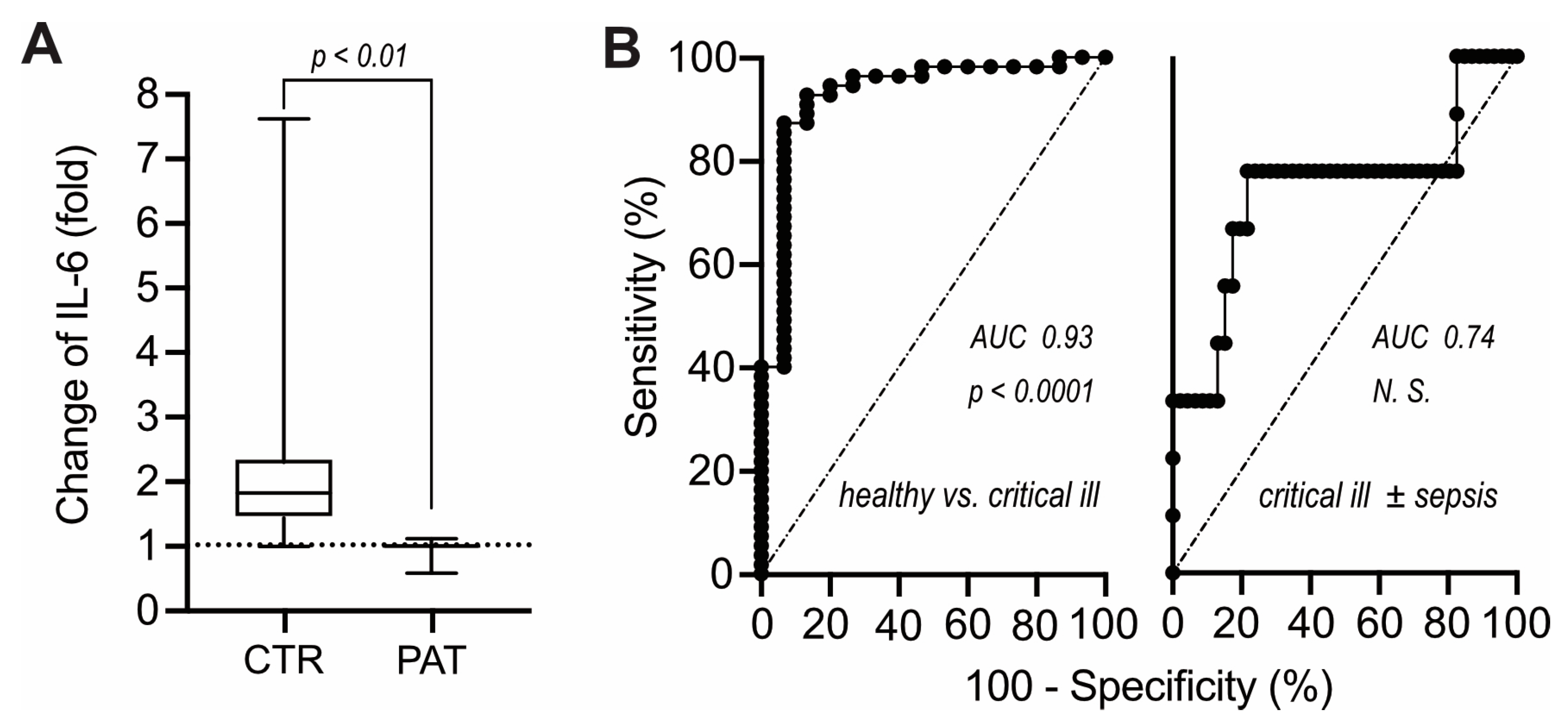
Given the responsiveness of IL-6 production in heparin plasma to exogenous LPS, we considered that pro-inflammatory stimuli present in blood samples of critical ill patients could possibly be detected via increases of IL-6 following preincubation before centrifugation. However, contrary to expectations, heparin plasma of critical ill patients failed to exhibit any increases in IL-6 upon pre- incubation for 24 h (
Figure 3A). That lack of IL-6 response clearly discriminated critical ill patients from healthy individuals (
Figure 3B, left) and conceivably reflects sepsis-associated T-cell dysfunction [
14,
15]. However, critical ill patients with and without septicemia could not be discriminated in this fashion (
Figure 3B, right) and lack of increase in IL-6 upon pre-incubation was not correlated to diminished leukocyte counts and/or lowered lymphocyte fraction (not shown).
3.4. IL-6 Determination in EDTA-Plasma Is Unaffected by Pre-Centrifugation Delay up to 24 h
Similar IL-6 increases as observed here in heparin-plasma upon prolonged pre-centrifugation delay (
Figure 1), have recently also been observed in serum, whereas IL-6 determination in EDTA-plasma seemed less affected by pre-centrifugation delay [
11]. Therefore, the type of blood specimen used for determination of circulating IL-6 could play a role for the validity of the results in cases where centrifugation has been delayed. To follow up on this notion, the impact of pre-centrifugation delay (20 °C, 24 h) on IL-6 determination was compared between the various types of blood specimen commonly employed in german health care (serum, heparin-plasma, EDTA-plasma, and EDTA-plasma containing 1 mM NaF).
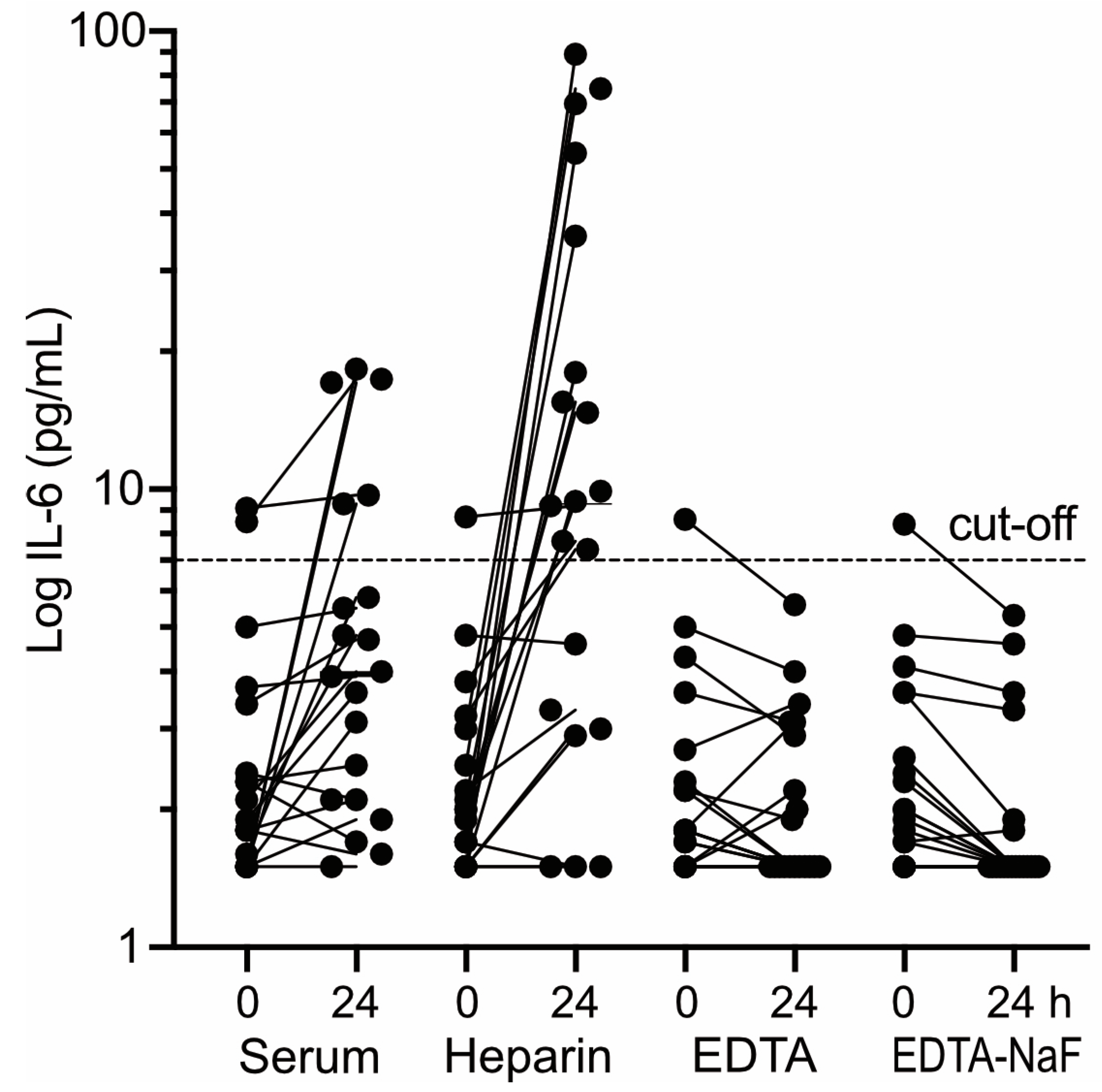
The comparison was performed on blood samples of presumably healthy controls (10 males, 14 females, age 29 – 65, median 43) from whom all four types of blood specimen had been gathered from a single vein puncture. Aliquots of the samples were either centrifuged immediately (within 20 min) or after preincubation (20 °C, 24 h). Subsequently, IL-6 was determined in the supernatants. The impact of the simulated centrifugation delay on IL-6 determination differed markedly between the four types of blood specimen (
Figure 4). Most pronounced effects were observed in heparin plasma (
Figure 4, second panel from left): In 17/20 heparin plasma samples tested, IL-6 increased up to 50-fold following preincubation. Moreover, in 12/20 heparin plasma samples preincubation lifted initially normal IL-6 values above the confidence limit. A similar albeit less pronounced effect was seen in serum (
Figure 4, leftmost panel): In 15/20 sera tested IL-6 values increased several-fold upon preincubation. However, only in four of these cases initially normal values were lifted by preincubation above the confidence limit. A completely opposite observation was made with EDTA-plasma (
Figure 4, third panel from left): Preincubation caused only very minor alterations of IL-6 and in none of the EDTA-plasma samples tested normal IL-6 values were raised above the confidence limit due to the simulated pre-centrifugation delay. Similar results were obtained with NaF-stabilized EDTA-plasma (
Figure 4, rightmost panel).
4. Discussion
4.1. Salient Findings
Lymphocytes retain in heparin plasma a considerable capability to produce IL-6 in response to microbial stimulators;
Several-fold increases in IL-6 in heparin plasma after prolonged centrifugation delay are most probably caused by ongoing in vitro-synthesis;
IL-6-increases due to centrifugation delay are less frequent/pronounced in heparin-plasma of critical ill patients;
IL-6-increases due to centrifugation delay are less frequent/pronounced in serum;
Pre-incubation of EDTA-plasma at 20 °C for 24 h can entail a drop of IL-6;
4.2. Limitations
The number of patients and controls studied was comparatively small, however, the observed effects were sufficiently large to allow unambiguous conclusions;
IL-6 increases following extended centrifugation are assumed due to ongoing in-vitro synthesis. That assumption is not supported by direct evidence such as pulse-chase experiments, but it seems to be the most plausible explanation for the observed phenomena;
Pre-incubation induced IL-6 decreases in EDTA-plasma were not studied in samples exhibiting increased levels of IL-6;
4.3. Discussion
It is known for some time that results of IL-6 determination in peripheral blood can vary considerably depending on the time span between sample collection and centrifugation, which prompted the recommendation to “centrifuge tubes quickly following collection“ [
10] and to adhere to “a standard blood sample handling procedure” [
11] when measuring IL-6 in human blood for diagnostic purposes. These recommendations are valuable and worth respecting. However, they are of little help when pre-centrifugation delay cannot be controlled or avoided, for instance because a distant laboratory is commissioned with the measurements. To obtain a handling guideline for the latter situation, we have investigated the impact of pre-centrifugation delay on IL-6 values in various specimen of peripheral venous blood.
The results here presented suggest that the risk of confounding IL-6 values by extensions of pre-analytic time-span can be avoided or minimized by choosing an appropriate type of blood specimen. In heparin-plasma or serum, extended pre-centrifugation delay induced increases in IL-6 in about 85 % of the cases. The increases were quite substantial (up to 50-fold and 12-fold in heparin-plasma and serum, respectively) and caused normal IL-6 values to increase above the confidence limit in 12/20 heparin plasma samples and 4/20 sera. Thus, IL-6 determinations in heparin-plasma and serum carry a substantial risk of yielding false-positive results, if time-lapses between sample collection and centrifugation are uncertain. Moreover, that risk is higher in healthy subjects than in critical ill patients, which further compromises the disease-discriminative power of the marker.
In EDTA-plasma, the risk of false-positive IL-6 results due to extended pre-centrifugation delay appears negligible. Preincubation (20 °C, 24 h) before centrifugation increased IL-6 values only moderately (less than 2-fold) and infrequently (4/20 samples). In no case normal IL-6 values were thereby pushed above the confidence limit. However, more than half of the EDTA-plasma samples (12/20) exhibited a notable drop of IL-6 (up to 30 %) following preincubation, which in one case moved an increased IL-6 value below the confidence limit. Tthese observations suggest that extended pre-centrifugation delay possibly enhances the risk of false-negative IL-6 determinations in EDTA-plasma. So far, that issue has not been definitely resolved, since in this and previous studies [
10,
11] the impact of preincubation on IL-6 values has only been investigated in EDTA-plasma exhibiting normal IL-6 levels. Moreover, in one of the previous studies [
11] preincubation had no effect on subsequent IL-6 determinations. Consequently, such investigations would need to be repeated with EDTA-plasma from patients suffering from chronic inflammatory disease or severe illness, in order to properly gauge the clinically relevant risk to provoke by prolonged pre-centrifugation delay false-negative IL-6 results in EDTA plasma.
5. Conclusions
When the time span between collection and centrifugation of blood samples is extended to several hours, IL-6 frequently becomes vastly overestimated in serum or heparin-plasma entailing a high rate of false positive results. In contrast, IL-6 can be mildly underestimated in EDTA-plasma upon delayed centrifugation, which however entails false-negative results in < 10% of cases. The lesser confounding effect of delayed centrifugation suggests EDTA-plasma as the blood specimen of choice for IL-6 determination, most notably when the time to centrifugation is unknown or inevitably prolonged.
Author Contributions
Conceptualization, Hannah Sauerwein, Derik Hermsen and Fritz Boege; Data curation, Hannah Sauerwein, Derik Hermsen, Johannes Fischer and Fritz Boege; Formal analysis, Hannah Sauerwein, Derik Hermsen, Detlef KIndgen-Milles, Erik Michael, Johannes Fischer and Fritz Boege; Investigation, Hannah Sauerwein and Derik Hermsen; Methodology, Hannah Sauerwein, Derik Hermsen, Erik Michael, Johannes Fischer and Fritz Boege; Project administration, Derik Hermsen and Fritz Boege; Resources, Hannah Sauerwein, Derik Hermsen, Detlef KIndgen-Milles, Erik Michael, Johannes Fischer and Fritz Boege; Supervision, Detlef KIndgen-Milles, Erik Michael, Johannes Fischer and Fritz Boege; Validation, Hannah Sauerwein, Derik Hermsen, Detlef KIndgen-Milles, Erik Michael, Johannes Fischer and Fritz Boege; Visualization, Derik Hermsen and Fritz Boege; Writing – original draft, Fritz Boege; Writing – review & editing, Hannah Sauerwein, Derik Hermsen, Detlef KIndgen-Milles, Erik Michael, Johannes Fischer and Fritz Boege.
Institutional Review Board Statement
Clinical trial protocols were approved by the local ethics board of Heinrich-Heine University Düsseldorf (study number 2023-2681). The investigation conforms with the principles outlined in the World´s Medical Association Declaration of Helsinki.
Informed Consent Statement
In agreement with the local ethics board of Heinrich-Heine University Düsseldorf, patient consent was waived because the study serves a quality control measure according to DIN EN ISO 12189 as stipulated by the German accreditation agency (DAkks), which was performed with leftovers of diagnostic samples completely anonymized before analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Technical help is gratefully acknowledged to Olexander Weinstein and Cengül Caglar.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EDTA |
Ethylenediamine tetraacetic acid |
| IL-6 |
Interleukin 6 |
| LPS |
Lipopolysacharids |
| PHA |
Phytohemagglutinin |
References
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006, 8 Suppl 2, S3. [CrossRef]
- Rose-John, S. Local and systemic effects of interleukin-6 (IL-6) in inflammation and cancer. FEBS Lett 2022, 596, 557-566. [CrossRef]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev 2014, 22, 147-151. [CrossRef]
- Sebbar, E.H.; Choukri, M. Interleukin 6: A biomarker for COVID-19 progression. Mater Today Proc 2023, 72, 3351-3355. [CrossRef]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis. J Clin Immunol 2021, 41, 11-22. [CrossRef]
- Mundorf, A.K.; Semmler, A.; Heidecke, H.; Schott, M.; Steffen, F.; Bittner, S.; Lackner, K.J.; Schulze-Bosse, K.; Pawlitzki, M.; Meuth, S.G.; et al. Clinical and Diagnostic Features of Post-Acute COVID-19 Vaccination Syndrome (PACVS). Vaccines (Basel) 2024, 12. [CrossRef]
- Majdinasab, M.; Lamy de la Chapelle, M.; Marty, J.L. Recent Progresses in Optical Biosensors for Interleukin 6 Detection. Biosensors (Basel) 2023, 13. [CrossRef]
- Szymanska, B.; Lukaszewski, Z.; Oldak, L.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. Two Biosensors for the Determination of Interleukin-6 in Blood Plasma by Array SPRi. Biosensors (Basel) 2022, 12. [CrossRef]
- Kenis, G.; Teunissen, C.; De Jongh, R.; Bosmans, E.; Steinbusch, H.; Maes, M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine 2002, 19, 228-235.
- Verberk, I.M.; Nossent, E.J.; Bontkes, H.J.; Teunissen, C.E. Pre-analytical sample handling effects on blood cytokine levels: quality control of a COVID-19 biobank. Biomark Med 2021, 15, 987-997. [CrossRef]
- Gong, Y.; Liang, S.; Zeng, L.; Ni, Y.; Zhou, S.; Yuan, X. Effects of blood sample handling procedures on measurable interleukin 6 in plasma and serum. J Clin Lab Anal 2019, 33, e22924. [CrossRef]
- Reinsberg, J.; Dembinski, J.; Dorn, C.; Behrendt, D.; Bartmann, P.; van Der Ven, H. Determination of total interleukin-8 in whole blood after cell lysis. Clin Chem 2000, 46, 1387-1394.
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40-47.
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013, 13, 862-874. [CrossRef]
- Wang, Z.; Zhang, W.; Chen, L.; Lu, X.; Tu, Y. Lymphopenia in sepsis: a narrative review. Crit Care 2024, 28, 315. [CrossRef]
- Morris, M.C.; Gilliam, E.A.; Button, J.; Li, L. Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J Biol Chem 2014, 289, 21584-21590. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).