Introduction
When emphasizing the value of planktonic foraminifera for studies in (paleo)oceanography,
Brummer and Kučera (
2022) remarked on the need for robust operational taxonomies and the value of benchmarking morphological species concepts. ‘Operational taxonomy’ was a primary concept at the inception of the numerical approach to taxonomy (e.g.,
Sokal and Sneath 1963;
Sokal and Camin 1965). Those authors viewed operational taxonomy as one providing contestable statements about species and higher-level groups. How this translates in practice rests with a taxonomist’s view of species. Their taxonomy (e.g.,
Sneath and Sokal 1963) operated on relationships of discrete characters of individuals subjectively selected. It is advocated here that the primary step in an operational taxonomy for a morphospecies is to investigate whether a given named sample of a taxon represents a single population (
Scott 2024) based on its important traits. This interpretation is applied to
Globorotalia (
Turborotalia)
oceanica Cushman and Bermudez 1949 which was proposed for specimens found in a seafloor sample off Cuba. Currently, this taxonomic species is rejected by
Brummer and Kučera (
2022) as a member of the modern planktonic fauna on the ground that it is a synonym of
Truncorotalia crassaformis. That assessment is reviewed from an analysis of the axial shape of living and Holocene specimens from the tropical Atlantic Ocean and Caribbean Sea previously identified as
Truncorotalia crassaformis. Also noted is the axial shape of early Pliocene specimens. Holocene tropical Atlantic samples are compared with one from the Holocene southwest Pacific taken to represent
Truncorotalia crassaformis in the sense of Scott (2023).
Material & Methods
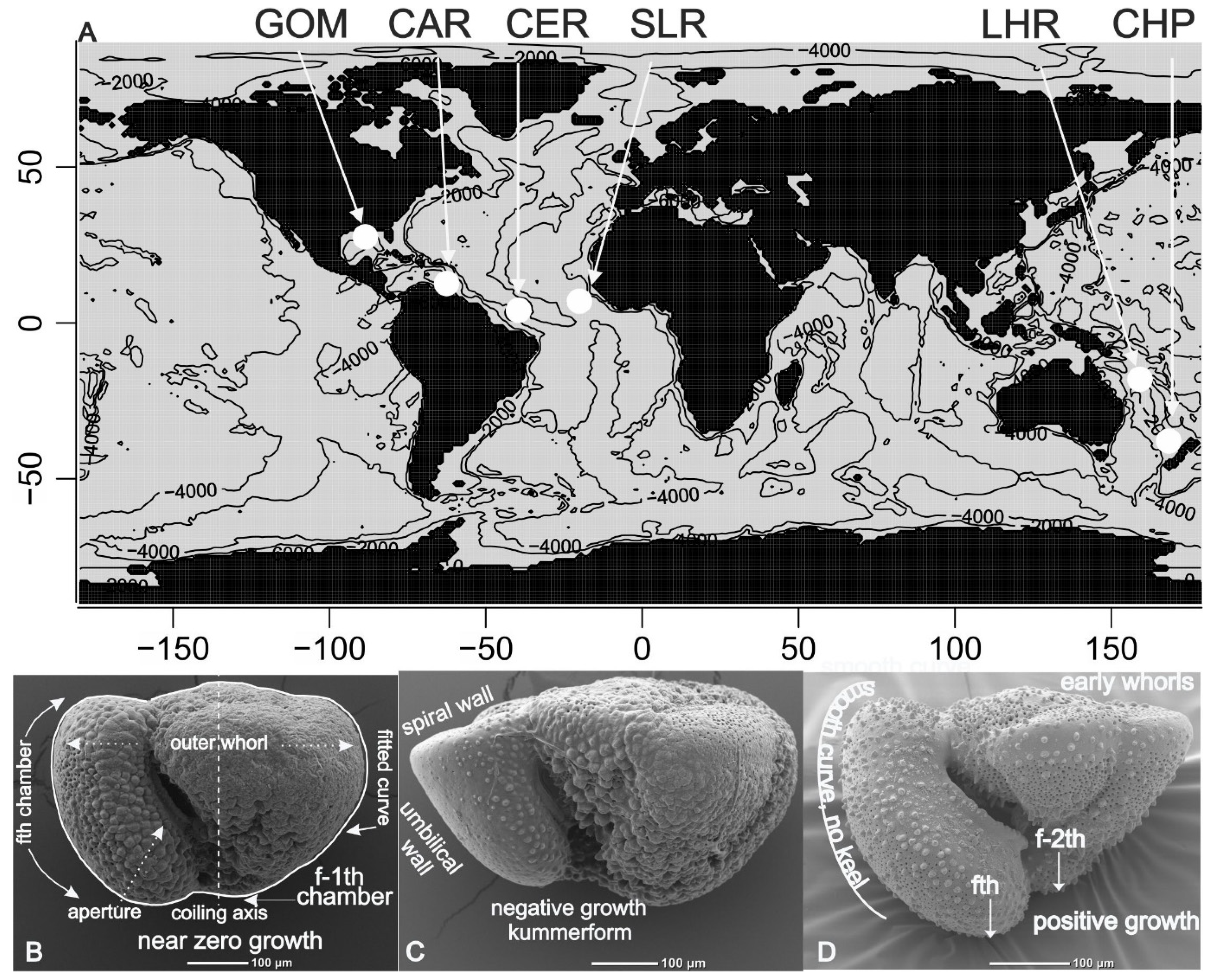
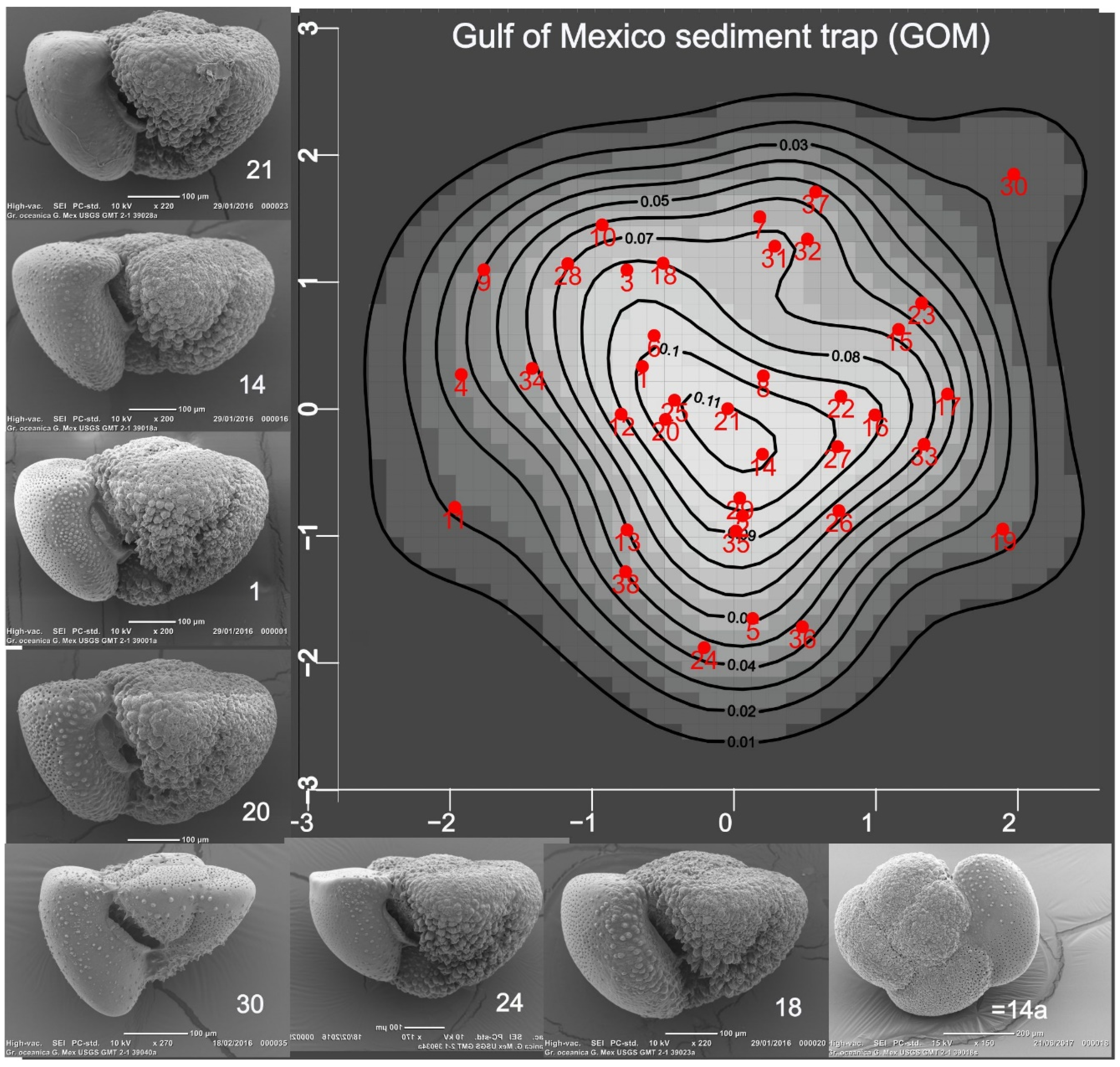
Gulf of Mexico sediment trap (GOM): This trap (
Figure 1) is a time series (2008–2012) of foraminiferal and particulate flux at 700 m on the northern Gulf of Mexico continental shelf (27.5° N; 90.3° W). Dr. Caitlin Reynolds supplied 38 specimens (212 μm–425 μm fraction) from the GMT21 sediment trap (
Richey et al. 2014). Specimens were collected between 21–27 April, 2008.
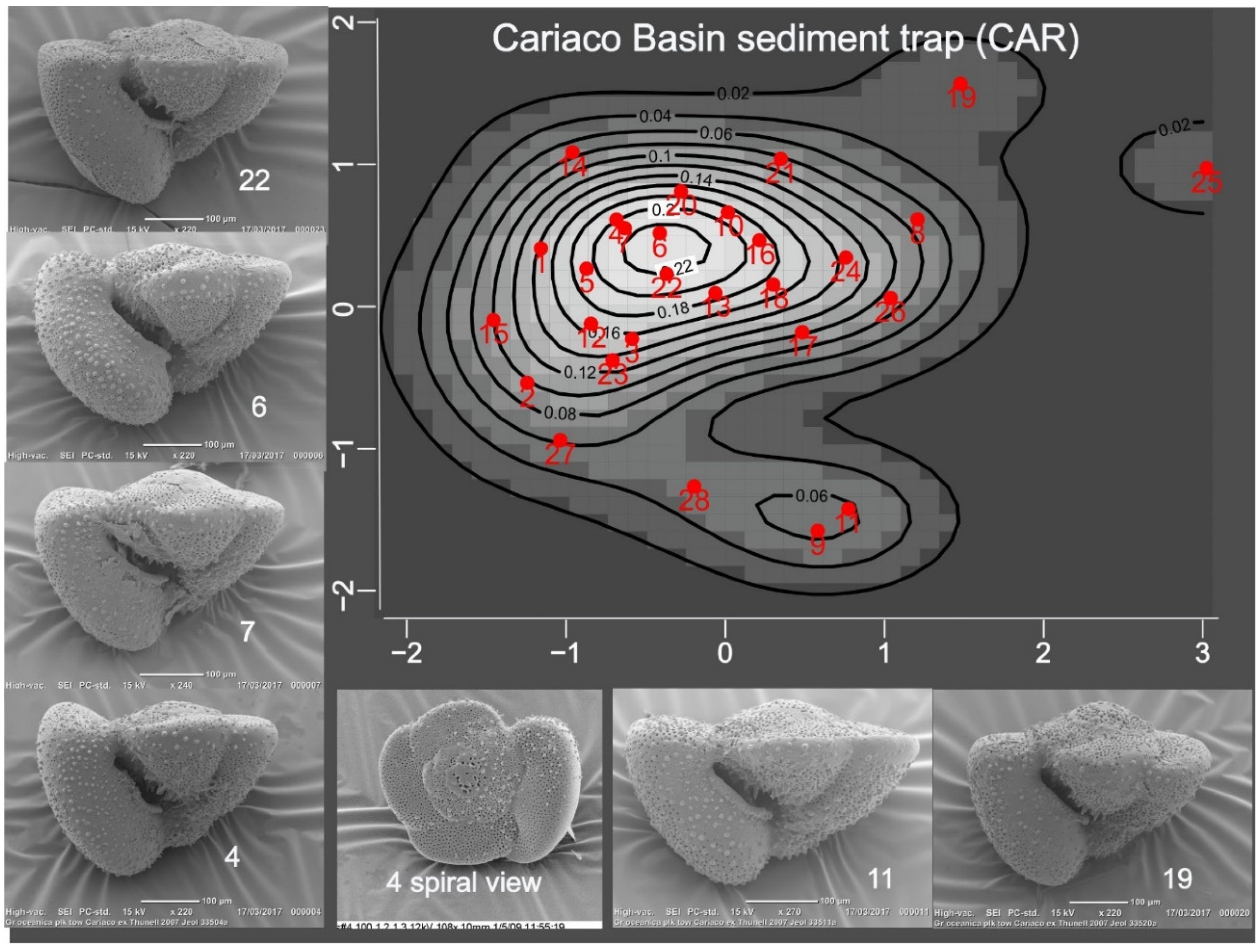
Cariaco Basin sediment trap (CAR): Dr. Robert Thunell supplied 29 specimens of
Truncorotalia crassaformis collected in a sediment trap at 150 m in Cariaco Basin (
Figure 1) in January 2007.
Tedesco and Thunell (
2003) give details of its location, sampling procedures, and species fluxes. This anoxic basin is on the continental shelf, separated from the Caribbean by a sill at about 150 m.
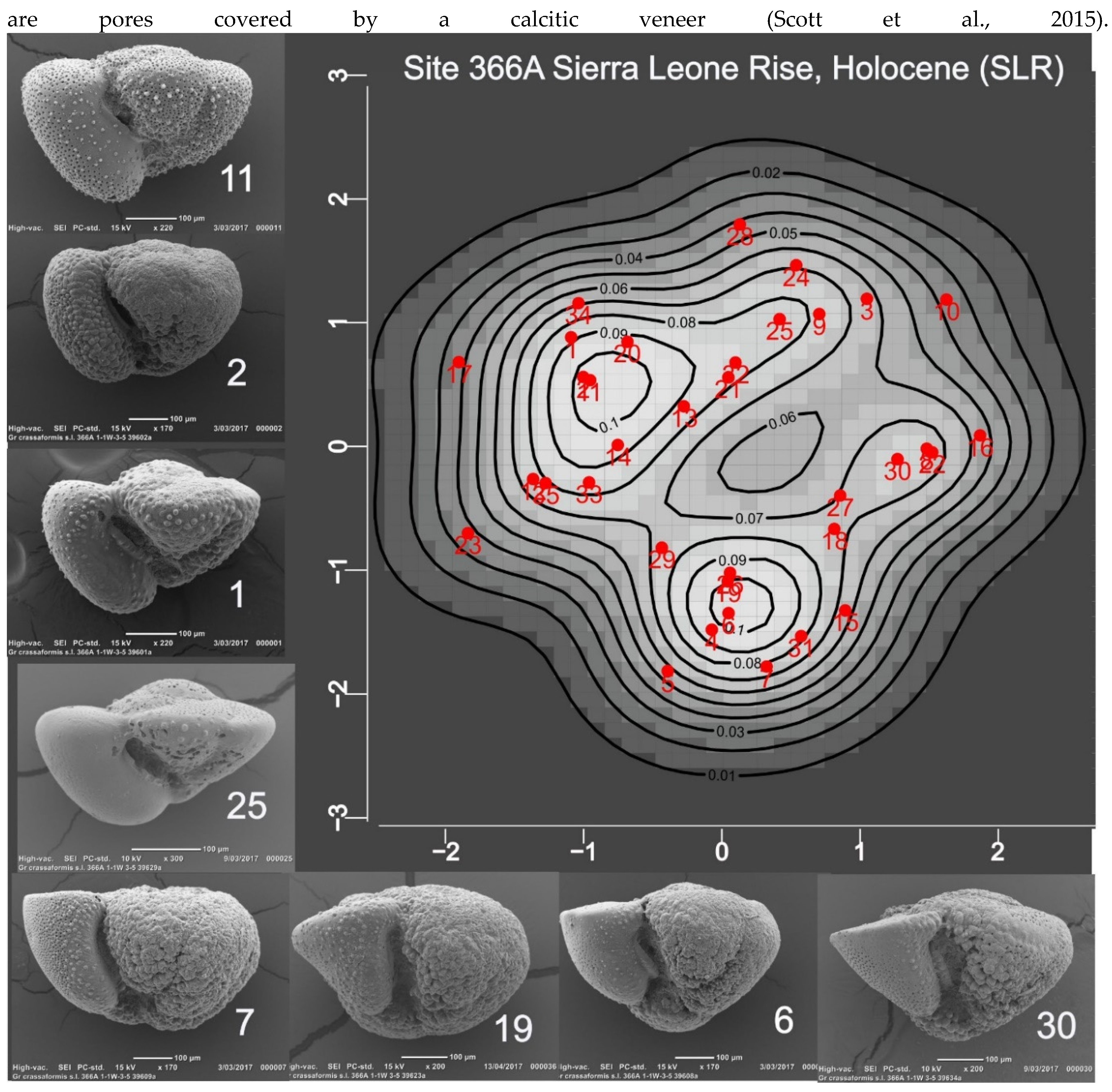
DSDP Site 366A 1-1W-3-5 cm. (SLR): This site (05 40.7° N; 19 51° W; 2853 mbsf,
Figure 1) is on the Sierra Leone Rise in the eastern tropical Atlantic and lies under the Equatorial Counter Current. It is near Core 234 examined by Lidz (1972). From the model of Lazarus et. al. (1995), the age of the sampled horizons is <3 kyr. Thirty-five specimens of
Truncorotalia crassaformis s.l. from the >149 μm fraction were randomly sampled.
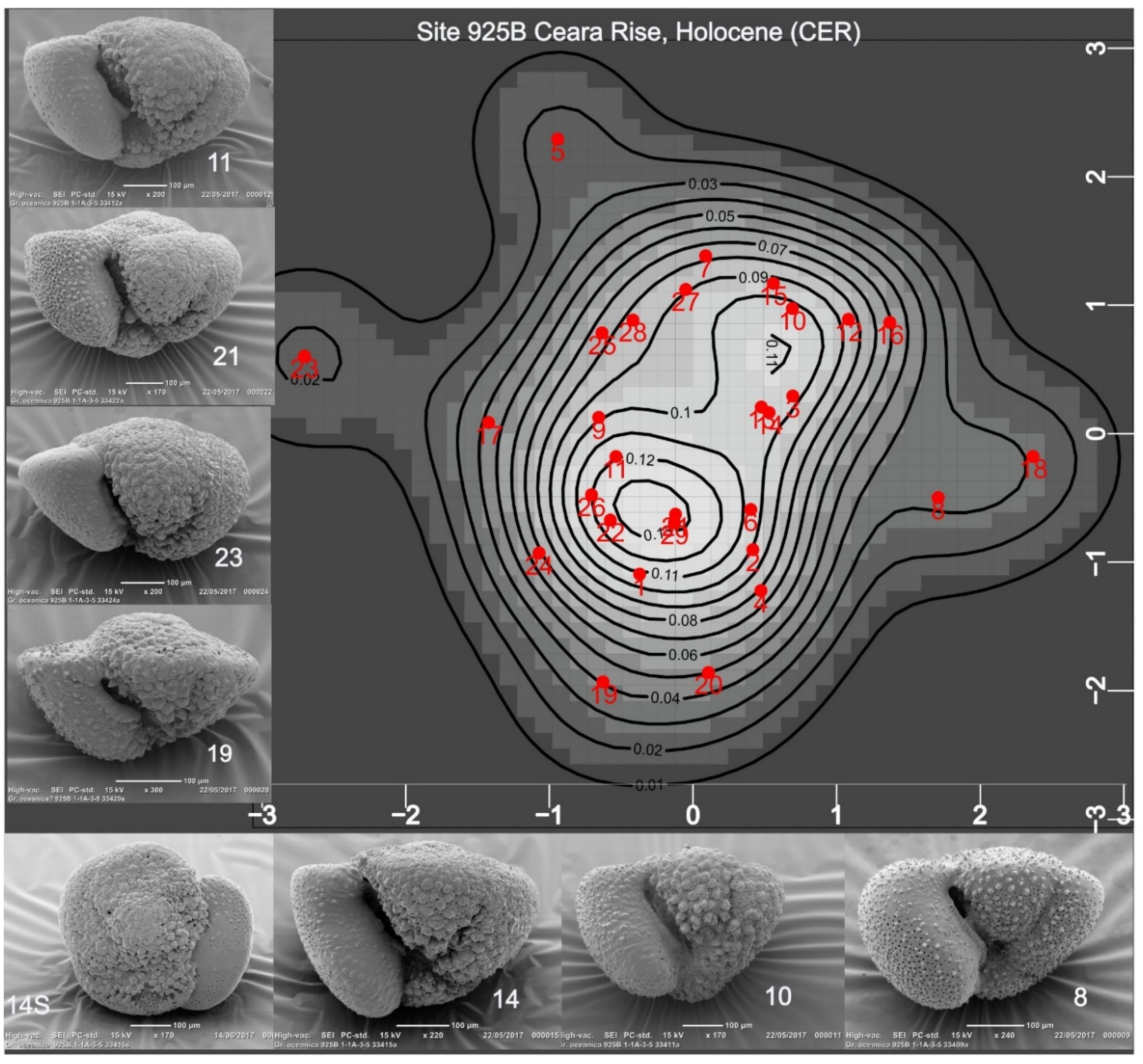
ODP Site 925B (CER) is on the Ceara Rise in the western equatorial Atlantic Ocean (04 12.12.2° N; 43 29.3° W; 3053 mbsf,
Figure 1). A random sample of 29 specimens was taken by the author from the >149 µm fraction of core 1H-1A-3–5. From the model of
Chaisson and Pearson (
1997, Table 1) the age of the sampled horizons is c. 2 kyr.
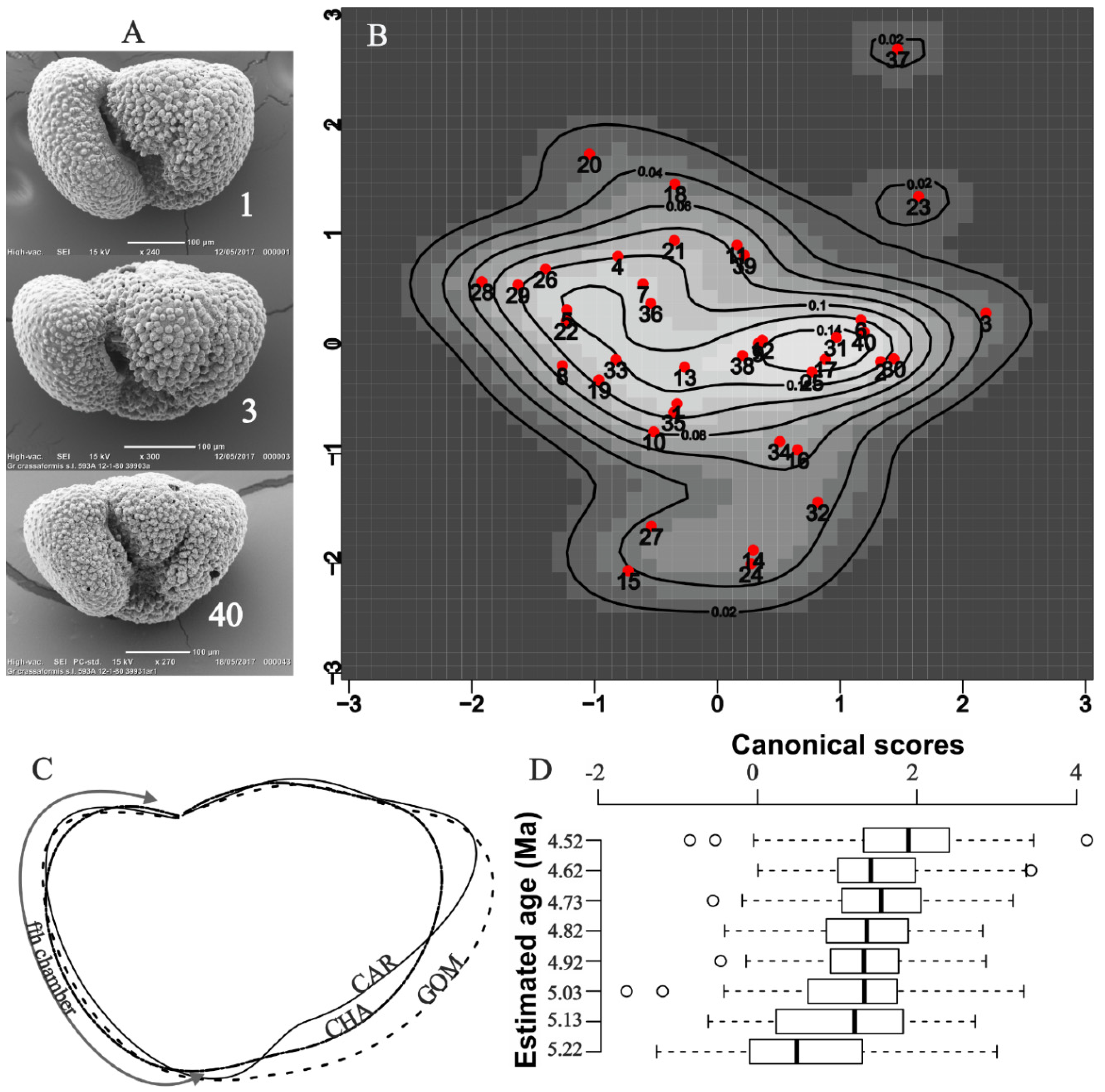
DSDP Site 593A (CHP) is on the Southwest Pacific Challenger Plateau (40 30.47° S; 167 40.47° E; 1079 mbsf,
Figure 1) west of New Zealand. A random sample of 40 specimens was taken by the author from the >149 µm fraction of core 12-1-80. From integrated biostratigraphic and magnetostratigraphic data Cooke (2002) dated this horizon at 4.73 Ma.
DSDP Site 588 (LHR) is on the Southwest Pacific Lord Howe Rise (26°06.7'S; 161°13.6'E), 1533 mbsf,
Figure 1) east of Queensland, Australia. A sample of 26 specimens was taken by the author from the >149 µm fraction of core 588-1-1-7-9. From the model of
Barton and Bloemendal (
1986) the age of the sampled horizon is <5 kyr.
Methods
Truncorotalia crassaformis s.l. builds a trochoidal shell by incremental addition of ~15 chambers that expand isometrically and are arranged in a low spiral of about 3 whorls. A view of the outline in the plane of the coiling axis (
Figure 1F–H) is highly informative as it encapsulates much of the ontogeny, including rate of whorl translation (height of early whorls), gross radial/axial dimensions and the axial extension of late-formed chambers (conical form). The significance of this trait is shown by its iterative evolution over the past 65 Myr (
Cifelli 1969).
As the primary focus of the investigation is the integrity of samples drawn from single populations, two-dimensional density maps (
Weglarczyk 2018; function kde2d in R package MASS,
https://CRAN.R-project.org/package=MASS) show the distribution of specimens in the PC1:2 shape space. The function fits a bivariate normal probability model to each individual and their distribution in the sample is contoured.
Results
The density (=heat) maps for outline data (
Figure 2,
Figure 3,
Figure 4 and
Figure 5 ) provide graphical evidence based on frequency data (as in a histogram) of the structure of each collection, considered as a sample from a bivariate normal shape population. The analysis is sensitive to gross dimensions, axially and radially, and to changes in curvature around the periphery. Tests for conformity with a normal model (
https://CRAN.R-project.org/package=MVN) show that PC1 and PC2 distributions are univariate normal (p>0.05) for all samples, but only GOM and CER are bivariate normal at that level. Although CAR has several distant outliers, its shape most closely resembles that of a bivariate normal population. The dead specimen samples (CER, SLR) are characterized by multiple, weakly defined, lower probability peaks. Placement of specimens within each shape model are discussed in the captions for
Figure 2,
Figure 3,
Figure 4 and
Figure 5. The pooled samples are analysed in
Figure 6.
Discussion
The axial shape of trochoidal planktonic foraminiferal shells is a highly informative trait for representing their morphology. It encapsulates the entire growth history of individuals, whereas the spiral view provides only a cross-section of the shell at the termination of growth. Although its value was recognized by Cifelli (1969), who documented the iterative evolution of axial shape over the past 65 Myr, its functional role is still poorly understood.
Caromel et al. (
2014) related shell shape to position in the water column but it might also be a trophic strategy that aids positioning of rhizopodial nets for capture of sinking particles (Slomka et al. 2020). A focus on the normal model is justified because conformity suggests that axial shape is under selection for an optimal form. Nevertheless, specimens flagged by the normal model as unlikely to belong to the statistical shape population might be accepted as part of the taxonomic concept.
Much of the axial shape of specimens refers to growth of the 4 chambers forming the outer whorl of the shell and particularly the last (fth) chamber relative to its predecessors in the outer whorl (f-1:3th), A simple ad hoc terminology applicable to the specimen orientation of this study is used (
Figure 1). In all CAR specimens the umbilical face of the fth chamber extends beyond the f-1th: this is designated as representing normal (~isometric) growth. Zero growth refers to specimens in which axial extension of the fth chamber is approximately that of the f-1th. This occurs commonly in GOM, CER and SLR but not in CAR, Negative growth refers to specimens in which axial extension of the fth chamber is clearly smaller than that of the f-1th chamber: often the profile of the fth chamber is distorted and the junction of its umbilical and spiral faces may be angular. This state includes kummerforms of Berger (1969). This condition is also restricted to the deep water GOM, CER and SLR samples.
How these states are interpreted is equivocal. Zero growth suggests that the individual is at, or near, its inflection point in chamber growth (
Hohenegger 2018). Positive growth in individuals with c. 3 whorls might identify adults but whether they are at the inflection point, or pre- or post- reproductive is undetermined. A negative condition indicates growth following the inflection point: it might identify post-reproductive growth (
Morad et al. 2019) but generally it is related to a decline in metabolic rate (
Hohenegger 2018) which might arise from adverse changes in the environment, e.g., oxygen, food, temperature. Perhaps the GOM data are the most informative: at 700 m most specimens show zero or negative growth; those that retain positive growth are rare; many are partially encrusted. From this level up to the chlorophyll maximum may be optimal for this species.
Systematics
Family GLOBOROTALIIDAE Cushman, 1927
Genus Truncorotalia Cushman & Bermúdez, 1949
Type species: Rotalina truncatulinoides d’Orbigny, 1839
Globorotalia (Turborotalia) oceanica Cushman and Bermudez, 1949
Pl. 8, Figs. 13-15
Truncorotalia crassaformis Blow, 1969, pl. 37, fig. 3.
Truncorotalia crassaformis Blow, 1969, pl. 37, fig. 6.
Truncorotalia crassaformis Postuma, 1971, p. 319.
Globorotalia crassaformis Rogl and Bolli, 1973, pl. 7, fig. 7.
Globorotalia crassaformis Be, 1977, pl. 31, fig. 30b.
Globorotalia oceanica Saito, Thompson & Breger, 1981, pl. 44, fig. 1b.
Globorotalia (Truncorotalia) crassaformis Kennett and Srinivasan, 1982, pl. 34, fig. 7.
Globorotalia crassaformis Arnold, 1983, fig. 1 (top).
Globorotalia crassaformis Scott et al., 1990, fig. 62.
Globorotalia crassaformis Bylinskaya, 2005, fig. 5, 5.
Truncorotalia oceanica Boudagher-Fardel, 2012, fig. 6.13.
Globorotalia crassaformis Schiebel & Hemleben, 2017, pl. 2.2.
Truncorotalia crassaformis Bicknell et al., 2018 fig. 1B.
Truncorotalia crassaformis Scott, 2019, fig. 2–5.
Diagnosis
Critical to the recognition of
Truncorotalia oceanica as a taxonomic species is its discrimination from
Truncorotalia crassaformis, described by
Galloway and Wissler (
1927. p. 41) as: “Test rotaliform, dorsal side flat, ventral side convexly rounded, umbilicate, periphery rounded, lobated; chambers, few (usually about four) in the last formed coil, inflated, rapidly increasing in size; sutures distinct, curved, deep, not limbate; very finely perforate; aperture an elongate opening extending from the umbilicus, where it is widest, almost to the peripheral margin and sometimes provided with a narrow lip”.
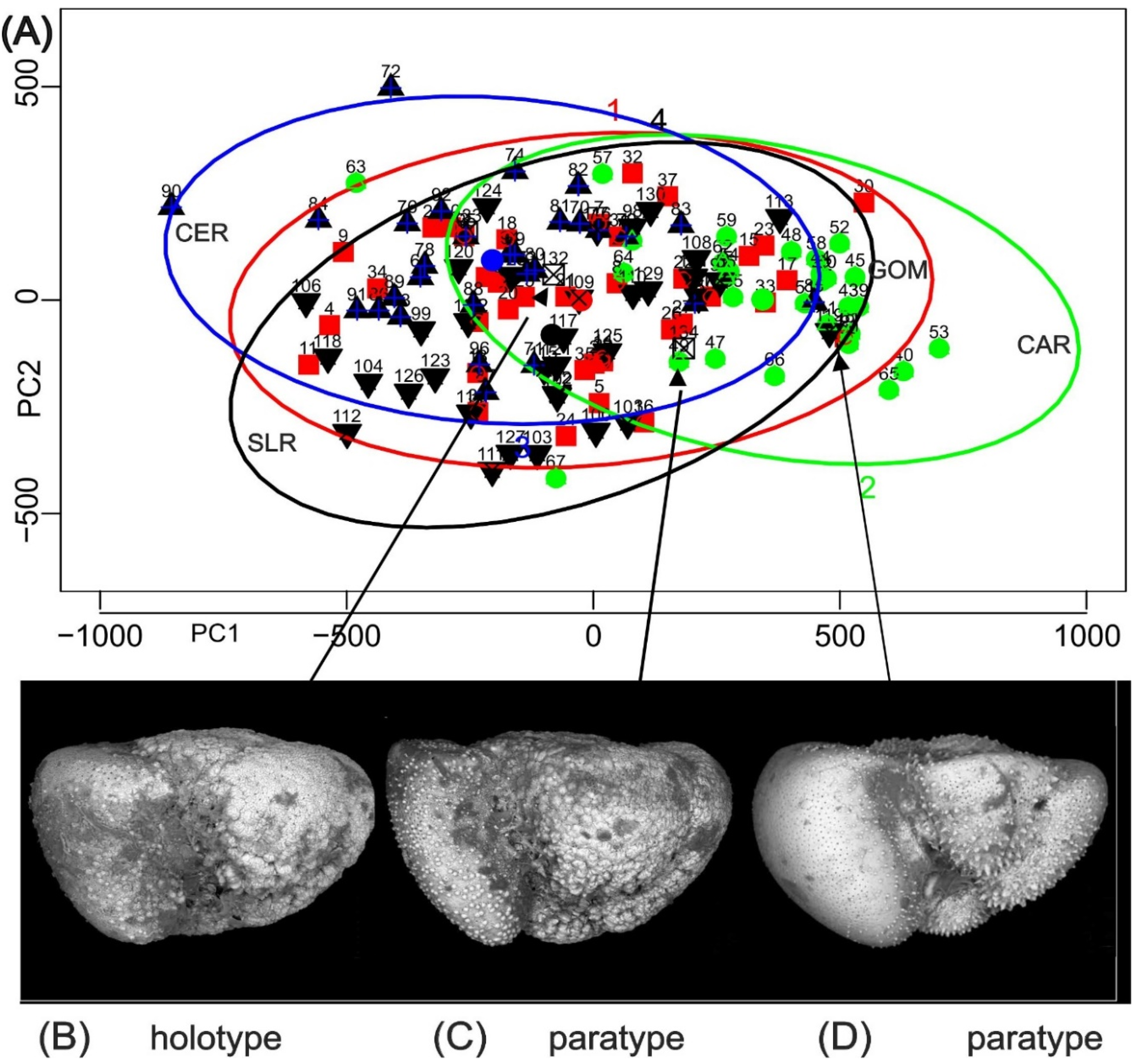
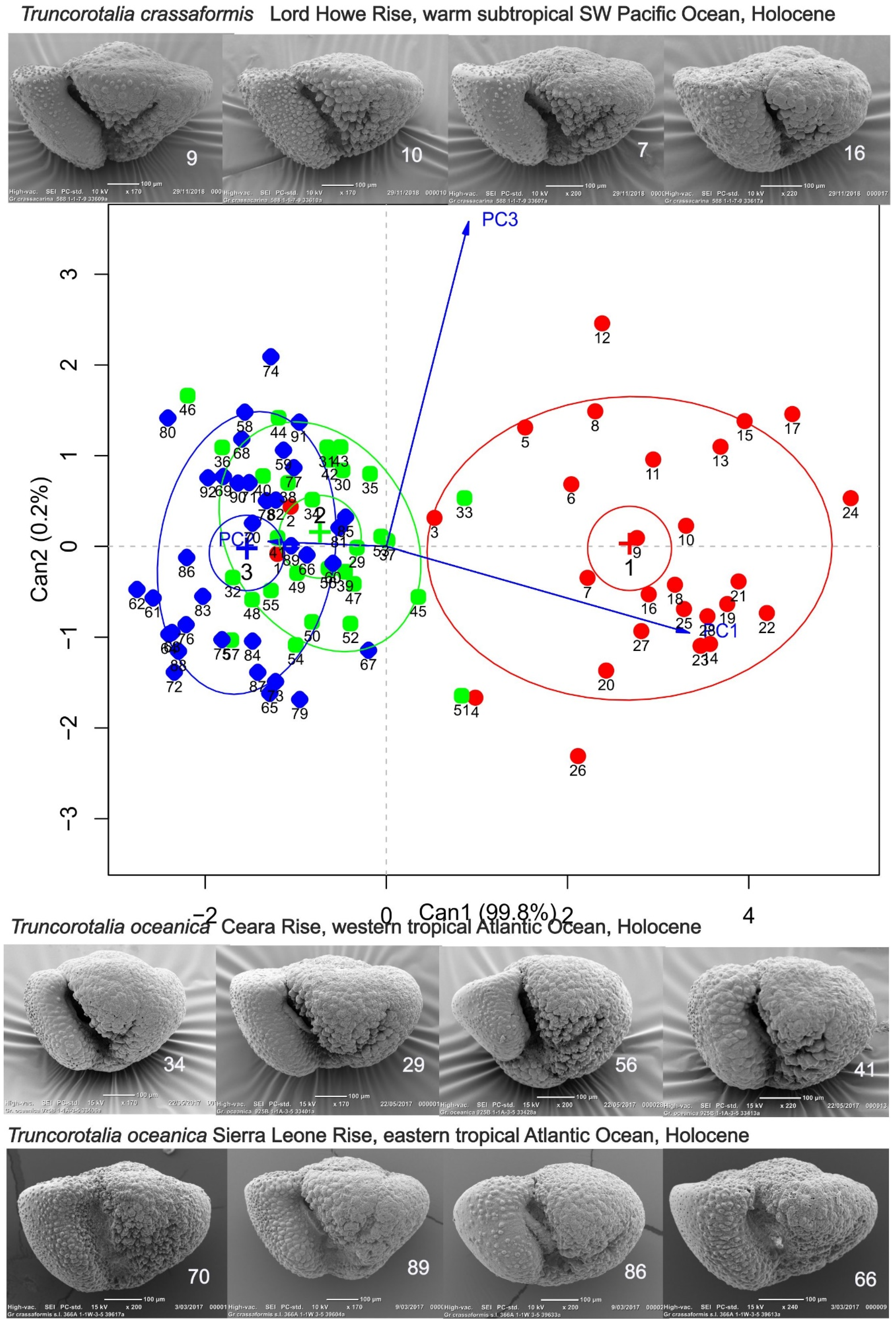
The use of the type locality of
Truncorotalia crassaformis as a standard of comparison is compromised by its allochthonous assemblage (Scott et al., (2015). Here, a Holocene sample from DSDP Site 588 in the warm subtropical Southwest Pacific, which has specimens closely resembling the neotype, is taken to exemplify the species. A discriminant function analysis (
Figure 8) demonstrates the marked contrast in axial shape between Holocene Atlantic and Pacific specimens. The latter are conelike, due to strongly positive growth axially, with a wider angle of coiling; the spiral and umbilical faces meet at an angular junction where a keel forms a topographic ridge.
Conclusion
The morphospecies Truncorotalia oceanica is widely distributed in the Holocene tropical Atlantic Ocean and Caribbean Sea; it has been misidentified as Truncorotalia crassaformis. Although local population integrity is demonstrated, variation in axial shape is complex and is related to growth of the last-formed chamber, and to the depth at which samples are sourced.
The rounded axial profile of late-formed chambers, a feature of early Pliocene populations of Truncorotalia oceanica, is maintained in Holocene – modern tropical Atlantic and Caribbean modern populations, These include a wider range of terminal growth strategies which are reflected in architectures and include specimens with angular malformed terminal chambers, particularly in bathyal populations. Truncorotalia oceanica is promoted as the stem species of the Truncorotalia truncatulinoides clade on the basis of its Pliocene–Holocene record of relatively stable morphology.
Truncorotalia crassaformis is distinguished primarily by its cone-like axial shape and the common presence of a keel at the junction of spiral and umbilical walls of chambers in the outer whorl. Its presence in the Southwest Pacific Holocene suggests that there is much to learn about the biogeography of both species both within (depthwise) and between water masses.
Although data presented here extend the validation of a taxonomic species beyond the basic level of benchmarking, molecular and further morphometric studies have much to contribute to the taxonomic interpretation of Truncorotalia oceanica and Truncorotalia crassaformis in the Holocene: they are closely related morphospecies.
Acknowledgments
I thank Caitlin Reynolds, Julie Ritchie, and the late Robert Thunell for specimens.
Conflicts of Interest
The author declares no conflict of interest.
References
- Arnold, A. J. 1983, Phyletic evolution in the Globorotalia crassaformis (Galloway and Wissler) lineage: a preliminary report: Paleobiology, v. 9, p. 390–397.
- Aze, T., T. G. Ezard, A. Purvis, H. K. Coxall, D. R. M. Stewart, B. S. Wade, and P. N. Pearson. 2011, A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data: Biological Reviews v. 86, p. 900–927.
- Barton, C. B., and J. Bloemendal. 1986, Paleomagnetism of sediments during Leg 90, Southwest Pacific: in Kennett, J. P., von der Borsch, C. C., et al. (eds.), Initial reports of the Deep Sea Drilling Project: U.S. Government Printing Office, Washington, D.C. v. 90, p. 1273–1316.
- Bé, A. W. H. 1977, An ecological, zoogeographic and taxonomic review of Recent planktonic Foraminifera: in Ramsay, A. T. S. (ed.), Oceanic Micropalaeontology: Academic Press, London, vol. 1, p. 1–100.
- Berger, W. H. 1969, Kummerform foraminifera as clues to oceanic environments: American Association of Petroleum Geologists, v. 53, p. 706.
- Bicknell, R. D. C., K. C. Collins, M. P. Crundwell, M. Hannah, and J. S. Crampton. 2018, Evolutionary transition in the late Neogene planktonic foraminiferal genus Truncorotalia: iScience, v. 8, p. 295–303.
- Blow, W. H., 1969, Late middle Eocene to Recent planktonic foraminiferal biostratigraphy: in Brönnimann, P., and Renz, H. H. (eds.), Proceedings of the First International Conference on Planktonic Microfossils: Brill, Leiden, v. 1, p. 199–422.
- Brummer, G. A., and M. Kučera. 2022, Taxonomic review of living planktonic foraminifera: Journal of Micropalaeontology, v. 41, p. 29–74.
- BouDagher-Fadel, M. 2012, Biostratigraphic and geological significance of planktonic foraminifera: Developments in Palaeontology and Stratigraphy, v. 22: Elsevier, New York, 312 p.
- Bylinskaya, M. E. 2005, Range and stratigraphic significance of the Globorotalia crassaformis plexus: Journal of Iberian Geology, v. 31, p. 51–63.
- Caromel, A. G. M., D. N. Schmidt, J. C. Phillips, and E. J. Rayfield. 2014, Hydrodynamic constraints on the evolution and ecology of planktic foraminifera: Marine Micropaleontology, v. 106, p. 69–78.
- Chaisson, W. P., and P. N. Pearson. 1997, Planktonic foraminifera biostratigraphy at Site 925: middle Miocene-Pleistocene: in Shackleton, N. J., Curry, W. B., Richter, C., and Bralower, T. J., (eds.) Proceedings of the Ocean Drilling Project, Scientific Results, U.S. Government Printing Office, Washington, D.C, v. 154, p. 3–32.
- Cifelli, R. 1969, Radiation of Cenozoic planktonic foraminifera: Systematic Zoology, v.18, p.154–168.
- Ph.D. dissertation, lodged in the Library, University of Waikato, Hamilton, New Zealand.
- Cushman, J. A., and P. J. Bermudez. 1949, Some Cuban species of Globorotalia: Contributions from the Cushman Laboratory for Foraminiferal Research, v. 25, p. 26–45.
- Galloway, J. J., and S. W. Wissler. 1927, Pleistocene foraminifera from the Lomita Quarry, Palos Verde Hills, California: Journal of. Paleontology, v. 1, p. 35–87.
- Hohenegger, J. 2018, Foraminiferal growth and test development: Earth-Science Reviews, v. 185, p. 140–162.
- Kennett, J. P., and M. S. Srinivasan. 1983, Neogene Planktonic Foraminifera: Hutchinson Ross, Stroudsburg, 265 p.
- Lazarus, D., H. Hilbrecht, C. Spencer-Cervato, and H. Thierstein. 1995, Sympatric speciation and phyletic change in Globorotalia truncatulinoides: Paleobiology, v. 21, p. 28–51.
- Lidz, B. 1972, Globorotalia crassaformis morphotype variations in Atlantic and Caribbean deep-sea cores: Micropaleontology, v. 18, p. 194–211.
- Morad, R., N. M. Vollmar, M. Greco, and M. Kucera. 2019. Unassigned diversity of planktonic foraminifera from environmental sequencing revealed as known but neglected species: Plos One, v. 14, e0213936.
- Postuma, J. A. 1971, Manual of Planktonic Foraminifera: Elsevier, Amsterdam, 420 p.
- Richey, J. N., C. E. Reynolds, E. Tappa, and R. Thunell. 2014, Weekly resolution particulate flux from a sediment trap in the northern Gulf of Mexico, 2008–2012: United States Geological Survey Open-File Report 2014-1035, 9 p.
- Rogl, F., and H. M. Bolli. 1973, Holocene to Pleistocene planktonic foraminifera of Leg 15, Site 147 (Cariaco Basin (Trench), Caribbean Sea) and their climatic interpretation: in Edgar, N. T., Kaneps, A. G., and Herring, J. R. (eds), Initial Reports of the Deep Sea Drilling Project v. 15, Washington, U.S. Government Printing Office, p. 533–573.
- Saito, T., P. R. Thompson, and D. Breger. 1981, Systematic Index of Recent and Pleistocene Planktonic Foraminifera: University of Tokyo Press, Tokyo, 190 p.
- Schiebel, R., and C. Hemleben. 2017, Planktic Foraminifers in the Modern Ocean: Springer, Berlin, 358 p.
- Scott, G. H., J. C. Ingle, B. McCane, C. L. Powell, and R. C. Thunell. 2015, Truncorotalia crassaformis from its type locality: Comparison with Caribbean plankton and Pliocene relatives: Marine Micropaleontology, v. 117, p. 1–12.
- Scott, G. H. 2019, Recognition of Truncorotalia crassaformis as a modern planktonic foraminiferal morphospecies in the Caribbean and equatorial Atlantic Ocean and proposal of a neotype: Journal of Foraminiferal Research, v. 49, p. 94–102.
- Scott, G. H. 2023, A replacement neotype for Globigerina crassaformis Galloway and Wissler, 1927: Journal of Foraminiferal Research, v. 53, p. 397–402.
- Scott, G. H. 2024, Voucher specimens in taxonomy and Simpsons’s hypodigm: Diversity, v. 16, 666.
- Scott, G. H., S. Bishop, and B. J. Burt. 1990, Guide to some Neogene Globorotalids (Foraminiferida) from New Zealand: Lower Hutt, New Zealand Geological Survey Paleontological Bulletin 61. 135 p. (2013 Digital Edition ISBN 978-1-972192-18-4.
- Słomka, J., U. Alcolombri, E. Secchi, R. Stocker, and V.I. Fernandez. 2020, Encounter rates between bacteria and small sinking particles: New Journal of Physics, v. 22, 043016.
- Sokal, R. R., and J. H. Camin. 1965, The two taxonomies: areas of agreement and conflict: Systematic Zoology, v. 14, p. 176–192.
- Sokal, R. R., and P. H. A. Sneath. 1963, Principles of numerical taxonomy: W. H. Freeman, San Francisco, 359 p.
- Tedesco, K. A., and R. C. Thunell. 2003, Seasonal and interannual variations in planktonic foraminiferal flux and assemblage composition in the Cariaco Basin, Venezuela: Journal of Foraminiferal Research, v. 33, p. 192–210.
- Webster, M., and H. D. Sheets. 2010, A practical introduction to landmark-based geometric morphometrics: Paleontological. Society Papers, v.16, p. 163–188.
- Weglarczyk, S. 2018, Kernel density estimation and its application: ITM Web of Conferences, 2018, v. 23, 00037.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).