Introduction
Mucopolysaccharidosis (MPS) is a group of rare lysosomal storage disorders characterized by deficient lysosomal enzymes resulting in the accumulation of glycosaminoglycans throughout the body [
1]. This accumulation leads to progressive dysfunction of multiple organs, including the bones, heart, liver, and spleen [
2]. Without early diagnosis and treatment, patients may develop severe complications that significantly impact their quality of life and prognosis [
3].
Recent advances in newborn screening technology have made early detection of MPS possible. Since 2015, Taiwan has incorporated MPS into its newborn screening program, utilizing tandem mass spectrometry to measure enzymatic activities in blood samples. This enables the identification of potential patients before symptom onset [
4,
5,
6,
7]. Early diagnosis is crucial for patients with MPS, as timely intervention can prevent or slow disease progression and improve outcomes [
8].
Imaging studies play a vital role in the diagnostic process of MPS. Skeletal radiography can assess the extent and distribution of bone abnormalities [
9], echocardiography can detect early cardiac valve disease and myocardial dysfunction [
10], and abdominal ultrasonography can evaluate hepatosplenomegaly [
11]. However, the current literature on imaging findings in newborns with positive MPS-screening is relatively limited, and it is particularly lacking in integrated analyses of these three imaging modalities [
12].
This study aimed to systematically analyze skeletal radiographic and cardiac and abdominal ultrasonographic findings in newborns with positive MPS screening to investigate the value of these imaging studies for early diagnosis and disease evaluation. The hope was to establish a more comprehensive imaging assessment model that could assist clinicians in making more accurate diagnostic decisions and treatment plans for screening-positive newborns.
Materials and Methods
Study Population and Design
This retrospective study included patients referred to MacKay Memorial Hospital, Taipei, following positive newborn screening results for MPS between January 2015 and December 2024. Inclusion criteria were newborns with reduced enzyme activity detected through tandem mass spectrometry screening who were subsequently referred to our hospital for confirmatory testing and clinical evaluation [
13].
Imaging Studies
All patients underwent comprehensive imaging evaluations including skeletal radiography, cardiac ultrasonography, and abdominal ultrasonography. Imaging studies were conducted and interpreted according to standardized protocols by experienced radiologists and specialists.
Skeletal Radiography
Complete skeletal surveys were conducted using standardized positioning and exposure parameters. Radiographic assessment included:
Skull morphology and thickness
Vertebral body shape and alignment
Joint morphology focusing on hip dysplasia and genu valgum
Hand and wrist structures, including carpal and metacarpal bones
Bone density and cortical thickness
Presence of a J-shaped sella turcica
Cardiac Ultrasonography
Transthoracic echocardiography was performed using standardized views and measurements. The following were assessed:
Cardiac valve morphology and function
Left ventricular dimensions and systolic function
Right ventricular size and function
Presence of valve regurgitation or stenosis
Wall thickness and myocardial texture
Presence of pericardial effusion
Abdominal Ultrasonography
Abdominal ultrasound was performed after an appropriate fasting period. The evaluation included:
Liver size and parenchymal texture
Spleen size and echotexture
Presence of hepatosplenomegaly
Gallbladder and biliary tract assessment
Kidney size and echogenicity
Statistical Analysis
Statistical analyses were performed using MedCalc® version 23.0.9 (MedCalc Software Ltd, Ostend, Belgium). Descriptive statistics are presented as means ± standard deviations for continuous variables and frequencies (percentages) for categorical variables. Comparisons between groups were conducted using Student’s t-test for continuous variables and chi-square or Fisher’s exact test for categorical variables. Statistical significance was defined as p < 0.05.
Results
Patient Demographics and Baseline Characteristics
A total of 277 infants with 4 different types of MPS identified through newborn screening between 2015 and 2024 (
Table 1) were included. The breakdown by MPS type included MPS I (n = 15), MPS II (n = 113), MPS IVA (n = 127), and MPS VI (n = 22). The cohort was predominantly male (80.9%) with a median age at initial evaluation of 2 months (range, 1–5 months).
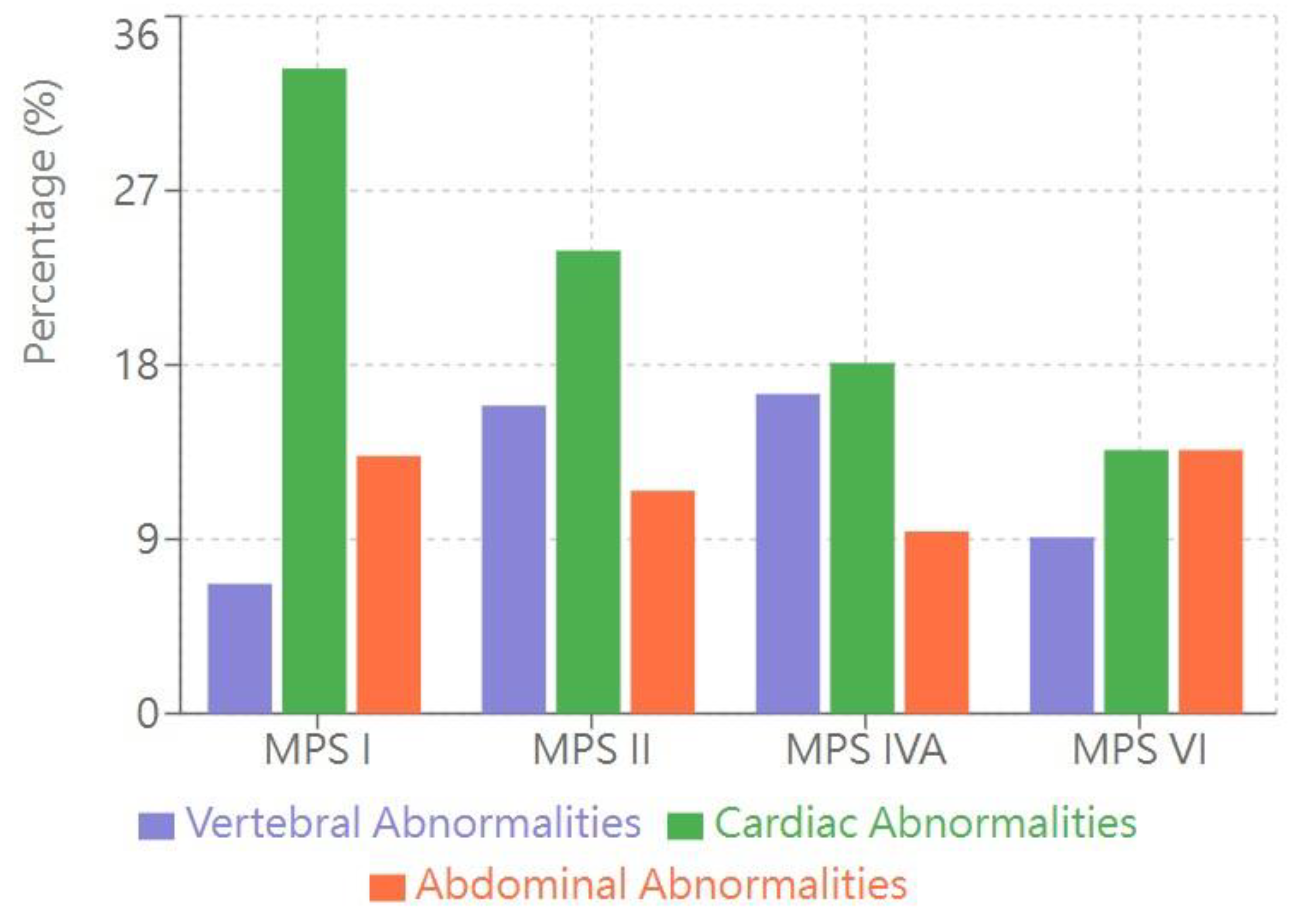
Skeletal Radiographic Findings (Table 2, Figure 1)
The most common abnormality in each MPS type was:
MPS I: Proximal pointing of the metacarpal bones and bullet-shaped phalanges (13.3%)
MPS II: Mild anterior vertebral beaking, particularly at the T12–L5 levels (15.9%)
MPS IVA: Anterior vertebral beaking and posterior scalloping (16.5%)
MPS VI: Vertebral body rounding (13.6%)
Cardiac Ultrasonographic Findings (Table 2, Figure 1)
The most common cardiac anomaly in each MPS type was:
Abdominal Ultrasonographic Findings (Table 2, Figure 1)
The most common abdominal imaging abnormalities was:
MPS I: Hepatomegaly (13.3%)
MPS II: Renal pelvic dilation (11.5%)
MPS IVA: Renal pelvic dilation (9.4%)
- Ovarian cysts in females (5.5% of female patients)
MPS VI: Gastric stasis (13.6%)
Bar graph showing the percentage of patients with abnormalities detected through three imaging modalities across MPS types I, II, IVA, and VI. Vertebral abnormalities (purple bars) represent skeletal radiographic findings including anterior vertebral beaking and posterior scalloping. Cardiac abnormalities (green bars) primarily consist of atrial septal defects/patent foramen ovale (ASD/PFO) and valvular abnormalities. Abdominal abnormalities (orange bars) include hepatomegaly, renal pelvic dilation, and other organ involvement. Data are presented as percentages of affected patients within each MPS type.
Association Between Imaging Findings and Clinical Parameters
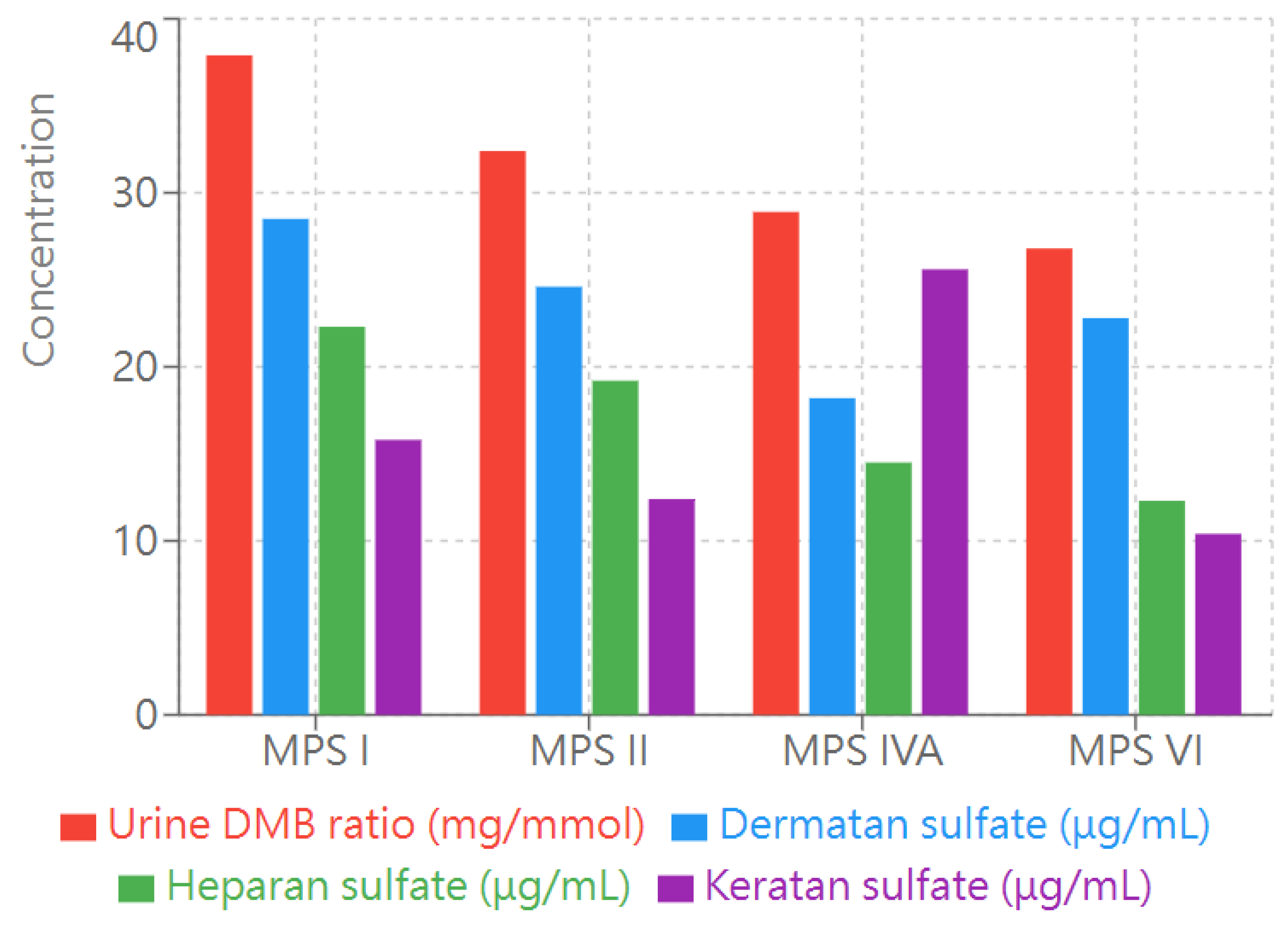
The analysis of imaging findings and their correlation with clinical parameters revealed several notable patterns across the different types of MPS (
Figure 2). Vertebral abnormalities had the strongest correlation with biochemical markers, particularly in patients with MPS IVA and II. Patients with elevated keratan sulfate (KS) levels (>10 μg/mL) demonstrated a higher frequency of vertebral body changes, especially anterior breakage and posterior scalloping
(Figure 2). This association was most common in patients with MPS IVA where 16.5% exhibited vertebral abnormalities.
Bar graph illustrating the levels of four key biochemical markers in patients with different types of MPS (I, II, IVA, and VI). Urine dimethylene blue (DMB) ratio (red bars) is measured in mg/mmol creatinine. Dermatan sulfate (blue bars), heparan sulfate (green bars), and keratan sulfate (purple bars) concentrations are measured in μg/mL. These markers represent key diagnostic indicators and disease monitoring parameters for MPS. The height of each bar represents the mean value for each marker in the respective MPS type. .
Cardiac manifestations demonstrated a correlation with dermatan sulfate (DS) levels. Patients with MPS I, who typically presented with higher DS concentrations, had the highest prevalence of cardiac abnormalities (33.3% with ASD/PFO and 13.3% with valvular anomalies). This relationship between DS levels and cardiac involvement was particularly notable, suggesting a potential mechanistic link between glycosaminoglycan accumulation and cardiac pathology.
Abdominal imaging findings correlated with both enzyme activity levels and urinary dimethylene blue (DMB) ratios. Hepatomegaly was most prevalent in MPS I (13.3%) and had an inverse correlation with enzyme activity levels. The presence of renal abnormalities, particularly pelvic dilatation, was observed across all MPS types but had the highest frequency in MPS II (11.5%), correlating with elevated urinary DMB ratios.
Discussion
Comprehensive analysis of radiographic and ultrasonographic findings in newborns with positive MPS screening results provides valuable insights into the role of multimodal imaging in early diagnosis and disease evaluation. Our findings demonstrate that even when patients are presymptomatic, subtle but distinctive imaging abnormalities can be detected across multiple organ systems, highlighting the importance of a systematic imaging approach.
Although the classic features of dysostosis multiplex may not be immediately apparent in the early stages of MPS, subtle skeletal changes can be observed. Patients with MPS commonly develop abnormalities in the vertebrae and acetabular region [
14]. These pathological changes include thoracolumbar kyphosis/scoliosis, odontoid hypoplasia, and dysplastic acetabuli, which can be detected through radiological examination. These early skeletal manifestations are important indicators for the diagnosis and monitoring of disease progression. These early changes align with previous studies suggesting that bone abnormalities begin during fetal life [
15], making radiographic evaluation a sensitive tool for early disease detection.
Cardiac ultrasonography is essential for evaluating cardiovascular involvement in MPS, with the most common findings being cardiac valve thickening and dysfunction (occurring in 60–90% of patients) [
10,
16]. Progressive valve pathology, particularly affecting left-sided valves, is the most prominent cardiac manifestation. The mitral valve is most frequently involved, with thickened leaflets and regurgitation seen in up to 80% of MPS I patients. In addition, left ventricular hypertrophy and diastolic dysfunction often emerge early on. These findings align with previous research demonstrating that cardiac manifestations are common and early features of MPS, particularly in types I, II and VI. This highlights the importance of regular cardiac screening and monitoring starting at the time of diagnosis.
Abdominal ultrasound can detect visceral manifestations such as hepatosplenomegaly and renal pelvic dilation in MPS [
17]. These findings support the fact that visceral symptoms can be detected in the early stages of the disease, and abdominal ultrasound examination should be included in the initial evaluation of patients with MPS. This supports the emphasis of the current study on early diagnosis, especially the importance of newborn screening to detect MPS.
The integration of multiple imaging modalities offers significant advantages for the early diagnosis and monitoring of MPS II, a progressive multisystem disorder [
18]. By combining different imaging techniques, clinicians can comprehensively assess the burden of disease throughout the entire patient, enabling more accurate prognostication and personalized treatment planning. For asymptomatic newborns who screen positive, multimodal imaging provides increased sensitivity to detect subtle abnormalities that may elude any single modality alone, especially when biochemical markers are inconclusive. This allows for timely intervention before irreversible organ damage occurs.
Furthermore, obtaining comprehensive baseline imaging at diagnosis establishes a reference point for tracking disease progression over time in these patients whose clinical course can be highly variable [
18]. Serial imaging allows objective assessment of changes to specific organs which can guide management more reliably than subjective clinical findings alone. Although prospective studies quantifying the benefits of multimodal imaging in MPS II are needed, current understanding suggests that this approach enhances the accuracy of early diagnosis, disease staging, and treatment monitoring. As such, the use of multimodal imaging in MPS II patient care warrants strong consideration and further research.
The findings of this study revealed distinctive imaging and biochemical characteristics across different MPS types. In MPS IVA, patients exhibit a strong correlation between vertebral abnormalities and elevated serum and urine KS levels [
19]. Similarly, cardiac manifestations in patients with MPS I are closely associated with dermatan sulfate levels [
10]. These observations suggest potential genotype-phenotype correlations that merit comprehensive investigation. By systematically studying these associations, researchers may refine diagnostic algorithms in the future for patients who screen positive.
Understanding genotype-phenotype correlations through integrated imaging analysis is crucial for improving patient care. Our findings demonstrate that early imaging changes correlate with specific biochemical markers, suggesting a potential predictive value for disease progression [
10,
19]. This aligns with previous studies that found early intervention before significant organ involvement could lead to better outcomes [
20].
The implementation of newborn screening for MPS has revolutionized early detection, but it also presents new challenges in defining appropriate diagnostic and monitoring protocols [
5,
21,
22]. This study demonstrates that integrated imaging assessment can provide valuable information even in presymptomatic stages, potentially helping to identify patients who would benefit most from early therapeutic intervention [
23].
A significant advantage of our multimodal imaging approach is its ability to detect subtle organ involvement that might be missed by single-modality assessment. This comprehensive evaluation allows for better disease staging and more informed treatment decisions [
18]. Furthermore, our findings suggest that certain imaging patterns may be predictive of disease severity and progression rate, although longer follow-up studies are needed to confirm these associations.
This study does have several limitations. First, the follow-up period was relatively short, and long-term outcomes data would be valuable for validating the prognostic significance of early imaging findings. Second, the number of patients in some MPS subtypes, particularly types I and VI, was relatively small, which may limit the generalizability of these findings. Finally, the cost-effectiveness of comprehensive imaging protocols in asymptomatic newborns needs further evaluation.
Conclusions
To the best of our knowledge, this is the first systematic analysis of multiple imaging modalities in patients who screened positive for MPS. It demonstrates the value of integrated imaging assessment in early disease evaluation. The study found that subtle but significant abnormalities can be detected across different organ systems even in presymptomatic stages thus supporting the role of comprehensive imaging protocols during the initial patient evaluation. The correlation between imaging findings and biochemical markers provides new insights into disease mechanisms and potential prognostic indicators. These findings can contribute to the optimization of diagnostic algorithms and monitoring strategies for patients with MPS identified through newborn screening programs.
Author Contributions
The manuscript was primarily authored by C-LL and S-WC. S-PL, and H-YL made significant contributions through their work in patient follow-up and assistance with manuscript preparation. Manuscript revisions were carefully conducted by H-HF, C-KC, Y-RT, Y-TL, Y-HC, J-YW and H-CC. All authors thoroughly reviewed the manuscript and provided their formal approval of the final version prior to submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by multiple grants from two major institutions. MacKay Memorial Hospital provided funding through grants MMH-E-114-13, MMH-MM-113-13, MMH-E-113-13, MMH-MM-112-14, and MMH-E-112-13. Additional support was received from the Ministry of Science and Technology, Executive Yuan, Taiwan through several research grants: NSTC-113-2314-B-195-003, NSTC-113-2314-B-195-004, NSTC-113-2314-B-715-002, NSTC-113-2314-B-195-021, NSTC-113-2811-B-195-001, NSTC-112-2314-B-195-014-MY3, NSTC-112-2811-B-195-001, NSTC-112-2314-B-195-003, NSTC-111-2314-B-195-017, NSTC-111-2811-B-195-002, NSTC-111-2811-B-195-001, NSTC-110-2314-B-195-014, NSTC-110-2314-B-195-010-MY3, and NSTC-110-2314-B-195-029.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki. It was approved by the Mackay Memorial Hospital Institutional Review Board (Reference number: 21MMHIS109e, approval date: 1 October 2021) and was permitted to be published.
Informed Consent Statement
Written informed consent to have patient details and accompanying images published was obtained from the patients’ legal guardians.
Data Availability Statement
All datas are present within the article.
Acknowledgments
The successful completion of this research would not have been possible without the exceptional dedication and expertise of our clinical staff and research laboratory personnel. Their tireless commitment to excellence in both patient care and laboratory procedures was invaluable to this study.
Conflicts of Interest
The authors confirm that there are no competing interests or conflicts of interest, financial or non-financial, that could have influenced this study.
References
- Muenzer, J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J. Pediatr. 2004, 144, S27–S34. [CrossRef]
- Clarke, L.A. Mucopolysaccharidosis Type I. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., et al., Eds.; University of Washington: Seattle, USA, updated 11 April, 2024; pp. 1993–2025.
- Muenzer, J. Overview of the mucopolysaccharidoses. Rheumatol. (Oxf. Engl.) 2011, 50, v4–v12. [CrossRef]
- Chan, M.J.; Liao, H.C.; Gelb, M.H.; Chuang, C.K.; Liu, M.Y.; Chen, H.J.; Kao, S.M.; Lin, H.Y.; Huang, Y.H.; Kumar, A.B.; et al. Taiwan national newborn screening program by tandem mass spectrometry for mucopolysaccharidoses Types I, II, and VI. J. Pediatr. 2019, 205, 176–182. [CrossRef]
- Chuang, C.K.; Lee, C.L.; Tu, R.Y.; Lo, Y.T.; Sisca, F.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; et al. Nationwide Newborn Screening Program for Mucopolysaccharidoses in Taiwan And an update of the "gold standard" criteria required to make a confirmatory diagnosis. Diagnostics 2021, 11, 1583. [CrossRef]
- Chuang, C.K.; Tu, Y.R.; Lee, C.L.; Lo, Y.T.; Chang, Y.H.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; Kao, S.M.; Wang, L.Y.; et al. Updated Confirmatory Diagnosis for Mucopolysaccharidoses in Taiwanese Infants and the Application of Gene Variants. Int. J. Mol. Sci. 2022, 23, 9979. [CrossRef]
- Chuang, C.K.; Lin, H.Y.; Wang, T.J.; Huang, Y.H.; Chan, M.J.; Liao, H.C.; Lo, Y.T.; Wang, L.Y.; Tu, R.Y.; Fang, Y.Y.; et al. Status of newborn screening and follow up investigations for Mucopolysaccharidoses I and II in Taiwan. Orphanet J. Rare Dis. 2018, 13, 84. [CrossRef]
- Giugliani, R.; Lampe, C.; Guffon, N.; Ketteridge, D.; Leão-Teles, E.; Wraith, J.E.; Jones, S.A.; Piscia-Nichols, C.; Lin, P.; Quartel, A.; et al. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)—10-year follow-up of patients who previously participated in an MPS VI Survey Study. Am. J. Med. Genet. A 2014, 164A, 1953–1964. [CrossRef]
- Morishita, K.; Petty, R.E. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatol. (Oxf. Engl.) 2011, 50, v19–v25. [CrossRef]
- Braunlin, E.A.; Harmatz, P.R.; Scarpa, M.; Furlanetto, B.; Kampmann, C.; Loehr, J.P.; Ponder, K.P.; Roberts, W.C.; Rosenfeld, H.M.; Giugliani, R. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management. Presentation. J. Inherit. Metab. Dis. 2011, 34, 1183–1197. [CrossRef]
- Jerves Serrano, T.; Gold, J.; Cooper, J.A.; Church, H.J.; Tylee, K.L.; Wu, H.Y.; Kim, S.Y.; Stepien, K.M. Hepatomegaly and splenomegaly: an approach to the diagnosis of lysosomal storage diseases. J. Clin. Med. 2024, 13, 1465. [CrossRef]
- Zhou, J.; Lin, J.; Leung, W.T.; Wang, L. A basic understanding of mucopolysaccharidosis: incidence, clinical features, diagnosis, and management. Intractable Rare Dis. Res. 2020, 9, 1–9. [CrossRef]
- Lin, H.Y.; Lee, C.L.; Chang, Y.H.; Tu, Y.R.; Lo, Y.T.; Wu, J.Y.; Niu, D.M.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; et al. Implementation of Newborn Screening for Mucopolysaccharidosis Type IVA and Long-Term Monitoring in Taiwan. Genet. Med. 2024, 26, 101286. [CrossRef]
- Oussoren, E.; Brands, M.M.; Ruijter, G.J.; der Ploeg, A.T.; Reuser, A.J. Bone, joint and tooth development in mucopolysaccharidoses: relevance to therapeutic options. Biochim. Biophys. Acta 2011, 1812, 1542–1556. [CrossRef]
- Tomatsu, S.; Montaño, A.M.; Oikawa, H.; Smith, M.; Barrera, L.; Chinen, Y.; Thacker, M.M.; Mackenzie, W.G.; Suzuki, Y.; Orii, T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [CrossRef]
- Lin, H.-Y.; Chuang, C.K.; Chen, M.R.; et al. Cardiac structure and function and effects of enzyme replacement therapy in patients with mucopolysaccharidoses I, II, IVA and VI. Mol. Genet. Metab. 2016, 117, 431-437. [CrossRef]
- Lin, S.P.; Lin, H.Y.; Wang, T.J.; Chang, C.Y.; Lin, C.H.; Huang, S.F.; Tsai, C.C.; Liu, H.L.; Keutzer, J.; Chuang, C.K. A pilot newborn screening program for mucopolysaccharidosis type I in Taiwan. Orphanet J. Rare Dis. 2013, 8, 147. [CrossRef]
- Mao, S.J.; Chen, Q.Q.; Dai, Y.L.; Dong, G.P.; Zou, C.C. The diagnosis and management of mucopolysaccharidosis type II. Ital. J. Pediatr. 2024, 50, 207. [CrossRef]
- Hendriksz, C.J.; Harmatz, P.; Beck, M.; Jones, S.; Wood, T.; Lachman, R.; Gravance, C.G.; Orii, T.; Tomatsu, S. Review of clinical presentation and diagnosis of mucopolysaccharidosis IVA. Mol. Genet. Metab. 2013, 110, 54-64. [CrossRef]
- Giugliani, R.; Federhen, A.; Rojas, M.V.; Vieira, T.; Artigalás, O.; Pinto, L.L.; Azevedo, A.C.; Acosta, A.; Bonfim, C.; Lourenço, C.M.; et al. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment. Genet. Mol. Biol. 2010, 33, 589-604. [CrossRef]
- Lin, H.-Y.; Chuang, C.K.; Lee, C.L.; Chen, M.R.; Sung, K.T.; Lin, S.M.; Hou, C.J.; Niu, D.M.; Chang, T.M.; Hung, C.L.; et al. Cardiac Evaluation using Two-dimensional Speckle-tracking Echocardiography and Conventional Echocardiography in Taiwanese Patients with Mucopolysaccharidoses. Diagnostics 2020, 10, 62. [CrossRef]
- Lin, H.-Y.; Lee, C.L.; Chang, C.Y.; Chiu, P.C.; Chien, Y.H.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; Lin, S.J.; Lin, J.L.; et al. Survival and diagnostic age of 175 Taiwanese patients with mucopolysaccharidoses (1985-2019). Orphanet J. Rare Dis. 2020, 15, 314. [CrossRef]
- Lin, H.-Y.; Chang, Y.H.; Lee, C.L.; Tu, Y.R.; Lo, Y.T.; Hung, P.W.; Niu, D.M.; Liu, M.Y.; Liu, H.Y.; Chen, H.J.; et al. Newborn Screening Program for Mucopolysaccharidosis Type II and Long-Term Follow-up of the Screen-Positive Subjects in Taiwan. J. Pers. Med. 2022, 12, 102. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).