1. Introduction
The diagnosis of Chronic Obstructive Pulmonary Disease (COPD) has evolved over the years alongside the use of Non-invasive Mechanical Ventilation (NIMV). As early as 1990, it was shown that bilevel pressure applied non-invasively via a nasobuccal interface in patients with COPD exacerbated by respiratory acidosis reduces the need for orotracheal intubation, admission to the Intensive Care Unit (ICU), hospital mortality as well as the average length of stay, with improved blood gas and correction of pH [
1,
2]. In the succeeding years, NIV has become the standard of care for this type of patient: the Global Initiative for Chronic Obstructive Lung Diseases (GOLD) establishes the criteria for its indication with a level of evidence A [
3].
Currently, Long-term Home Non-Invasive Mechanical Ventilation (LTH-NIV) is conditionally recommended with low evidence. The Spanish COPD Guide (GesEPOC 2021) [
3] proposes a personalized approach to treatable traits: among these, LTH-NIV. It is observed that patients with persistent hypercapnia after a COPD exacerbation who require acute NIV have a higher probability of hospital readmission and mortality than those who normalize arterial blood carbon dioxide levels (PaCO
2). Thus, long-term LYH-NIV is indicated in hypercapnic patients with stable COPD and a history of previous acidotic exacerbations due to its survival benefits, as well as in those who remain hypercapnic 2-4 weeks after an episode of hypercapnic respiratory failure that has required hospital ventilatory support [
3]. In turn, the European Respiratory Society offered conditional recommendations on LTH-NIV: the use of LTH-NIV in COPD with stable hypercapnia, after an exacerbation requiring acute NIV, and the use of LTH-NIV configurations aimed at reducing PaCO
2 [
4]. The American Thoracic Society recommends the use of nocturnal LTH-NIV (with respiratory polygraphs) and advises against starting therapy during a hospital stay but rather doing a re-evaluation 2 to 4 weeks after the resolution of the exacerbation: the consensus being that the main objective in these patients is the normalization of PaCO
2 to improve survival [
4,
5,
6].
COPD is one of the most prevalent respiratory diseases worldwide, representing a substantial economic burden for healthcare systems. Annual direct costs per patient vary significantly depending on the severity of the disease, from €1,047 in mild cases to €38,820 in the most severe cases [
6]. COPD exacerbations represent a substantial proportion of these costs, with values ranging from €194 to €449 in moderate exacerbations, and between €2,373 and €13,046 in severe cases requiring hospitalization [
7]. Proper medical control can prevent frequent hospital visits, difficult-to-manage admissions and therefore potentially avoidable health costs [
8,
9,
10,
11,
12].
In this context, this study describes the cohort of COPD patients treated with LTH-NIV at the Germans Trias y Pujol University Hospital, assesses the economic impact of the intervention on the healthcare provider and seeks to determine the existence of clinical or ventilatory variables that are related to the success of the therapy.
2. Methods
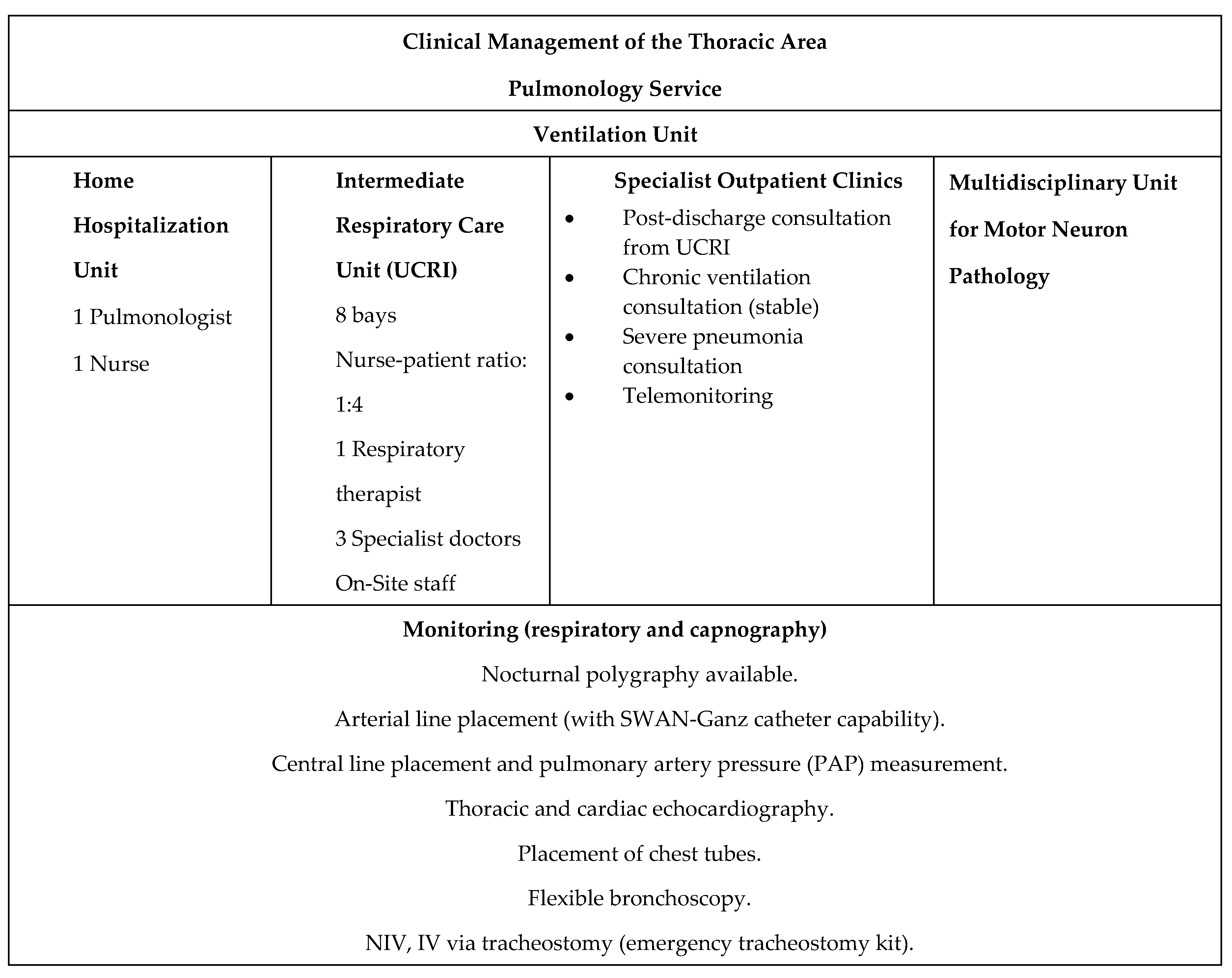
The Ventilation Unit at the Germans Trias y Pujol University Hospital is comprised of three outpatient clinics, complemented by telematic monitoring of ventilators and their variables using the Airview Resmed® and Care Orchestrator Phillips Respironics® platforms. In turn, the Intermediate Respiratory Care Unit (UCRI), consisting of 8 individual bays with advanced hemodynamic monitoring, is available for acute patients. NIV titrations and re-titrations in home patients are carried out according to complexity protocols in the pulmonology day hospital, the Multidisciplinary Sleep Unit or during scheduled inpatient admission (
Figure 1).
A retrospective descriptive observational study of 68 COPD patients with LTH-NIV monitored via telemonitoring between the years 2019 and 2022 inclusive. The analysis was carried out using data from the telemonitoring platforms and the patients’ medical history. Sociodemographic variables (age, sex); clinical phenotype (presence of comorbidities: bronchiectasis in imaging tests, Obesity Hypoventilation Syndrome, arterial hypertension, chronic kidney disease, as well as heart failure and presence of obstructive sleep apnea); pulmonary function tests (FEV1 and FVC post-bronchodilator test); arterial blood gas before the start of treatment and in a stable condition; home ventilator titration parameters (respirator model, ventilator mode, date of therapy prescription, IPAP (inspiratory positive airway pressure) and EPAP (expiratory positive airway pressure) parameters, unintentional leas, and type of interface; variables related to the patient’s treatment with inhalation therapy; as well as variables related to the care process (hospital admissions, number of visits to the emergency room, outpatient clinic check-ups and mortality) with their corresponding cost (approximated according to the prices published by the health provider).
In the descriptive analysis, means, range (maximum and minimum) and standard deviation were used for the quantitative variables, while the qualitative variables were expressed as percentages. A logistic regression was performed and the relationship between 2 qualitative variables was obtained (taking into account comorbidities as dependent variables to find a direct relationship with associated pathologies) using contingency tables. The Odds Ratio was calculated with its confidence intervals (CI). The significance level was set at p<0.05. Statistical analysis was performed using SPSS version 15.0 for Windows.
3. Results
Patient characteristics: The study included 68 patients diagnosed with COPD, of which 50 (73.5%) were male. The mean age was 71 years (SD 10.1) with a range between 55 and 86 years. Emphysema was the predominant clinical phenotype (62.5%) and the mean degree of obstruction of the sample was 42.3% (SD 18%) with a range of 15%-117%; the most prevalent associated comorbidities were OSA and AHT, chronic kidney disease was present, though not significant, as were congestive heart failure (CHF), obesity and bronchiectasis (
Table 1).
Arterial blood gas characteristics: In the initial review of arterial blood gas at the time of Home Mechanical Ventilation (HMV) indication, the mean PaCO2 prior to initiating was 57 mmHg (SD 19) [38-81] compared to 46 mmHg (SD 22) [36–68] in a stable situation post-initiation. A significant improvement, defined as a decrease of as a reduction of more than 20% in PaCO2 with respect to the initial arterial blood gas, was observed in 51.5% of patients.
Characteristics of the non-invasive mechanical ventilation mode: The predominant ventilatory mode used was ST, mostly with the Lumis 150 Resmed® device. In 98.5% of patients, the mean IPAP was 17.04 cmH
2O (SD 5.2) [10 - 24]; The mean EPAP was 8.91 cmH
2O (SD 2.3) [5-13] with no significant differences between clinical phenotypes. The mean compliance was 7.66 hours (SD 7.3) [1.17-13.46] and the mean leak was 26.65 L/min (SD 57.2) [0-116]) (
Table 2).
Predictive capacity of the variables subject to telemonitoring: A relationship was found to be statistically nonsignificant between the degree of obstruction and the improvement of pCO
2 after initiating ventilation (
Table 3). Only AHT has been significantly associated with the failure of HMV, in turn a close but non-significant relationship is found with OSA. Variables such as pressures used, mean leaks, and compliance were not significantly associated with gasometric improvement after HMV initiation. However, a significant direct relationship was noted between the need for increased IPAP and EPAP and the number of emergency room visits (correlation coefficient of 0.35) and hospital admissions (correlation coefficient of 0.48) with no correlation to mortality.
Pharmacological characteristics: The specific inhaled treatment of each patient has been assessed, taking into account the use of dual therapy, triple therapy and the need for associated biological drugs. 24 patients are on triple therapy and 44 on dual therapy. No significant relationship was found between dual or triple inhaled therapy and the need to increase ventilatory pressures (IPAP or EPAP) as well as the need for re-titration of these variables. Similarly, no significant relationship was observed with patient survival when inhaled pharmacological treatment was combined with HMV therapy. It was found that those individuals with a lower forced expiratory volume in the first second post-bronchodilator test (FEV1 post) present greater use of triple therapy and a higher number of visits to the emergency room (p 0.013).
Table 1.
Economic impact: The study sample recorded 61 hospital admissions due to pulmonary causes, accounting for 48.5% of all hospital admissions to the Pulmonology Department. Likewise, 53 emergency consultations related to pulmonary causes have been recorded, equivalent to 64.7% of the department as a whole. In addition, 489 specialized outpatient consultations were carried out, including the areas of ventilation, COPD and pulmonary hypertension. Two patients died during the study period.
The average cost per patient in the sample analysed was €8,548 (SD €14,662) (
Table 4), with a high variability in individual costs: the patient with the highest cost was approximately €91,426, while the lowest cost was €6,614. This difference is mainly attributed to the need for hospital admission, which represents the highest average cost, followed by outpatient consultations and emergency visits.
4. Conclusions
Non-invasive Mechanical Ventilation is currently the standard of care for the treatment of patients with acute COPD. The Global Initiative for Chronic Obstructive Lung Diseases clearly establishes the indications for therapy with an evidence level of A.
In contrast, LTH-NIV in COPD patients is recommended conditionally with low-level evidence. The GesEPOC guidelines propose it for patients with persistent hypercapnia following an acute exacerbation requiring NIV or for stable hypercapnic patients. Achieving normocapnia implies a reduction in hospital readmissions and mortality, making it thus far the only variable that has been shown to be representative. In addition, both the ERS and the ATS recommend initiating therapy 2-4 weeks post-hospital discharge, rather than during the admission due to acute exacerbation.
The lack of clinical and functional variables associated with the LTH-NIV indication is evident in current guidelines. In our study, we propose chronic obstruction or increased dyspnea as potential indicators for LTH-NIV, rather than gasometric variables alone. Likewise, comorbidities should also be taken into account, and identifying clusters of patients would be an efficient way to indicate the therapy. As discussed below, some of the patients studied in our group did not initially present with marked hypercapnia, but rather associated dyspnea symptoms, improving their quality of life with treatment and night support.
COPD remains one of the most prevalent respiratory pathologies globally and imposes a significant economic burden on healthcare systems. Hospitalisation due to lack of stability, can incur direct annual costs of up to €40,000. Proper medical management and precise therapeutic indications can reduce potentially avoidable health costs, highlighting the importance of follow-up and control protocols.
During our study of COPD patients being monitored via telemonitoring, we observed that the majority are older adult men who show signs of emphysema and moderate obstruction. Almost all of them presented with hypercapnia greater than 54 mmHg at the start of therapy, although some emphysematous individuals initiated therapy without elevated PaCO2 but with clinical signs such as tachypnea and dyspnea on exertion. OSA and AHT are significant associated comorbidities, and chronic kidney disease, congestive heart failure, obesity and bronchiectasis stand out. AHT indicates the high failure of HMV. Patients with a lower failure rate showed a good response to therapy with LTH-NIV exhibiting a reduction of more than 20% in PaCO2, as observed in over half the sample.
In terms of healthcare resource usage, nearly half of the patients experienced at least one hospital admission for pulmonary causes, while over half had emergency visits related to pulmonary conditions. Additionally, nearly 500 specialised outpatient consultations were conducted. Mortality has been very low.
The average cost of the patients included in the sample has been €8,548, with a high variability in individual costs: the patient with the highest cost was approximately €91,426, while the lowest cost generated was €66. Given this significant economic cost, the associated morbidity and mortality and COPD’s prevalence, it is worth considering different therapies that can improve stability and increased survival while reducing health costs. The current guidelines focus on gasometric variables alone to indicate LTH-NIV, but this study highlights the importance of considering broader patient characteristics when initiating therapy. The patient is a set of circumstances and each individual may require support at different stages of the disease. Our study indicates that the PaCO2 variable is not the only manifestation that must be taken into account and that it is important to expand our registry in terms of the start and indication of LTH-NIV in COPD.
References
- Engstrom, C.G. Treatment of severe cases of respiratory paralysis by the Engström universal respirator. Br Med J. 1954, 2, 666–669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brochard, L.; Isabey, D.; Piquet, J.; Amaro, P.; Mancebo, J.; Messadi, A.A.; et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990, 323, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Diseases. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2016 report).
- Cosío, B.G.; Hernández, C.; Chiner, E.; Gimeno-Santos, E.; Pleguezuelos, E.; Seijas, N.; Rigau, D.; López-Campos, J.L.; Soler-Cataluñaj, J.J.; Calle, M.; Miravitlles, M.; Casanova, C.; en nombre del equipo de trabajo de GesEPOC 2021. Actualización 2021 de la Guía Española de la EPOC (GesEPOC). Tratamiento no farmacológico. Spanish COPD Guidelines (GesEPOC 2021): Non-pharmacological Treatment Update. Arch. Bronconeumol. 2022, 58, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Repir J 2019, 54, 1901003. [Google Scholar] [CrossRef] [PubMed]
- Macrea, M.; Oczkowski, S.; Rochwerg, B.; Branson, R.D.; Celli, B.; Coleman, J.M., III.; Hess, D.R.; Knight, S.L.; Ohar, J.A.; Orr, J.E.; Piper, A.J.; Punjabi, N.M.; Rahangdale, S.; Wijkstra, P.J.; Yim-Yeh, S.; Drummond, M.B.; Owens, R.L.; on behalf of the American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. Long-Term Noninvasive Ventilation in Chronic Stable Hypercapnic Chronic Obstructive Pulmonary Disease An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020, 202, e74–e87. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int J Chron Obstruct Pulmon Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Alvear, S.; Canteros, J.; Jara, J.; Rodríguez, P. Costos reales de tratamientos intensivos por paciente y día cama [Real daily costs of patients admitted to public intensive care units]. Rev Med Chil. 2013, 141, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Elpern, E.H.; Silver, M.R.; Rosen, R.L.; Bone, R.C. The noninvasive respiratory care unit. Patterns of use and financial implications. Chest. 1991, 99, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Galdeano Lozano, M.; Alfaro Álvarez, J.C.; Heili Frades, S.; Parra Macías, N.; Parra Ordaz, O. Mejora en la eficiencia del manejo del paciente ingresado en una unidad de cuidados respiratorios intermedios tras la introducción de un proceso asistencial integrado [Improvement in the efficiency of patient management in an intermediate respiratory care unit following the introduction of an integrated care process. ]. Journal Healthcare Quality Reserach. 2021, 36, 211–216. [Google Scholar] [CrossRef]
- Duiverman, M.L.; Maagh, P.; Magnet, F.S.; et al. Impact of high-intensity-NIV on the heart in stable COPD: A randomised crossover pilot study. Respir Res 2017, 18, 76, 37. [Google Scholar] [CrossRef] [PubMed]
- Duiverman, M.L.; Wempe, J.B.; Bladder, G.; et al. Two-year home-based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: A randomized controlled trial. Respir Res 2011, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Kohnlein, T.; Windisch, W.; Kohler, D.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2014, 2, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Order of Public Prices in the ICS - Germans Trias. Last viewed: 10/2023.
- Adler, D.; Perrig, S.; Takahashi, H.; et al. Polysomnography in stable COPD under non-invasive ventilation to reduce patient ventilator asynchrony and morning breathlessness. Sleep Breath 2012, 16, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Navalesi, P. Similowski, T., Whitelaw, W., Derenne, J., Eds.; Domiciliary noninvasive ventilatory support. In Clinical Management of chronic obstructive pulmonary disease; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 813–813. [Google Scholar]
- Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation. A consensus conference report. Chest 1999, 116, 521–534. [CrossRef] [PubMed]
- Actualisation 2003 des recommandations de la SPLF sur la prise en charge de la BPCO. Rev Mal Respir 2003; 20: 4S1-4S68. 44. Muir J, Levi-Valensi P. When should patients with COPD be ventilated? Eur J Respir Dis 1987, 70, 135–139.
- Tuggey, J.M.; Plant, P.K.; Elliott, M.W. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: An economic analysis. Thorax 2003, 58, 867–871. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).