Submitted:
14 February 2025
Posted:
18 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fungal Dimorphism Regulators
2.1. Temperature-Responsive Genes
2.2. Mating is Associated with Dimorphism
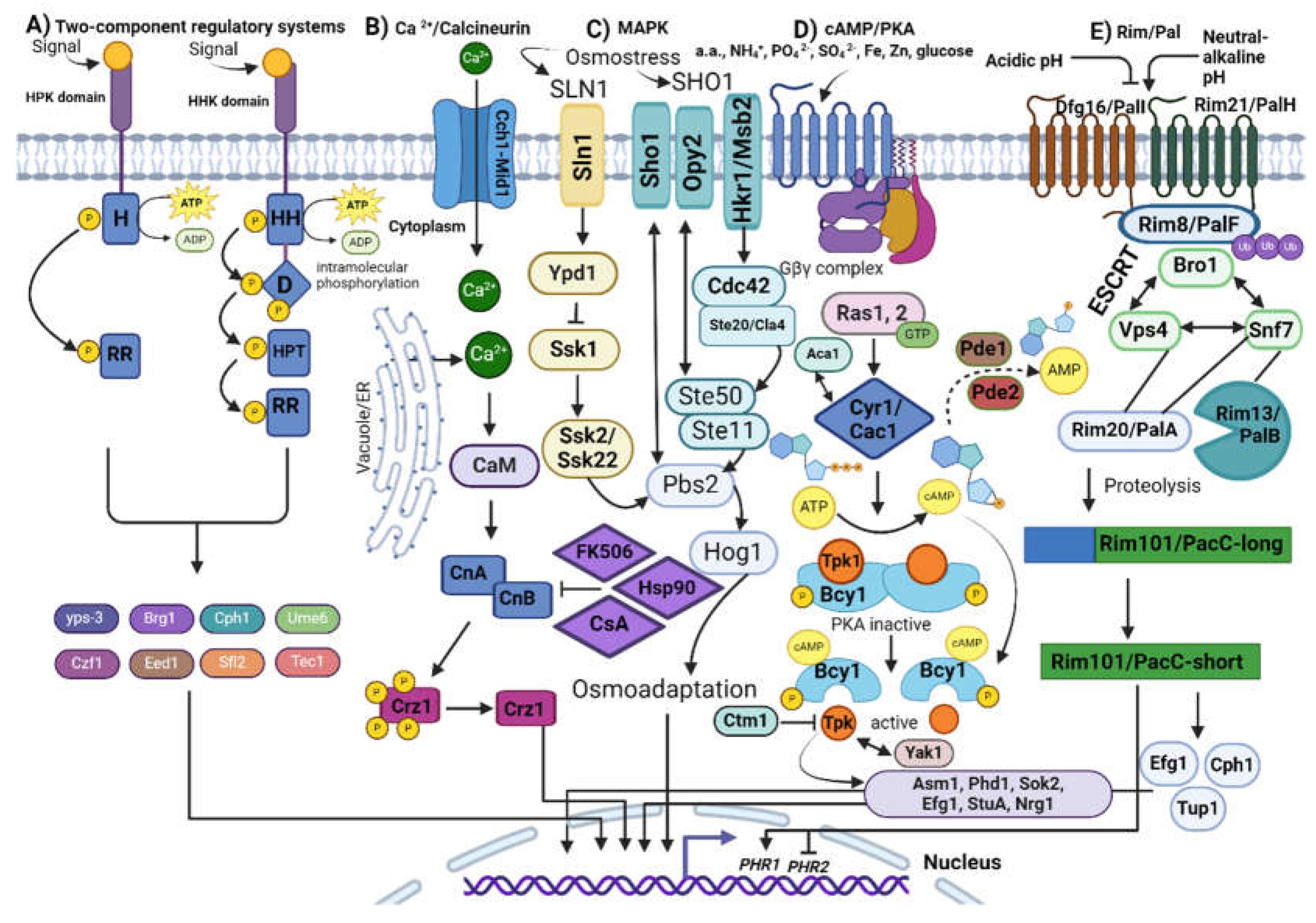
2.3. Signalling Cascades in Dimorphic Fungi
2.3.1. Two-Component Regulatory Systems
2.3.2. Calcium/Calcineurin Pathway
2.3.3. Mitogen-Activated Protein Kinase (MAPK)
2.3.4. Cyclic AMP-Dependent Protein Kinase A
2.3.5. Pal/Rim Pathway
3. Transcriptional Regulation of Fungal Dimorphism
4. Dimorphism Regulators with Therapeutic Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement:
Data Availability Statement
Conflicts of Interest
References
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog 2015, 11, e1004608. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.S.; Govender, N.P.; Corcoran, C.; Dlamini, S.; Prozesky, H.; Burton, R.; Mendelson, M.; Taljaard, J.; Lehloenya, R.; Calligaro, G.; et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: a retrospective case series. Clin Infect Dis 2015, 61, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.M. Fungal dimorphism and virulence: molecular mechanisms for temperature adaptation, immune evasion, and in vivo survival. Mediators Inflamm 2017, 2017, 8491383. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.; Klein, B.S. Insights into fungal morphogenesis and immune evasion: fungal conidia, when situated in mammalian lungs, may switch from mold to pathogenic yeasts or spore-forming spherules. Microbe Wash DC 2008, 3, 416–423. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin Microbiol Rev 2000, 13, 236–301. [Google Scholar] [CrossRef]
- Lee, S.C.; Li, A.; Calo, S.; Heitman, J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathog 2013, 9, e1003625. [Google Scholar] [CrossRef]
- Jiménez Mdel, P.; Restrepo, A.; Radzioch, D.; Cano, L.E.; García, L.F. Importance of complement 3 and mannose receptors in phagocytosis of Paracoccidioides brasiliensis conidia by Nramp1 congenic macrophages lines. FEMS Immunol Med Microbiol 2006, 47, 56–66. [Google Scholar] [CrossRef]
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect 2012, 14, 1093–1101. [Google Scholar] [CrossRef]
- McKenzie, C.G.; Koser, U.; Lewis, L.E.; Bain, J.M.; Mora-Montes, H.M.; Barker, R.N.; Gow, N.A.; Erwig, L.P. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun 2010, 78, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.F.; Bain, J.M.; Erwig, L.P.; Brown, A.J.P.; Gow, N.A.R. Hyphal swelling induced in the phagosome of macrophages. Fungal Biology 2024, 128, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Martínez-Duncker, I.; Gómez-Gaviria, M.; Mora-Montes, H.M. Differential recognition of clinically relevant Sporothrix species by human mononuclear cells. J Fungi (Basel) 2023, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol 2017, 8, 843. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans yeast, pseudohyphal, and hyphal morphogenesis differentially affects immune recognition. Front Immunol 2017, 8, 629. [Google Scholar] [CrossRef]
- Villalobos-Duno, H.L.; Barreto, L.A.; Alvarez-Aular, Á.; Mora-Montes, H.M.; Lozoya-Pérez, N.E.; Franco, B.; Lopes-Bezerra, L.M.; Niño-Vega, G.A. Comparison of cell wall polysaccharide composition and structure between strains of Sporothrix schenckii and Sporothrix brasiliensis. Front Microbiol 2021, 12, 726958. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 2005, 24, 1277–1286. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Naseem, S.; Konopka, J.B.; Sil, A. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet 2013, 9, e1003799. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Sil, A. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc Natl Acad Sci U S A 2008, 105, 4880–4885. [Google Scholar] [CrossRef]
- Webster, R.H.; Sil, A. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc Natl Acad Sci U S A 2008, 105, 14573–14578. [Google Scholar] [CrossRef]
- Gauthier, G.M.; Sullivan, T.D.; Gallardo, S.S.; Brandhorst, T.T.; Vanden Wymelenberg, A.J.; Cuomo, C.A.; Suen, G.; Currie, C.R.; Klein, B.S. SREB, a GATA transcription factor that directs disparate fates in Blastomyces dermatitidis including morphogenesis and siderophore biosynthesis. PLoS Pathog 2010, 6, e1000846. [Google Scholar] [CrossRef]
- Chao, L.Y.; Marletta, M.A.; Rine, J. Sre1, an iron-modulated GATA DNA-binding protein of iron-uptake genes in the fungal pathogen Histoplasma capsulatum. Biochemistry 2008, 47, 7274–7283. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Konopka, J.B. A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis. Eukaryot Cell 2012, 11, 1210–1218. [Google Scholar] [CrossRef]
- González-Novo, A.; Correa-Bordes, J.; Labrador, L.; Sánchez, M.; Vázquez de Aldana, C.R.; Jiménez, J. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol Biol Cell 2008, 19, 1509–1518. [Google Scholar] [CrossRef]
- Warenda, A.J.; Konopka, J.B. Septin function in Candida albicans morphogenesis. Mol Biol Cell 2002, 13, 2732–2746. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Sellam, A.; Tebbji, F.; Whiteway, M.; Nantel, A.; Cowen, L.E. Pho85, Pcl1, and Hms1 signaling governs Candida albicans morphogenesis induced by high temperature or Hsp90 compromise. Curr Biol 2012, 22, 461–470. [Google Scholar] [CrossRef]
- Robbins, N.; Cowen, L.E. Roles of Hsp90 in Candida albicans morphogenesis and virulence. Curr Opin Microbiol 2023, 75, 102351. [Google Scholar] [CrossRef]
- Saraswat, D.; Kumar, R.; Pande, T.; Edgerton, M.; Cullen, P.J. Signalling mucin Msb2 regulates adaptation to thermal stress in Candida albicans. Mol Microbiol 2016, 100, 425–441. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiology Reviews 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Beyhan, S.; Gutierrez, M.; Voorhies, M.; Sil, A. A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol 2013, 11, e1001614. [Google Scholar] [CrossRef]
- Cleare, L.G.; Zamith-Miranda, D.; Nosanchuk, J.D. Heat shock proteins in Histoplasma and Paracoccidioides. Clin Vaccine Immunol 2017, 24, e00221–00217. [Google Scholar] [CrossRef]
- Caruso, M.; Sacco, M.; Medoff, G.; Maresca, B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatum strains with different levels of thermotolerance and pathogenicity. Mol Microbiol 1987, 1, 151–158. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.P.; Borges-Walmsley, M.I.; Pereira, I.S.; Soares, C.M.; Walmsley, A.R.; Felipe, M.S. Differential expression of an hsp70 gene during transition from the mycelial to the infective yeast form of the human pathogenic fungus Paracoccidioides brasiliensis. Mol Microbiol 1999, 31, 1039–1050. [Google Scholar] [CrossRef]
- Minchiotti, G.; Gargano, S.; Maresca, B. The intron-containing hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum is properly spliced in severe heat shock conditions. Mol Cell Biol 1991, 11, 5624–5630. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.A.; Zemska, O.; Rappleye, C.A. Discovery of a role for Hsp82 in Histoplasma virulence through a quantitative screen for macrophage lethality. Infect Immun 2011, 79, 3348–3357. [Google Scholar] [CrossRef]
- Rocha, M.C.; Minari, K.; Fabri, J.; Kerkaert, J.D.; Gava, L.M.; da Cunha, A.F.; Cramer, R.A.; Borges, J.C.; Malavazi, I. Aspergillus fumigatus Hsp90 interacts with the main components of the cell wall integrity pathway and cooperates in heat shock and cell wall stress adaptation. Cell Microbiol 2021, 23, e13273. [Google Scholar] [CrossRef]
- Nicola, A.M.; Andrade, R.V.; Dantas, A.S.; Andrade, P.A.; Arraes, F.B.; Fernandes, L.; Silva-Pereira, I.; Felipe, M.S. The stress responsive and morphologically regulated hsp90 gene from Paracoccidioides brasiliensis is essential to cell viability. BMC Microbiol 2008, 8, 158. [Google Scholar] [CrossRef]
- Matos, T.G.; Morais, F.V.; Campos, C.B. Hsp90 regulates Paracoccidioides brasiliensis proliferation and ROS levels under thermal stress and cooperates with calcineurin to control yeast to mycelium dimorphism. Med Mycol 2013, 51, 413–421. [Google Scholar] [CrossRef]
- Morrow, C.A.; Fraser, J.A. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res 2009, 9, 161–177. [Google Scholar] [CrossRef]
- Lee, H.; Chang, Y.C.; Kwon-Chung, K.J. TUP1 disruption reveals biological differences between MATa and MATα strains of Cryptococcus neoformans. Mol Microbiol 2005, 55, 1222–1232. [Google Scholar] [CrossRef]
- Fraser, J.A.; Heitman, J. Fungal mating-type loci. Curr Biol 2003, 13, R792–R795. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc Natl Acad Sci U S A 2001, 98, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol 2009, 9, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Fink, G.R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev 1994, 8, 2974–2985. [Google Scholar] [CrossRef]
- Chang, Y.C.; Wickes, B.L.; Miller, G.F.; Penoyer, L.A.; Kwon-Chung, K.J. Cryptococcus neoformans STE12alpha regulates virulence but is not essential for mating. J Exp Med 2000, 191, 871–882. [Google Scholar] [CrossRef]
- Hull, C.M.; Boily, M.J.; Heitman, J. Sex-specific homeodomain proteins Sxi1alpha and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot Cell 2005, 4, 526–535. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef]
- Bennett, R.J.; Turgeon, B.G. Fungal sex: the Ascomycota. Microbiol Spectr 2016, 4. [Google Scholar] [CrossRef]
- Reedy, J.L.; Floyd, A.M.; Heitman, J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr Biol 2009, 19, 891–899. [Google Scholar] [CrossRef]
- Sherwood, R.K.; Scaduto, C.M.; Torres, S.E.; Bennett, R.J. Convergent evolution of a fused sexual cycle promotes the haploid lifestyle. Nature 2014, 506, 387–390. [Google Scholar] [CrossRef]
- Kvaal, C.A.; Srikantha, T.; Soll, D.R. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun 1997, 65, 4468–4475. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One 2008, 3, e1473. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Hernday, A.D.; Hirakawa, M.P.; Johnson, A.D.; Bennett, R.J. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog 2013, 9, e1003210. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Nicolet, J. Specificity models in MAPK cascade signaling. FEBS Open Bio 2023, 13, 1177–1192. [Google Scholar] [CrossRef]
- Braunsdorf, C.; Mailänder-Sánchez, D.; Schaller, M. Fungal sensing of host environment. Cell Microbiol 2016, 18, 1188–1200. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Xue, C.; Idnurm, A.; Rutherford, J.C.; Heitman, J.; Cardenas, M.E. Sensing the environment: lessons from fungi. Nat Rev Microbiol 2007, 5, 57–69. [Google Scholar] [CrossRef]
- Xue, C.; Hsueh, Y.P.; Heitman, J. Magnificent seven: roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol Rev 2008, 32, 1010–1032. [Google Scholar] [CrossRef]
- Basso, V.; Znaidi, S.; Lagage, V.; Cabral, V.; Schoenherr, F.; LeibundGut-Landmann, S.; d'Enfert, C.; Bachellier-Bassi, S. The two-component response regulator Skn7 belongs to a network of transcription factors regulating morphogenesis in Candida albicans and independently limits morphogenesis-induced ROS accumulation. Mol Microbiol 2017, 106, 157–182. [Google Scholar] [CrossRef]
- Liao, B.; Ye, X.; Chen, X.; Zhou, Y.; Cheng, L.; Zhou, X.; Ren, B. The two-component signal transduction system and its regulation in Candida albicans. Virulence 2021, 12, 1884–1899. [Google Scholar] [CrossRef]
- Chaves, A.F.; Navarro, M.V.; Castilho, D.G.; Calado, J.C.; Conceição, P.M.; Batista, W.L. A conserved dimorphism-regulating histidine kinase controls the dimorphic switching in Paracoccidioides brasiliensis. FEMS Yeast Res 2016, 16, fow047. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, Z.; Zheng, F.; Liu, X. Molecular cloning, characterization and differential expression of DRK1 in Sporothrix schenckii. Int J Mol Med 2013, 31, 99–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, B.; Wu, Y.Z.; Wang, Y.; Liu, X.; Han, S. Two-component histidine kinase DRK1 is required for pathogenesis in Sporothrix schenckii. Mol Med Rep 2018, 17, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.; Mavrianos, J.; Chauhan, N. Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot Cell 2011, 10, 1370–1374. [Google Scholar] [CrossRef]
- Mavrianos, J.; Desai, C.; Chauhan, N. Two-component histidine phosphotransfer protein Ypd1 is not essential for viability in Candida albicans. Eukaryot Cell 2014, 13, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Calderone, R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res 2006, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Okabe, T.; Mio, T.; Ono, N.; Kashima, Y.; Matsui, M.; Arisawa, M.; Yamada-Okabe, H. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol 1999, 181, 7243–7247. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, Y.J.; Kim, S.Y.; Bahn, Y.S. Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryot Cell 2011, 10, 998–1002. [Google Scholar] [CrossRef]
- Li, S.; Ault, A.; Malone, C.L.; Raitt, D.; Dean, S.; Johnston, L.H.; Deschenes, R.J.; Fassler, J.S. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. Embo j 1998, 17, 6952–6962. [Google Scholar] [CrossRef]
- Chauvel, M.; Nesseir, A.; Cabral, V.; Znaidi, S.; Goyard, S.; Bachellier-Bassi, S.; Firon, A.; Legrand, M.; Diogo, D.; Naulleau, C.; et al. A versatile overexpression strategy in the pathogenic yeast Candida albicans: identification of regulators of morphogenesis and fitness. PLoS One 2012, 7, e45912. [Google Scholar] [CrossRef]
- Capra, E.J.; Laub, M.T. Evolution of two-component signal transduction systems. Annu Rev Microbiol 2012, 66, 325–347. [Google Scholar] [CrossRef]
- Tebbets, B.; Yu, Z.; Stewart, D.; Zhao, L.X.; Jiang, Y.; Xu, L.H.; Andes, D.; Shen, B.; Klein, B. Identification of antifungal natural products via Saccharomyces cerevisiae bioassay: insights into macrotetrolide drug spectrum, potency and mode of action. Med Mycol 2013, 51, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.N.; Menon, S.K.; West, A.H. Extended N-terminal region of the essential phosphorelay signaling protein Ypd1 from Cryptococcus neoformans contributes to structural stability, phosphostability and binding of calcium ions. FEMS Yeast Res 2016, 16, fow068. [Google Scholar] [CrossRef] [PubMed]
- Colinet, A.S.; Sengottaiyan, P.; Deschamps, A.; Colsoul, M.L.; Thines, L.; Demaegd, D.; Duchêne, M.C.; Foulquier, F.; Hols, P.; Morsomme, P. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci Rep 2016, 6, 24282. [Google Scholar] [CrossRef]
- Xu, J.R. Map kinases in fungal pathogens. Fungal Genet Biol 2000, 31, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Miskei, M.; Karányi, Z.; Pócsi, I. Annotation of stress-response proteins in the aspergilli. Fungal Genet Biol 2009, 46 Suppl 1, S105–S120. [Google Scholar] [CrossRef]
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J Biol Chem 2002, 277, 33075–33080. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Wu, J.; Han, J.; Li, S.; Shao, S. Transcriptomic analysis reveals genes mediating salt tolerance through calcineurin/CchA-Independent signaling in Aspergillus nidulans. Biomed Res Int 2017, 2017, 4378627. [Google Scholar] [CrossRef]
- Mendoza, I.; Rubio, F.; Rodriguez-Navarro, A.; Pardo, J.M. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem 1994, 269, 8792–8796. [Google Scholar] [CrossRef]
- Fox, D.S.; Cruz, M.C.; Sia, R.A.; Ke, H.; Cox, G.M.; Cardenas, M.E.; Heitman, J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol Microbiol 2001, 39, 835–849. [Google Scholar] [CrossRef]
- Stie, J.; Fox, D. Calcineurin regulation in fungi and beyond. Eukaryot Cell 2008, 7, 177–186. [Google Scholar] [CrossRef]
- Kothe, G.O.; Free, S.J. Calcineurin subunit B is required for normal vegetative growth in Neurospora crassa. Fungal Genet Biol 1998, 23, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.C.; Goldstein, A.L.; Blankenship, J.R.; Del Poeta, M.; Davis, D.; Cardenas, M.E.; Perfect, J.R.; McCusker, J.H.; Heitman, J. Calcineurin is essential for survival during membrane stress in Candida albicans. Embo j 2002, 21, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Chávez, J.A.; Ali, S.; Bakkeren, G. Response to environmental stresses, cell-wall integrity, and virulence are orchestrated through the calcineurin pathway in Ustilago hordei. Mol Plant Microbe Interact 2011, 24, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Garfoot, A.L.; Rappleye, C.A. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. Febs j 2016, 283, 619–633. [Google Scholar] [CrossRef]
- Li, W.; Shrivastava, M.; Lu, H.; Jiang, Y. Calcium-calcineurin signaling pathway in Candida albicans: A potential drug target. Microbiol Res 2021, 249, 126786. [Google Scholar] [CrossRef]
- Bader, T.; Bodendorfer, B.; Schröppel, K.; Morschhäuser, J. Calcineurin is essential for virulence in Candida albicans. Infect Immun 2003, 71, 5344–5354. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: a specialized but essential protein-folding tool. J Cell Biol 2001, 154, 267–273. [Google Scholar] [CrossRef]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother 2003, 47, 956–964. [Google Scholar] [CrossRef]
- Li, L.; An, M.; Shen, H.; Huang, X.; Yao, X.; Liu, J.; Zhu, F.; Zhang, S.; Chen, S.; He, L.; et al. The non-Geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole. Am J Transl Res 2015, 7, 2589–2602. [Google Scholar]
- Yu, Q.; Wang, H.; Cheng, X.; Xu, N.; Ding, X.; Xing, L.; Li, M. Roles of Cch1 and Mid1 in morphogenesis, oxidative stress response and virulence in Candida albicans. Mycopathologia 2012, 174, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1. FEBS Lett 2010, 584, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mallick, J.; Maqnas, A.; Sun, Y.; Choudhury, B.I.; Côte, P.; Yan, L.; Ni, T.J.; Li, Y.; Zhang, D.; et al. Chemogenomic profiling of the fungal pathogen Candida albicans. Antimicrob Agents Chemother 2018, 62, e02365–02317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, D.; Hameed, A.; Fang, T.; Bakr Ahmad Fazili, A.; Asghar, F. The plasma membrane protein Rch1 and the Golgi/ER calcium pump Pmr1 have an additive effect on filamentation in Candida albicans. Fungal Genet Biol 2018, 115, 1–8. [Google Scholar] [CrossRef]
- Luna-Tapia, A.; DeJarnette, C.; Sansevere, E.; Reitler, P.; Butts, A.; Hevener, K.E.; Palmer, G.E. The vacuolar Ca(2+) ATPase pump Pmc1p is required for Candida albicans pathogenesis. mSphere 2019, 4, e00715–00718. [Google Scholar] [CrossRef]
- Karababa, M.; Valentino, E.; Pardini, G.; Coste, A.T.; Bille, J.; Sanglard, D. CRZ1, a target of the calcineurin pathway in Candida albicans. Mol Microbiol 2006, 59, 1429–1451. [Google Scholar] [CrossRef]
- Blankenship, J.R.; Wormley, F.L.; Boyce, M.K.; Schell, W.A.; Filler, S.G.; Perfect, J.R.; Heitman, J. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell 2003, 2, 422–430. [Google Scholar] [CrossRef]
- Chen, Y.L.; Brand, A.; Morrison, E.L.; Silao, F.G.; Bigol, U.G.; Malbas, F.F., Jr.; Nett, J.E.; Andes, D.R.; Solis, N.V.; Filler, S.G.; et al. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot Cell 2011, 10, 803–819. [Google Scholar] [CrossRef]
- Odom, A.; Muir, S.; Lim, E.; Toffaletti, D.L.; Perfect, J.; Heitman, J. Calcineurin is required for virulence of Cryptococcus neoformans. Embo j 1997, 16, 2576–2589. [Google Scholar] [CrossRef]
- Kraus, P.R.; Nichols, C.B.; Heitman, J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot Cell 2005, 4, 1079–1087. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Cramer, R.A., Jr.; Perfect, B.Z.; Asfaw, Y.G.; Sauer, T.C.; Najvar, L.K.; Kirkpatrick, W.R.; Patterson, T.F.; Benjamin, D.K., Jr.; Heitman, J.; et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell 2006, 5, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E.; Burns, K.A.; Randell, S.H.; Steinbach, W.J. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Mol Microbiol 2011, 82, 1235–1259. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Lamoth, F.; Steinbach, W.J. Calcineurin as a multifunctional regulator: unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol Rev 2014, 28, 56–69. [Google Scholar] [CrossRef]
- Campos, C.B.; Di Benedette, J.P.; Morais, F.V.; Ovalle, R.; Nobrega, M.P. Evidence for the role of calcineurin in morphogenesis and calcium homeostasis during mycelium-to-yeast dimorphism of Paracoccidioides brasiliensis. Eukaryot Cell 2008, 7, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- García-Carnero, L.C.; Martínez-Álvarez, J.A. Virulence factors of Sporothrix schenckii. J Fungi (Basel) 2022, 8, 318. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.R. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef]
- May, G.S.; Xue, T.; Kontoyiannis, D.P.; Gustin, M.C. Mitogen activated protein kinases of Aspergillus fumigatus. Medical Mycology 2005, 43, S83–S86. [Google Scholar] [CrossRef]
- Takayama, T.; Yamamoto, K.; Saito, H.; Tatebayashi, K. Interaction between the transmembrane domains of Sho1 and Opy2 enhances the signaling efficiency of the Hog1 MAP kinase cascade in Saccharomyces cerevisiae. PLoS One 2019, 14, e0211380. [Google Scholar] [CrossRef]
- Monge, R.A.; Román, E.; Nombela, C.; Pla, J. The MAP kinase signal transduction network in Candida albicans. Microbiology (Reading) 2006, 152, 905–912. [Google Scholar] [CrossRef]
- Lo, H.J.; Köhler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Braun, B.R.; Head, W.S.; Wang, M.X.; Johnson, A.D. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 2000, 156, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Csank, C.; Schröppel, K.; Leberer, E.; Harcus, D.; Mohamed, O.; Meloche, S.; Thomas, D.Y.; Whiteway, M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun 1998, 66, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, K.; Kim, J.; Yee, S.; Kim, W.; Choi, W. A MAP kinase pathway is implicated in the pseudohyphal induction by hydrogen peroxide in Candica albicans. Mol Cells 2012, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Nombela, C.; Alonso-Monge, R.; Pla, J. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology (Reading) 2005, 151, 1033–1049. [Google Scholar] [CrossRef]
- Román, E.; Nombela, C.; Pla, J. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol Cell Biol 2005, 25, 10611–10627. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Navarro-García, F.; Molero, G.; Diez-Orejas, R.; Gustin, M.; Pla, J.; Sánchez, M.; Nombela, C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol 1999, 181, 3058–3068. [Google Scholar] [CrossRef]
- Du, C.; Sarfati, J.; Latge, J.P.; Calderone, R. The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med Mycol 2006, 44, 211–218. [Google Scholar] [CrossRef]
- Ma, D.; Li, R. Current understanding of HOG-MAPK pathway in Aspergillus fumigatus. Mycopathologia 2013, 175, 13–23. [Google Scholar] [CrossRef]
- Jang, Y.-B.; Kim, J.-Y.; Bahn, Y.-S. Unraveling the cryptic functions of mitogen-activated protein kinases Cpk2 and Mpk2 in Cryptococcus neoformans. mBio 2024, 15, e01156–01124. [Google Scholar] [CrossRef]
- González-Rubio, G.; Fernández-Acero, T.; Martín, H.; Molina, M. Mitogen-activated protein kinase phosphatases (MKPs) in fungal signaling: conservation, function, and regulation. Int J Mol Sci 2019, 20, 1709. [Google Scholar] [CrossRef]
- Rodriguez, L.; Voorhies, M.; Gilmore, S.; Beyhan, S.; Myint, A.; Sil, A. Opposing signaling pathways regulate morphology in response to temperature in the fungal pathogen Histoplasma capsulatum. PLoS Biol 2019, 17, e3000168. [Google Scholar] [CrossRef] [PubMed]
- Sil, A. Molecular regulation of Histoplasma dimorphism. Curr Opin Microbiol 2019, 52, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jung, W.H.; Kronstad, J.W. The cAMP/protein kinase A signaling pathway in pathogenic basidiomycete fungi: Connections with iron homeostasis. J Microbiol 2015, 53, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Leberer, E.; Harcus, D.; Dignard, D.; Johnson, L.; Ushinsky, S.; Thomas, D.Y.; Schröppel, K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol 2001, 42, 673–687. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/protein kinase a pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front Cell Infect Microbiol 2019, 9, 212. [Google Scholar] [CrossRef]
- Chen, D.; Janganan, T.K.; Chen, G.; Marques, E.R.; Kress, M.R.; Goldman, G.H.; Walmsley, A.R.; Borges-Walmsley, M.I. The cAMP pathway is important for controlling the morphological switch to the pathogenic yeast form of Paracoccidioides brasiliensis. Mol Microbiol 2007, 65, 761–779. [Google Scholar] [CrossRef]
- Zaremberg, V.; Donella-Deana, A.; Moreno, S. Mechanism of activation of cAMP-dependent protein kinase: in Mucor rouxii the apparent specific activity of the cAMP-activated holoenzyme is different than that of its free catalytic subunit. Arch Biochem Biophys 2000, 381, 74–82. [Google Scholar] [CrossRef]
- Zeng, G.; Xu, X.; Kok, Y.J.; Deng, F.S.; Ling Chow, E.W.; Gao, J.; Bi, X.; Wang, Y. Cytochrome c regulates hyphal morphogenesis by interfering with cAMP-PKA signaling in Candida albicans. Cell Rep 2023, 42, 113473. [Google Scholar] [CrossRef]
- Zuber, S.; Hynes, M.J.; Andrianopoulos, A. G-protein signaling mediates asexual development at 25 degrees C but has no effect on yeast-like growth at 37 degrees C in the dimorphic fungus Penicillium mameffei. Eukaryot Cell 2002, 1, 440–447. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Q.; Wei, Y.; Wang, Y.; Du, H. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Mol Microbiol 2019, 111, 6–16. [Google Scholar] [CrossRef]
- Wijnants, S.; Vreys, J.; Nysten, J.; Van Dijck, P. The Cdc25 and Ras1 proteins of Candida albicans Influence epithelial toxicity in a niche-specific way. J Fungi (Basel) 2023, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Piispanen, A.E.; Bonnefoi, O.; Carden, S.; Deveau, A.; Bassilana, M.; Hogan, D.A. Roles of Ras1 membrane localization during Candida albicans hyphal growth and farnesol response. Eukaryot Cell 2011, 10, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fang, H.M.; Wang, Y.M.; Zeng, G.S.; Zheng, X.D.; Wang, Y. Ras1 and Ras2 play antagonistic roles in regulating cellular cAMP level, stationary-phase entry and stress response in Candida albicans. Mol Microbiol 2009, 74, 862–875. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Ramírez-Sotelo, U.; Mora-Montes, H.M. Non-albicans Candida species: immune response, evasion mechanisms, and new plant-derived alternative therapies. J Fungi (Basel) 2022, 9, 11. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Hicks, J.K.; Giles, S.S.; Cox, G.M.; Heitman, J. Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot Cell 2004, 3, 1476–1491. [Google Scholar] [CrossRef]
- Mogensen, E.G.; Janbon, G.; Chaloupka, J.; Steegborn, C.; Fu, M.S.; Moyrand, F.; Klengel, T.; Pearson, D.S.; Geeves, M.A.; Buck, J.; et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell 2006, 5, 103–111. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bahn, Y.S.; Heitman, J. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot Cell 2005, 4, 1971–1981. [Google Scholar] [CrossRef]
- Davis-Hanna, A.; Piispanen, A.E.; Stateva, L.I.; Hogan, D.A. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 2008, 67, 47–62. [Google Scholar] [CrossRef]
- MacAlpine, J.; Liu, Z.; Hossain, S.; Whitesell, L.; Robbins, N.; Cowen, L.E. DYRK-family kinases regulate Candida albicans morphogenesis and virulence through the Ras1/PKA pathway. mBio 2023, 14, e0218323. [Google Scholar] [CrossRef]
- Giusani, A.D.; Vinces, M.; Kumamoto, C.A. Invasive filamentous growth of Candida albicans is promoted by Czf1p-dependent relief of Efg1p-mediated repression. Genetics 2002, 160, 1749–1753. [Google Scholar] [CrossRef]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 2007, 71, 348–376. [Google Scholar] [CrossRef] [PubMed]
- Glazier, V.E. EFG1, everyone's favorite gene in Candida albicans: a comprehensive literature review. Front Cell Infect Microbiol 2022, 12, 855229. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yu, J.; Lu, Y. Hyphal development in Candida albicans from different cell states. Curr Genet 2018, 64, 1239–1243. [Google Scholar] [CrossRef]
- Maeng, S.; Ko, Y.J.; Kim, G.B.; Jung, K.W.; Floyd, A.; Heitman, J.; Bahn, Y.S. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot Cell 2010, 9, 360–378. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Jung, K.W. Stress signaling pathways for the pathogenicity of Cryptococcus. Eukaryot Cell 2013, 12, 1564–1577. [Google Scholar] [CrossRef]
- Maliehe, M.; Ntoi, M.A.; Lahiri, S.; Folorunso, O.S.; Ogundeji, A.O.; Pohl, C.H.; Sebolai, O.M. Environmental factors that contribute to the maintenance of Cryptococcus neoformans pathogenesis. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Selvig, K.; Alspaugh, J.A. pH Response pathways in fungi: adapting to host-derived and environmental signals. Mycobiology 2011, 39, 249–256. [Google Scholar] [CrossRef]
- Cervantes-Chávez, J.A.; Ortiz-Castellanos, L.; Tejeda-Sartorius, M.; Gold, S.; Ruiz-Herrera, J. Functional analysis of the pH responsive pathway Pal/Rim in the phytopathogenic basidiomycete Ustilago maydis. Fungal Genet Biol 2010, 47, 446–457. [Google Scholar] [CrossRef]
- Davis, D.; Wilson, R.B.; Mitchell, A.P. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol 2000, 20, 971–978. [Google Scholar] [CrossRef]
- Davis, D.; Edwards, J.E., Jr.; Mitchell, A.P.; Ibrahim, A.S. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun 2000, 68, 5953–5959. [Google Scholar] [CrossRef]
- Ost, K.S.; O'Meara, T.R.; Huda, N.; Esher, S.K.; Alspaugh, J.A. The Cryptococcus neoformans alkaline response pathway: identification of a novel rim pathway activator. PLoS Genet 2015, 11, e1005159. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr Opin Microbiol 2009, 12, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; He, X.Y.; Chen, J.W.; Mao, Y.S.; Gao, X.D. The pH-responsive transcription factors YlRim101 and Mhy1 regulate alkaline pH-induced filamentation in the dimorphic yeast Yarrowia lipolytica. mSphere 2021, 6, e00179–00121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Wang, Y.; Zhang, Z.B.; Yang, H.L.; Yan, R.M.; Zhu, D. Influence of environmental and nutritional conditions on yeast–mycelial dimorphic transition in Trichosporon cutaneum. Biotechnology & Biotechnological Equipment 2017, 31, 516–526. [Google Scholar] [CrossRef]
- Kullas, A.L.; Li, M.; Davis, D.A. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot Cell 2004, 3, 1609–1618. [Google Scholar] [CrossRef]
- O'Meara, T.R.; Norton, D.; Price, M.S.; Hay, C.; Clements, M.F.; Nichols, C.B.; Alspaugh, J.A. Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 2010, 6, e1000776. [Google Scholar] [CrossRef]
- Odorizzi, G.; Katzmann, D.J.; Babst, M.; Audhya, A.; Emr, S.D. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J Cell Sci 2003, 116, 1893–1903. [Google Scholar] [CrossRef]
- Sentandreu, M.; Elorza, M.V.; Sentandreu, R.; Fonzi, W.A. Cloning and characterization of PRA1, a gene encoding a novel pH-regulated antigen of Candida albicans. J Bacteriol 1998, 180, 282–289. [Google Scholar] [CrossRef]
- Saporito-Irwin, S.M.; Birse, C.E.; Sypherd, P.S.; Fonzi, W.A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol 1995, 15, 601–613. [Google Scholar] [CrossRef]
- Liu, H. Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol 2001, 4, 728–735. [Google Scholar] [CrossRef]
- Cornet, M.; Gaillardin, C. pH signaling in human fungal pathogens: a new target for antifungal strategies. Eukaryot Cell 2014, 13, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lockhart, S.R.; Daniels, K.; Soll, D.R. Roles of TUP1 in switching, phase maintenance, and phase-specific gene expression in Candida albicans. Eukaryot Cell 2002, 1, 353–365. [Google Scholar] [CrossRef]
- Marcos, C.M.; de Oliveira, H.C.; Assato, P.A.; de Oliveira, L.T.; Fregonezi, N.; Dos Santos, K.S.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Polypeptides targeting Paracoccidioides brasiliensis Drk1. J Fungi (Basel) 2023, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Bezerra, B.T.; Rodrigues, M.L. Antifungal development and the urgency of minimizing the impact of fungal diseases on public health. ACS Bio Med Chem Au 2023, 3, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.M.; de Oliveira, H.C.; Assato, P.A.; Castelli, R.F.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Drk1, a Dimorphism Histidine Kinase, contributes to morphology, virulence, and stress adaptation in Paracoccidioides brasiliensis. J Fungi (Basel) 2021, 7, 852. [Google Scholar] [CrossRef]
- Navarro, M.V.; de Barros, Y.N.; Segura, W.D.; Chaves, A.F.A.; Jannuzzi, G.P.; Ferreira, K.S.; Xander, P.; Batista, W.L. The Role of Dimorphism Regulating Histidine Kinase (Drk1) in the pathogenic fungus Paracoccidioides brasiliensis cell wall. J Fungi (Basel) 2021, 7, 1014. [Google Scholar] [CrossRef]
- Martin, D.C.; Kim, H.; Mackin, N.A.; Maldonado-Báez, L.; Evangelista, C.C., Jr.; Beaudry, V.G.; Dudgeon, D.D.; Naiman, D.Q.; Erdman, S.E.; Cunningham, K.W. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J Biol Chem 2011, 286, 10744–10754. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 2003, 48, 959–976. [Google Scholar] [CrossRef]
- Whitesell, L.; Robbins, N.; Huang, D.S.; McLellan, C.A.; Shekhar-Guturja, T.; LeBlanc, E.V.; Nation, C.S.; Hui, R.; Hutchinson, A.; Collins, C.; et al. Structural basis for species-selective targeting of Hsp90 in a pathogenic fungus. Nat Commun 2019, 10, 402. [Google Scholar] [CrossRef]
- Cornet, M.; Gaillardin, C.; Richard, M.L. Deletions of the endocytic components VPS28 and VPS32 in Candida albicans lead to echinocandin and azole hypersensitivity. Antimicrob Agents Chemother 2006, 50, 3492–3495. [Google Scholar] [CrossRef]
- Garnaud, C.; García-Oliver, E.; Wang, Y.; Maubon, D.; Bailly, S.; Despinasse, Q.; Champleboux, M.; Govin, J.; Cornet, M. The Rim pathway mediates antifungal tolerance in Candida albicans through newly identified Rim101 transcriptional targets, including Hsp90 and Ipt1. Antimicrob Agents Chemother 2018, 62, e01785–01717. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Robbins, N.; Cowen, L.E. The role of Candida albicans stress response pathways in antifungal tolerance and resistance. iScience 2022, 25, 103953. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, K.T.; Lee, M.H.; Cheong, E.; Bahn, Y.S. Adenylyl cyclase and protein kinase A play redundant and distinct roles in growth, differentiation, antifungal drug resistance, and pathogenicity of Candida auris. mBio 2021, 12, e0272921. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




