Highlights:
the challenges associated with surgical management of desmoid tumors, particularly the difficulty in achieving microscopically negative margins, which contributes to high recurrence rates
the need to critically evaluate the role of invasive treatments and advocates for conservative approaches, such as active surveillance or systemic therapy, as primary strategies before resorting to surgical intervention
1. Introduction
Desmoid tumors (DTs), also known as aggressive fibromatoses, are rare mesenchymal neoplasms, accounting for approximately 3% of all soft tissue tumors and 0.03% of all neoplasms [
1,
4]. Despite their lack of metastatic potential, DTs exhibit aggressive local infiltration and can compress surrounding structures, leading to significant morbidity. Their high recurrence rates pose a major challenge, particularly when they arise in anatomically complex regions, where complete surgical resection is difficult [
2,
3,
5].
These tumors predominantly affect women of reproductive age, with a marked association with hormonal factors. They are rare after menopause and have been observed to increase in size during pregnancy. Management strategies remain controversial, ranging from active surveillance to aggressive surgical and systemic interventions, depending on tumor location, progression, and symptom severity.
We present the case of a 39-year-old woman, who initially developed a tumor in the left hypochondrium extending to the left hemithorax. After undergoing partial excision, she experienced a more aggressive recurrence that required extensive resection and thoracic wall reconstruction. This case highlights the challenges of managing DTs and raises questions regarding the role of surgical intervention versus conservative therapeutic approaches. Informed consent was obtained from the patient prior to the study.
2. Case Description
A 39-year-old female presented with a firm, painless, and well-defined left inframammary thoracic mass, adherent to adjacent anatomical structures. The mass was located at the superior margin of the left rectus abdominis muscle, extending to the left hemithorax. She also reported epigastric pain, pyrosis, and vomiting. Her medical history was notable for cervical intraepithelial neoplasia (CIN III), which had been treated with conization one month prior to presentation.
2.1. Initial Diagnostic Workup
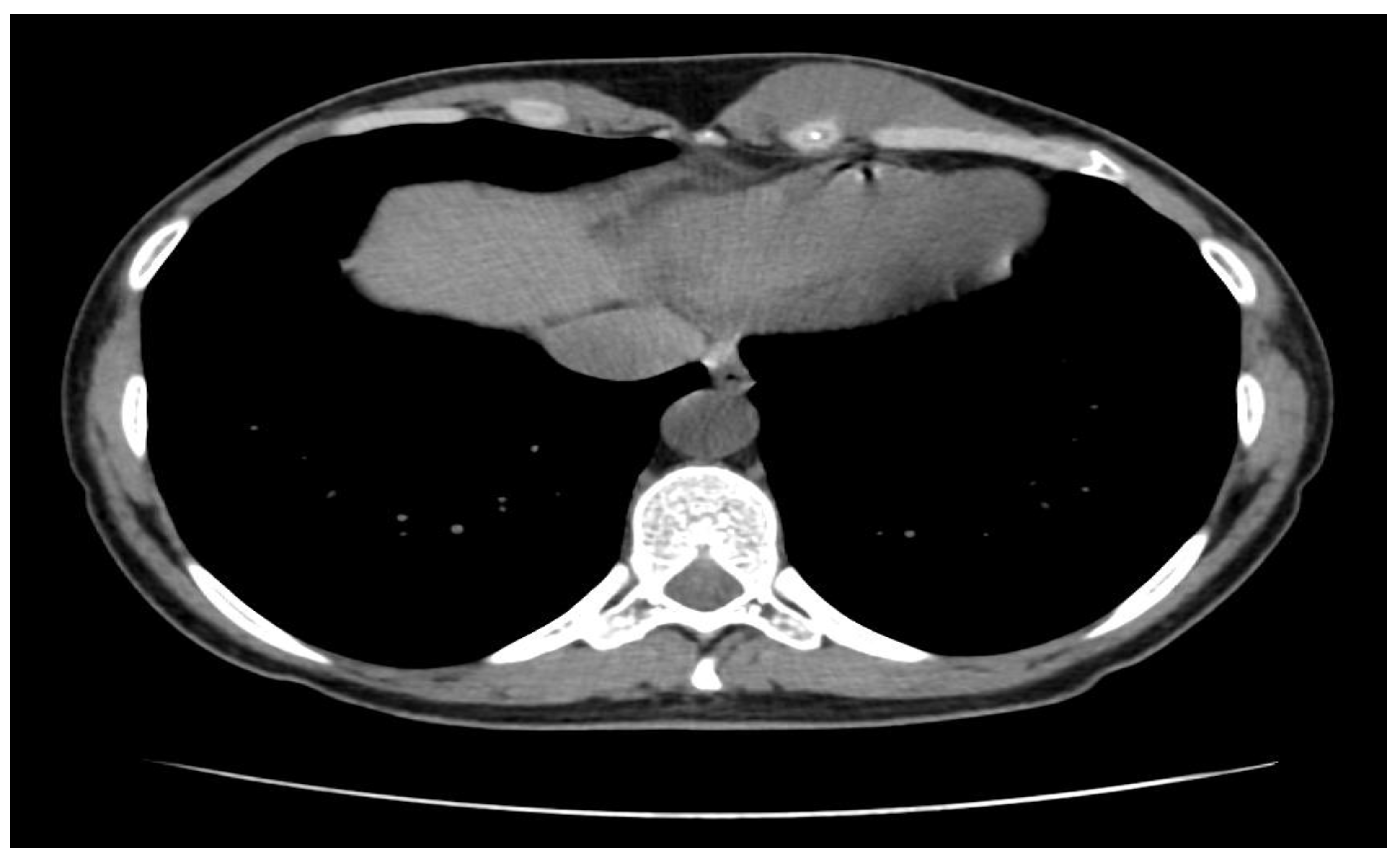
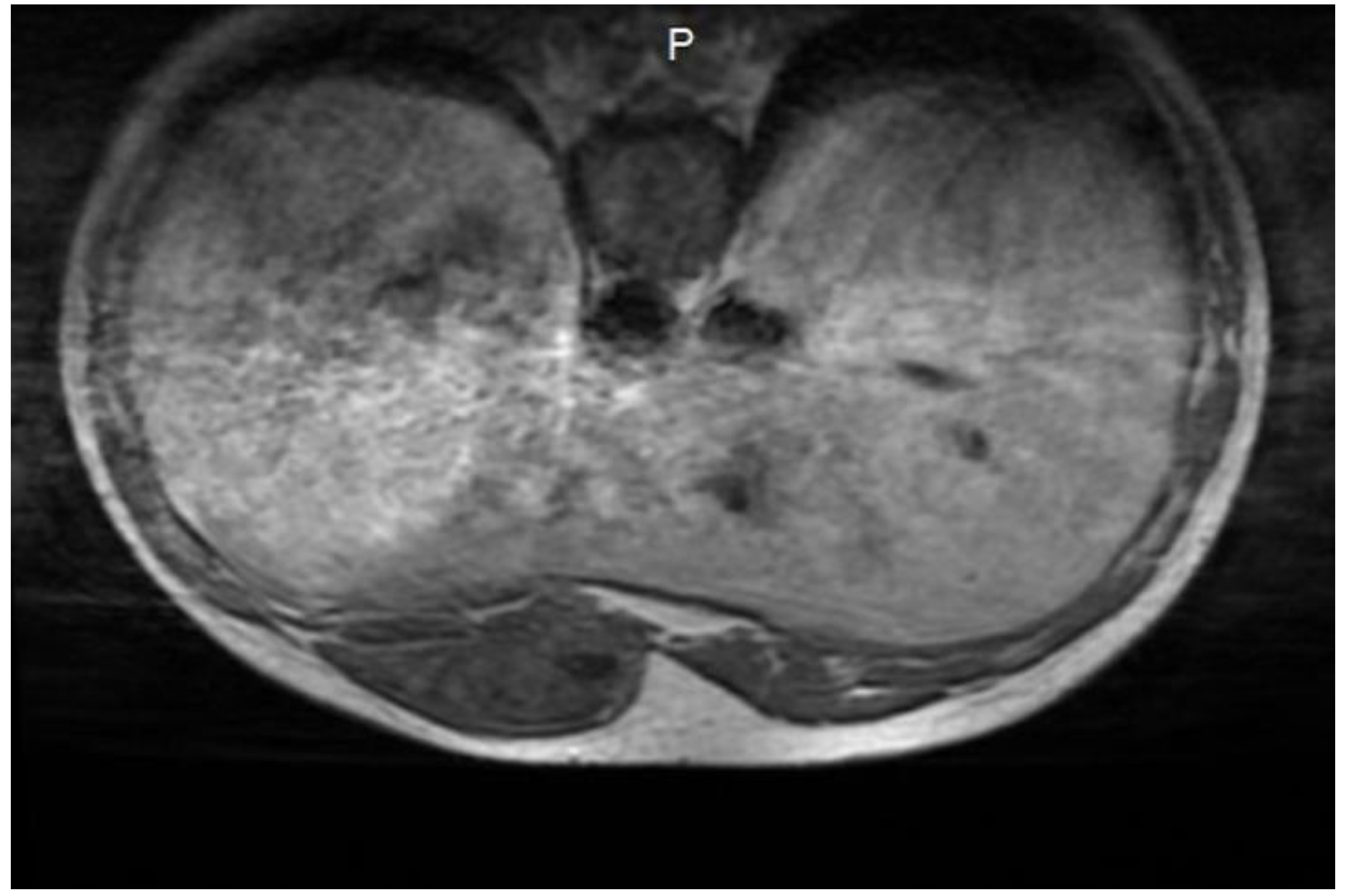
A soft-tissue ultrasound revealed a left paramedian parenchymatous mass measuring 4 × 2.7 cm, situated at the base of the hypochondrium, corresponding to the anterior XI-XII intercostal spaces. A contrast-enhanced thoraco-abdominal CT scan (
Figure 1) further identified a well-defined, oval, intramuscular mass (22 × 48 × 55 mm, AP/LL/CC) within the proximal segment of the left rectus abdominis muscle, anterior to the VI-VII-VIII costal cartilages. There was no evidence of intra-abdominal or intrathoracic extension, adjacent tissue invasion, bone lysis, or suspicious adenopathies.
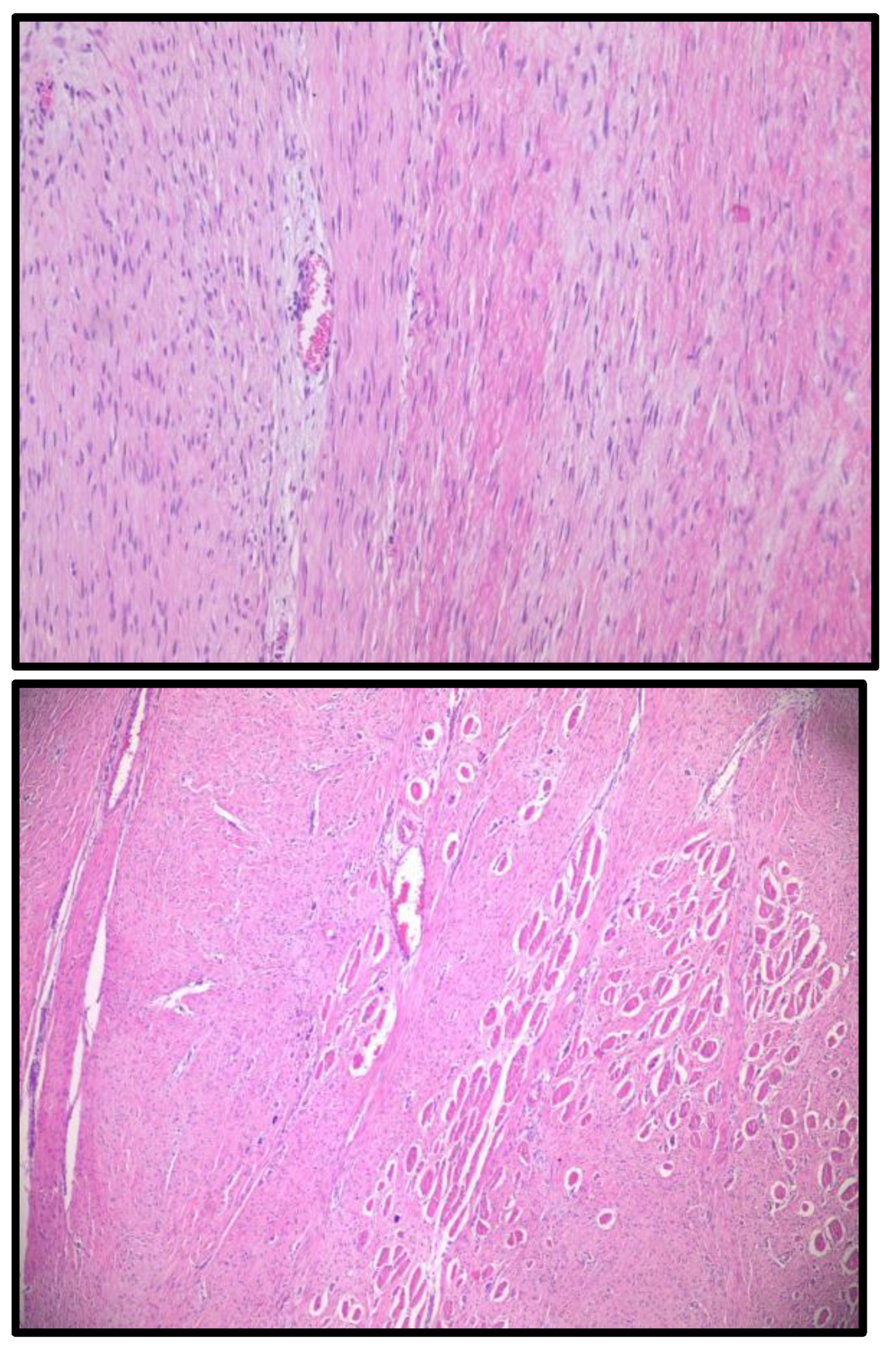
A tru-cut biopsy was subsequently performed under ultrasound guidance. Histopathological examination revealed a spindle-cell tumor composed of elongated or oval-shaped nuclei, moderate eosinophilic cytoplasm, and bundles intersecting at various angles, compressing the surrounding muscle fibers. These findings suggested a mesenchymal tumor with uncertain malignant potential, with differential diagnoses including desmoid tumor and low-grade myofibroblastic sarcoma. (
Figure 2)
2.2. Surgical Intervention and Initial Outcomes
Given the uncertainty of the diagnosis, a surgical evaluation was recommended to establish a definitive diagnosis. Following consultation, the decision was made to proceed with tumor excision. The mass was circumferentially delineated and resected down to the costal plane, resulting in partial tumor removal (specimen size: 6 × 5 × 4 cm).
Immunohistochemical evaluation was performed, revealing focal positivity for SMA and Desmin, while STAT6 and S100 were negative. The Ki-67 proliferative index was <1%, indicating low proliferative activity. Due to the exhaustion of biopsy material, β-catenin staining was not performed. The final diagnosis confirmed a desmoid tumor, but with positive resection margins.
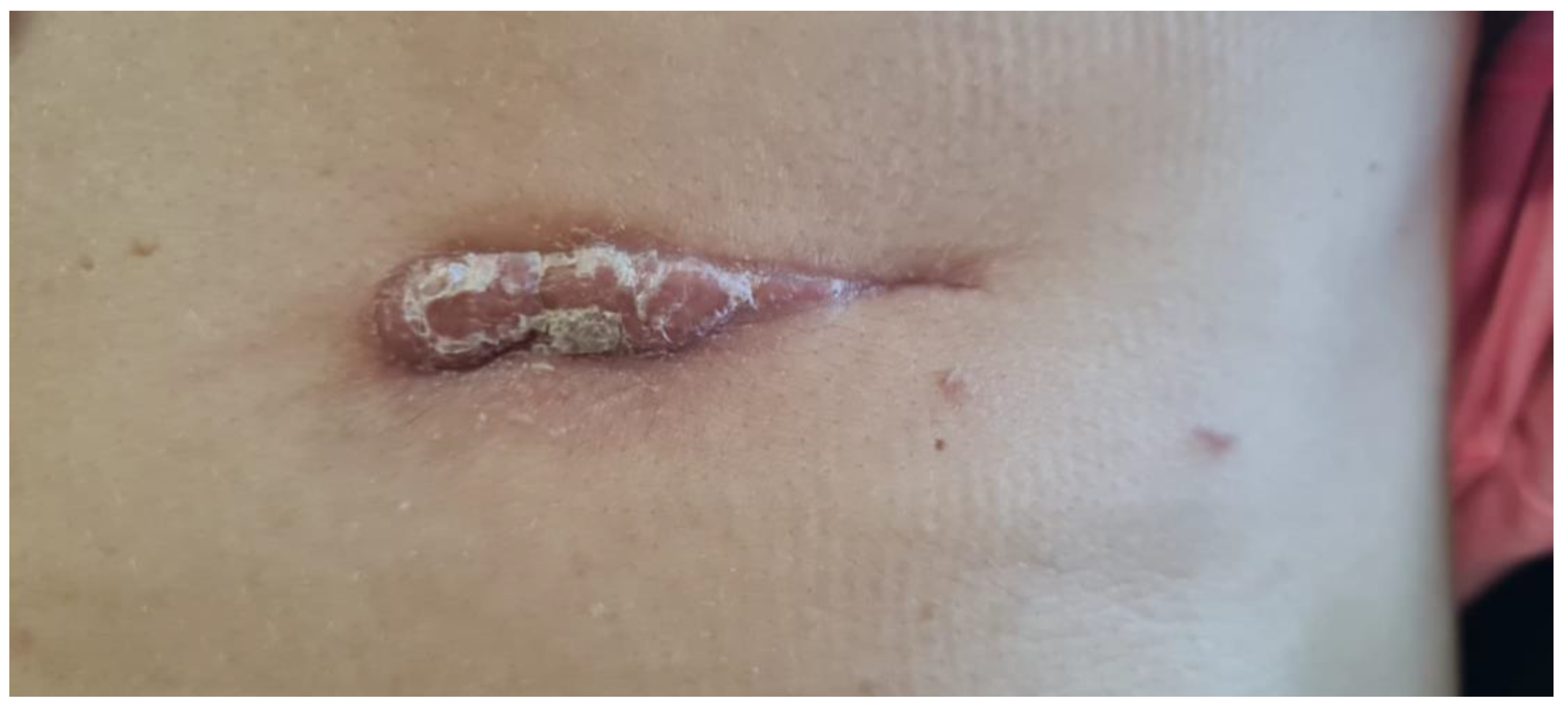
Postoperatively, there were no immediate complications, though the patient developed a keloid scar at the surgical site. (
Figure 3)
2.3. Tumor Recurrence and Further Management
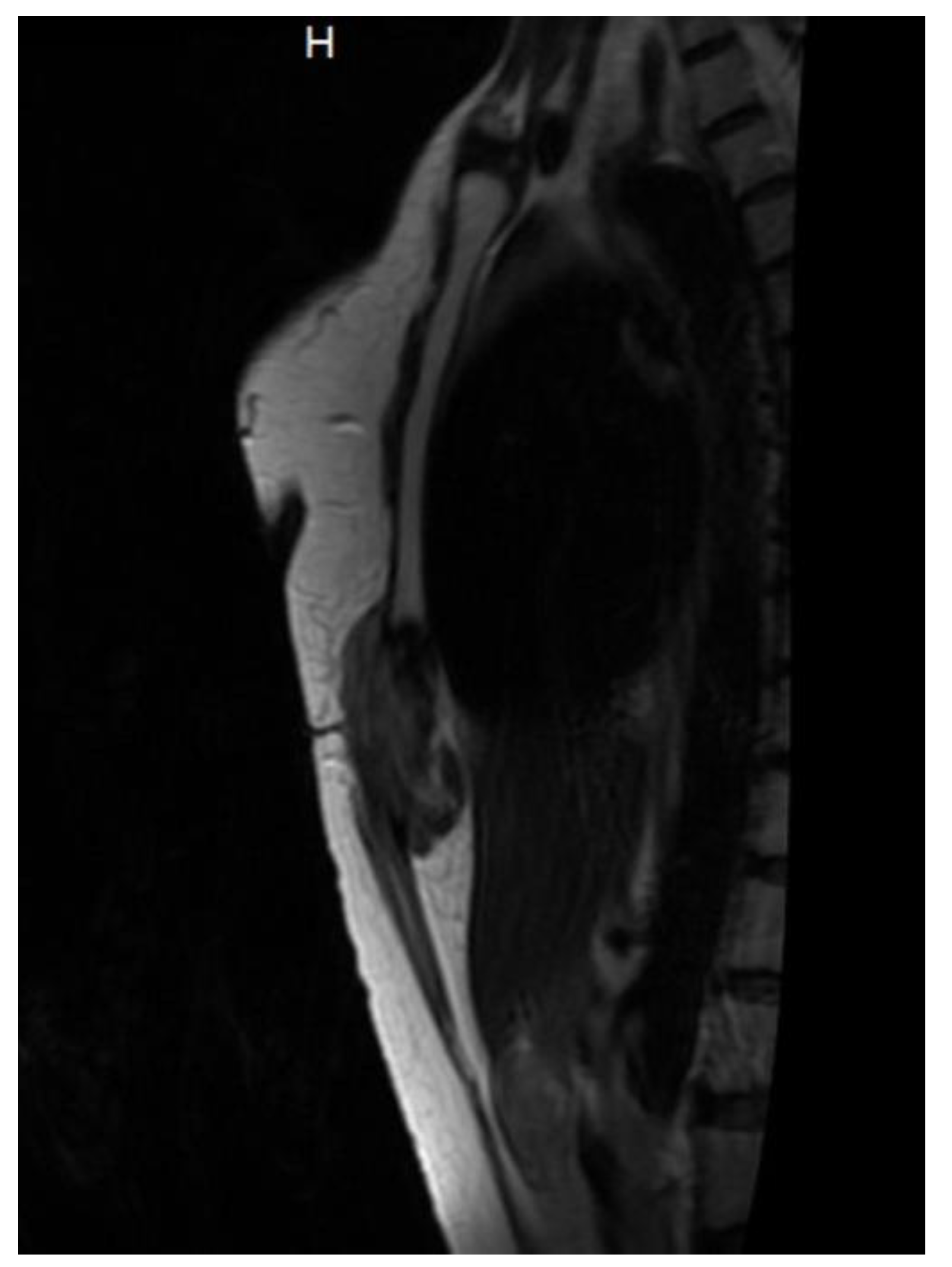
By May 2022, MRI imaging revealed tumor recurrence (28 × 50 × 84 mm, AP/LL/CC). Due to the progressive nature of the recurrence, an oncological evaluation was recommended. The patient sought further assessment at the
Fondazione IRCCS Istituto Nazionale dei Tumori in Italy, where the recurrence was confirmed, with a maximum tumor diameter of 8 cm. (
Figure 4 and
Figure 5). The medical team advised against further surgery and recommended conservative management, including chemotherapy with methotrexate, vinorelbine, VEGF inhibitors, and anthracyclines. However, the patient declined chemotherapy and opted for systemic treatment.
By September 2022, a CT scan revealed further tumor progression (36 × 76 × 75 mm, AP/LL/CC), with continued growth documented in January 2023 (47 × 74 × 91 mm). The tumor now featured intrathoracic and intra-abdominal extension, infiltrating the chondro-sternal cartilages of the VI and VII ribs, and contacting the homolateral V rib cartilage, without malignant transformation.
2.4. Second Surgical Intervention and Postoperative Course
Despite the oncologist's recommendation to avoid surgery, the patient chose to undergo a second surgical intervention. An en bloc resection of the tumor was performed, involving removal of the affected sternum and ribs, followed by thoracic wall reconstruction using a myocutaneous flap.
On postoperative day one, the venous anastomosis of the flap thrombosed, necessitating an emergency microsurgical reconstruction.
2.5. Long-Term Follow-up and Disease Progression
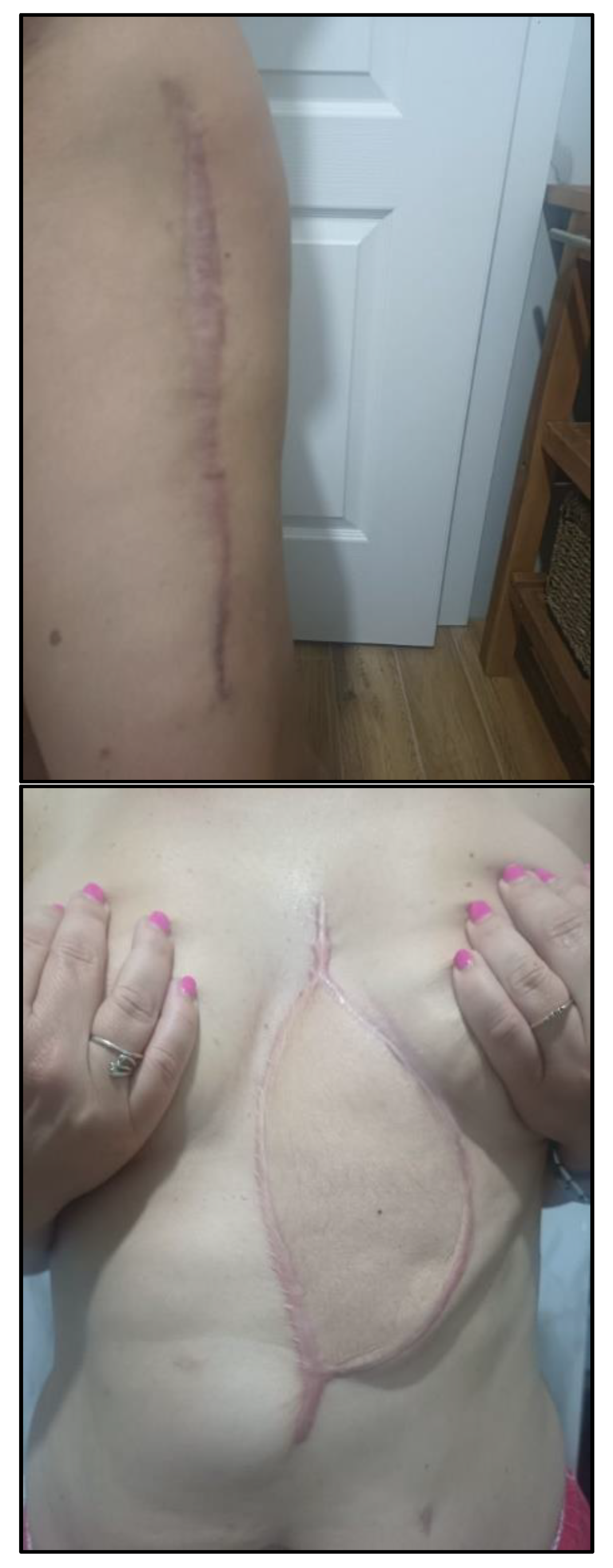
Over the two-year period following the initial diagnosis, the patient underwent multiple imaging studies (CT, MRI, ultrasound) and numerous medical consultations. Despite aggressive surgical treatments, she developed two postoperative scars:
a hypertrophic scar on the left thigh (from flap harvesting) (
Figure 6A)
a thoraco-abdominal scar from the second surgical resection (
Figure 6B)
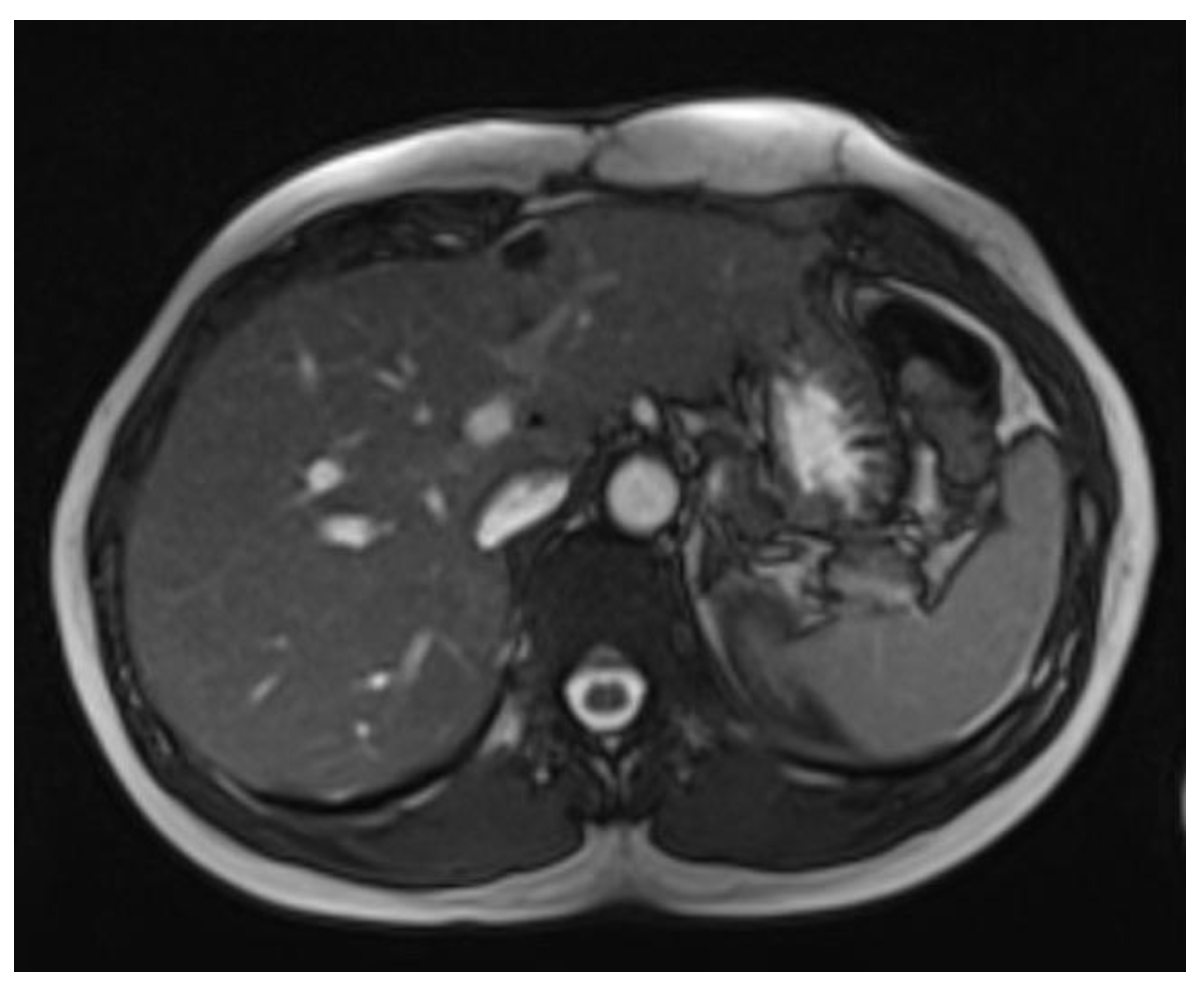
In April 2024, a thoracic MRI confirmed tumor recurrence at the same location, reinforcing the chronic, recurrent nature of the disease. (
Figure 7)
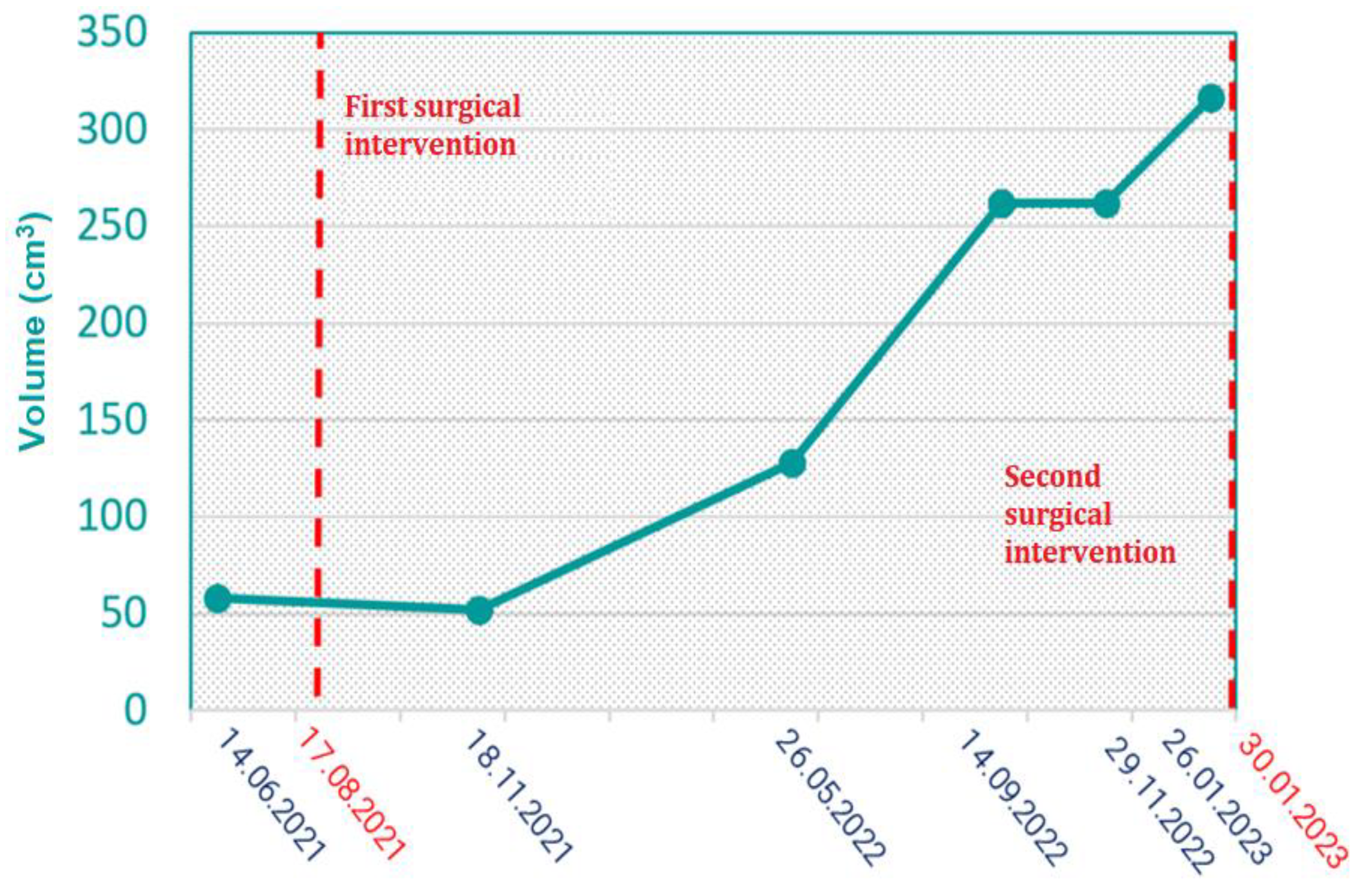
Initially, the tumor showed a slow increase in size; however, following the first surgical intervention on August 17, 2021, a steady growth pattern was observed, leading to a significant increase in volume. Despite this, the tumor continued to progress, necessitating a second surgical intervention on January 30, 2023. This trend highlights the high recurrence rate of desmoid tumors, particularly when complete resection with negative margins is challenging to achieve. (
Figure 8)
2.6. Clinical Implications
This case exemplifies the high recurrence rate of desmoid tumors, particularly when negative resection margins are not achieved. Despite oncological recommendations favoring conservative therapy, the patient opted for surgical interventions, which led to multiple recurrences and increased morbidity.
The case highlights the importance of a multidisciplinary approach, involving oncologists, surgeons, and radiologists, to balance the risks and benefits of surgical versus non-invasive management strategies.
3. Discussions
Desmoid tumors (DTs) are rare, locally aggressive mesenchymal neoplasms characterized by infiltrative growth and a high recurrence rate despite their lack of metastatic potential [
1,
8]. These tumors primarily affect the abdominal and intra-abdominal regions, with thoracic wall and retroperitoneal localizations being uncommon [
8]. Other possible sites include the shoulders, upper arms, and thighs [
1].
DTs predominantly occur in individuals aged 15 to 60 years, with a higher prevalence in females due to hormonal influences, particularly pregnancy. Approximately 20% of desmoid tumors are associated with familial adenomatous polyposis (FAP) and mutations in the APC gene, with an estimated 11% mortality rate in these cases [
20]. Additionally, 5-10% of desmoid tumors have a hereditary component, often linked to Gardner syndrome, which significantly increases the risk of colorectal cancer and desmoid tumor development [
1].
Our patient’s clinical presentation and medical history were atypical, as she had no prior pregnancies, surgical trauma, or genetic syndromes, yet developed an extensive tumor involving the left hypochondrium and hemithorax.
3.1. Diagnosis and Imaging Characteristics
A combination of ultrasonography, CT, and MRI is typically used for DT diagnosis. Ultrasound findings vary, but generally show well-defined, hypoechoic masses with minimal vascularity. Contrast-enhanced CT scans often demonstrate high-attenuation tumors with well-defined or infiltrative borders [
10]. In our patient, serial contrast-enhanced CT scans were instrumental in assessing tumor recurrence, progression rate, and extent, while also ruling out distant metastasis.
However, imaging alone is insufficient for definitive diagnosis, as DTs can mimic other mesenchymal tumors. In our case, a tru-cut biopsy was essential for differentiating between desmoid tumor and low-grade myofibroblastic sarcoma. Histopathological analysis revealed spindle cells arranged in intersecting bundles, confirming a mesenchymal tumor with uncertain malignant potential. Immunohistochemical evaluation further supported the DT diagnosis, with tumor cells showing focal positivity for SMA and Desmin, negativity for STAT6 and S100, and a low Ki-67 proliferation index (<1%). Unfortunately, β-catenin staining could not be performed due to limited biopsy material, which could have provided additional diagnostic confirmation.
3.2. Molecular Aspects and Prognostic Markers
At the molecular level, desmoid tumors are associated with mutations in the β-catenin (CTNNB1) gene or APC gene. Identifying a CTNNB1 mutation can aid in differential diagnosis and predict the likelihood of recurrence [
9]. In our case, molecular testing was unavailable, which may have impacted the treatment strategy.
Despite their benign histological appearance, DTs are locally invasive and often necessitate surgical resection [
7]. However, local recurrence rates range from 45% to 70%, largely depending on tumor size, treatment modality, and resection margin status [
6,
7]. In our patient, incomplete surgical excision (positive margins) led to an aggressive recurrence within nine months, further reinforcing the importance of achieving negative resection margins.
3.3. Management Strategies: Surgery vs. Conservative Approaches
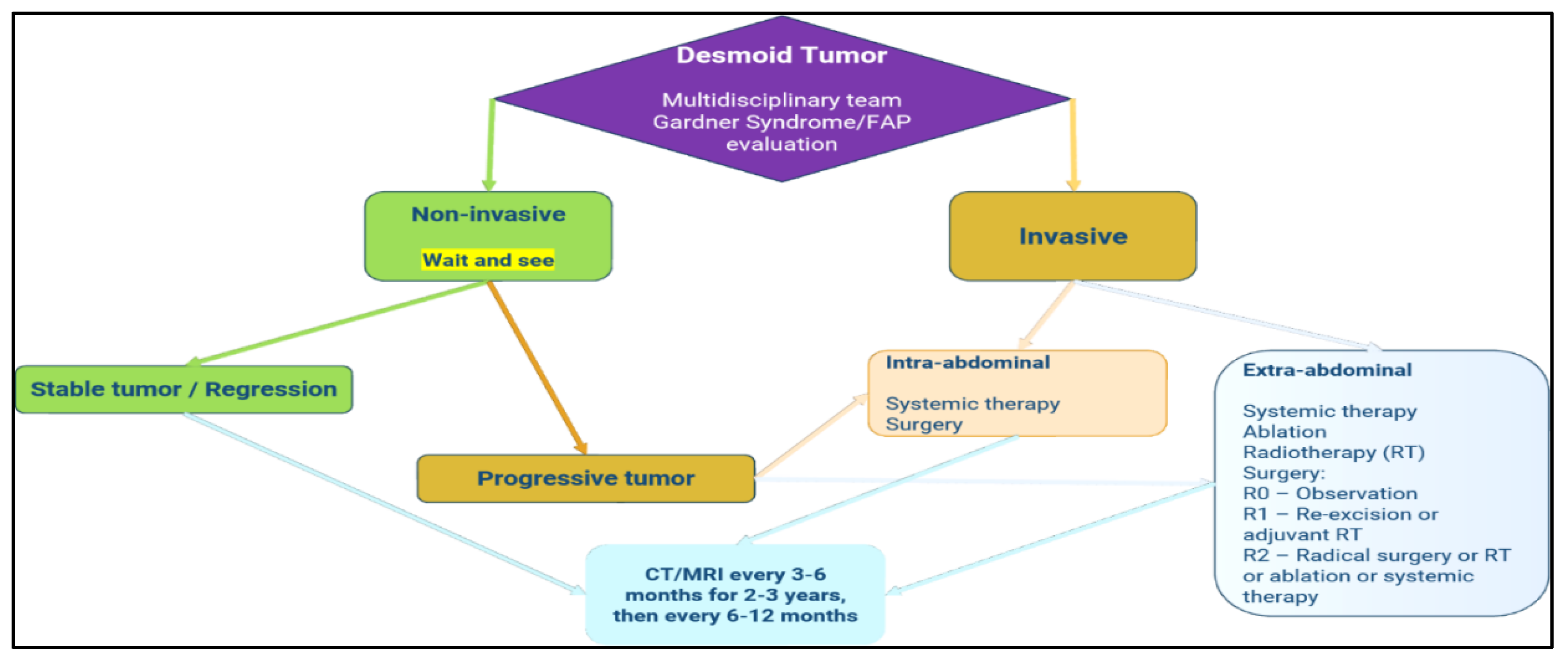
There is no universal consensus on the optimal treatment strategy for DTs. Management (
Figure 2). Treatment strategies can be categorized into active and conservative approaches. Active management encompasses surgical intervention, radiotherapy (RT), systemic therapies such as chemotherapy and targeted therapy, as well as combination treatments. In contrast, conservative approaches involve active surveillance, commonly referred to as the "watch-and-wait" strategy, alongside locoregional therapies, including cryoablation, high-intensity focused ultrasound (HIFU), and radiofrequency ablation.
Figure 9.
Desmoid Tumor Management (according to the National Comprehensive Cancer Network) [
21].
Figure 9.
Desmoid Tumor Management (according to the National Comprehensive Cancer Network) [
21].
Surgical resection has historically been the gold standard for DTs, particularly when the tumor is localized and resectable [
5]. The primary goal of surgery is to preserve critical structures while achieving complete tumor removal [
2]. However, due to high recurrence rates (15-40.6%), particularly in extra-abdominal DTs (29.6-45.5%), an increasing number of studies suggest non-surgical management strategies [
21]. Incomplete resection (positive margins) is associated with a 1.78-fold increase in recurrence risk [
21].
Our patient’s initial surgical intervention resulted in a more aggressive recurrence, with increased tumor size and invasion. Despite the oncologist’s recommendation for non-surgical management, the patient opted for a second surgery, which included an en-bloc resection of the sternum and ribs, followed by thoracic wall reconstruction. Although negative margins were achieved in the second surgery, tumor recurrence still occurred after a few months.
3.4. The Role of Watch-and-Wait in Desmoid Tumors
A "watch-and-wait" approach is increasingly advocated for asymptomatic, stable, or minimally symptomatic DTs. Bonvolot et al. proposed that active surveillance could be the preferred initial strategy, allowing clinicians to distinguish aggressive from indolent tumors [
11,
16]. Bektas et al. also emphasized that patients with non-progressive or critical-location tumors may benefit more from conservative management [
16,
21].
In our patient’s case, oncologists recommended systemic therapy (methotrexate, vinorelbine, VEGF inhibitors, and gamma-secretase inhibitors) to avoid repeat surgery, but the patient refused medical treatment, ultimately leading to further morbidity and tumor recurrence.
3.5. Radiotherapy as an Alternative or Adjuvant Treatment
Radiotherapy (RT) is a valuable option for recurrent DTs, particularly in cases where the patient is not a candidate for surgery, when the surgical morbidity is excessive or when positive surgical margins are present.
When used as an adjuvant therapy after surgery, tumor control rates reach 85% compared to 78% with RT alone. The local recurrence rate for RT monotherapy ranges from 14.1% to 37.5% [
21]. Studies suggest that postoperative RT significantly improves progression-free survival compared to surgery alone [
13,
14]. However, the optimal RT dose remains uncertain, with recommendations ranging between 50-60 Gy [
15]. Higher doses (>56 Gy) do not significantly improve outcomes but are associated with increased radiation-induced complications [
12].
3.6. Systemic Therapy and Locoregional Treatments
For rapidly progressing or symptomatic desmoid tumors (DTs), systemic therapy may be considered. Cytotoxic chemotherapy, such as doxorubicin combined with dacarbazine, is one potential option [
17]. Additionally, low-dose methotrexate in combination with vinca alkaloids has demonstrated a 50% response rate over five years [
18]. Tyrosine kinase inhibitors (TKIs), including imatinib—particularly effective in CTNNB1-mutated DTs—along with sorafenib and pazopanib, have also been utilized [
16,
19].
Minimally invasive locoregional therapies have been explored as alternative treatment modalities. Cryoablation has shown a tumor control rate of 85.1% at one year and 77.3% at three years [
22]. High-intensity focused ultrasound (HIFU) has demonstrated a tumor volume reduction of up to 63% in extra-abdominal DTs [
21]. Similarly, radiofrequency ablation has yielded promising outcomes, particularly in small to moderate-sized DTs [
21].
3.7. Final Considerations and Clinical Lessons
This case underscores the complexity of DT management and the need for personalized treatment strategies. While surgical intervention remains an option, particularly for localized tumors, the high recurrence rates and surgical morbidity highlight the importance of multidisciplinary decision-making involving oncologists, surgeons, and radiologists.
Key takeaways from this case emphasize the optimal management of desmoid tumors. Surgical excision with positive margins is associated with a high risk of recurrence, emphasizing the need for careful patient selection. In certain cases, a watch-and-wait approach should be prioritized to avoid unnecessary interventions. Additionally, radiotherapy and systemic therapy serve as valuable alternatives to surgery, offering effective treatment options with potentially lower morbidity. Ultimately, optimal management requires a multidisciplinary approach, integrating the expertise of surgeons, oncologists, and other specialists to tailor treatment strategies to individual patient needs. Ultimately, treatment decisions should balance tumor progression, patient preference, and morbidity risks, aiming for the best long-term outcome while minimizing unnecessary interventions.
4. Conclusion
Desmoid tumors (DTs) pose significant challenges in treatment selection due to their high recurrence rates, local invasiveness, and unpredictable behavior. The heterogeneous nature of DTs necessitates an individualized, multidisciplinary approach, balancing the risks and benefits of invasive versus conservative management.
This case highlights the limitations of surgical intervention, particularly in cases where negative resection margins are difficult to achieve. The patient's recurrent tumor growth following incomplete excision reinforces the importance of carefully assessing the necessity of surgery before proceeding with invasive treatment.
Current scientific literature supports a "watch-and-wait" approach as the preferred initial strategy for asymptomatic or minimally symptomatic tumors, as it can help avoid unnecessary procedures, surgical morbidity, and increased recurrence risk. In cases where intervention is warranted, radiotherapy, systemic therapy, and locoregional treatments (e.g., cryoablation, HIFU, radiofrequency ablation) offer viable alternatives or adjuncts to surgery, particularly in recurrent or unresectable DTs.
Ultimately, the optimal management of DTs requires a patient-centered approach, guided by tumor behavior, patient preference, and multidisciplinary expertise, to achieve the best long-term outcomes while minimizing treatment-related morbidity.
Acronyms and Abbreviations
CIN III- cervical intraepithelial neoplasia
AP/LL/CC- antero-posterior/latero-lateral/cranio-caudal
CT- contrast tomography
MRI- magnetic resonance imaging
VEGF- vascular endothelial growth factor
APC- adenomatous polyposis coli
Author Contributions
Conceptualization, M.G.P. and T.G.A.; Data curation, V.Z., S.B. and V.R.; Formal analysis, M.V.M. .and C.V.V.; Funding acquisition, N.V.L.; Investigation, R.D.T., G.E.P. and I.Ș.; Methodology, O.H.O.; Supervision, L.C., O.H.O. and T.G.A.; Writing—original draft, M.G.P. and T.G.A.; Writing—review and editing, M.V.M. and O.H.O. All authors have read and agreed to the published version of the manuscript.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
References
- Wang Z, Wu J, Lv A, Tian X, Hao C. En bloc resection for intra-abdominal/retroperitoneal desmoid-type fibromatosis with adjacent organ involvement: A case series and literature review. Biosci Trends. 2018;12(6):620-626. [CrossRef] [PubMed]
- Moore D, Burns L, Creavin B, et al. Surgical management of abdominal desmoids: a systematic review and meta-analysis [published correction appears in Ir J Med Sci. 2023 Apr;192(2):967. doi: 10.1007/s11845-022-03025-7.]. Ir J Med Sci. 2023;192(2):549-560. [CrossRef]
- Kiel KD, Suit HD. Radiation therapy in the treatment of aggressive fibromatoses (desmoid tumors). Cancer. 1984;54(10):2051-2055. [CrossRef]
- Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86(10):2045-2052.
- Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16(5):682-693. [CrossRef]
- Huang PW, Tzen CY. Prognostic factors in desmoid-type fibromatosis: a clinicopathological and immunohistochemical analysis of 46 cases. Pathology. 2010;42(2):147-150. [CrossRef]
- Zhao CX, Dombrowski ND, Perez-Atayde AR, et al. Desmoid tumors of the head and neck in the pediatric population: Has anything changed? Int J Pediatr Otorhinolaryngol. 2021; 140:110511. [CrossRef]
- Overhaus M, Decker P, Fischer HP, Textor HJ, Hirner A. Desmoid tumors of the abdominal wall: A case report. World J Surg Oncol. 2003;1(1):11. Published 2003 Jul 9. [CrossRef]
- Ghert M, Yao X, Corbett T, et al. Treatment and follow-up strategies in desmoid tumours: a practice guideline. Curr Oncol. 2014;21(4):e642-e649. [CrossRef]
- Ma JH, Ma ZH, Dong XF, Yin H, Zhao YF. Abdominal wall desmoid tumors: A case report. Oncol Lett. 2013;5(6):1976-1978. [CrossRef]
- Bonvalot S, Eldweny H, Haddad V, et al. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34(4):462-468. [CrossRef]
- Guadagnolo BA, Zagars GK, Ballo MT. Long-term outcomes for desmoid tumors treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2008;71(2):441-447. [CrossRef]
- Baumert, B.G., Spahr, M.O., Hochstetter, A.V. et al. The impact of radiotherapy in the treatment of desmoid tumours. An international survey of 110 patients. A study of the Rare Cancer Network. Radiat Oncol 2, 12 (2007). [CrossRef]
- Ergen ŞA, Tiken EE, Öksüz DÇ, et al. The Role of Radiotherapy in the Treatment of Primary or Recurrent Desmoid Tumors and Long-Term Results. Balkan Med J. 2016;33(3):316-321. [CrossRef]
- Bonvalot S, Desai A, Coppola S, et al. The treatment of desmoid tumors: a stepwise clinical approach. Ann Oncol. 2012;23 Suppl 10:x158-x166. [CrossRef]
- Napolitano A, Mazzocca A, Spalato Ceruso M, et al. Recent Advances in Desmoid Tumor Therapy. Cancers (Basel). 2020;12(8):2135. Published 2020 Aug 1. [CrossRef]
- Gega M, Yanagi H, Yoshikawa R, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24(1):102-105. [CrossRef]
- Palassini E, Frezza AM, Mariani L, et al. Long-term Efficacy of Methotrexate Plus Vinblastine/Vinorelbine in a Large Series of Patients Affected by Desmoid-Type Fibromatosis. Cancer J. 2017;23(2):86-91. [CrossRef]
- Szucs Z, Messiou C, Wong HH, et al. Pazopanib, a promising option for the treatment of aggressive fibromatosis. Anticancer Drugs. 2017;28(4):421-426. [CrossRef]
- Slowik V, Attard T, Dai H, Shah R, Septer S. Desmoid tumors complicating Familial Adenomatous Polyposis: a meta-analysis mutation spectrum of affected individuals. BMC Gastroenterol. 2015;15:84. Published 2015 Jul 16. [CrossRef]
- Bektas M, Bell T, Khan S, et al. Desmoid Tumors: A Comprehensive Review. Adv Ther. 2023;40(9):3697-3722. [CrossRef]
- Auloge P, Garnon J, Robinson JM, et al. Percutaneous cryoablation for advanced and refractory extra-abdominal desmoid tumors. Int J Clin Oncol. 2021;26(6):1147-1158. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).