Submitted:
13 February 2025
Posted:
14 February 2025
Read the latest preprint version here
Abstract
Keywords:
1.1. Background
1.2. Introduction
2. Synthesis and Medical Applications of Carbon Nanomaterials
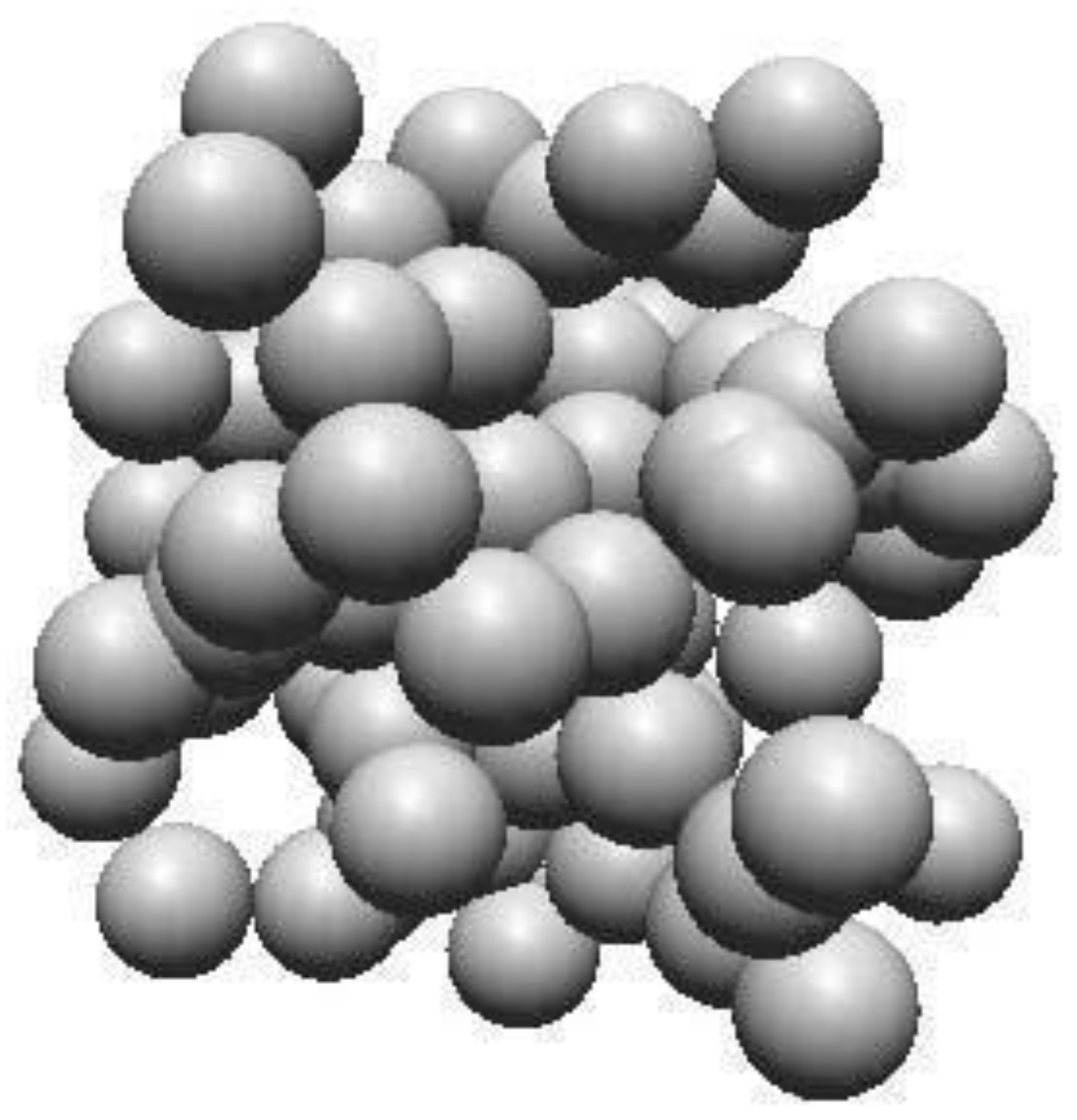
2.1. Amorphous Carbon
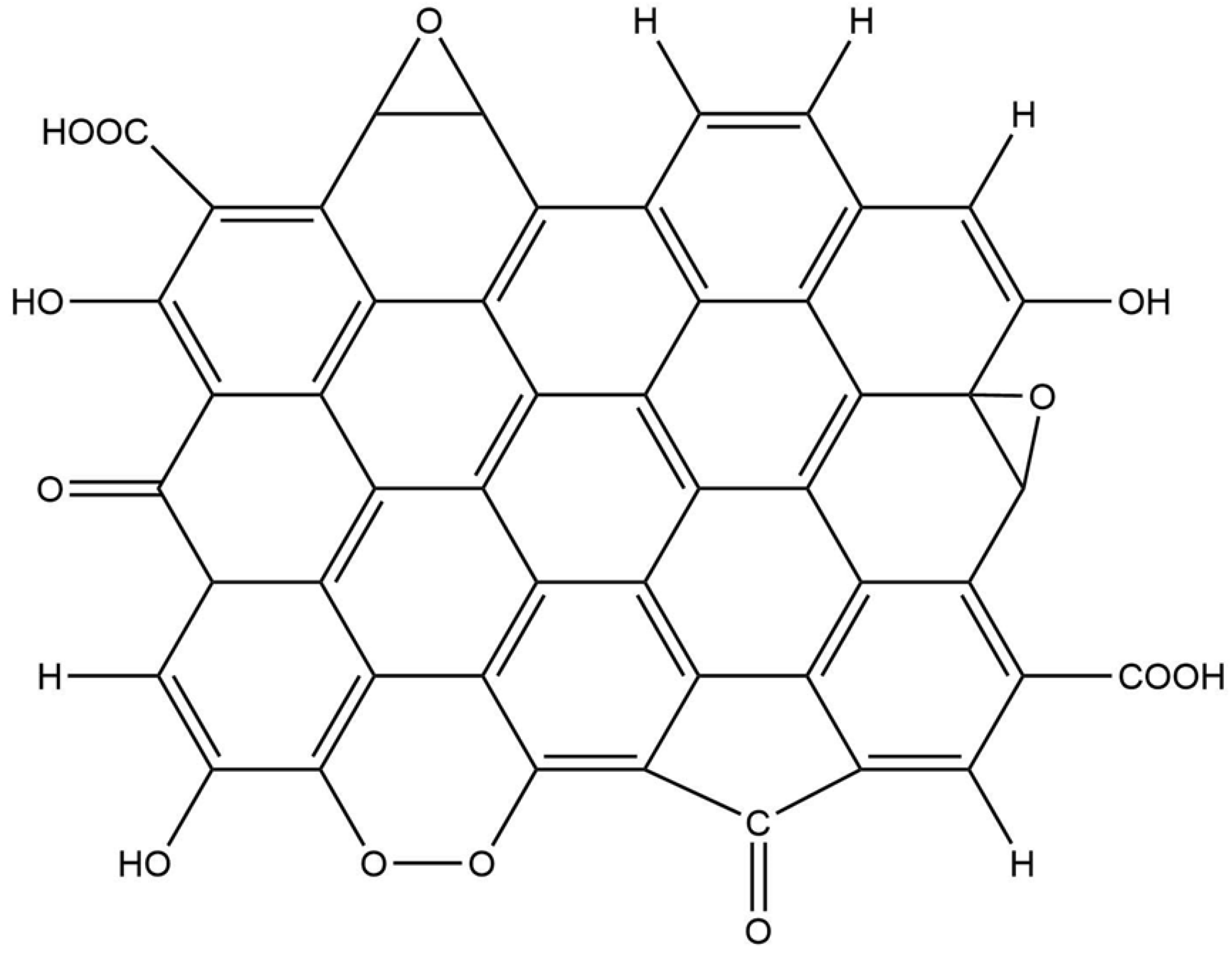
2.1.1. Structure of Amorphous Carbon
2.1.2. Synthesis of Amorphous Carbon
2.1.3. Uses of Amorphous Carbon in Medicine
2.2. Graphite
2.2.1. Discovery of Graphite
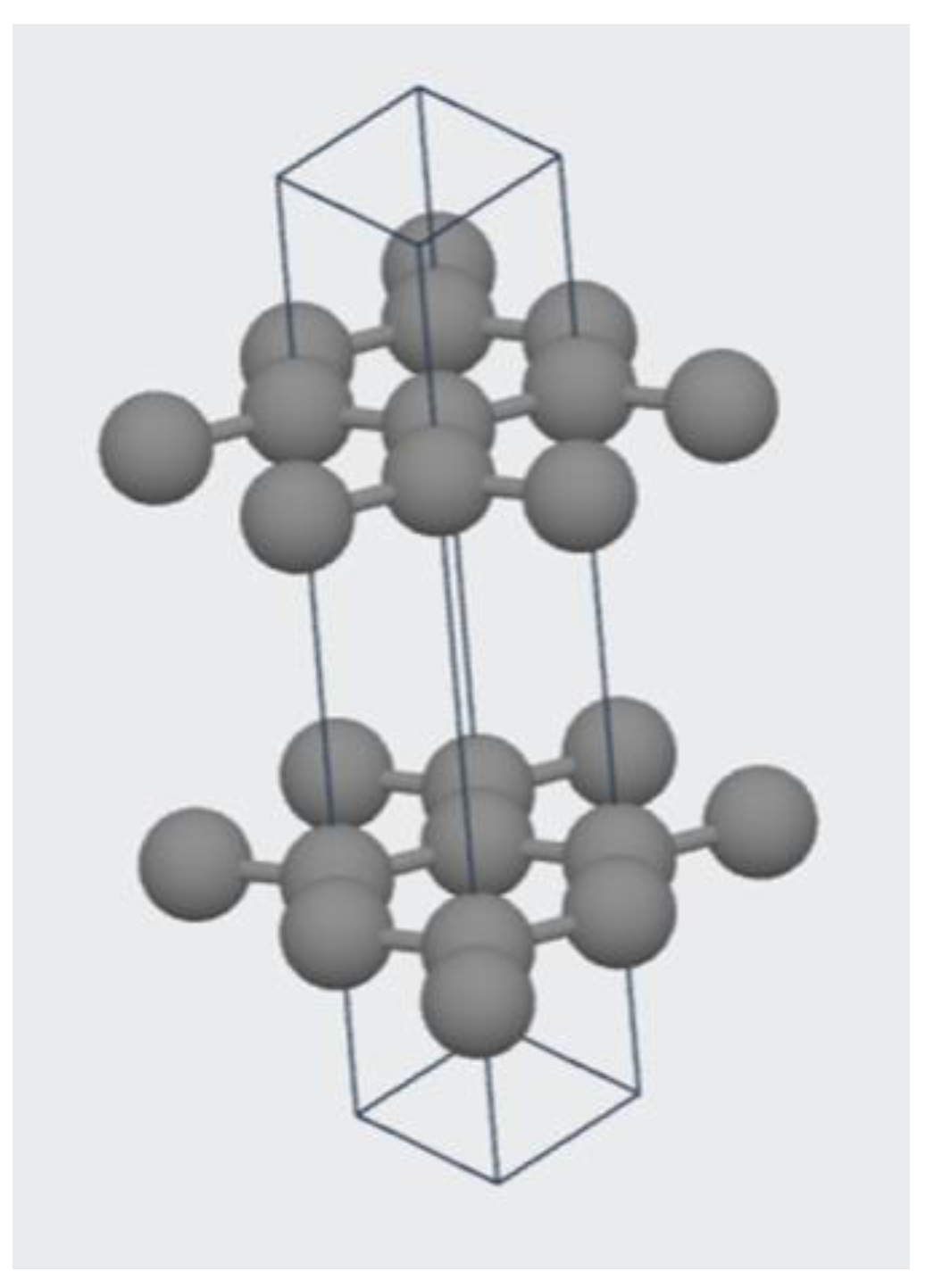
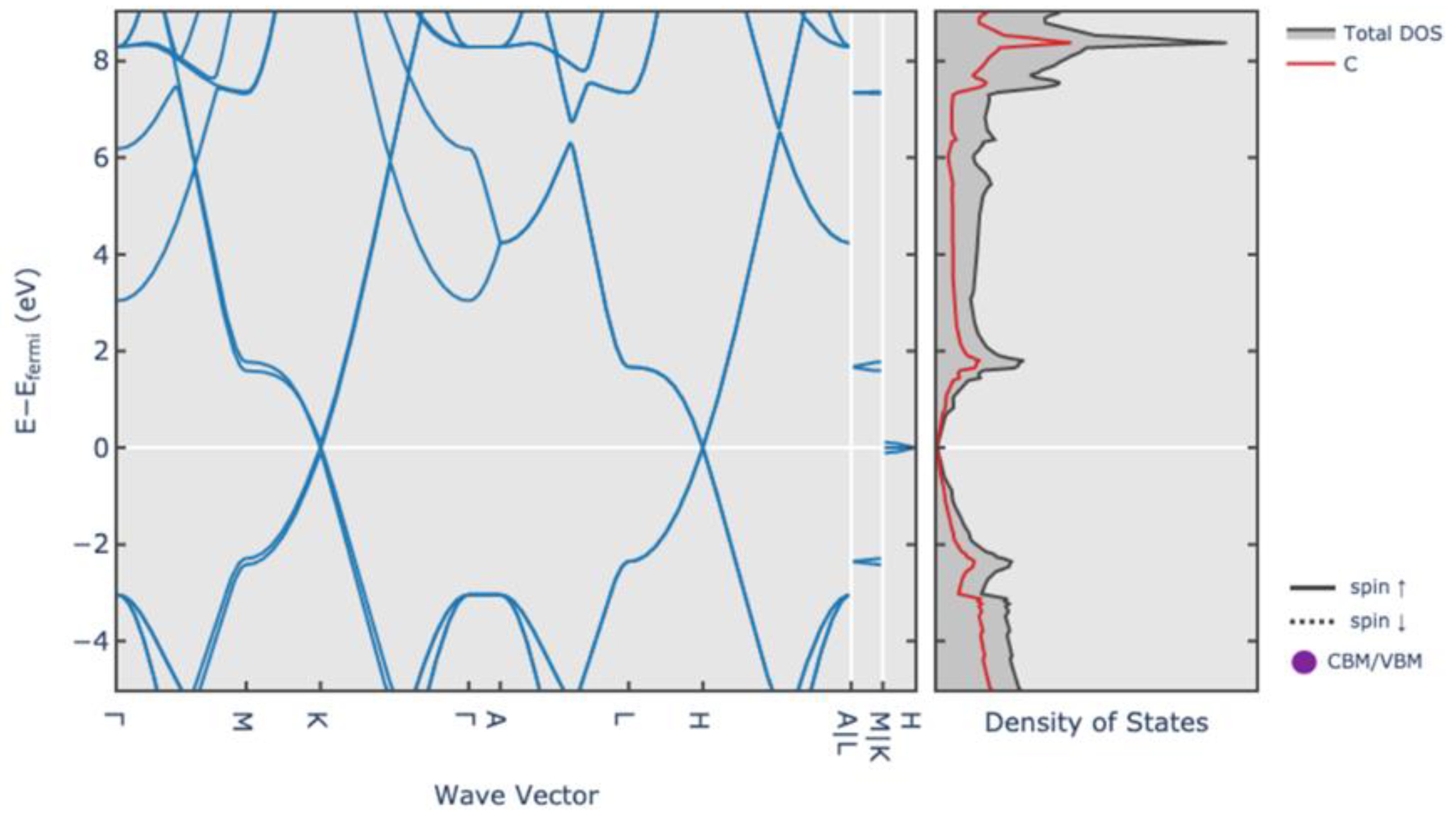
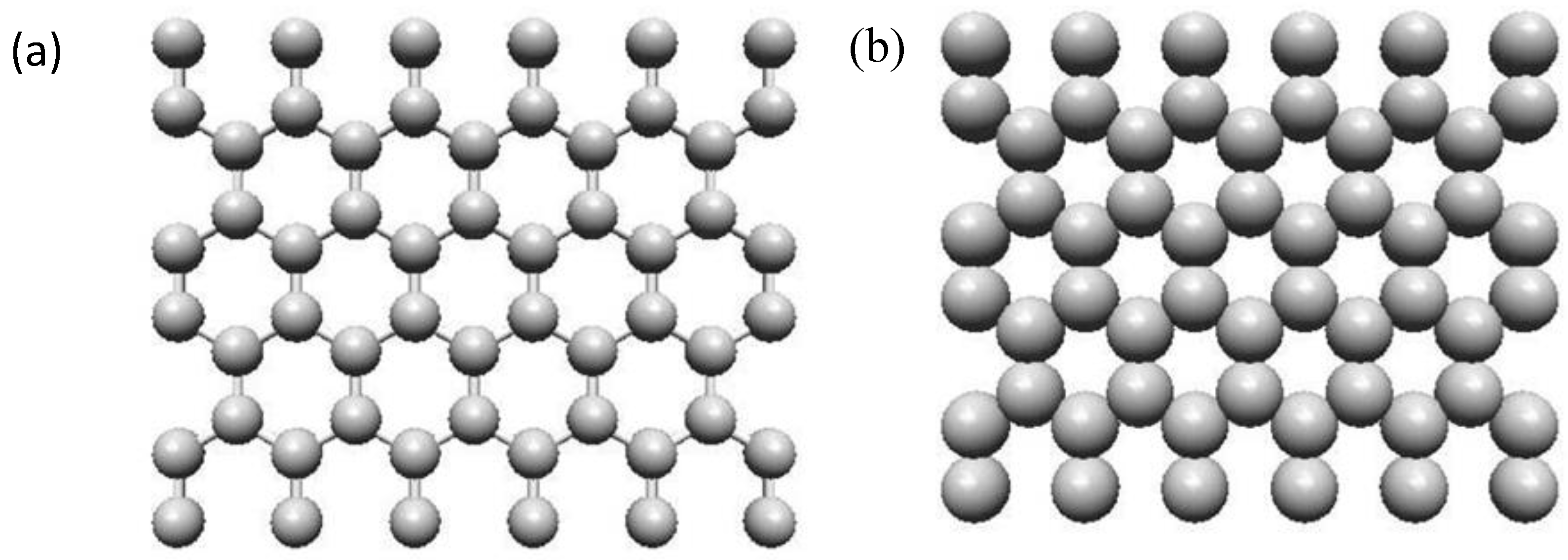
2.2.2. Structure of Graphite
2.2.3. Synthesis of Graphite
2.2.4. Uses of Graphite in Medicine
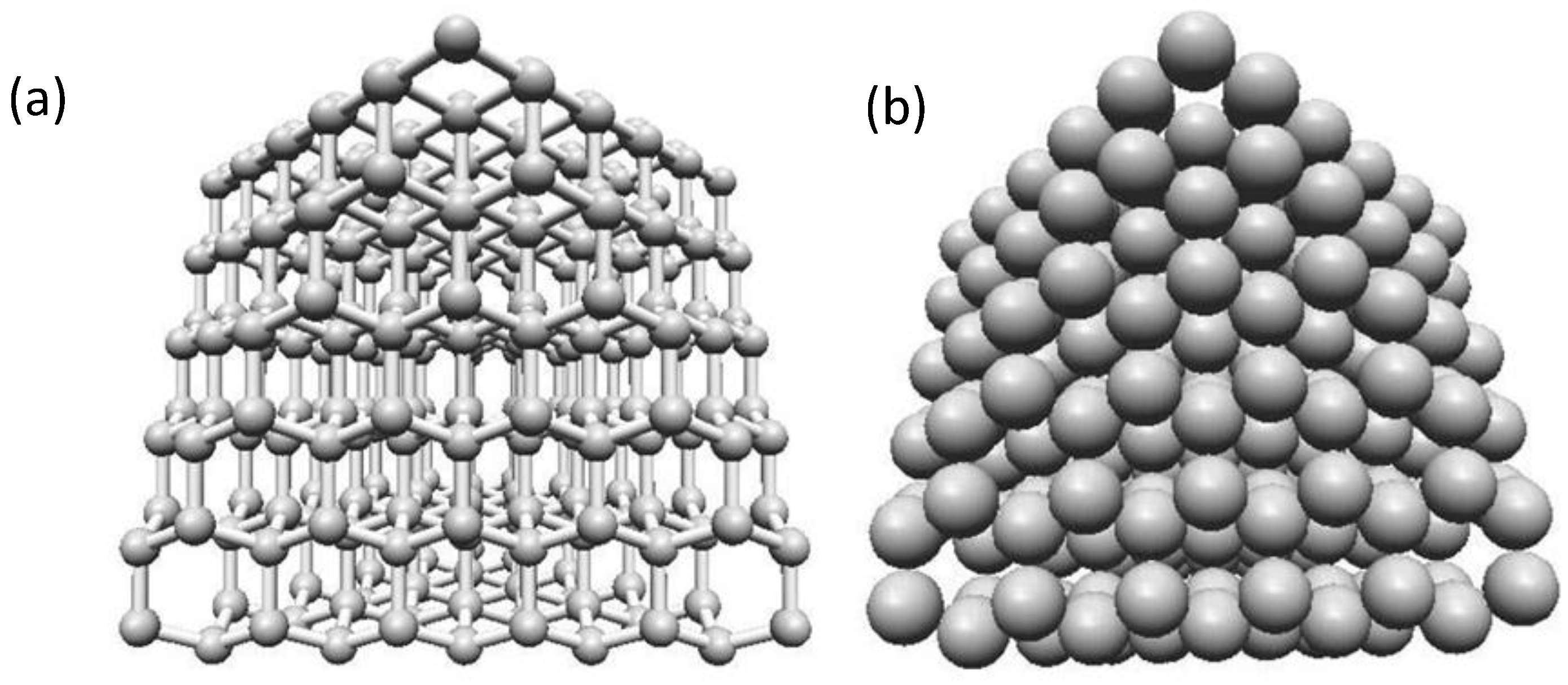
2.3. Carbon Nanocones
2.3.1. Discovery of Carbon Nanocones
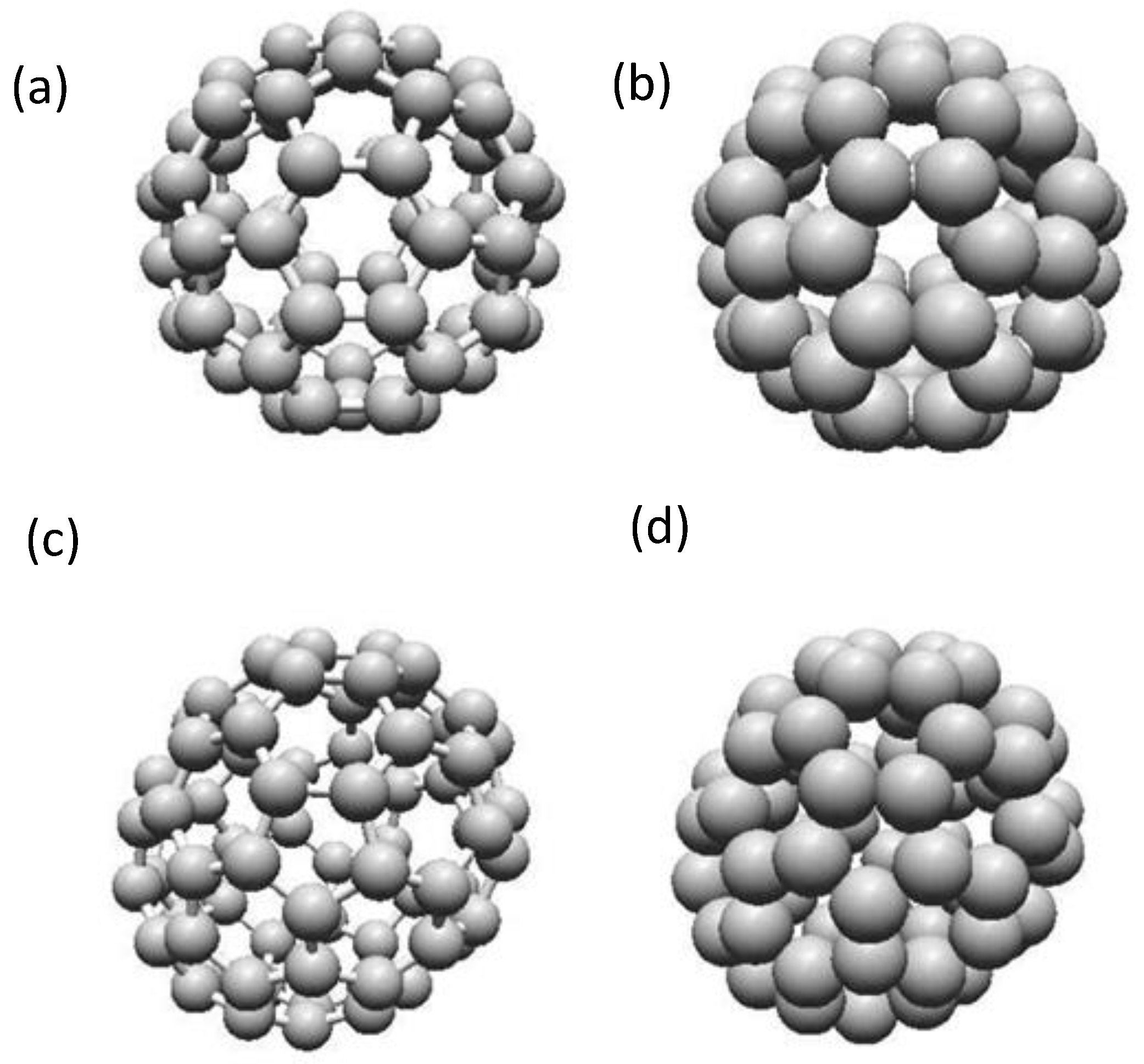
2.3.2. Structure of Carbon Nanocones
2.3.3. Synthesis of Carbon Nanocones
2.3.4. Uses of Carbon Nanocones in Medicine
2.4. Fullerene (C60)
2.4.1. Discovery of Fullerene
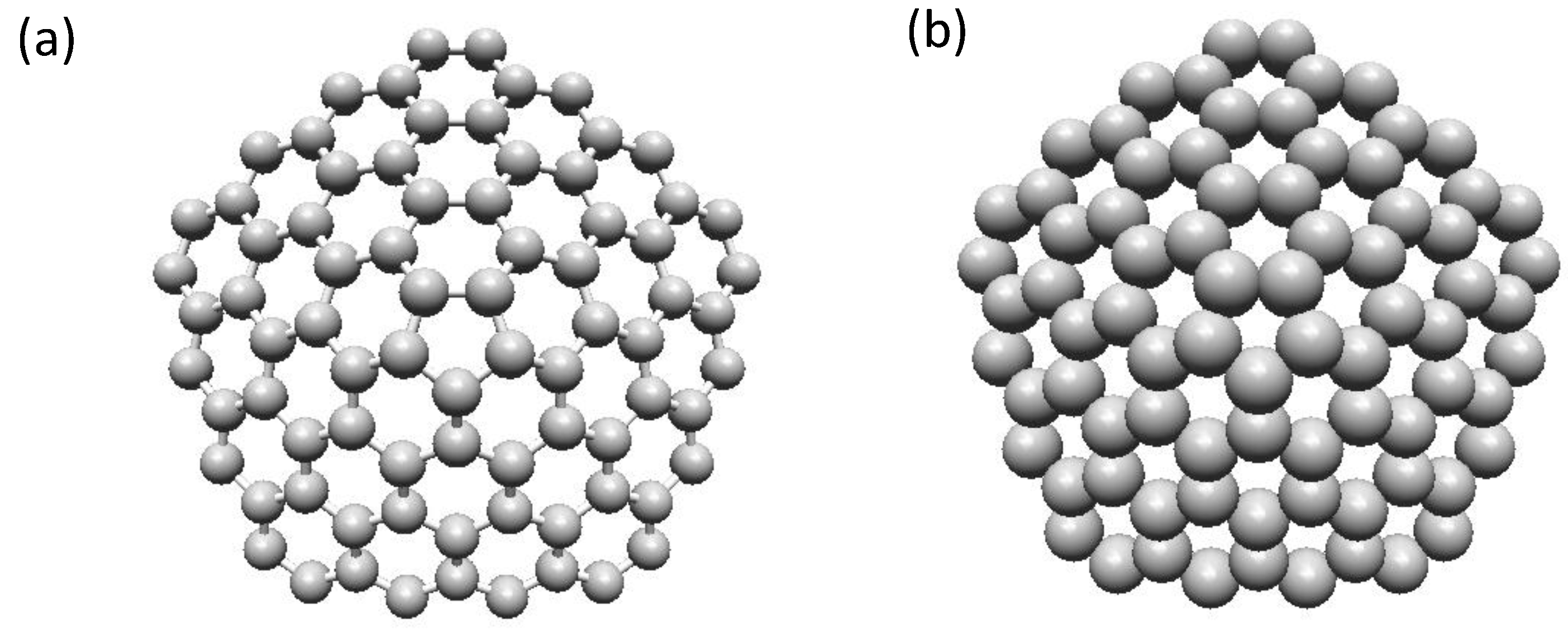
2.4.2. Structure of Fullerene
2.4.3. Synthesis of Fullerene
2.4.4. Uses of Fullerene in Medicine
2.5. Graphene
2.5.1. Discovery and Structure of Graphene
2.5.2. Synthesis of Graphene
2.5.3. Uses of Graphene in Medicine
2.6. Reduced Graphene/Graphite Oxide (RGO)
2.6.1. Synthesis of Reduced Graphene/Graphite Oxides
2.6.2. Structure of Graphene Oxide
2.6.3. Synthesis of Reduced Graphite Oxide
2.6.4. Uses of Graphite Oxide in Medicine
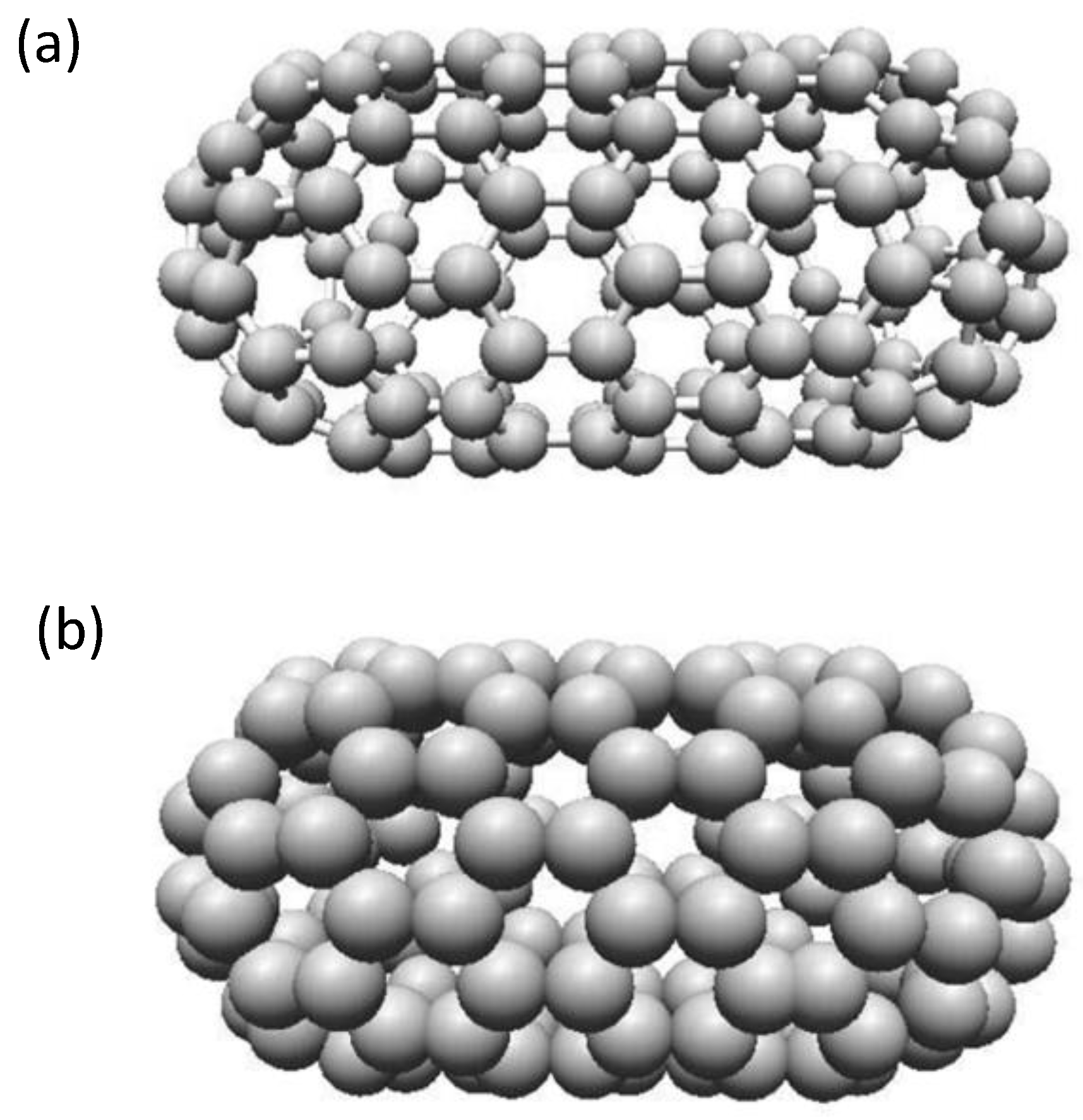
2.7. Single-Walled Carbon Nanotubes (SWCNT) and Multi-Walled Carbon Nanotubes (MWCNTs)
2.7.1. Discovery of Carbon Nanotubes
2.7.2. Structure of Carbon Nanotubes
2.7.3. Synthesis of Carbon Nanotubes
2.7.4. Uses of Carbon Nanotubes in Medicine
2.8. Nanodiamond
2.8.1. Discovery of Nanodiamond
2.8.2. Structure of Nanodiamond
2.8.3. Synthesis of Nanodiamond
2.8.4. Uses of Nanodiamond in Medicine
Challenges
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeinalipour-Yazdi, C. D., and D. P. Pullman,. Journal of Physical Chemistry B 2008, 112, 7377-7386.). “Quantitative Structure - Property Relationships for Longitudinal, Transverse, and Molecular Static Polarizabilities in Polyynes.” Journal of Physical Chemistry B 112 (2008): 7377-86. [CrossRef]
- Zeinalipour-Yazdi, C. D., and C. Christofides. “Linear Correlation between Binding Energy and Young’s Modulus in Graphene Nanoribbons.” Journal of Applied Physics 106 (2009): 054318-23. [CrossRef]
- Zeinalipour-Yazdi, CD. “Electronic Structure and Interlayer Binding Energy of Graphite.” University of California, San Diego and San Diego State University, San Diego, Digital Dissertations (2006): 1-183.
- Akase, Z. Murakami, Y. Nakamura, T. Sergiienko, R. Shibata, E. Shindo, and H. D. Suwa. “Synthesis of Amorphous Carbon Nanoparticles and Carbon Encapsulated Metal Nanoparticles in Liquid Benzene by an Electric Plasma Discharge in Ultrasonic Cavitation Field.” Ultrasonics Sonochemistry 13, no. 1 (2006): 6-12. [CrossRef]
- Falcao, E., and F. Wudl. “Carbon Allotropes: Beyond Graphite and Diamond.” Chemical Technology and Biotechnology 82, no. 6 (2007): 524-41. [CrossRef]
- Rafique, Irum, Ayesha Kausar, Zanib Anwar, and Bakhtiar Muhammad. “Exploration of Epoxy Resins, Hardening Systems and Epoxy/Carbon Nanotube Composite Designed for High Performance Materials: A Review.” Polymer-Plastics Technology and Engineering 55, no. 3 (2019): 312-33. [CrossRef]
- Cai, M, D Thorpe, DH Adamson, and HC Schniepp. “Methods of Graphite Exfoliation.” Journal of Materials Chemistry 22 (2012): 24992-5000. [CrossRef]
- Eid, M. E-S. “Polyethylenimine-Functionalized Magnetic Amorphous Carbon Fabricated from Oil Palm Leaves as a Novel Adsorbent for Hg(Ii) from Aqueous Solutions.” Egyptian Journal of Petroleum 27, no. 4 (2018): 1051-60. [CrossRef]
- Clough, F, J., and S. Paul. “Use of Amorphous Carbon as a Gate Insulator for Gaas and Related Compounds.” Microelectric Engineering 70, no. 1 (2003): 78-82. [CrossRef]
- Zeinalipour-Yazdi, C.D. “A Dft Study of the Interaction of Aspirin, Paracetamol and Caffeine with One Water Molecule.” Journal of Molecular Modeling 28, no. 9 (2022): 285. [CrossRef]
- Miriyala, N, D Ouyang, Y Perrie, D Lowry, and DJ Kirby. “Activated Carbon as a Carrier for Amorphous Drug Delivery: Effect of Drug Characteristics and Carrier Wettability.” Eur J Pharm Biopharm. 115 (2017): 197-205. [CrossRef]
- Barlow, A.J., D.G. McCulloch, D.R. McKenzie, B.J. Murdoch, J.G. Partridge, T.J. Raeber, and Z. C. Zhao. “Light-Gated Amorphous Carbon Memristors with Indium-Free Transparent Electrodes.” Carbon 152 (2019): 59-65. [CrossRef]
- Zhang, Meng, Xuexhang Xiao, Jianfeng Mao, Zhenyun Lan, Xu Huang, Yunhao Lu, Bosang Luo, Meijia Liu, Man Chen, and Lixin Chen. “Synergistic Catalysis in Monodispersed Transition Metal Oxide Nanoparticles Anchored on Amorphous Carbon for Excellent Low-Temperature Dehydrogenation of Magnesium Hydride.” Materials Today Energy 12 (2019): 146-54. [CrossRef]
- Zeinalipour-Yazdi, Constantinos. Supervisor: David P. Pullman Electronic Structure and Interlayer Binding Energy of Graphite. Digital Dissertations, 2006. [CrossRef]
- CD Zeinalipour-Yazdi, DP Pullman. “A New Interpretation of the Scanning Tunneling Microscope Image of Graphite.” Chemical Physics 348, no. 1-3 (2008): 233-36. [CrossRef]
- Zeinalipour-Yazdi, C. D., and D. P. Pullman. “Study of Rhombohedral Graphite X-Ray Filter Using the Sphere-in-Contact Model.” Chem. Phys. Lett. 734 (2019): 136717. [CrossRef]
- “Persson, Kristin, Materials Data on C (Sg:194) by Materials Project, 7/2014, Https://Materialsproject.Org/Docs/Calculations. [CrossRef]
- Jain, A., S.P. Ong, G. Hautier, W. Chen, W.D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder, and K.A. Persson. “The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation.” APL Materials 1, no. 1 (2013): 011002. [CrossRef]
- Binnig, Gerd, and Heinrich Rohrer. “Scanning Tunneling Microscopy—from Birth to Adolescence.” Rev. Mod. Phys. 59 (1987): 615. [CrossRef]
- Zhang, J.-r., Y.-s. Kan, L.-l. Gu, C.-y. Wang, and Y. Zhang. “Graphite Carbon Nitride and Its Composites for Medicine and Health Applications.” Chem. Asian J. 16 (2021): 2003. [CrossRef]
- Narjabadifam, Ali, Farid Vakili-Tahami, and Mohammad Zehsaz. “Modal Analysis of Multi-Walled Carbon Nanocones Using Molecular Dynamics Simulation.” Computational Materials Science 137 (2017): 55-66. [CrossRef]
- Naess, Stine Nalum, Arnljot Elgsaeter, Geir Helgesen, and Kenneth D Knudsen. “Carbon Nanocones: Wall Structure and Morphology.” Science and Technology of Advanced Materials 10, no. 6 (2009): 065002. [CrossRef]
- Ge, Sattler. “Observation of Fullerene Cones.” Chemical Physics Letters 220 (1994): 192-96. [CrossRef]
- Ardeshana, Bhavik A., Umang B. Jani, Ajay M. Patel, and Anand Y. Joshi. “Characterizing the Vibration Behavior of Double Walled Carbon Nano Cones for Sensing Applications.” Materials Technology 33 (2018): 451-66. [CrossRef]
- Charlier, Jean-Christophe, and Gian-Marco Rignanese. “Electronic Structure of Carbon Nanocones.” Physical Review Letters 86 (2001): 5970-73. [CrossRef]
- El-Barbary, A.A., M.A. Kamel, M.A. Eid, H.O. Taha, R.A. Mohamed, and M.A. Al-Khateeb. “The Surface Reactivity of Pure and Monohydrogenated Nanocones Formed from Graphene Sheets.” Graphene 4 (2015): 75-83. [CrossRef]
- Ansari, Mahmoudinezhad. “Characterizing the Mechanical Properties of Carbon Nanocones Using an Accurate Spring-Mass Model.” Computational Materials Science 101 (2015): 260-66. [CrossRef]
- Kroto, H. W., J. R. Heath, S. C. O’Brien, R. F. Curl, and R. E. Smalley. “C60: Buckminsterfullerene.” Nature 318 (1985): 162-63.
- Smalley, R. E. “Discovering the Fullerenes, Nobel Lecture. Chemistry.” (1996): 89-103. [CrossRef]
- Krätschmer, W., Lowell D. Lamb, K. Fostiropoulos, and Donald R. Huffman. “Solid C60: A New Form of Carbon.” Nature 347 (1990): 354-58. [CrossRef]
- Voicu, I., X. Armand, M. Cauchetier, N. Herlin, and S. Bourcier. “Laser Synthesis of Fullerenes from Benzene-Oxygen Mixtures.” Chemical Physics Letters 256, no. 3 (1996): 261-68. [CrossRef]
- Howard, J.B., J.T. McKinnon, Y. Makarovsky, A.L. Lafleur, and M.E. Johnson. “Fullerenes C60 and C70 in Flames.” Nature 352, no. 6331 (1991): 139-41. [CrossRef]
- R, Bakry, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, and Bonn GK. “Medicinal Applications of Fullerenes.” Int J Nanomedicine 2, no. 4 (2007): 639-49.
- Chen, Z., Mao R., and Liu Y. “Fullerenes for Cancer Diagnosis and Therapy: Preparation, Biological and Clinical Perspectives.” Current Drug Metabolism 13, no. 8 (2012): 1035-45. [CrossRef]
- Somani, Rakesh, and Madhura Gokhale. “Fullerenes: Chemistry and It’s Applications.” Mini Reviews in Organic Chemistry 12 (2015): 1-12. [CrossRef]
- Grushko, Y. S., V. P. Sedov, and V. A. Shilin. “Technology for Manufacture of Pure Fullerenes C60, C70 and a Concentrate of Higher Fullerenes.” Russian Journal of Applied Chemistry (2007): 448-55. [CrossRef]
- Vidal, S., J. Marco-Martínez, S. Filippone, and N. Martín. “Fullerenes for Catalysis: Metallofullerenes in Hydrogen Transfer Reactions.” Chem. Commun. 53 (2017): 4842-44. [CrossRef]
- Lieber, C. M., and C.-C. Chen. “Preparation of Fullerenes and Fullerene-Based Materials.” Solid State Physics 48 (1994): 109-48. [CrossRef]
- Nimibofa, Ayawei, Ebelegi Augustus Newton, Abasi Yameso Cyprain, and Wankasi Donbebe. “Fullerenes: Synthesis and Applications.” Journal of Materials Science Research 7 (2018): 22-36. [CrossRef]
- Yang, S., C. Pettiette, J. Conceição, O. Cheshnovsky, and R. Smalley. “Ups of Buckminsterfullerene and Other Large Clusters of Carbon.” Chemical Physics Letters 589 (2013): 31-34. [CrossRef]
- Casadei, N., M. Mireille, Y. Guillaume, and C. André. “A Humic Acid Stationary Phase for the High Performance Liquid Chromatography Separation of Buckminsterfullerenes: Theoretical and Practical Aspects.” Analytica Chimica Acta 588 (2007): 268-73. [CrossRef]
- Kroto, W. H. “The Stability of the Fullerenes Cn, with N = 24, 28, 32, 36, 50, 60 and 70.” Nature 329 (1987): 529-31.
- Dreyer, D.R., R.S. Ruoff, and C.W. Bielawski. “From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future.” Angew. Chem. Int. Ed. 49 (2010): 9336-44. [CrossRef]
- Prekodravac, Jovana R., Dejan P. Kepić, Juan Carlos Colmenares, Dimitrios A Giannakoudakis, and Svetlana P. Jovanović. “A Comprehensive Review on Selected Graphene Synthesis Methods: From Electrochemical Exfoliation through Rapid Thermal Annealing Towards Biomass Pyrolysis.” J. Mater. Chem. C 9, no. 21 (2021): 6722-48. [CrossRef]
- Yuan, L., J. Ge, X. Peng, Q. Zhang, Z. Wu, Y. Jian, X. Xiong, H. Yin, and J. Han. “A Reliable Way of Mechanical Exfoliation of Large Scale Two Dimensional Materials with High Quality.” AIP advances 6 (2016): 125201. [CrossRef]
- Bharech, S., and R. Kumar. “A Review on the Properties and Applications of Graphene.” Journal of Material Science and Mechanical Engineering 2 (2015): 70-73.
- Neto, A. H. Castro, F. Guinea, N. M. R. Peres, K. S. Novoselov, and A. K. Geim. “The Electronic Properties of Graphene.” Review of modern physics 81 (2009): 109.
- Balandin, Alexander A. “Thermal Properties of Graphene and Nanostructured Carbon Materials.” Nature Materials 10 (2011): 569-81. [CrossRef]
- Ferrari, AC, and DM Basko. “Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene.” Nature nanotechnology 8 (2013): 235-46. [CrossRef]
- Homaeigohar, S., and M. Elbahri. “Graphene Membranes for Water Desalination.” NPG Asia materials 9 (2017): e417. [CrossRef]
- Kuila, T, S Bose, AK Mishra, P Khanra, NH Kim, and JH Lee. “Chemical Functionalization of Graphene and Its Applications, Progress in Materials Science.” Progress in Materials Science 57 (2012): 1061-105. [CrossRef]
- Georgakilas, V, M Otyepka, AB Bourlinos, V Chandra, N Kim, KC Kemp, P Hobza, R Zboril, and KS Kim. “Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications.” Chemical Reviews 112 (2012): 6156-214. [CrossRef]
- Wei, W, and X Qu. “Extraordinary Physical Properties of Functionalized Graphene.” Small 8, no. 14 (2012): 2138-51. [CrossRef]
- Lü, P., Y. Feng, X. Zhang, Y. Li, and W. Feng. “Recent Progresses in Application of Functionalized Graphene Sheets.” Science China Technological Sciences 53, no. 9 (2010): 2311-19. [CrossRef]
- Abdullaeva, Z., Z. Kelgenbaeva, T. Masayuki, M. Hirano, S. Nagaoka, and T. Shirosaki. “Graphene Sheets with Modified Surface by Sodium Lauryl Sulfate Surfactant for Biomedical Applications.” Graphene 5, no. 4 (2016). [CrossRef]
- Gadakh, D., P. Dashora, and G. Wadhankar. “A Review Paper on Graphene Coated Fibres.” Graphene 8, no. 4 (2019): 53-74. [CrossRef]
- Atif, R., and F. Inam. “The Dissimilarities between Graphene and Frame-Like Structures.” Graphene 5 (2016): 55-72. [CrossRef]
- Randviir, E. P., D. A. C. Brownson, and C. E. Banks. “A Decade of Graphene Research: Production, Applications and Outlook.” Materials Today Energy 17, no. 9 (2014): 426-32.
- Dimitrakopoulos, C., and P. Avouris. “Graphene: Synthesis and Applications.” Materials Today Energy 15, no. 3 (2012): 86-97. [CrossRef]
- Adetayo, A., and D. Runsewe. “Synthesis and Fabrication of Graphene and Graphene Oxide: A Review.” Open Journal of Composite Materials, 9 (2019): 207-29. [CrossRef]
- Reshma, S., and P. Mohanan. “Graphene: A Multifaceted Nanomaterial for Cutting Edge Biomedical Application.” International Journal of Medical Nano Research, no. 1 (2014): 1-6.
- Cai, X., L. Lai, Z. Shen, and J. Lin. “Graphene and Graphene-Based Composites as Li-Ion Battery Electrode Materials and Their Application in Fuel Cells.” Journal of Materials Chemistry A 5 (2017): 15423-46. [CrossRef]
- Pumera, M. “Graphene in Biosensing.” Materials Today 14, no. 7-8 (2011): 308-15. [CrossRef]
- Avouris, P., and F. Xia. “Graphene Applications in Electronics and Photonics. Graphene Fundamentals and Functionality.” Cambridge University Press 37, no. 12 (2012): 1225-34. [CrossRef]
- Dreyer, D.R., R.S. Ruoff, and C.W. Bielawski. “From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future.” Angew. Chem. Int. Ed. 49 (2010): 9336-44. [CrossRef]
- Cote, L., F. Kim, and J. Huang. “Langmuir−Blodgett Assembly of Graphite Oxide Single Layers.” Journal of the American Chemical Society 131, no. 3 (2009): 1043-49.
- Wu, S., T. Shi, and L. Zhang. “Preparation and Properties of Amine-Functionalized Reduced Graphene Oxide/Waterborne Polyurethane Nanocomposites.” High Performance Polymers 28, no. 4 (2015): 453-65. [CrossRef]
- Marcano, D., D. Kosynkin, J. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. Alemany, W. Lu, and J. Tour. “Improved Synthesis of Graphene Oxide.” ACS Nano 4, no. 8 (2010): 4806-14. [CrossRef]
- Marcano, Daniela C., Dmitry V. Kosynkin, Jacob M. Berlin, Alexander Sinitskii, Zhengzong Sun, Alexander S. Slesarev, Lawrence B. Alemany, Wei Lu, and James M. Tour. “Correction to Improved Synthesis of Graphene Oxide.” ACS Nano 12, no. 2 (2018): 2078-78. [CrossRef]
- Pei, S., and H. Cheng. “The Reduction of Graphene Oxide.” Carbon 50, no. 9 (2012): 3210-28.
- Dideikin, A., and A. Vul’. “Graphene Oxide and Derivatives: The Place in Graphene Family.” Frontiers in Physics 6 (2019): 1-13. [CrossRef]
- Gómez-Navarro, C., J. Meyer, R. Sundaram, A. Chuvilin, S. Kurasch, M. Burghard, K. Kern, and U. Kaiser. “Atomic Structure of Reduced Graphene Oxide.” Nano Letters 10, no. 4 (2010): 1144-48. [CrossRef]
- Hidayah, N., W. Liu, C. Lai, N. Noriman, C. Khe, U. Hashim, and H. Lee. “Comparison on Graphite, Graphene Oxide and Reduced Graphene Oxide: Synthesis and Characterization.” AIP Conf. Proc. 1892 (2017): 150002. [CrossRef]
- Yang, H., Y. Cao, J. He, Y. Zhang, B. Jin, J. Sun, Y. Wang, and Z. Zhao. “Highly Conductive Free-Standing Reduced Graphene Oxide Thin Films for Fast Photoelectric Devices.” Carbon 115 (2017): 561-70. [CrossRef]
- Smith, A., A. LaChance, S. Zeng, B. Liu, and L. Sun. “Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites.” Nano Materials Science 1, no. 1 (2019): 31-47. [CrossRef]
- Iijima, S. “Helical Microtubules of Graphitic Carbon.” Nature 354 (1991): 56-58. [CrossRef]
- Radushkevich, L.V., and V.M. Lukyanovich. “The Structure of Carbon Forming in Thermal Decomposition of Carbon Monoxide on an Iron Catalyst.” Russian Journal of Physical Chemistry 26 (1952): 88-95.
- Iijima, S., and T. Ichihashi. “Single-Shell Carbon Nanotubes of 1-Nm Diameter.” Nature 363 (1993): 603-05. [CrossRef]
- Bethune, D. S., C. H. Kiang, M. S. de Vries, G. Gorman, R. Savoy, J. Vazquez, and R. Beyers. “Cobalt-Catalysed Growth of Carbon Nanotubes with Single-Atomic-Layer Walls.” Nature 363 (1993): 605-07. [CrossRef]
- Monthioux, M., and V. Kuznetsov. “Who Should Be Given the Credit for the Discovery of Carbon Nanotubes?” Carbon (2006): 1621-23.
- Deshpande, P., and A. Mahendru. “A Review of Single Wall Carbon Nanotube: Structure and Preparation.” International Journal of Scientific and Technology Research (2018): 132-34.
- S, Karthikeyan, Mahalingam P, and Karthik M. “Large Scale Synthesis of Carbon Nanotubes.” Journal of chemistry 6, no. 1 (2008): 1-12. [CrossRef]
- Sun, D. L., R. Y. Hong, J. Y. Liu, F. Wang, and Y. F. Wang. “Preparation of Carbon Nanomaterials Using Two-Group Arc Discharge Plasma.” Chemical Engineering Journal 303 (2016): 217–30. [CrossRef]
- Venkataraman, A., E. Victoria Amadi, Y. Chen, and C. Papadopoulos. “Carbon Nanotube Assembly and Integration for Applications.” Nanoscale Res. Lett. 14, no. 220 (2019): 1-47. [CrossRef]
- X, Zhu, Xie Y, Zhang Y, Huang H, Huang S, Hou L, Zhang H, Li Z, Shi J, and Zhang Z. “Thermo-Sensitive Liposomes Loaded with Doxorubicin and Lysine Modified Single-Walled Carbon Nanotubes as Tumor-Targeting Drug Delivery System.” J Biomater Appl. 29, no. 5 (2014): 769-79.
- Anzar, Nigar, Rahil Hasan, Manshi Tyagi, Neelam Yadav, and Jagriti Narang. “Carbon Nanotube - a Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science.” Sensors International 1 (2020). [CrossRef]
- A, Burke, Ding X, Singh R, Kraft RA, Levi-Polyachenko N, Rylander MN, Szot C, Buchanan C, Whitney J, Fisher J, Hatcher HC, D’Agostino R Jr, Kock ND, Ajayan PM, Carroll DL, Akman S, Torti FM, and Torti SV. “Long-Term Survival Following a Single Treatment of Kidney Tumors with Multiwalled Carbon Nanotubes and near-Infrared Radiation.” Proc Natl Acad Sci USA 106, no. 31 (2009): 12897-902. [CrossRef]
- H, He, Pham-Huy LA, Dramou P, Xiao D, Zuo P, and Pham-Huy C. “Carbon Nanotubes: Applications in Pharmacy and Medicine.” Biomed Res Int. 578290 (2013): 1-12.
- Danilenko, V.V. “On the History of the Discovery of Nanodiamond Synthesis.” Phys. Solid State 46 (2004): 595-99. [CrossRef]
- Narayan, J., and A. Bhaumik. “Research Update: Direct Conversion of Amorphous Carbon into Diamond at Ambient Pressures and Temperatures in Air.” APL Materials 3, no. 10 (2015): 100702. [CrossRef]
- Xu, Jingru, and Edward Kai-Hua Chow. “Biomedical Applications of Nanodiamonds: From Drug-Delivery to Diagnostics.” SLAS Technology 28, no. 4 (2023): 214-22. [CrossRef]
- Malode, S.J., Pandiaraj S., Alodhayb A., Shetti N.P., “Carbon Nanomaterials for Biomedical Applications: Progress and Outlook” ACS Applied Bio Materials 7, no. 2 (2024): 752-777. [CrossRef]
| Synthesis Method | Key Features | Cost | Scalability | Purity | Relevance to Medical Applications | Challenges |
|---|---|---|---|---|---|---|
| Chemical Vapor Deposition (CVD) | Produces high-quality carbon nanotubes (CNTs) and graphene with precise control over material properties. | High | Moderate | High | Ideal for biomedical applications requiring high purity, such as biosensors and advanced neural interfaces. | High cost and precise control required for optimal results. |
| Pyrolysis of Hydrocarbons | Thermal decomposition of hydrocarbons (e.g., methane) to produce amorphous carbon or graphene at scale. | Low | High | Moderate | Cost-effective approach for creating nanomaterials for coatings, drug delivery systems, and composite materials. | Impurities and limited control over structure. |
| Laser Ablation | Uses high-intensity laser pulses to vaporize graphite, yielding high-purity graphene or nanotubes. | Very High | Low | Very High | Suitable for advanced medical applications, such as prosthetics, imaging agents, and sensors requiring high purity. | High cost and low scalability limit industrial use. |
| Scotch Tape Technique | Mechanical exfoliation of graphite layers to produce graphene manually in small quantities. | Very Low | Very Low | Very High | Historically significant and used for research-level applications, such as early biosensing studies. | Labor-intensive and impractical for large-scale production. |
| Graphite Intercalation | Inserting molecules (e.g., acids, potassium chlorate) between graphite layers to create graphite oxide. | Moderate | Low to Moderate | Moderate to High | Early method for creating graphene oxide, paving the way for biomedical coatings, functional materials, and composites. | Chemical reactions can generate hazardous byproducts. |
| Electric Arc Discharge | High-energy discharge between graphite electrodes produces carbon nanotubes and fullerene structures. | Moderate | Low | High | Used for imaging and diagnostics due to high-quality nanotubes, but applications are still in research stages. | Limited scalability and energy-intensive process. |
| Laser Vaporization | High-powered lasers vaporize graphite targets to produce nanotubes or graphene nanomaterials. | Very High | Low | Very High | Suitable for specific medical devices requiring precise structural purity and uniformity, such as advanced implants. | Expensive and unsuitable for large-scale production. |
| Solar Energy Focusing | Concentrates solar energy to achieve high temperatures for producing carbon nanomaterials sustainably. | Low | Low to Moderate | Moderate | A promising eco-friendly method for producing materials for drug delivery systems and medical composites. | Limited control over structure and requires constant sunlight. |
| Laser-Induced Melting | Melts and restructures carbon materials using high-intensity lasers, producing novel nanostructures. | High | Low | High | Can create specialized nanomaterials for high-end biomedical applications, such as optical imaging tools. | Requires advanced equipment and expertise. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




